Towards Automated and High-Throughput Quantitative Sizing and Isotopic Analysis of Nanoparticles via Single Particle-ICP-TOF-MS
Abstract
1. Introduction
2. Experimental
2.1. Materials & Reagents
2.2. Multi-Collector–Inductively Coupled Plasma–Mass Spectrometry
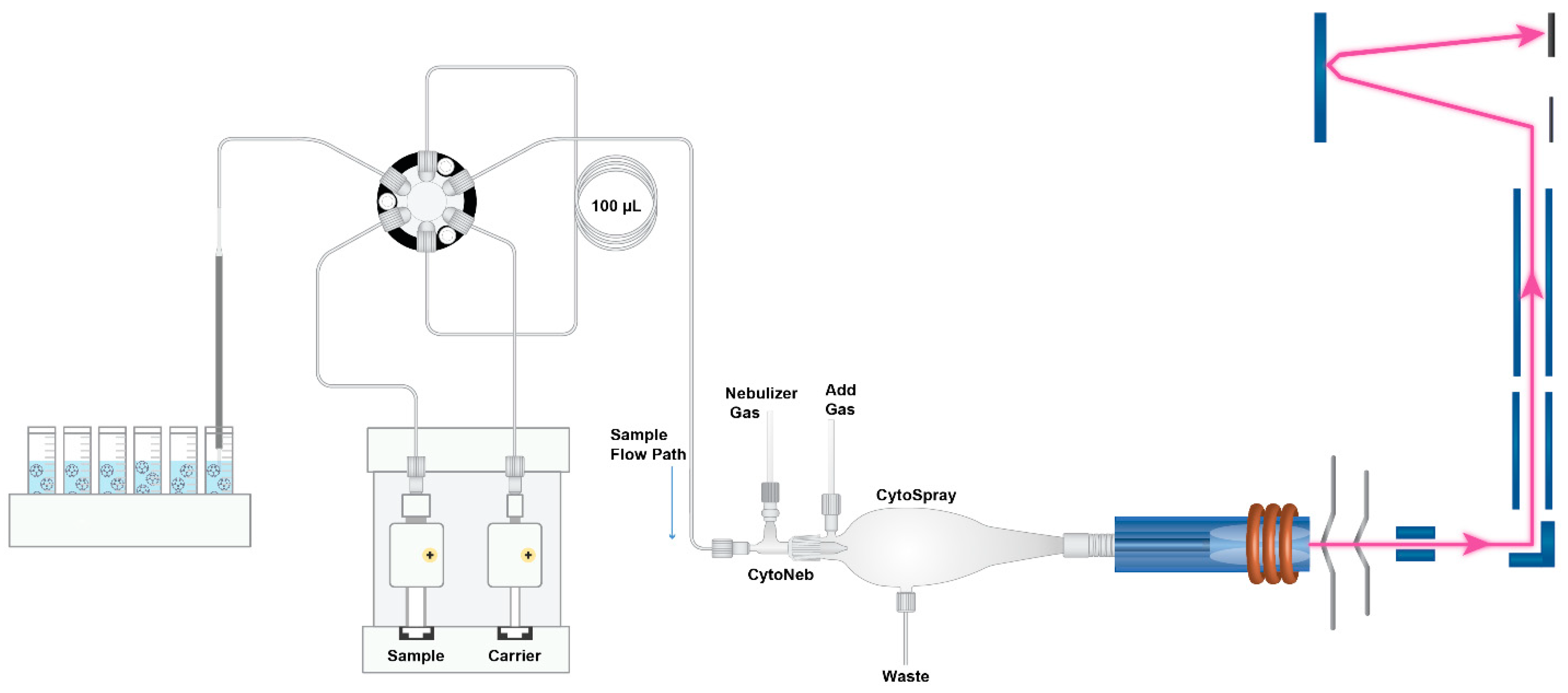
2.3. Single Particle–Inductively Coupled Plasma–Time of Flight–Mass Spectrometry
3. Results and Discussion
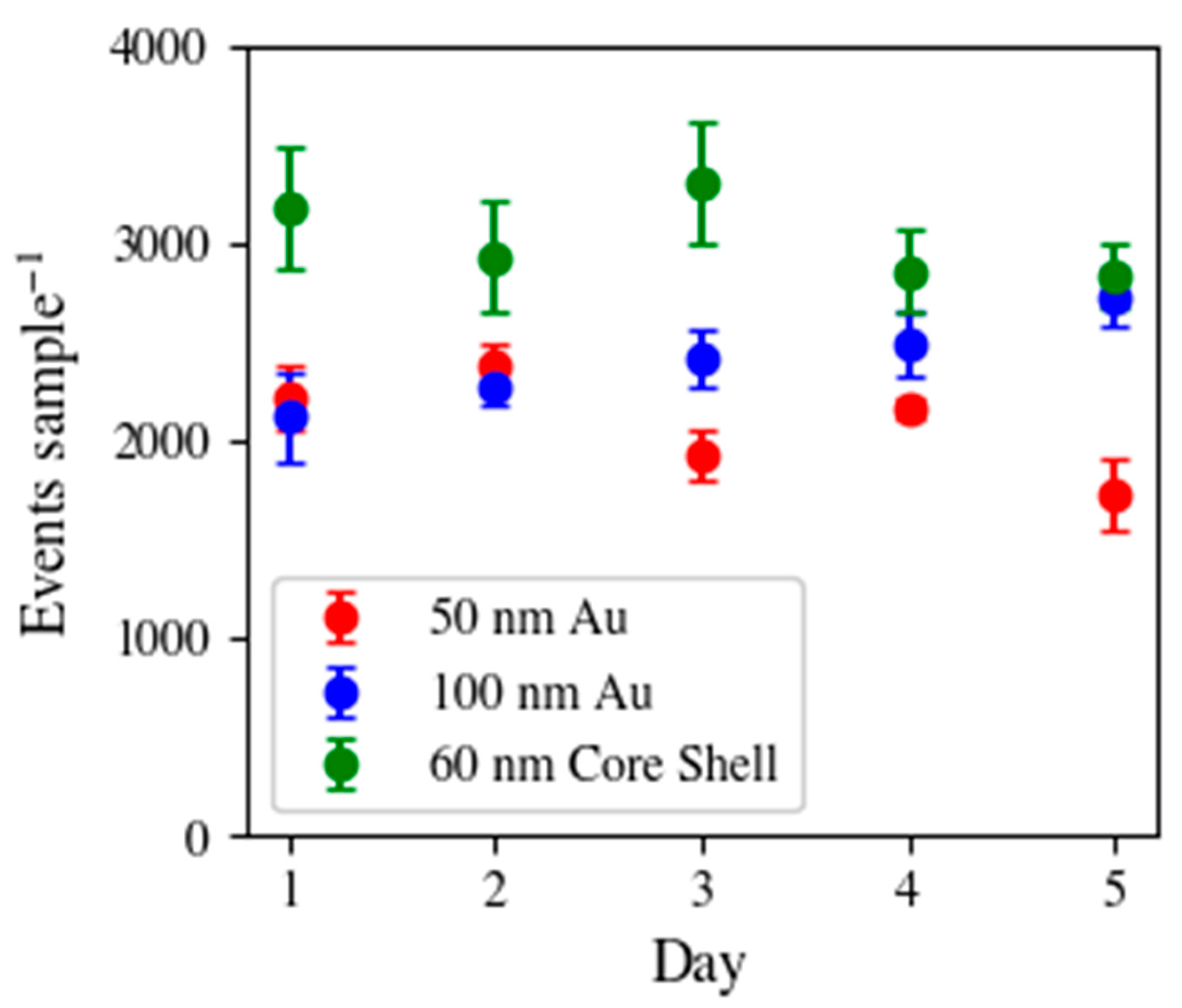
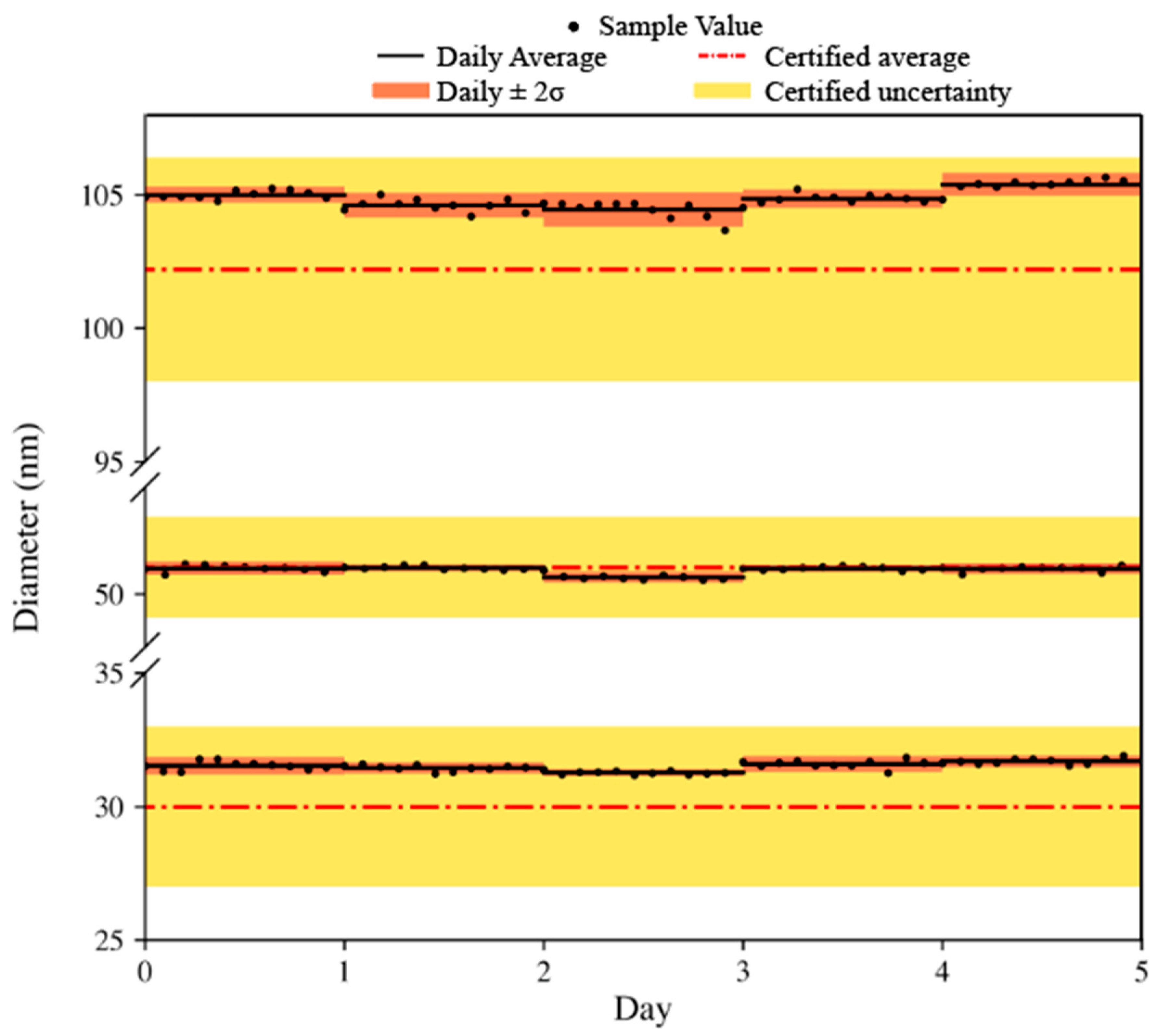
3.1. Reproducibility of Detected Particles
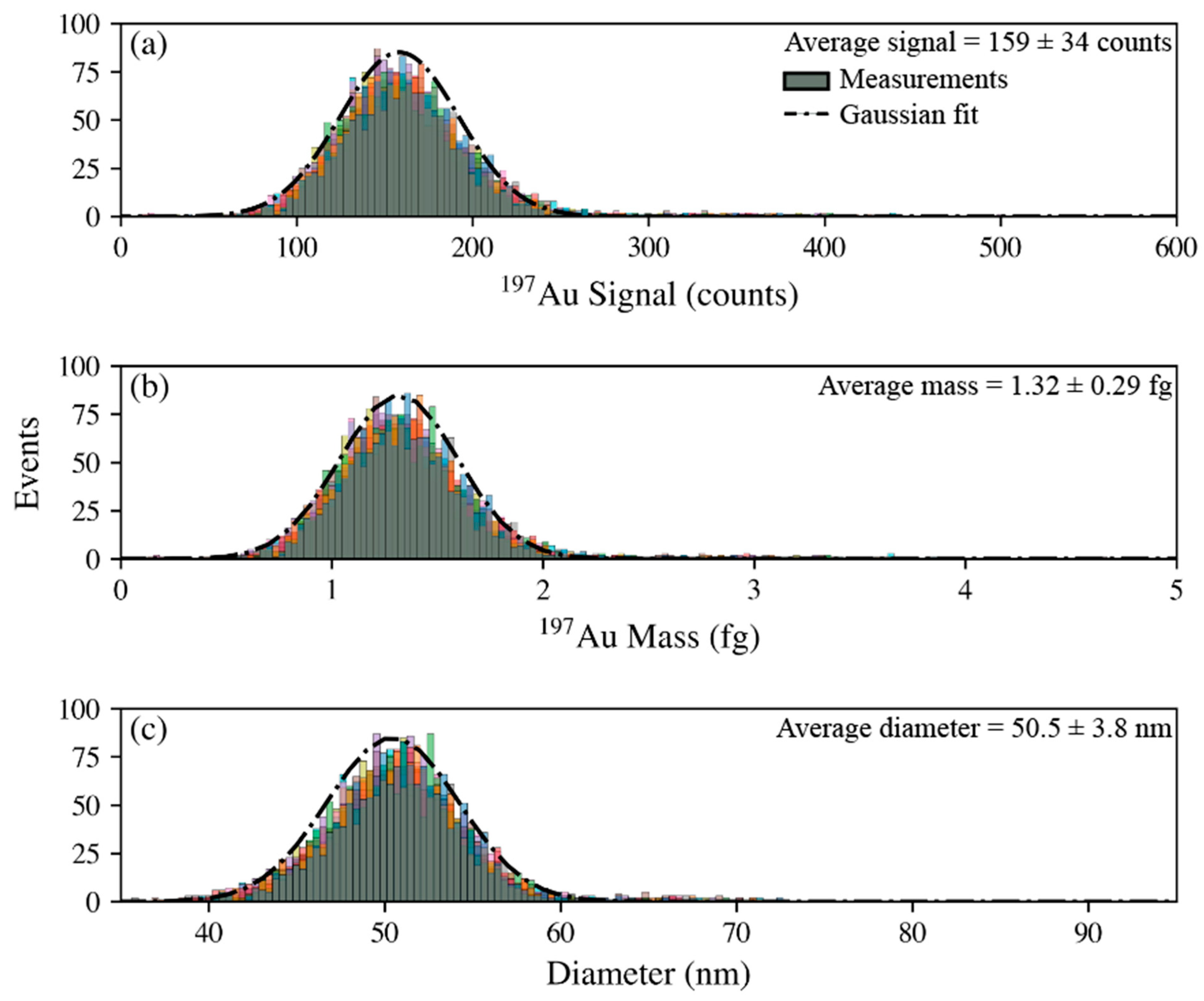
3.2. Mass and Size Characterization of Nanoparticles
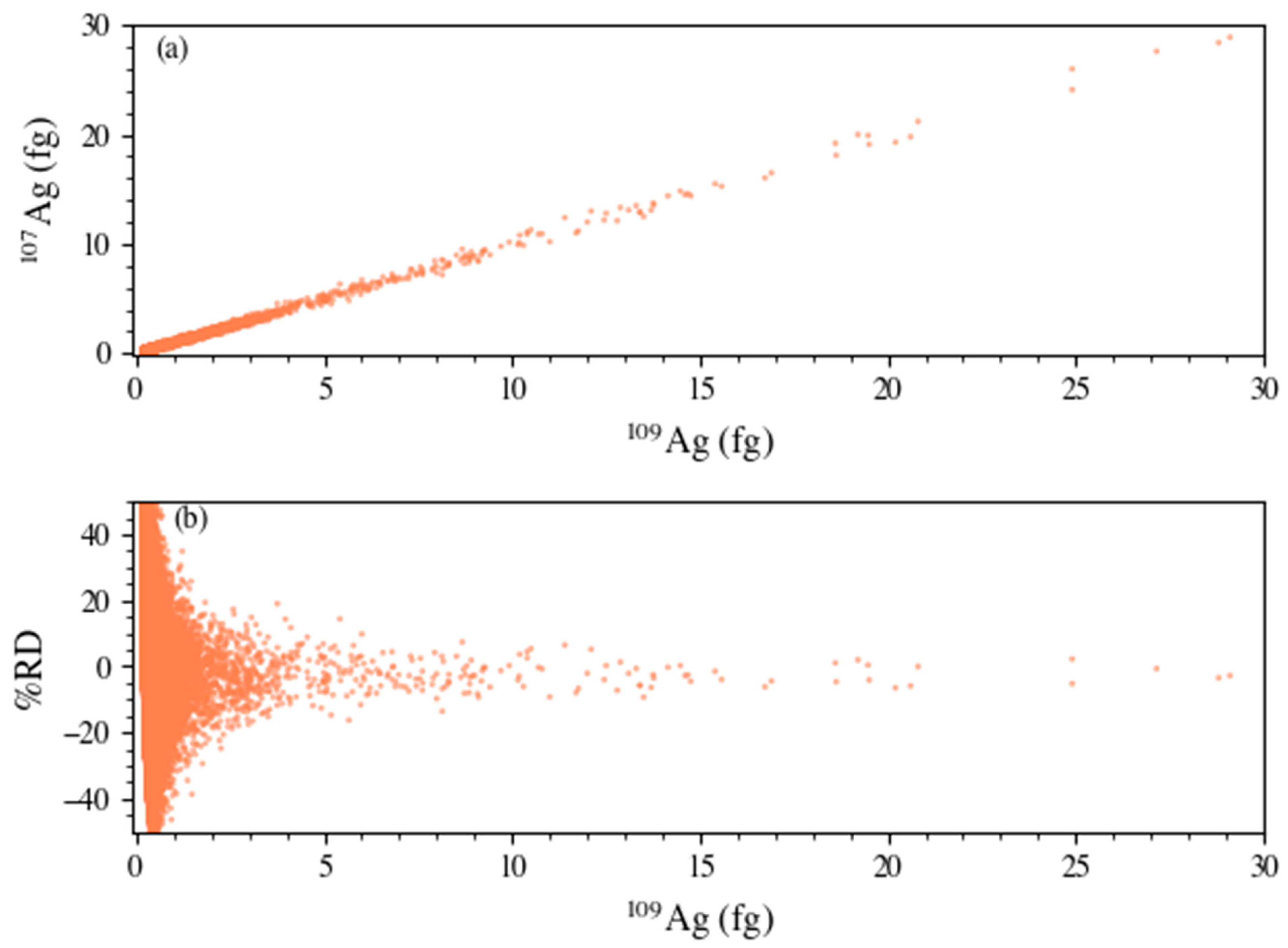
3.3. Isotopic Characterization of Nanoparticles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, R. A Practical Guide to ICP-MS, 3rd ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Louvat, P.; Moureau, J.; Paris, G.; Bouchez, J.; Noireaux, J.; Gaillardet, J. A fully automated direct injection nebulizer (d-DIHEN) for MC-ICP-MS isotope analysis: Application to boron isotope ratio measurements. J. Anal. At. Spectrom. 2014, 29, 1698–1707. [Google Scholar] [CrossRef]
- Takasaki, Y.; Inagaki, K.; Akhmad, S.; Fujii, S.-i.; Iwahata, D.; Takatsu, A.; Chiba, K.; Umemura, T. Multielement analysis of micro-volume biological samples by ICP-MS with highly efficient sample introduction system. Talanta 2001, 87, 24–29. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, J.; Tang, L.; Yu, C.; Mo, G.; Deng, B. A highly efficient introduction system for single cell- ICP-MS and its application to detection of copper in single human red blood cells. Talanta 2020, 206, 120174. [Google Scholar] [CrossRef]
- Sloop, J.T.; Bonilla, H.J.B.; Harville, T.; Jones, B.T.; Donati, G.L. Automated matrix-matching calibration using standard dilution analysis with two internal standards and a simple three-port mixing chamber. Talanta 2019, 205, 120160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Itoh, A.; Umemura, T.; Haraguchi, H.; Inagaki, K.; Chiba, K. Determination of REEs in natural water by ICP-MS with the aid of an automatic column changing system. J. Anal. At. Spectrom. 2010, 25, 1253–1258. [Google Scholar] [CrossRef]
- Manard, B.T.; Bostick, D.A.; Metzger, S.C.; Ticknor, B.W.; Zirakparvar, N.A.; Rogers, K.T.; Hexel, C.R. Rapid and automated separation of uranium ore concentrates for trace element analysis by inductively coupled plasma–optical emission spectroscopy/triple quadrupole mass spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2021, 179, 106097. [Google Scholar] [CrossRef]
- Metzger, S.C.; Ticknor, B.W.; Rogers, K.T.; Bostick, D.A.; McBay, E.H.; Hexel, C.R. Automated separation of uranium and plutonium from environmental swipe samples for multiple collector inductively coupled plasma mass spectrometry. Anal. Chem. 2018, 90, 9441–9448. [Google Scholar] [CrossRef] [PubMed]
- Montoya, D.P.; Manard, B.T.; Xu, N. Novel sample introduction system to reduce ICP-OES sample size for plutonium metal trace impurity determination. J. Radioanal. Nucl. Chem. 2016, 307, 2009–2014. [Google Scholar] [CrossRef]
- Wylie, E.M.; Manard, B.T.; Quarles, C.D., Jr.; Meyers, L.A.; Xu, N. An automated micro-separation system for the chromatographic removal of uranium matrix for trace element analysis by ICP-OES. Talanta 2018, 189, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Quarles, C.D., Jr.; Manard, B.T.; Wylie, E.M.; Xu, N. Trace elemental analysis of bulk uranium materials using an inline automated sample preparation technique for ICP-OES. Talanta 2018, 190, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Quarles, C.D.; Szoltysik, M.; Sullivan, P.; Reijnen, M. A fully automated total metals and chromium speciation single platform introduction system for ICP-MS. J. Anal. At. Spectrom. 2019, 34, 284–291. [Google Scholar] [CrossRef]
- Romaniello, S.J.; Field, M.P.; Smith, H.B.; Gordon, G.W.; Kim, M.H.; Anbar, A.D. Fully automated chromatographic purification of Sr and Ca for isotopic analysis. J. Anal. At. Spectrom. 2015, 30, 1906–1912. [Google Scholar] [CrossRef]
- Quarles, C.D.; Sullivan, P.; Bohlim, N.; Saetveit, N. Rapid automated total arsenic and arsenic speciation by inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2022, 37, 1240–1246. [Google Scholar] [CrossRef]
- Quarles, C.D.; Bohlim, N.; Wiederin, K.; Saetveit, N.; Sullivan, P. Evaluation of blood and synthetic matrix-matched calibrations using manual and inline sample preparation methods. J. Anal. At. Spectrom. 2022, 37, 1512–1521. [Google Scholar] [CrossRef]
- Meermann, B.; Nischwitz, V. ICP-MS for the analysis at the nanoscale—A tutorial review. J. Anal. At. Spectrom. 2018, 33, 1432–1468. [Google Scholar] [CrossRef]
- Montaño, M.D.; Olesik, J.W.; Barber, A.G.; Challis, K.; Ranville, J.F. Single particle ICP-MS: Advances toward routine analysis of nanomaterials. Anal. Bioanal. Chem. 2016, 408, 5053–5074. [Google Scholar] [CrossRef]
- Mozhayeva, D.; Engelhard, C. A critical review of single particle inductively coupled plasma mass spectrometry–A step towards an ideal method for nanomaterial characterization. J. Anal. At. Spectrom. 2020, 35, 1740–1783. [Google Scholar] [CrossRef]
- Wei, X.; Zheng, D.-H.; Cai, Y.; Jiang, R.; Chen, M.-L.; Yang, T.; Xu, Z.-R.; Yu, Y.-L.; Wang, J.-H. High-throughput/high-precision sampling of single cells into ICP-MS for elucidating cellular nanoparticles. Anal. Chem. 2018, 90, 14543–14550. [Google Scholar] [CrossRef] [PubMed]
- Koolen, C.D.; Torrent, L.; Agarwal, A.; Meili-Borovinskaya, O.; Gasilova, N.; Li, M.; Luo, W.; Zuttel, A. High-Throughput Sizing, Counting, and Elemental Analysis of Anisotropic Multimetallic Nanoparticles with Single-Particle Inductively Coupled Plasma Mass Spectrometry. ACS Nano 2022, 16, 11968–11978. [Google Scholar] [CrossRef]
- Von der Au, M.; Schwinn, M.; Kuhlmeier, K.; Büchel, C.; Meermann, B. Development of an automated on-line purification HPLC single cell-ICP-MS approach for fast diatom analysis. Anal. Chim. Acta 2019, 1077, 87–94. [Google Scholar]
- Kocic, J.; Günther, D.; Hattendorf, B. Improving detection capability for single particle inductively coupled plasma mass spectrometry with microdroplet sample introduction. J. Anal. At. Spectrom. 2021, 36, 233–242. [Google Scholar] [CrossRef]
- Chun, K.-H.; Lum, J.T.-S.; Leung, K.S.-Y. Dual-elemental analysis of single particles using quadrupole-based inductively coupled plasma-mass spectrometry. Anal. Chim. Acta 2022, 1192, 339389. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, L.; Gundlach-Graham, A.; Hattendorf, B.; Günther, D. Characterization of a new ICP-TOFMS instrument with continuous and discrete introduction of solutions. J. Anal. At. Spectrom. 2017, 32, 548–561. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, H.; Wang, M.; Cui, W.; Guo, Y.; Zheng, L.; Hu, L.; Qu, G.; Yin, Y.; Cai, Y. Exploring the performance of quadrupole, time-of-flight, and multi-collector ICP-MS for dual-isotope detection on single nanoparticles and cells. Anal. Chim. Acta 2023, 1240, 340756. [Google Scholar] [CrossRef]
- Menero-Valdés, P.; Lores-Padín, A.; Fernández, B.; Quarles, C.D., Jr.; García, M.; González-Iglesias, H.; Pereiro, R. Determination and localization of specific proteins in individual ARPE-19 cells by single cell and laser ablation ICP-MS using iridium nanoclusters as label. Talanta 2023, 253, 123974. [Google Scholar] [CrossRef] [PubMed]
- Gundlach-Graham, A.; Hendriks, L.; Mehrabi, K.; Günther, D. Monte Carlo simulation of low-count signals in time-of-flight mass spectrometry and its application to single-particle detection. Anal. Chem. 2018, 90, 11847–11855. [Google Scholar] [CrossRef]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Higgins, C.P.; Ranville, J.F. Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal. Chem. 2011, 83, 9361–9369. [Google Scholar] [CrossRef] [PubMed]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Gray, E.P.; Higgins, C.P.; Ranville, J.F. Single particle inductively coupled plasma-mass spectrometry: A performance evaluation and method comparison in the determination of nanoparticle size. Environ. Sci. Technol. 2012, 46, 12272–12280. [Google Scholar] [CrossRef]
- Montoro Bustos, A.R.; Petersen, E.J.; Possolo, A.; Winchester, M.R. Post hoc interlaboratory comparison of single particle ICP-MS size measurements of NIST gold nanoparticle reference materials. Anal. Chem. 2015, 87, 8809–8817. [Google Scholar] [CrossRef] [PubMed]
- Bland, G.D.; Zhang, P.; Valsami-Jones, E.; Lowry, G.V. Application of Isotopically Labeled Engineered Nanomaterials for Detection and Quantification in Soils via Single-Particle Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. Environ. Sci. Technol. 2022, 56, 15584–15593. [Google Scholar] [CrossRef]
- Su, Y.; Wang, W.; Li, Z.; Deng, H.; Zhou, G.; Xu, J.; Ren, X. Direct detection and isotope analysis of individual particles in suspension by single particle mode MC-ICP-MS for nuclear safety. J. Anal. At. Spectrom. 2015, 30, 1184–1190. [Google Scholar]
- Yamashita, S.; Ishida, M.; Suzuki, T.; Nakazato, M.; Hirata, T. Isotopic analysis of platinum from single nanoparticles using a high-time resolution multiple collector Inductively Coupled Plasma-Mass Spectroscopy. Spectrochim. Acta Part B 2020, 169, 105881. [Google Scholar] [CrossRef]
- Yamashita, S.; Yamamoto, K.; Takahashi, H.; Hirata, T. Size and isotopic ratio measurements of individual nanoparticles by a continuous ion-monitoring method using Faraday detectors equipped on a multi-collector-ICP-mass spectrometer. J. Anal. At. Spectrom. 2022, 37, 178–184. [Google Scholar] [CrossRef]

| Characteristic | 50 nm Au | 100 nm Au | 60 nm Ag/Au Core Shells |
|---|---|---|---|
| Diameter (nm) | 51.0 ± 1.9 | 102.2 ± 4.2 | 59 ± 6 (total) |
| Au = 30 ± 3 Ag = 15 (calculated) | |||
| Surface Area (m2g−1) | 6.1 | 3.0 | 8.5 |
| Mass Concentration (g L−1) | 0.053 | 0.053 | 0.8 (Ag); 0.24 (Au) |
| Particle Concentration (particles mL−1) | 3.9 × 1010 | 4.9 × 109 | 8 × 1011 |
| Working Particle Concentration (particles mL−1) | 3.9 × 104 | 4.9 × 104 | 8 × 104 |
| Step | Action | Volume (µL) | Speed (µL min−1) |
|---|---|---|---|
| 1 | Probe descends into sample vial | – | – |
| 2–5 | Sample mixing | – | 3000 |
| 6 | Fill sample loop | 150 | 2000 |
| 7 | Probe ascends | – | – |
| 8 | Probe moves to rinse | – | – |
| 9 | Probe descends into rinse | – | – |
| 10 | Dispense from sample loop to ICP-MS | 100 | 10 |
| 11 | Fill sample loop from rinse | 1000 | 25,000 |
| 12 | Probe moves to waste | – | – |
| 13 | Dispense | 1000 | 25,000 |
| 14 | Probe moves to rinse | – | – |
| 15 | Fill sample loop from rinse | 1000 | 25,000 |
| 16 | Probe moves to waste | – | – |
| 17 | Dispense | 1000 | 25,000 |
| Sample | Integration Time (ms) | Acquisition Time (s) |
|---|---|---|
| DI Water (×3) | 100 | 60 |
| Au standards (1, 5, 10 ng mL−1) | 100 | 60 |
| DI Water | 100 | 60 |
| Ag standards (1, 5, 10 ng mL−1) | 100 | 60 |
| DI Water | 100 | 60 |
| 50 nm Au NPs (×11) | 2 | 450 |
| DI Water | 100 | 60 |
| 60 nm Au/Ag core-shell NPs (×11) | 2 | 450 |
| DI Water | 100 | 60 |
| 100 nm Au NPs (×11) | 2 | 450 |
| DI Water | 100 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manard, B.T.; Bradley, V.C.; Quarles, C.D., Jr.; Hendriks, L.; Dunlap, D.R.; Hexel, C.R.; Sullivan, P.; Andrews, H.B. Towards Automated and High-Throughput Quantitative Sizing and Isotopic Analysis of Nanoparticles via Single Particle-ICP-TOF-MS. Nanomaterials 2023, 13, 1322. https://doi.org/10.3390/nano13081322
Manard BT, Bradley VC, Quarles CD Jr., Hendriks L, Dunlap DR, Hexel CR, Sullivan P, Andrews HB. Towards Automated and High-Throughput Quantitative Sizing and Isotopic Analysis of Nanoparticles via Single Particle-ICP-TOF-MS. Nanomaterials. 2023; 13(8):1322. https://doi.org/10.3390/nano13081322
Chicago/Turabian StyleManard, Benjamin T., Veronica C. Bradley, C. Derrick Quarles, Jr., Lyndsey Hendriks, Daniel R. Dunlap, Cole R. Hexel, Patrick Sullivan, and Hunter B. Andrews. 2023. "Towards Automated and High-Throughput Quantitative Sizing and Isotopic Analysis of Nanoparticles via Single Particle-ICP-TOF-MS" Nanomaterials 13, no. 8: 1322. https://doi.org/10.3390/nano13081322
APA StyleManard, B. T., Bradley, V. C., Quarles, C. D., Jr., Hendriks, L., Dunlap, D. R., Hexel, C. R., Sullivan, P., & Andrews, H. B. (2023). Towards Automated and High-Throughput Quantitative Sizing and Isotopic Analysis of Nanoparticles via Single Particle-ICP-TOF-MS. Nanomaterials, 13(8), 1322. https://doi.org/10.3390/nano13081322