Multiwalled Carbon Nanotubes-Modified Metallic Electrode Prepared Using Chemical Vapor Deposition as Sequential Injection Analysis Detector for Determination of Ascorbic Acid
Abstract
1. Introduction
2. Experimental
2.1. Chemicals, Reagents, and Preparations
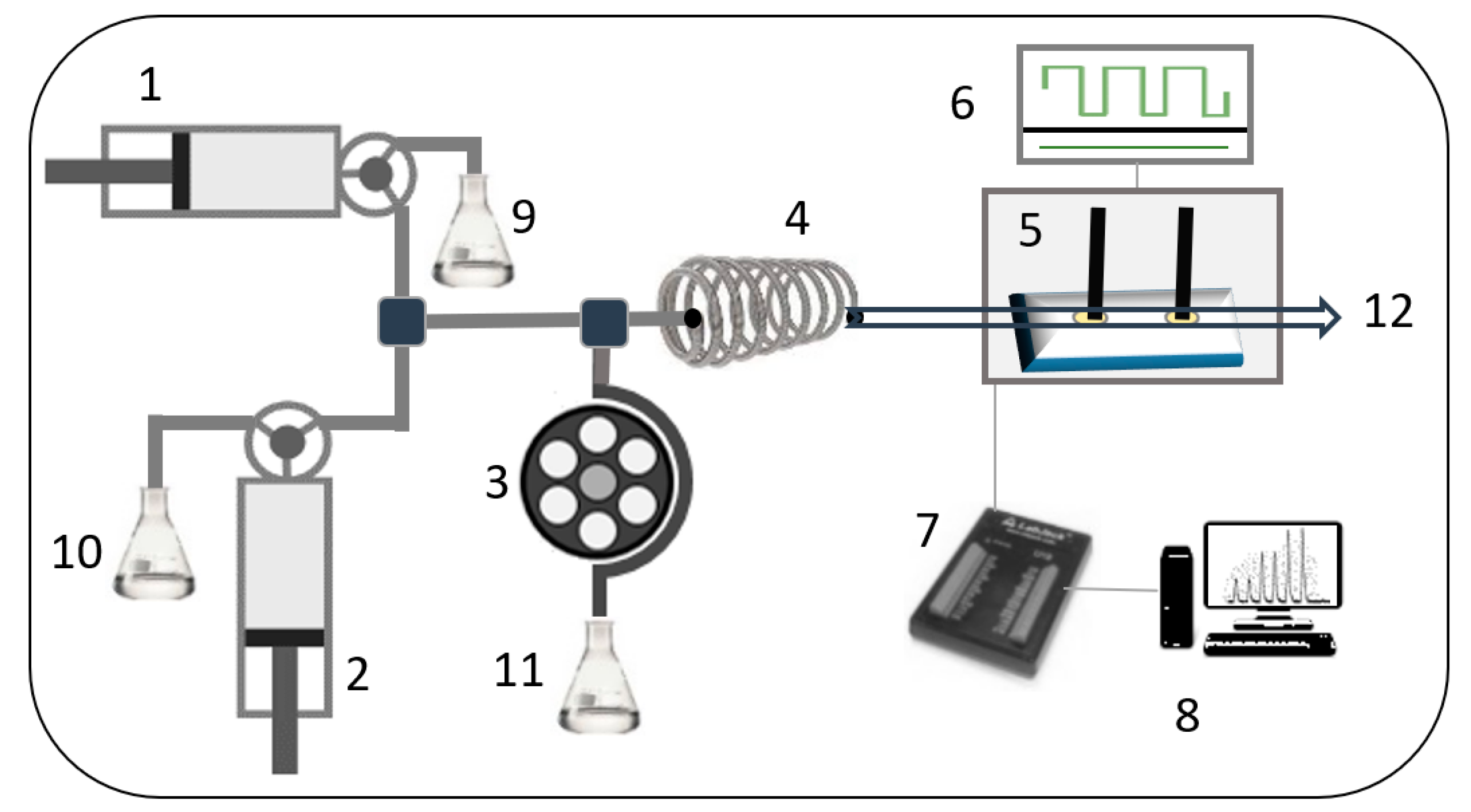
2.2. Instrumentation
2.3. Preparation and Characterization of CNTs-Coated Silver Electrode
2.4. SIA Configuration and Procedure
3. Results and Discussion
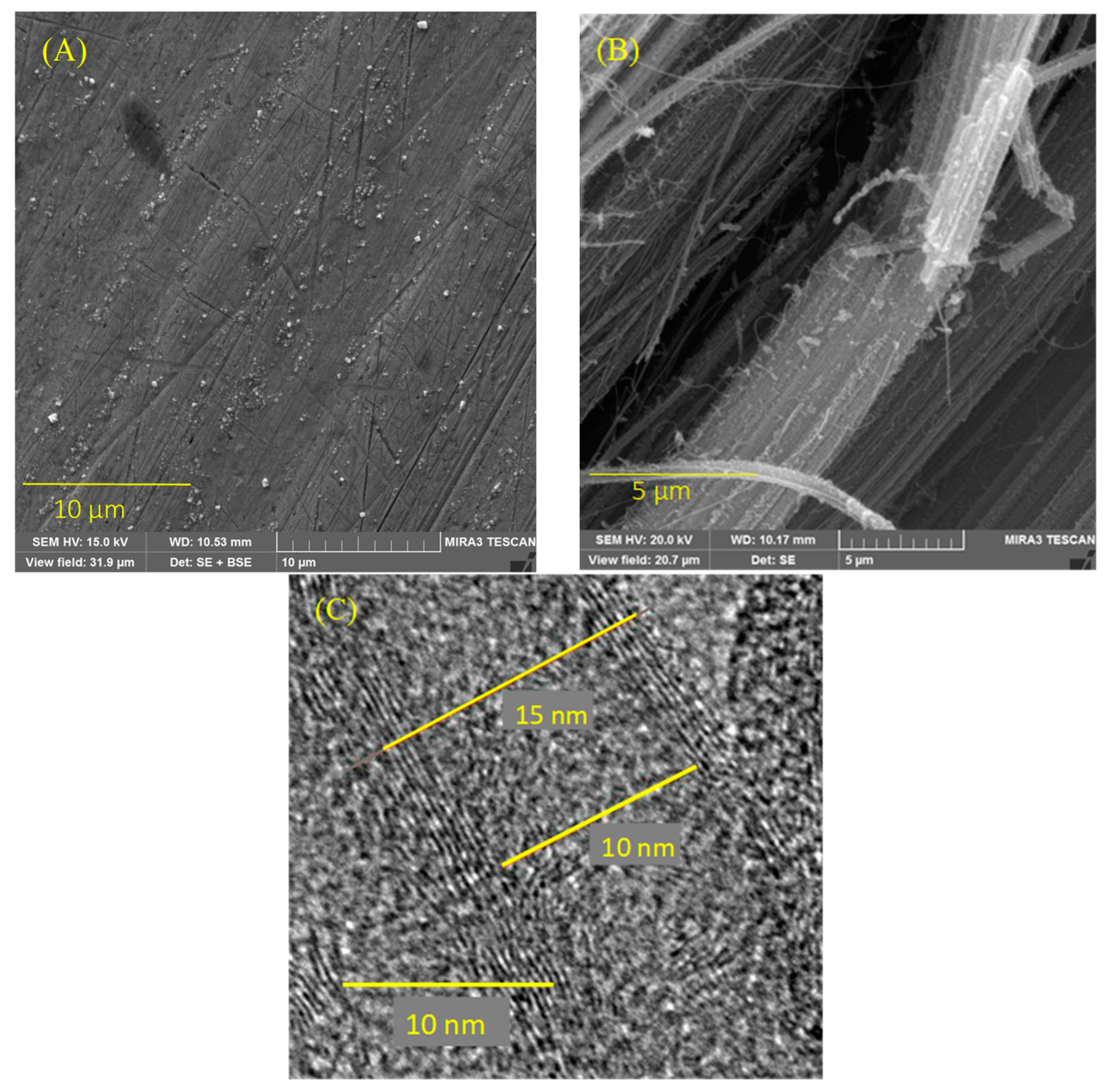
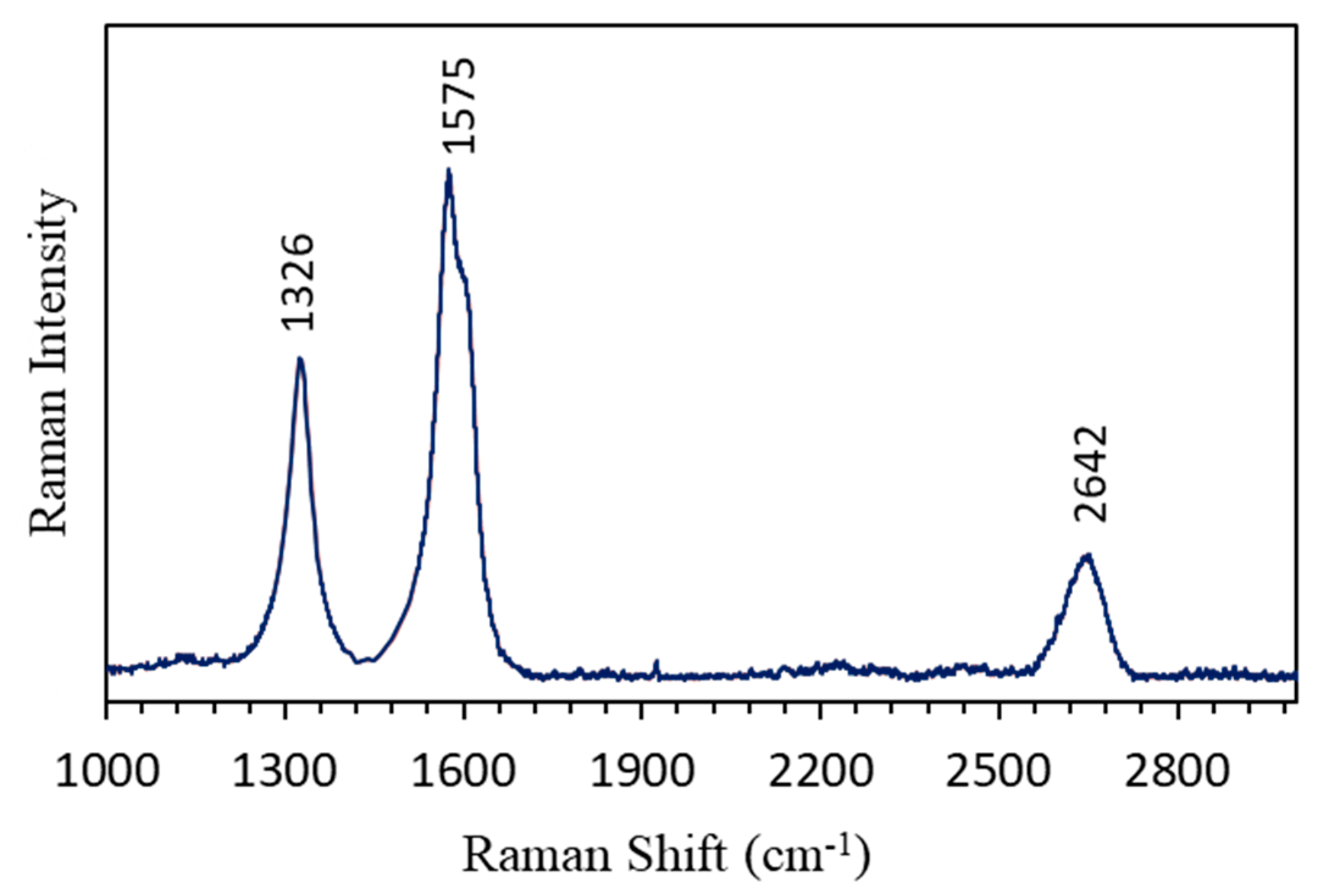
3.1. Morphological and Structural Characterizations of the CNTs Electrode
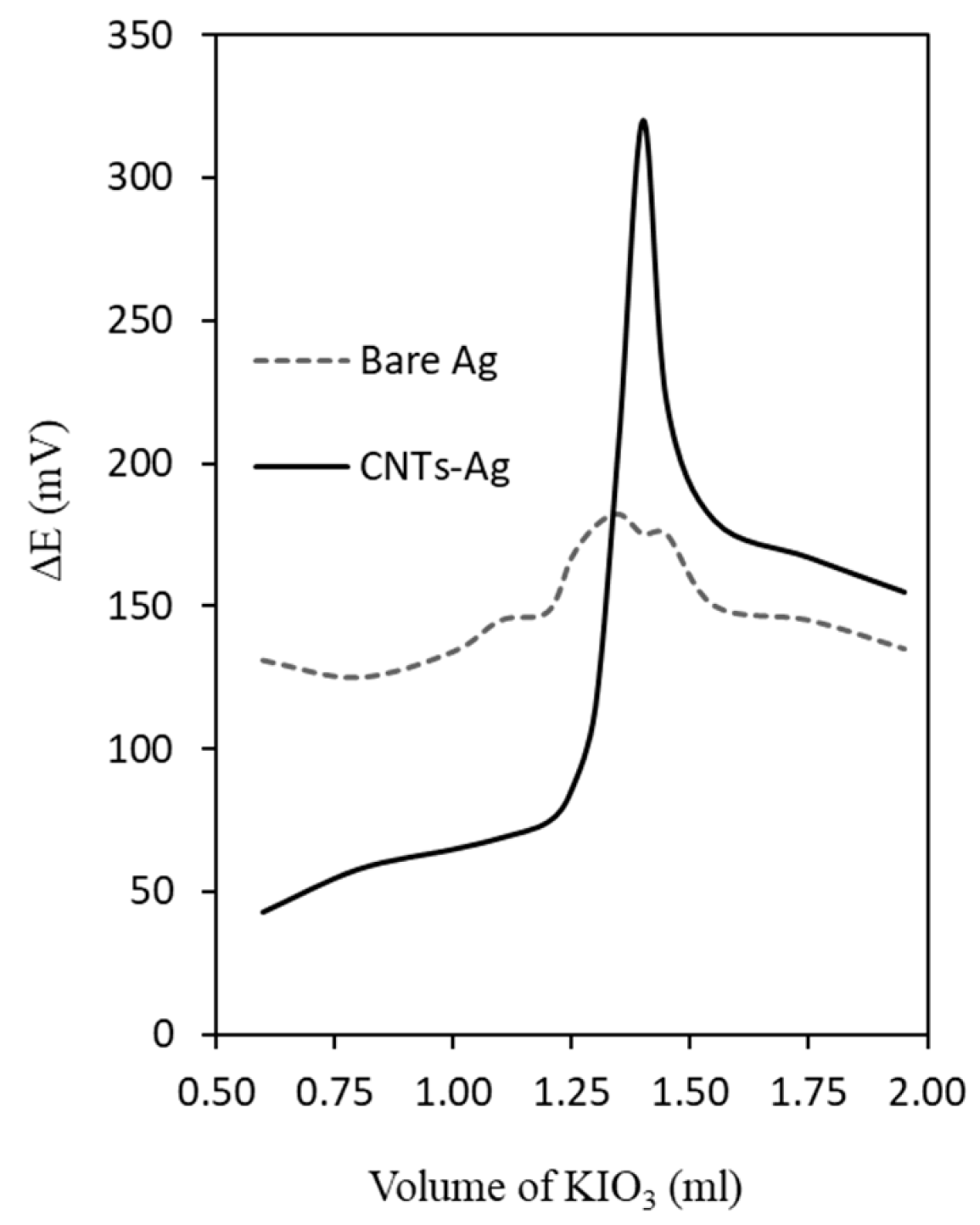
3.2. Performance of CNTs-Ag as SIA-DEP Indicator Electrodes
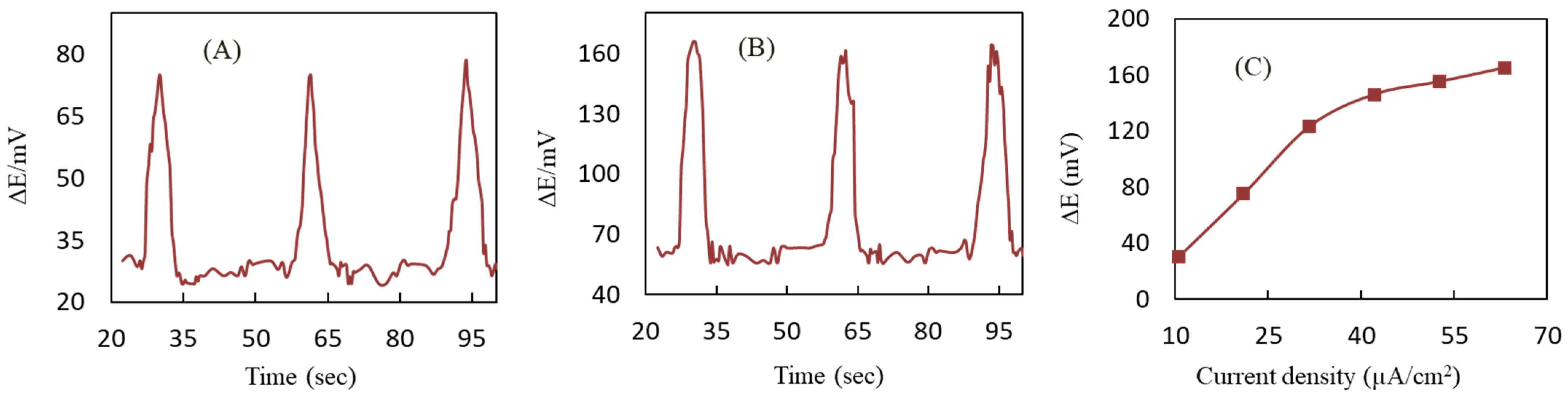
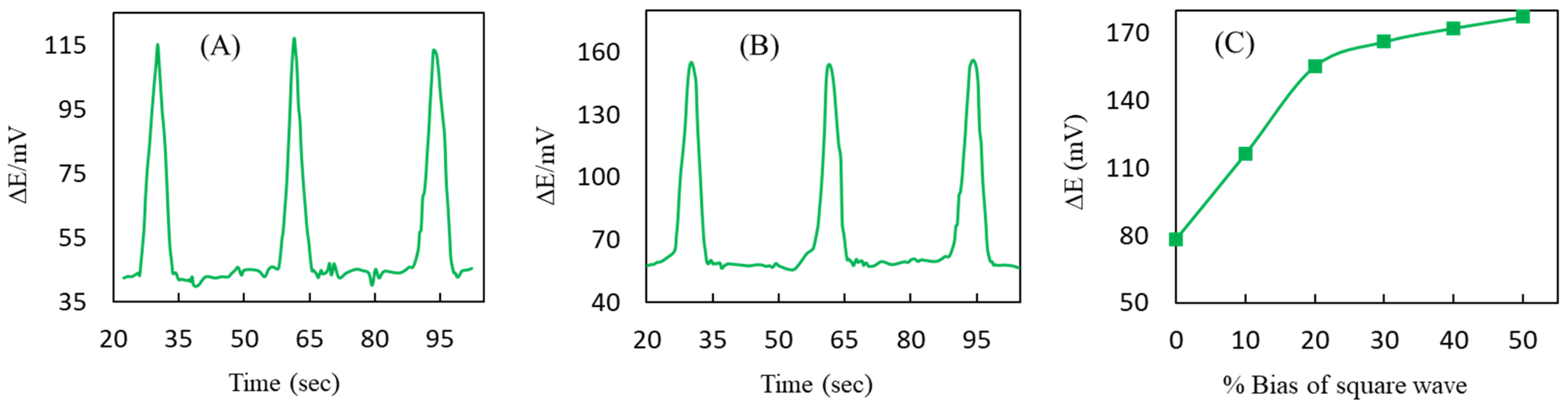
3.3. Optimization of DEP Parameters
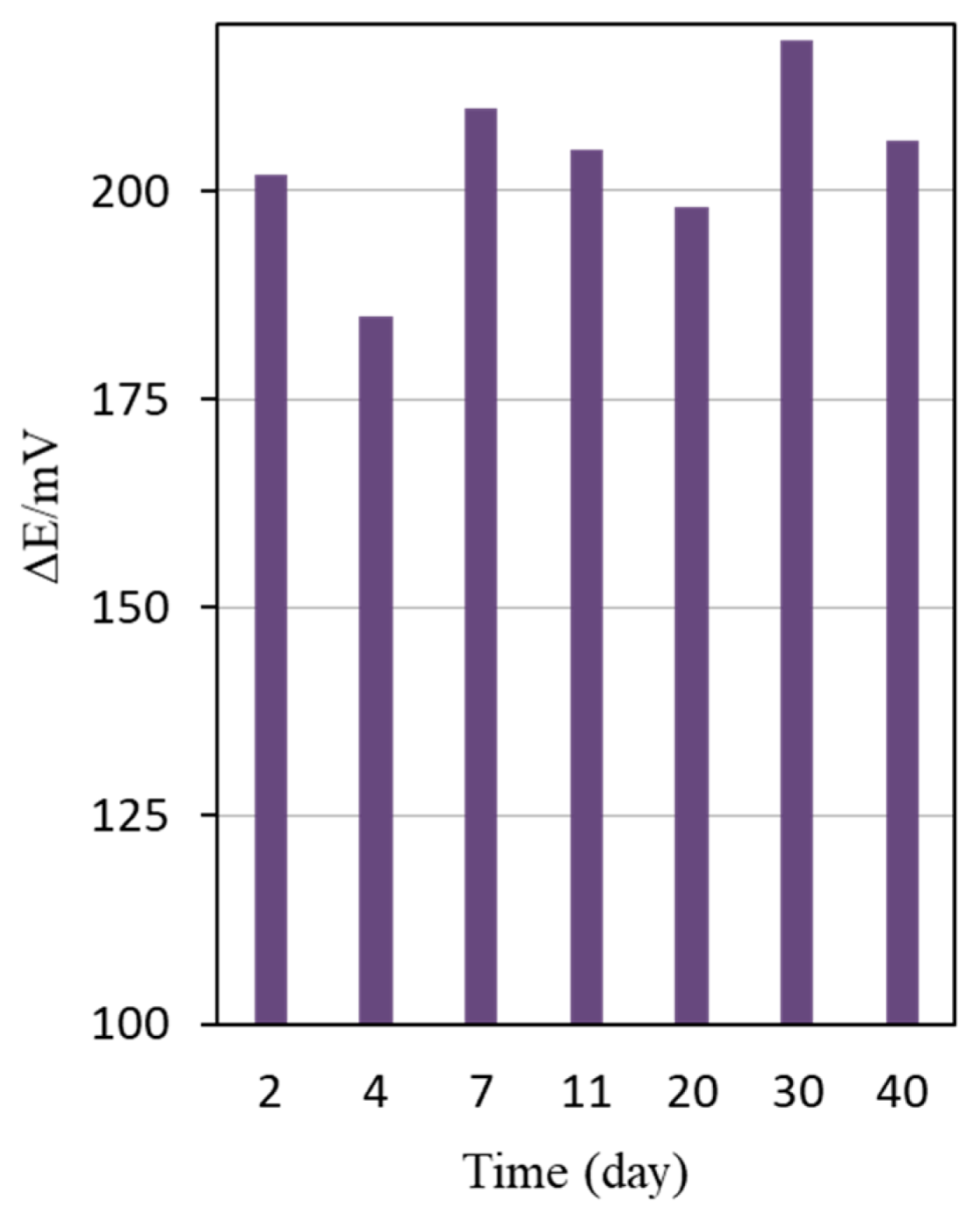
3.4. Characteristics of CNTs-Ag Electrode
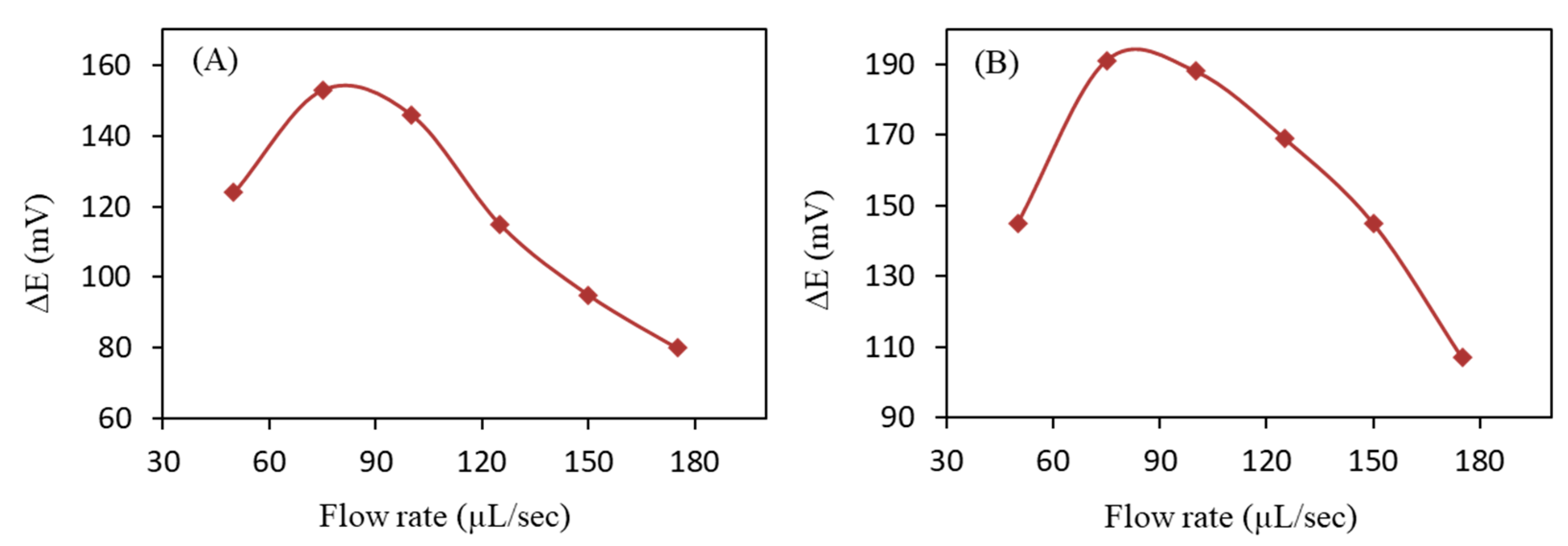
3.5. Optimization of SIA Parameters
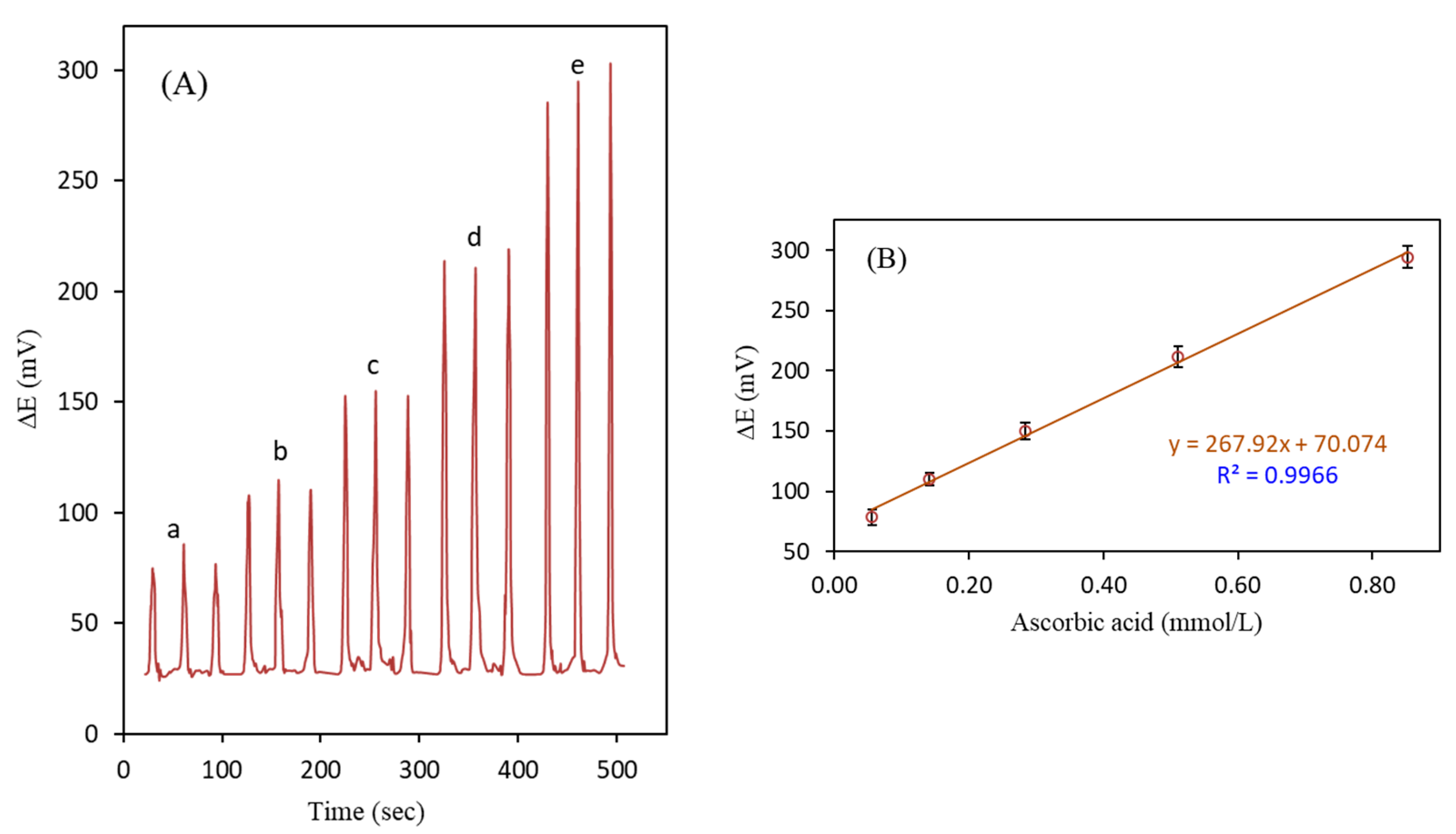
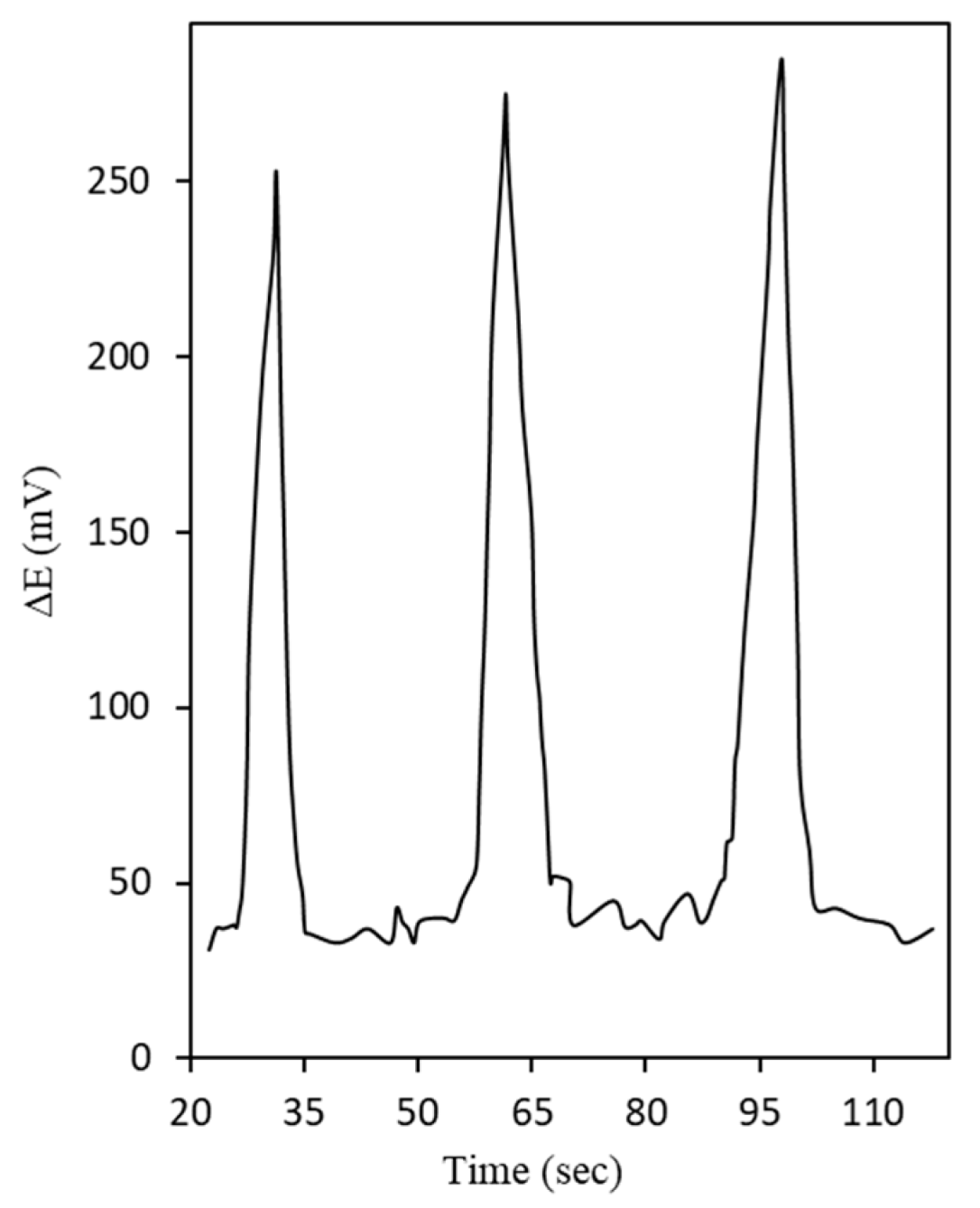
3.6. Analytical Performance for Ascorbic Acid Determination
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical Methods as a Tool for Determining the Antioxidant Capacity of Food and Beverages: A Review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef]
- Dodevska, T.; Hadzhiev, D.; Shterev, I. A Review on Electrochemical Microsensors for Ascorbic Acid Detection: Clinical, Pharmaceutical, and Food Safety Applications. Micromachines 2023, 14, 41. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Amine, A. Recent Advances in Electrochemical Sensors Based on Molecularly Imprinted Polymers and Nanomaterials. Electroanalysis 2019, 31, 188–201. [Google Scholar] [CrossRef]
- Qian, L.; Durairaj, S.; Prins, S.; Chen, A. Nanomaterial-Based Electrochemical Sensors and Biosensors for the Detection of Pharmaceutical Compounds. Biosens. Bioelectron. 2021, 175, 112836. [Google Scholar] [CrossRef]
- Kant, T.; Shrivas, K.; Dewangan, K.; Kumar, A.; Jaiswal, N.K.; Deb, M.K.; Pervez, S. Design and Development of Conductive Nanomaterials for Electrochemical Sensors: A Modern Approach. Mater. Today Chem. 2022, 24, 100769. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon Nanotubes: Synthesis, Properties and Engineering Applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Gergeroglu, H.; Yildirim, S.; Ebeoglugil, M.F. Nano-Carbons in Biosensor Applications: An Overview of Carbon Nanotubes (CNTs) and Fullerenes (C60). SN Appl. Sci. 2020, 2, 603. [Google Scholar] [CrossRef]
- Nugent, J.; Santhanam, K.; Rubio, A.; Ajayan, P. Fast Electron Transfer Kinetics on Multiwalled Carbon Nanotube Microbundle Electrodes. Nano Lett. 2001, 1, 87–91. [Google Scholar] [CrossRef]
- Trojanowicz, M. Analytical Applications of Carbon Nanotubes: A Review. TrAC Trends Anal. Chem. 2006, 25, 480–489. [Google Scholar] [CrossRef]
- Cho, G.; Azzouzi, S.; Zucchi, G.; Lebental, B. Electrical and Electrochemical Sensors Based on Carbon Nanotubes for the Monitoring of Chemicals in Water—A Review. Sensors 2022, 22, 218. [Google Scholar] [CrossRef]
- Baig, N.; Sajid, M.; Saleh, T.A. Recent Trends in Nanomaterial-Modified Electrodes for Electroanalytical Applications. TrAC Trends Anal. Chem. 2019, 111, 47–61. [Google Scholar] [CrossRef]
- Merkoçi, A.; Pumera, M.; Llopis, X.; Pérez, B.; Del Valle, M.; Alegret, S. New Materials for Electrochemical Sensing VI: Carbon Nanotubes. TrAC—Trends Anal. Chem. 2005, 24, 826–838. [Google Scholar] [CrossRef]
- Schnorr, J.M.; Swager, T.M. Emerging Applications of Carbon Nanotubes. Chem. Mater. 2011, 23, 646–657. [Google Scholar] [CrossRef]
- Barsan, M.M.; Ghica, M.E.; Brett, C.M.A. Electrochemical Sensors and Biosensors Based on Redox Polymer/Carbon Nanotube Modified Electrodes: A Review. Anal. Chim. Acta 2015, 881, 1–23. [Google Scholar] [CrossRef]
- Naqvi, S.T.R.; Rasheed, T.; Hussain, D.; Najam ul Haq, M.; Majeed, S.; Shafi, S.; Ahmed, N.; Nawaz, R. Modification Strategies for Improving the Solubility/Dispersion of Carbon Nanotubes. J. Mol. Liq. 2020, 297, 111919. [Google Scholar] [CrossRef]
- Basheer, B.V.; George, J.J.; Siengchin, S.; Parameswaranpillai, J. Polymer Grafted Carbon Nanotubes—Synthesis, Properties, and Applications: A Review. Nano-Struct. Nano-Objects 2020, 22, 100429. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-Generation Polymer Nanocomposite-Based Electrochemical Sensors and Biosensors: A Review. TrAC Trends Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- Gupta, S.; Murthy, C.N.; Prabha, C.R. Recent Advances in Carbon Nanotube Based Electrochemical Biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef]
- Yun, Y.; Dong, Z.; Shanov, V.; Heineman, W.R.; Halsall, H.B.; Bhattacharya, A.; Conforti, L.; Narayan, R.K.; Ball, W.S.; Schulz, M.J. Nanotube Electrodes and Biosensors This Article Reviews the State of the Art in Carbon Nanotube Electrode. Nano Today 2007, 2, 30–37. [Google Scholar] [CrossRef]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for Carbon Nanotubes Synthesis—Review. J. Mater. Chem. 2011, 21, 15872–15884. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Ihsanullah Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef]
- Pant, M.; Singh, R.; Negi, P.; Tiwari, K.; Singh, Y. A Comprehensive Review on Carbon Nano-Tube Synthesis Using Chemical Vapor Deposition. Mater. Today Proc. 2021, 46, 11250–11253. [Google Scholar] [CrossRef]
- Ahmad, M.; Silva, S.R.P. Low Temperature Growth of Carbon Nanotubes—A Review. Carbon N. Y. 2020, 158, 24–44. [Google Scholar] [CrossRef]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A Review on Carbon Nanotube: An Overview of Synthesis, Properties, Functionalization, Characterization, and the Application. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2021, 268, 115095. [Google Scholar] [CrossRef]
- Hu, Z.; Peng, W.; Tian, W.; Wang, F.; Kang, X.; Zhang, Y.X.; Yue, H.; Zhang, L.; Ji, J.; Wang, S. A General Strategy for In-Situ Fabrication of Uniform Carbon Nanotubes on Three-Dimensional Carbon Architectures for Electrochemical Application. Appl. Surf. Sci. 2019, 496, 143704. [Google Scholar] [CrossRef]
- Shah, K.A.; Tali, B.A. Synthesis of Carbon Nanotubes by Catalytic Chemical Vapour Deposition: A Review on Carbon Sources, Catalysts and Substrates. Mater. Sci. Semicond. Process. 2016, 41, 67–82. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Kołacińska, K. Recent Advances in Flow Injection Analysis. Analyst 2016, 141, 2085–2139. [Google Scholar] [CrossRef]
- Pimenta, A.M.; Montenegro, M.C.B.S.M.; Araújo, A.N.; Calatayud, J.M. Application of Sequential Injection Analysis to Pharmaceutical Analysis. J. Pharm. Biomed. Anal. 2006, 40, 16–34. [Google Scholar] [CrossRef]
- Pérez-Olmos, R.; Soto, J.C.; Zárate, N.; Araújo, A.N.; Lima, J.L.F.C.; Saraiva, M.L.M.F.S. Application of Sequential Injection Analysis (SIA) to Food Analysis. Food Chem. 2005, 90, 471–490. [Google Scholar] [CrossRef]
- Siddiqui, M.R.; AlOthman, Z.A.; Rahman, N. Analytical Techniques in Pharmaceutical Analysis: A Review. Arab. J. Chem. 2017, 10, S1409–S1421. [Google Scholar] [CrossRef]
- Lenehan, C.E.; Barnett, N.W.; Lewis, S.W. Sequential Injection Analysis. Analyst 2002, 127, 997–1020. [Google Scholar] [CrossRef]
- Pérez-Olmos, R.; Soto, J.C.; Zárate, N.; Araújo, A.N.; Montenegro, M.C.B.S.M. Sequential Injection Analysis Using Electrochemical Detection: A Review. Anal. Chim. Acta 2005, 554, 1–16. [Google Scholar] [CrossRef]
- Christian, G.D. Sequential Injection Analysis for Electrochemical Measurements and Process Analysis. Analyst 1994, 119, 2309–2314. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Fafaneata, C. Electrochemical Methods for Ascorbic Acid Determination. Electrochim. Acta 2014, 121, 443–460. [Google Scholar] [CrossRef]
- Beitollahi, H.; Movahedifar, F.; Tajik, S.; Jahani, S. A Review on the Effects of Introducing CNTs in the Modification Process of Electrochemical Sensors. Electroanalysis 2019, 31, 1195–1203. [Google Scholar] [CrossRef]
- Dhara, K.; Debiprosad, R.M. Review on Nanomaterials-Enabled Electrochemical Sensors for Ascorbic Acid Detection. Anal. Biochem. 2019, 586, 113415. [Google Scholar] [CrossRef]
- Al-Ghannam, S.M.; Al-Olyan, A.M. Differential Electrolytic Potentiometric Titration of Vitamin C in Pharmaceutical Preparations. J. Food Drug Anal. 2005, 13, 295–300. [Google Scholar] [CrossRef]
- Osman, A.M.; Abulkibash, A.M.; Atieh, M.A. Time-Biased Square Wave Differential Electrolytic Potentiometry for Determination of Ascorbic Acid in a Complex Matrix at Multi-Walled Carbon Nanotubes Modified Silver Electrodes. Arab. J. Chem. 2020, 13, 2955–2963. [Google Scholar] [CrossRef]
- Abulkibash, A.; Koken, M.; Khaled, M.; Sultan, S. Differential Electrolytic Potentiometry, a Detector in Flow Injection Analysis for Oxidation-Reduction Reactions. Talanta 2000, 52, 1139–1142. [Google Scholar] [CrossRef]
- Abulkibash, A.; Fraihat, S.; EL ALI, B. Flow Injection Determination of Vitamin C in Pharmaceutical Preparations by Differential Electrolytic Potentiometry. J. Flow Inject. Anal. 2009, 26, 121–125. [Google Scholar]
- Bishop, E. Differential Electrolytic Potentiometry with Periodic Polarisation Part XXII. Symmetrical Periodic Current Differential Electrolytic Potentiometry in Oxidation—Reduction Titrimetry. Analyst 1973, 98, 712–724. [Google Scholar] [CrossRef]
- United States Pharmacopoeia (USP). National Formulary. In USP-NF25; United States Pharmacopoeial Convention Inc.: Rockville, MD, USA, 2007; p. 1441. [Google Scholar]
- Osman, A.M.; Abulkibash, A.M.; Atieh, M.A. Fabrication of a CNT/Ag Potentiometric Sensor for Redox Reactions via Catalytic Chemical Vapor Deposition. Electrochem. Commun. 2020, 119, 106806. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, Y.; Zhou, M. AgNPs@CNTs/Ag Hybrid Films on Thiolated PET Substrate for Flexible Electronics. Chem. Eng. J. 2019, 368, 223–234. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Ogasawara, H.; Kim, I.S.; Ni, Q.Q. Cellulose Acetate/Multi-Wall Carbon Nanotube/Ag Nanofiber Composite for Antibacterial Applications. Mater. Sci. Eng. C 2020, 110, 110679. [Google Scholar] [CrossRef]
- Sivkov, D.; Nekipelov, S.; Petrova, O.; Vinogradov, A.; Mingaleva, A.; Isaenko, S.; Makarov, P.; Ob’edkov, A.; Kaverin, B.; Gusev, S.; et al. Studies of Buried Layers and Interfaces of Tungsten Carbide Coatings on the MWCNT Surface by XPS and NEXAFS Spectroscopy. Appl. Sci. 2020, 10, 4736. [Google Scholar] [CrossRef]
- Soni, S.K.; Thomas, B.; Kar, V.R. A Comprehensive Review on CNTs and CNT-Reinforced Composites: Syntheses, Characteristics and Applications. Mater. Today Commun. 2020, 25, 101546. [Google Scholar] [CrossRef]
- Piao, Y.; Tondare, V.N.; Davis, C.S.; Gorham, J.M.; Petersen, E.J.; Gilman, J.W.; Scott, K.; Vladár, A.E.; Hight Walker, A.R. Comparative Study of Multiwall Carbon Nanotube Nanocomposites by Raman, SEM, and XPS Measurement Techniques. Compos. Sci. Technol. 2021, 208. [Google Scholar] [CrossRef]
- DiLeo, R.; Landi, B.; Raffaelle, R. Purity Assessment of Multiwalled Carbon Nanotubes by Raman Spectroscopy. J. Appl. Phys. 2007, 101, 064307. [Google Scholar] [CrossRef]
- Heise, H.M.; Kuckuk, R.; Ojha, A.K.; Srivastava, A.; Srivastava, V.; Asthana, B.P. Characterisation of Carbonaceous Materials Using Raman Spectroscopy: A Comparison of Carbon Nanotube Filters, Single- And Multi-Walled Nanotubes, Graphitised Porous Carbon and Graphite. J. Raman Spectrosc. 2009, 40, 344–353. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the Characteristics of Multiwall Carbon Nanotubes. Carbon N. Y. 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Wang, C.; Zhang, Y.; Xu, L.; Zhu, K.; Xie, S. Raman Characterization of Aligned Carbon Nanotubes Produced by Thermal Decomposition of Hydrocarbon Vapor. Appl. Phys. Lett. 1997, 70, 2684–2686. [Google Scholar] [CrossRef]
- Hashmi, M.-U.-H. Assay of Vitamins in Pharmaceutical Preparations; John Wiley and Sons: London, UK, 1973. [Google Scholar]
- Bishop, B.Y.E. Differential Electrolytic Potentiometry with Periodic Polarisation Part XXI. Introduction and Instrumentation. Analyst 1973, 98, 769–776. [Google Scholar] [CrossRef]
- El-Shabasy, M. Perspective of Adhesion of Thin Films. Period. Polytech. Electr. Eng. 1981, 25, 123–134. [Google Scholar]
- Li, Y.; Ye, W.; Cui, Y.; Li, B.; Yang, Y.; Qian, G. A Metal-Organic Frameworks@ Carbon Nanotubes Based Electrochemical Sensor for Highly Sensitive and Selective Determination of Ascorbic Acid. J. Mol. Struct. 2020, 1209, 127986. [Google Scholar] [CrossRef]
- Iranmanesh, T.; Foroughi, M.M.; Jahani, S.; Shahidi Zandi, M.; Hassani Nadiki, H. Green and Facile Microwave Solvent-Free Synthesis of CeO2 Nanoparticle-Decorated CNTs as a Quadruplet Electrochemical Platform for Ultrasensitive and Simultaneous Detection of Ascorbic Acid, Dopamine, Uric Acid and Acetaminophen. Talanta 2020, 207, 120318. [Google Scholar] [CrossRef]
- Bayraktepe, D.E.; Yazan, Z.; Önal, M. Sensitive and Cost Effective Disposable Composite Electrode Based on Graphite, Nano-Smectite and Multiwall Carbon Nanotubes for the Simultaneous Trace Level Detection of Ascorbic Acid and Acetylsalicylic Acid in Pharmaceuticals. Talanta 2019, 203, 131–139. [Google Scholar] [CrossRef]
- Huang, Z.N.; Zou, J.; Teng, J.; Liu, Q.; Yuan, M.M.; Jiao, F.P.; Jiang, X.Y.; Yu, J.G. A Novel Electrochemical Sensor Based on Self-Assembled Platinum Nanochains—Multi-Walled Carbon Nanotubes-Graphene Nanoparticles Composite for Simultaneous Determination of Dopamine and Ascorbic Acid. Ecotoxicol. Environ. Saf. 2019, 172, 167–175. [Google Scholar] [CrossRef]
- Huang, D.; Li, X.; Chen, M.; Chen, F.; Wan, Z.; Rui, R.; Wang, R.; Fan, S.; Wu, H. An Electrochemical Sensor Based on a Porphyrin Dye-Functionalized Multi-Walled Carbon Nanotubes Hybrid for the Sensitive Determination of Ascorbic Acid. J. Electroanal. Chem. 2019, 841, 101–106. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; El-Gohary, A.R. Crown Ether Modified Poly(Hydroquinone)/Carbon Nanotubes Based Electrochemical Sensor for Simultaneous Determination of Levodopa, Uric Acid, Tyrosine and Ascorbic Acid in Biological Fluids. J. Electroanal. Chem. 2020, 863, 114032. [Google Scholar] [CrossRef]
- Baytak, A.K.; Aslanoglu, M. A Novel Sensitive Method for the Simultaneous Determination of Ascorbic Acid, Dopamine, Uric Acid and Tryptophan Using a Voltammetric Platform Based on Carbon Black Nanoballs. Arab. J. Chem. 2020, 13, 1702–1711. [Google Scholar] [CrossRef]
- Ma, C.; Xu, P.; Chen, H.; Cui, J.; Guo, M.; Zhao, J. An Electrochemical Sensor Based on Reduced Graphene Oxide/β-Cyclodextrin/Multiwall Carbon Nanotubes/ Polyoxometalate Tetracomponent Hybrid: Simultaneous Determination of Ascorbic Acid, Dopamine and Uric Acid. Microchem. J. 2022, 180, 107533. [Google Scholar] [CrossRef]



| Sample | Ascorbic Acid (µM) | Regression Parameters | Ascorbic Acid (µM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Added | DC | SW | SIA-DEP, DC Mode | SIA-DEP, SW Mode | Iodimetric | |||||||
| Avg. | (%RSD) | Avg. | (%RSD) | Slope | y-Intercept | R2 | Slope | y-Intercept | R2 | |||
| Pure ascorbic acid | 60.0 | 59.0 | 8.5 | 52.0 | 12.6 | 267.9 | 70.1 | 0.9966 | 491.8 | 74.0 | 0.9965 | 65.4 |
| 140.0 | 144.0 | 4.5 | 150.0 | 3.8 | 148.6 | |||||||
| 280.0 | 262.0 | 4.7 | 299.0 | 5.0 | 266.2 | |||||||
| 510.0 | 540.0 | 4.1 | 529 | 5.4 | 497.0 | |||||||
| 850.0 | 842.0 | 4.3 | 837 | 2.4 | 870.0 | |||||||
| Redoxon (304.0 mg/L) | - | 391.4 | 4.3 | 400.0 | 3.9 | 383.6 %RSD = 1.9 | ||||||
| Electrochemical Technique | Electrode Material | Linear Range (µM) | LOD (µM) | RSD (%) | Selectivity | Ref. |
|---|---|---|---|---|---|---|
| Differential pulse voltammetry | MOFs/CNTs@glassy carbon | 200–2267 | 1.03 | - | Yes | [56] |
| Differential pulse voltammetry | CeO2 NPs/CNTs | 0.01–900 | 0.003 | 2.4 | No | [57] |
| Adsorptive stripping differential puls voltammetry | Nano-smectite/MWCNT@pencil graphite | 0.353–60 | 0.096 | 0.106 | No | [58] |
| Differential pulse voltammetry | Platinum nanochains/MWCNTs/graphene NPs | 100–1200 | 10.0 | 0.62–2.9 | No | [59] |
| Amperometry | Porphyrin-functionalized MWCNTs@GCE | 18.7–1850 | 0.18 | - | Yes | [60] |
| Cyclic voltammetry | Crown ether- poly(hydroquinone)/CNTs | 0.1–50 | 0.0033 | - | No | [61] |
| Square wave voltammetry | Carbon black nanoballs/CNTs@GCE | 20–400 | 5.71 | - | No | [62] |
| Differential pulse voltammetry | RGO/β-cyclodextrin/MWCNTs/polyoxometalate | 5–2000 | 0.84 | - | No | [63] |
| FIA/DC-DEP | Pt | 5682–1704.5 | - | - | No | [39] |
| FIA/DC-DEP | Pt | 68.2–738.6 | 51.0 | - | No | [40] |
| SIA/SW-DEP | MWCNTs/Ag | 60.0–850.0 | 14 | 2.4–12.6 | No | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, A.M.; Hendi, A.; Osman, N.M.A. Multiwalled Carbon Nanotubes-Modified Metallic Electrode Prepared Using Chemical Vapor Deposition as Sequential Injection Analysis Detector for Determination of Ascorbic Acid. Nanomaterials 2023, 13, 1264. https://doi.org/10.3390/nano13071264
Osman AM, Hendi A, Osman NMA. Multiwalled Carbon Nanotubes-Modified Metallic Electrode Prepared Using Chemical Vapor Deposition as Sequential Injection Analysis Detector for Determination of Ascorbic Acid. Nanomaterials. 2023; 13(7):1264. https://doi.org/10.3390/nano13071264
Chicago/Turabian StyleOsman, Abdalghaffar M., Abdulmajeed Hendi, and Nadir M. A. Osman. 2023. "Multiwalled Carbon Nanotubes-Modified Metallic Electrode Prepared Using Chemical Vapor Deposition as Sequential Injection Analysis Detector for Determination of Ascorbic Acid" Nanomaterials 13, no. 7: 1264. https://doi.org/10.3390/nano13071264
APA StyleOsman, A. M., Hendi, A., & Osman, N. M. A. (2023). Multiwalled Carbon Nanotubes-Modified Metallic Electrode Prepared Using Chemical Vapor Deposition as Sequential Injection Analysis Detector for Determination of Ascorbic Acid. Nanomaterials, 13(7), 1264. https://doi.org/10.3390/nano13071264







