Co3O4 Supported on Graphene-like Carbon by One-Step Calcination of Cobalt Phthalocyanine for Efficient Oxygen Reduction Reaction under Alkaline Medium
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Co3O4/C
2.3. Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
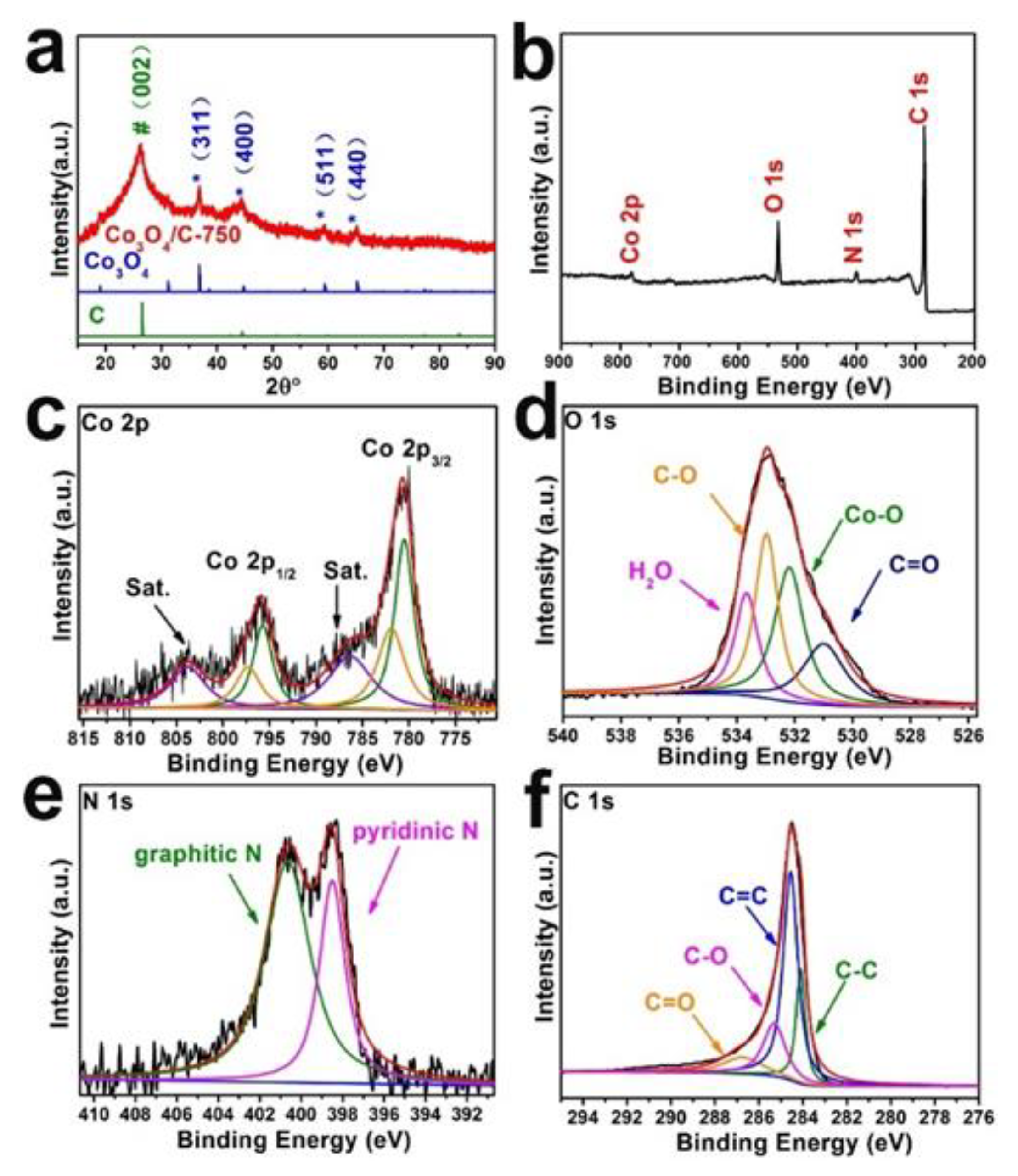
3.1. Morphological and Structural Characterization
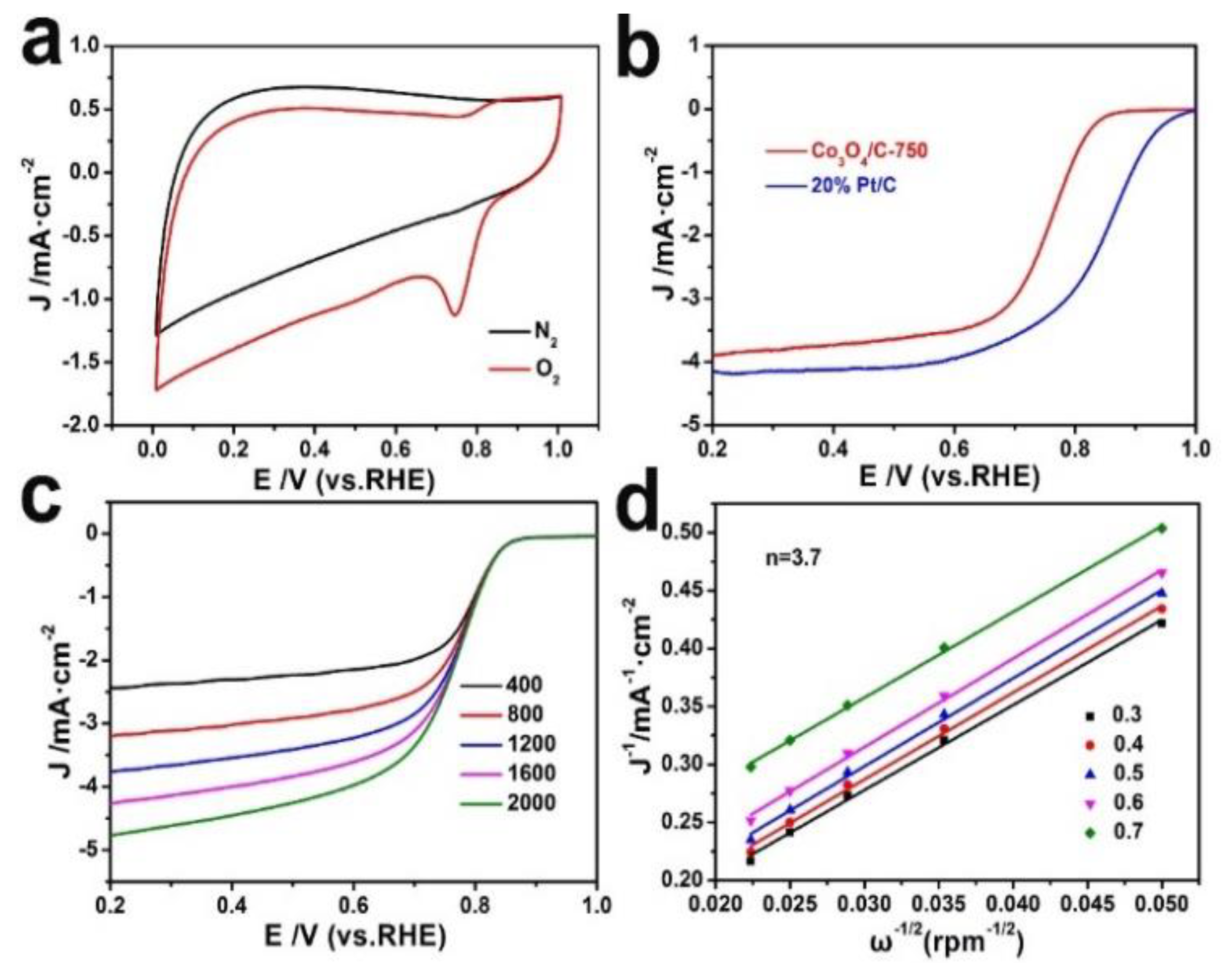
3.2. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guan, C.; Sumboja, A.; Wu, H.J.; Ren, W.N.; Liu, X.M.; Zhang, H.; Liu, Z.L.; Cheng, C.W.; Pennycook, S.J.; Wang, J. Hollow Co3O4 Nanosphere Embedded in Carbon Arrays for Stable and Flexible Solid-State Zinc–Air Batteries. Adv. Mater. 2017, 29, 1704117. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Chen, B.; Xu, H.R.; Cai, W.Z.; He, W.; Ni, M. In-situ growth of Co3O4 nanowire-assembled clusters on nickel foam for aqueous rechargeable Zn-Co3O4 and Zn-air batteries. Appl. Catal. B Environ. 2019, 241, 104–112. [Google Scholar] [CrossRef]
- Wang, Z.C.; Xu, W.J.; Chen, X.K.; Peng, Y.H.; Song, Y.Y.; Lv, C.X.; Liu, H.L.; Sun, J.W.; Yuan, D.; Li, X.Y.; et al. Defect-Rich Nitrogen Doped Co3O4/C Porous Nanocubes Enable High-Efficiency Bifunctional Oxygen Electrocatalysis. Adv. Funct. Mater. 2019, 29, 1902875. [Google Scholar] [CrossRef]
- Gao, R.; Yang, Z.Z.; Zheng, L.R.; Gu, L.; Liu, L.; Lee, Y.L.; Hu, Z.B.; Liu, X.F. Enhancing the Catalytic Activity of Co3O4 for Li−O2 Batteries through the Synergy of Surface/Interface/Doping Engineering. ACS Catal. 2018, 8, 1955–1963. [Google Scholar] [CrossRef]
- Han, P.; He, G.W.; He, Y.; Zheng, J.F.Z.X.R.; Li, L.L.; Zhong, C.; Hu, W.H.; Deng, Y.D.; Ma, T.-Y. Engineering Catalytic Active Sites on Cobalt Oxide Surface for Enhanced Oxygen Electrocatalysis. Adv. Energy Mater. 2018, 8, 1702222. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.Y.; Zhou, X.T.; Su, Y.H.; Riffat, S.; Liu, C.-J. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Lu, Y.; Du, S.; Steinberger-Wilckens, R. One-dimensional nanostructured electrocatalysts for polymer electrolyte membrane fuel cells—A review. Appl. Catal. B Environ. 2016, 199, 292–314. [Google Scholar] [CrossRef]
- Shao, M.H.; Chang, Q.W.; Dodelet, J.-P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Neburchilov, V.; Wang, H.; Martin, J.J.; Qu, W. A review on air cathodes for zinc-air fuel cells. J. Power Sources 2010, 195, 1271–1291. [Google Scholar] [CrossRef]
- Hamdani, M.; Singh, R.N.; Chartier, P. Co3O4 and Co-based spinel oxides bifunctional oxygen electrodes. Int. J. Electrochem. Sci. 2010, 5, 556–577. [Google Scholar] [CrossRef]
- Nikolova, V.; Iliev, P.; Petrov, K.; Vitanov, T.; Zhecheva, E.; Stoyanova, R.; Valov, I.; Stoychev, D. Electrocatalysts for bifunctional oxygen/air electrodes. J. Power Sources 2008, 185, 727–733. [Google Scholar] [CrossRef]
- Wang, B. Recent development of non-platinum catalysts for oxygen reduction reaction. J. Power Sources 2005, 152, 1–15. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metaleair batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Kawakita, K.; Abe, T.; Fukutsuka, T.; Kojima, K.; Ogumi, Z. Single-step synthesis of nano-sized perovskite-type oxide/carbon nanotube composites and their electrocatalytic oxygen-reduction activities. J. Mater. Chem. 2011, 21, 1913–1917. [Google Scholar] [CrossRef]
- Yuasa, M.; Nishida, M.; Kida, T.; Yamazoe, N.; Shimanoe, K. Bi-functional oxygen electrodes using LaMnO3/LaNiO3 for rechargeable metal-air batteries. J. Electrochem. Soc. 2011, 158, A605–A610. [Google Scholar] [CrossRef]
- Ríos, E.; Reyes, H.; Ortiz, J.; Gautier, J. Double channel electrode flow cell application to the study of HO2− production on MnxCo3−xO4 (0 ≤ x ≤ 1) spinel films. Electrochim. Acta 2005, 50, 2705–2711. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, J.; Zhang, J. NiCo2S4@graphene as a Bifunctional Electrocatalyst for Oxygen Reduction and Evolution Reactions. ACS Appl. Mater. Interfaces 2013, 5, 5002–5008. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shang, L.; Shi, R.; Zhang, X.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. 3D carbon nanoframe scaffold-immobilized Ni3FeN nanoparticle electrocatalysts for rechargeable zinc-air batteries’ cathodes. Nano Energy 2017, 40, 382–389. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Feng, Y.; Fang, L.; Wang, Y. Sandwich-like CoP/C nanocomposites as efficient and stable oxygen evolution catalysts. J. Mater. Chem. A 2016, 4, 9072–9079. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, X.Z.; Hin, J.N.C.; Wang, H.; Friedrich, K.A.; Schulz, M. A review of platinum-based catalyst layer degradation in proton exchange membrane fuel cells. J. Power Sources 2009, 194, 588–600. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, Y.; Wang, H.; Zhu, C.; Gao, J.; Wu, D.; Huang, H.; Liu, Y.; Kang, Z.H. A nitrogen and boron co-doped metal-free carbon electrocatalyst for an efficient oxygen reduction reaction. Inorg. Chem. Front. 2018, 5, 2985–2991. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Dhavale, V.M.; Kurungot, S. Surface-Tuned Co3O4 Nanoparticles Dispersed on Nitrogen-Doped Graphene as an Efficient Cathode Electrocatalyst for Mechanical Rechargeable Zinc–Air Battery. ACS Appl. Mater. Interfaces 2015, 7, 21138–21149. [Google Scholar] [CrossRef] [PubMed]
- Naoufal, B.; Ngamou, P.H.T.; Vincent, V.; Tilman, K.; Joachim, H.; Katharina, K.H.I. Tailoring the properties and the reactivity of the spinel cobalt oxide. Phys. Chem. Chem. Phys. 2009, 11, 9224–9232. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, B.; Liao, X.B.; Zhao, Y. Facet-dependent oxygen reduction reaction activity on the surfaces of Co3O4. Energy Environ. Mater. 2021, 4, 407–412. [Google Scholar] [CrossRef]
- He, X.B.; Yi, X.R.; Yin, F.X.; Chen, B.H.; Li, G.R.; Yin, H.Q. Less active CeO2 regulating bifunctional oxygen electrocatalytic activity of Co3O4@N-doped carbon for Zn–air batteries. J. Mater. Chem. A 2019, 7, 6753–6765. [Google Scholar] [CrossRef]
- Zhang, R.R.; Pan, L.; Guo, B.B.; Huang, Z.F.; Chen, Z.X.; Wang, L.; Zhang, X.W.; Guo, Z.Y.; Xu, W.; Loh, K.P.; et al. Tracking the role of defect types in Co3O4 structural evolution and active motifs during oxygen evolution reaction. J. Am. Chem. Soc. 2023, 145, 2271–2281. [Google Scholar] [CrossRef]
- Li, Y.G.; Gong, M.; Liang, Y.Y.; Feng, J.; Kim, J.-E.; Wang, H.L.; Hong, G.S.; Zhang, B.; Dai, H.J. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 2013, 4, 1805. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.G.; Wang, H.L.; Zhou, J.; Wang, J.; Regier, T.; Dai, H.J. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780. [Google Scholar] [CrossRef]
- Dou, S.; Li, X.Y.; Tao, L.; Huo, J.; Wang, S.Y. Cobalt nanoparticle-embedded carbon nanotube/porous carbon hybrid derived from MOF-encapsulated Co3O4 for oxygen electrocatalysis. Chem. Commun. 2016, 52, 9727–9730. [Google Scholar] [CrossRef]
- Illathvalappil, R.; Illathvalappil, S. Co9S8 nanoparticle-supported nitrogen-doped carbon as a robust catalyst for oxygen reduction reaction in both acidic and alkaline conditions. ChemElectroChem 2020, 7, 3123–3134. [Google Scholar] [CrossRef]
- Zhao, L.; Lan, Z.W.; Mo, W.H.; Su, J.Y.; Liang, H.Z.; Yao, J.Y.; Yang, W.H. High-level oxygen reduction catalysts derived from the compounds of high-specific-surface-area pine peel activated carbon and phthalocyanine cobalt. Nanomaterials 2021, 11, 3429–3440. [Google Scholar] [CrossRef] [PubMed]
- Ejsmont, A.; Kadela, K.; Grzybek, G.; Darvishzad, T.; Słowik, G.; Lofek, M.; Goscianska, J.; Kotarba, A.; Stelmachowski, P. Speciation of oxygen functional groups on the carbon support controls the electrocatalytic activity of cobalt oxide nanoparticles in the oxygen evolution reaction. ACS Appl. Mater. Interfaces 2023, 15, 5148–5160. [Google Scholar] [CrossRef]
- Jasinski, R. A New Fuel Cell Cathode Catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Guo, S.J.; Zhao, S.Q.; Wu, X.Q.; Li, H.; Zhou, Y.J.; Zhu, C.; Yang, N.J.; Jiang, X.; Gao, J.; Bai, L.; et al. A Co3O4-CDots-C3N4 three component electrocatalyst design concept for efficient and tunable CO2 reduction to syngas. Nat. Commun. 2017, 8, 1828. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Y.J.; Zhu, C.; Hu, L.L.; Han, M.M.; Wang, A.Q.; Huang, H.; Liu, Y.; Kang, Z.H. A Pt-Co3O4-CDs electrocatalyst with enhanced electrocatalytic performance and resistance to CO poisoning achieved by carbon dots and Co3O4 for direct methanol fuel cell. Nanoscale 2017, 9, 5467–5474. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Shang, C.Q.; Dong, S.M.; Wang, S.; Xiao, D.D.; Han, P.X.; Wang, X.G.; Gu, L.; Cui, G.L. Coaxial NixCo2x(OH)6x/TiN Nanotube Arrays as Supercapacitor Electrodes. ACS Nano 2013, 7, 5430–5436. [Google Scholar] [CrossRef]
- Xu, H.; Shi, Z.-X.; Tong, Y.-X.; Li, G.-R. Porous Microrod Arrays Constructed by Carbon-Confined NiCo@NiCoO2 Core@Shell Nanoparticles as Efficient Electrocatalysts for Oxygen Evolution. Adv. Mater. 2018, 30, 1705442. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Zhang, J.; Kou, Z.K.; Liu, X.B.; Asare, O.K.; Zhou, H.; Cheng, K.; Zhang, H.N.; Mai, L.Q.; Pan, M.; et al. Self-organized 3D porous graphene dual-doped with biomass-sponsored nitrogen and sulfur for oxygen reduction and evolution. ACS Appl. Mater. Interfaces 2016, 8, 29408–29418. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.; Zhu, Y.; Yang, X.; Li, C. Transition metals (Fe, Co, and Ni) encapsulated in nitrogen-doped carbon nanotubes as bi-functional catalysts for oxygen electrode reactions. J. Mater. Chem. A 2016, 4, 1694–1701. [Google Scholar] [CrossRef]
- Zhang, W.D.; Liu, X.M.; Gao, M.; Shang, H.; Liu, X.H. Co-Zn-MOFs derived N-doped carbon nanotubes with crystalline Co Nanoparticles embedded as effective oxygen electrocatalysts. Nanomaterials 2021, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Chutia, B.; Patowary, S.; Misra, A.; Rao, K.N.; Bharali, P. Morphology effect of Co3O4 nanooctahedron in boosting oxygen reduction and oxygen evolution reactions. Energy Fuels 2022, 36, 13863–13872. [Google Scholar] [CrossRef]
- Ashmath, S.; Kwon, H.J.; Peera, S.G.; Lee, T.G. Solid-state synthesis of cobalt/NCS electrocatalyst for oxygen reduction reaction in dual chamber microbial fuel cells. Nanomaterials 2022, 12, 4369. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.-X.; Wang, W.-J.; Song, R.-B.; Lv, J.-J.; Jiang, L.-P.; Zhu, J.-J. NaCl crystal tuning nitrogen self-doped porous graphitic carbon nanosheets for efficient oxygen reduction. ACS Sustain. Chem. Eng. 2017, 5, 10275–10282. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.; Liu, X.; Wang, M.; Huang, H.; Huang, P. Co3O4 Supported on Graphene-like Carbon by One-Step Calcination of Cobalt Phthalocyanine for Efficient Oxygen Reduction Reaction under Alkaline Medium. Nanomaterials 2023, 13, 1241. https://doi.org/10.3390/nano13071241
Tan H, Liu X, Wang M, Huang H, Huang P. Co3O4 Supported on Graphene-like Carbon by One-Step Calcination of Cobalt Phthalocyanine for Efficient Oxygen Reduction Reaction under Alkaline Medium. Nanomaterials. 2023; 13(7):1241. https://doi.org/10.3390/nano13071241
Chicago/Turabian StyleTan, Huang, Xunyu Liu, Miaohui Wang, Hui Huang, and Peipei Huang. 2023. "Co3O4 Supported on Graphene-like Carbon by One-Step Calcination of Cobalt Phthalocyanine for Efficient Oxygen Reduction Reaction under Alkaline Medium" Nanomaterials 13, no. 7: 1241. https://doi.org/10.3390/nano13071241
APA StyleTan, H., Liu, X., Wang, M., Huang, H., & Huang, P. (2023). Co3O4 Supported on Graphene-like Carbon by One-Step Calcination of Cobalt Phthalocyanine for Efficient Oxygen Reduction Reaction under Alkaline Medium. Nanomaterials, 13(7), 1241. https://doi.org/10.3390/nano13071241






