Alkali-Etched NiCoAl-LDH with Improved Electrochemical Performance for Asymmetric Supercapacitors
Abstract
1. Introduction
2. Materials and Methods
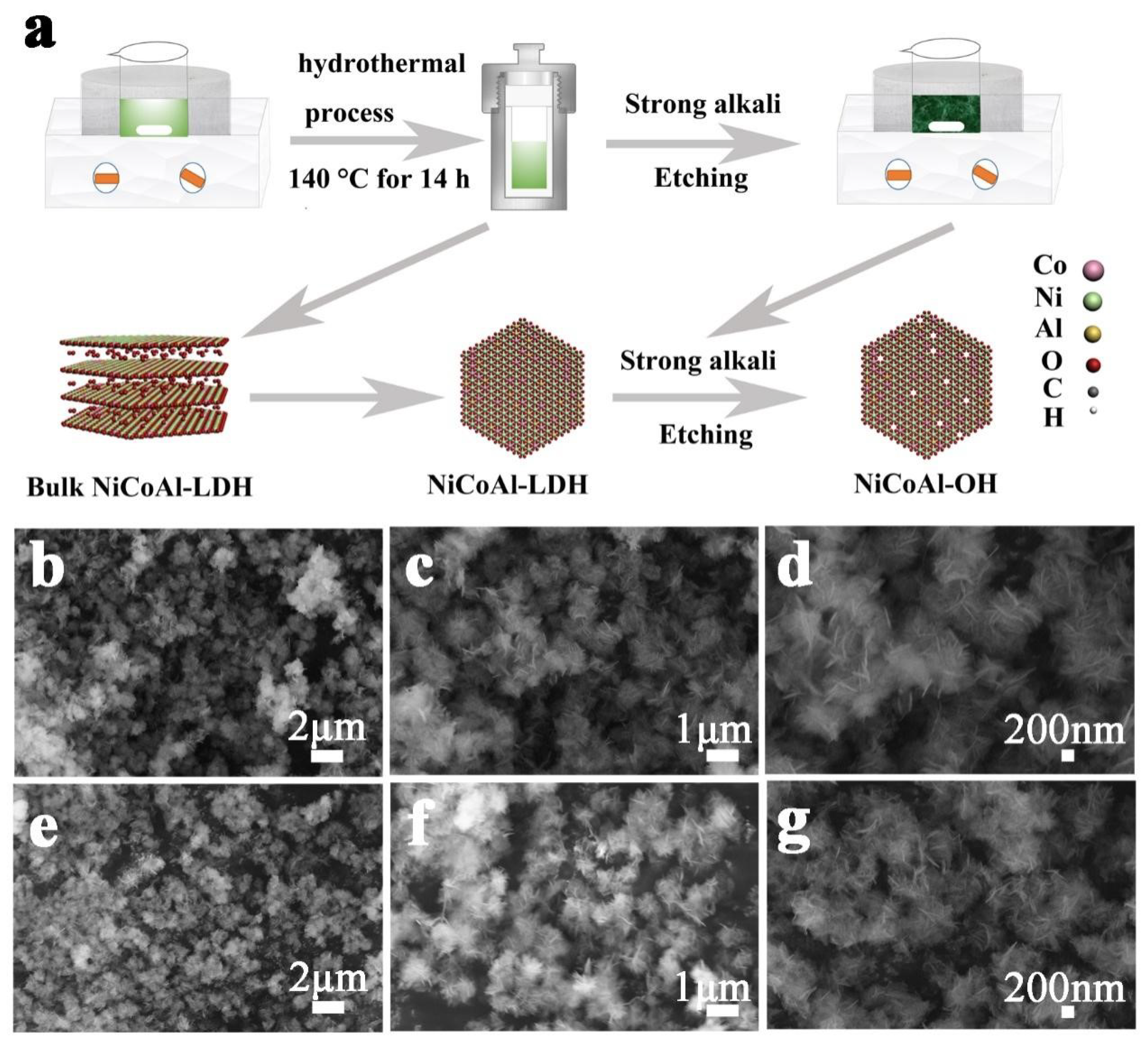
2.1. Preparation of NiCoAl-OH
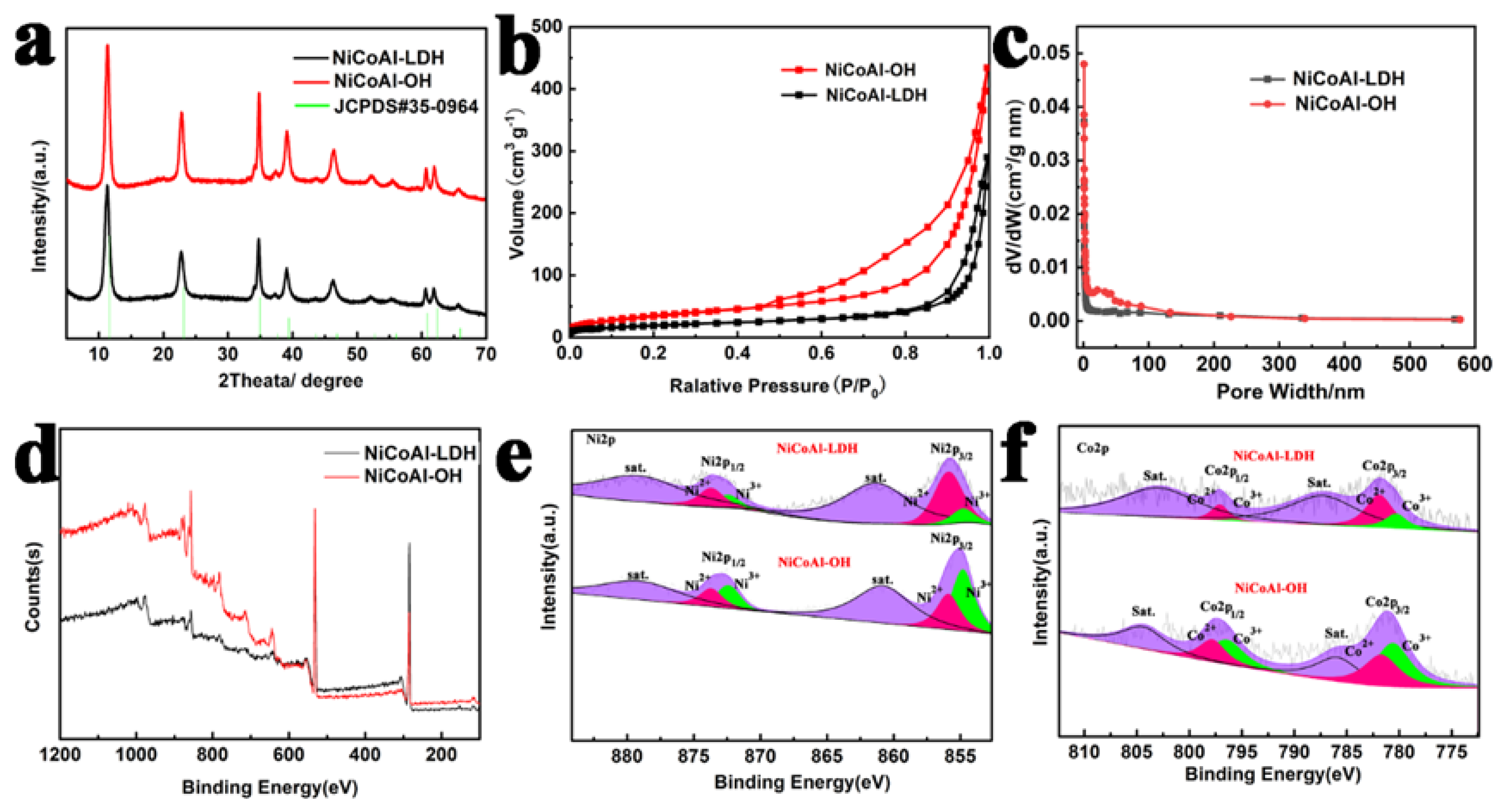
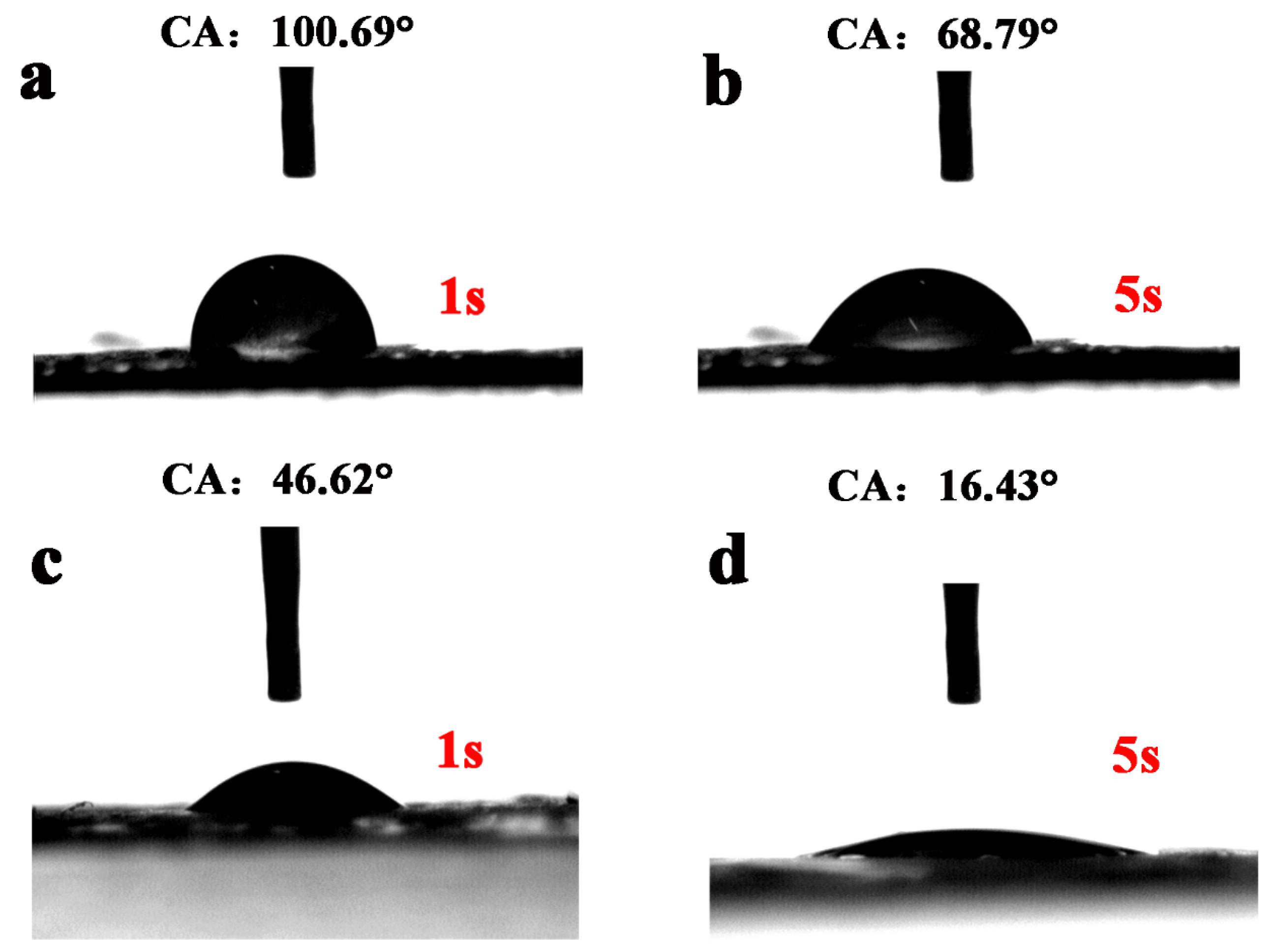
2.2. Structural and Morphological Characterization
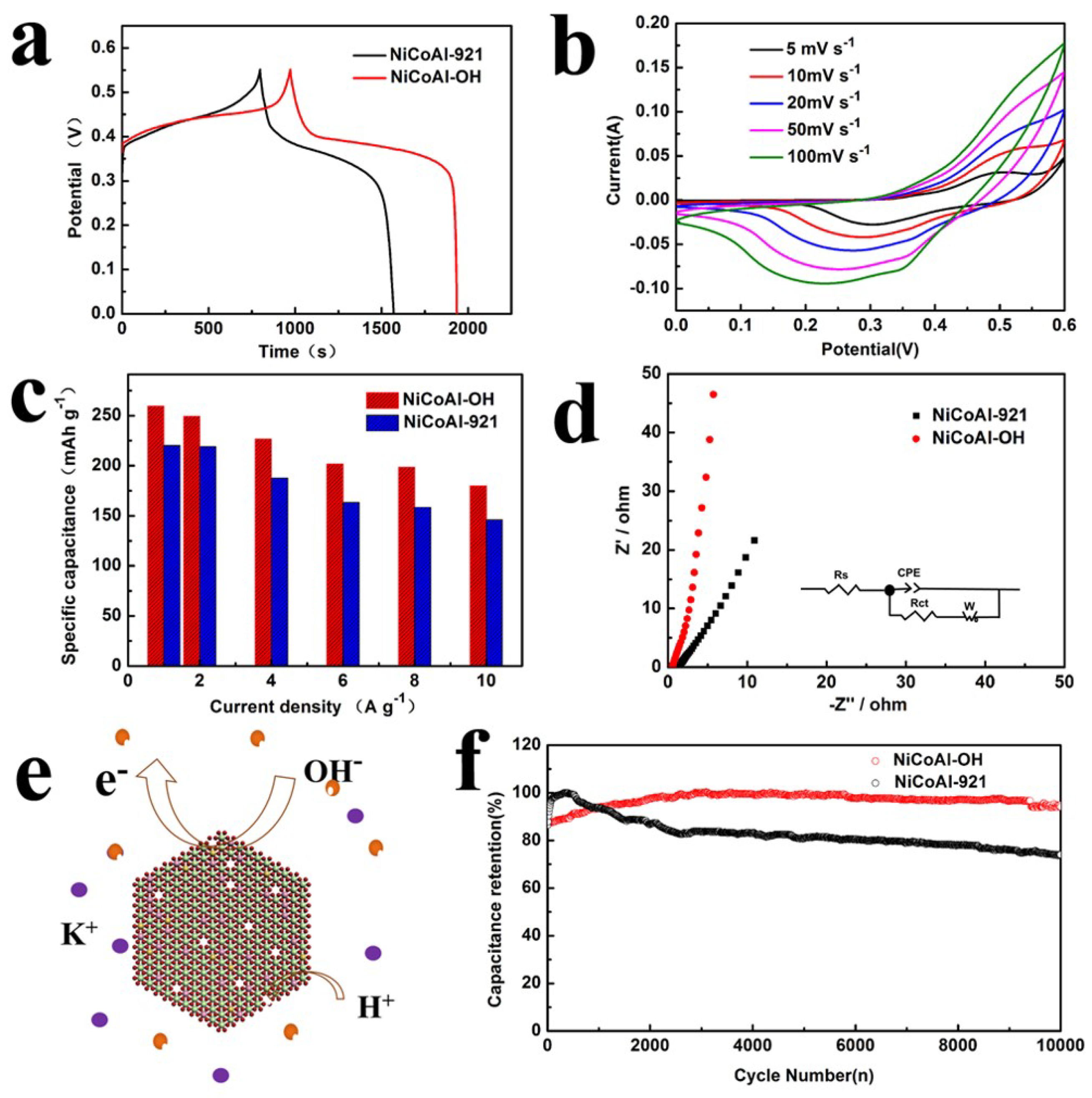
2.3. Electrochemical Measurements
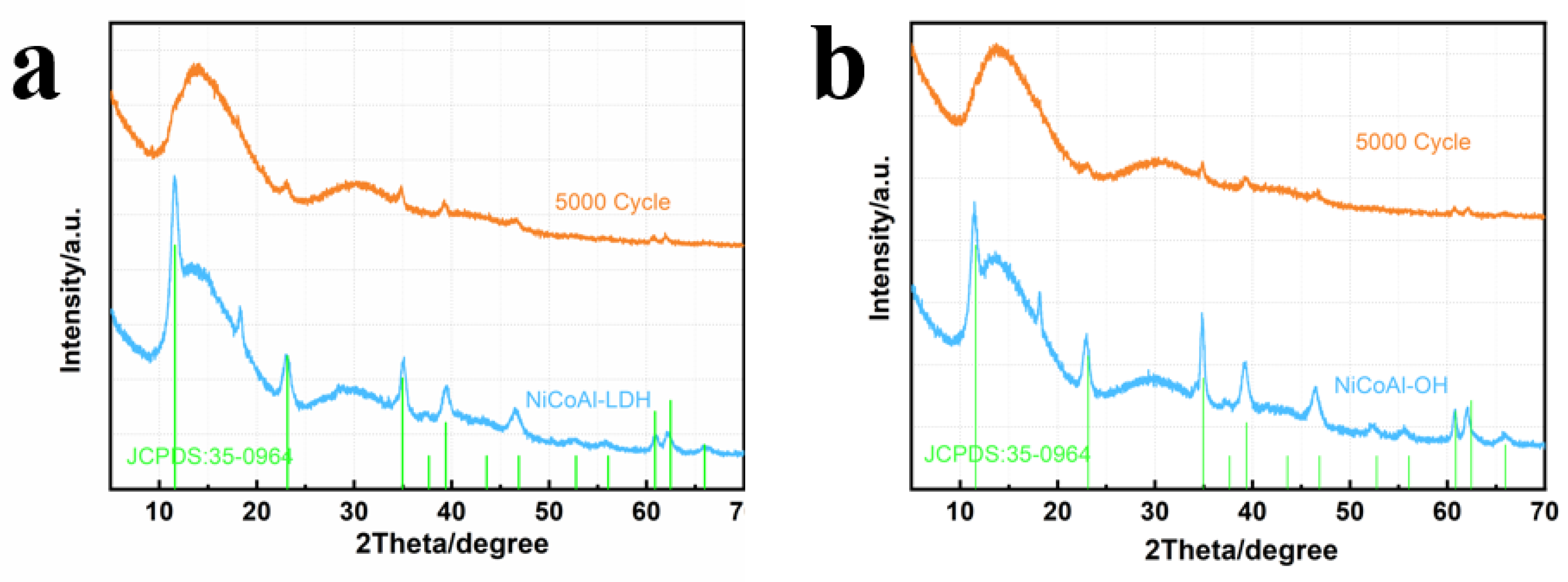
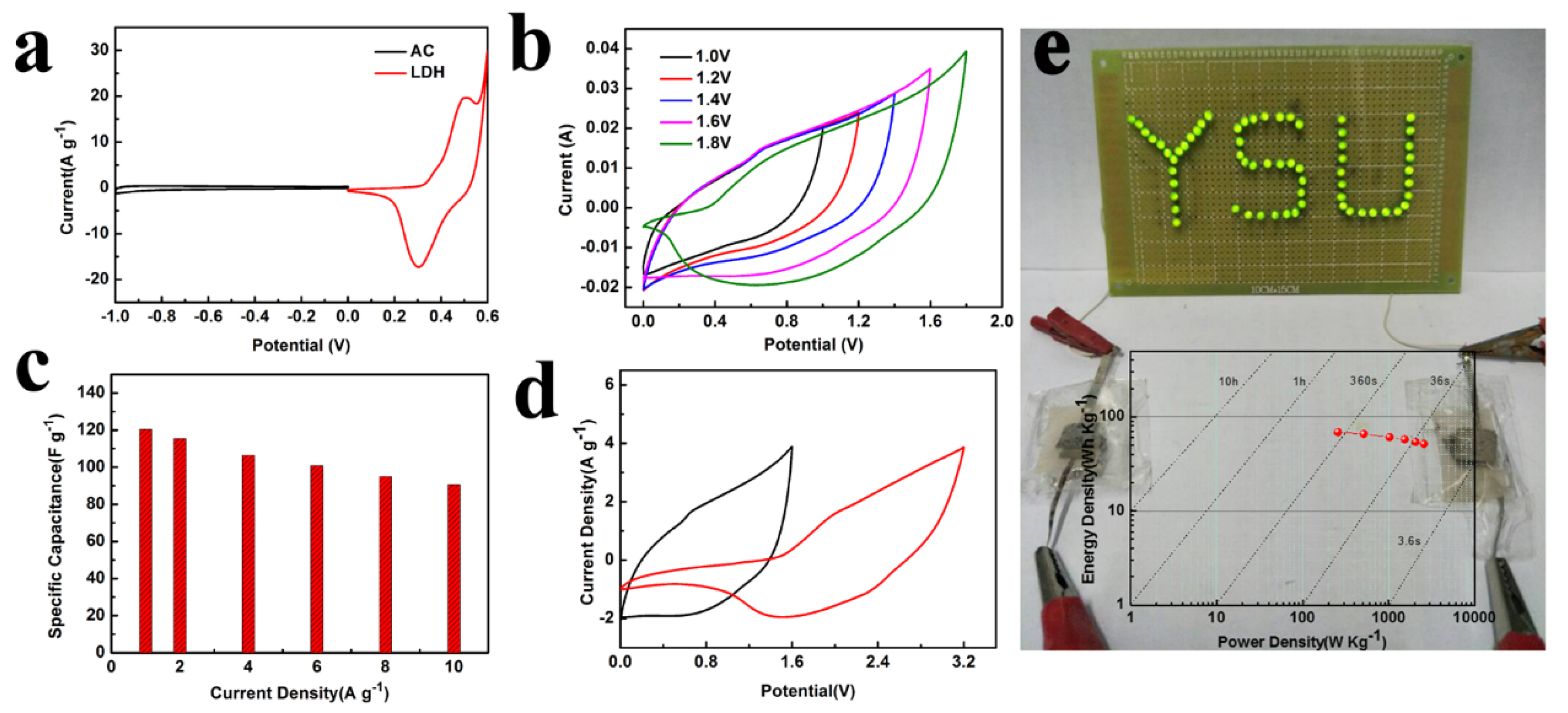
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Gao, L.; Du, Q.; Hou, L.; Ma, Z.; Qin, X.; Shao, G. Construction of NiCo2O4@ MnO2 nanosheet arrays for high-performance supercapacitor: Highly cross-linked porous heterostructure and worthy electrochemical double-layer capacitance contribution. J. Alloy. Compd. 2018, 749, 900–908. [Google Scholar] [CrossRef]
- Song, A.; Cao, L.; Yang, W.; Yang, W.; Wang, L.; Ma, Z.; Shao, G. In situ construction of nitrogen-doped graphene with surface-grown carbon nanotubes as a multifactorial synergistic catalyst for oxygen reduction. Carbon 2019, 142, 40–50. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, N.; Ye, Z.; Hong, Z.; Zhi, M. Synthesis of 3D hierarchical porous Ni–Co layered double hydroxide/N-doped reduced graphene oxide composites for supercapacitor electrodes. Inorg. Chem. Front. 2019, 6, 407–416. [Google Scholar] [CrossRef]
- Cao, L.; Ma, Y.; Song, A.; Bai, L.; Zhang, P.; Li, X.; Shao, G. Stable composite of flower-like NiFe-layered double hydroxide nucleated on graphene oxide as an effective catalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2019, 44, 5912–5920. [Google Scholar] [CrossRef]
- He, X.; Li, R.; Liu, J.; Liu, Q.; Song, D.; Wang, J. Hierarchical FeCo2O4@ NiCo layered double hydroxide core/shell nanowires for high performance flexible all-solid-state asymmetric supercapacitors. Chem. Eng. J. 2018, 334, 1573–1583. [Google Scholar] [CrossRef]
- Jayashree, R.; Kamath, P.V. Factors governing the electrochemical synthesis of α-nickel (II) hydroxide. J. Appl. Electrochem. 1999, 29, 449–454. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Huang, P.; Wang, H.; Cao, C.; Song, W. Carbonaceous aerogel and CoNiAl-LDH@ CA nanocomposites derived from biomass for high performance pseudo-supercapacitor. Sci. Bull. 2017, 62, 841–845. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, H.; Li, Z.; Sun, P.; Liu, F.; Dong, C.; Wang, J.; Li, Z.; Wu, M.; Zhang, C.; et al. Synthesis of delaminated layered double hydroxides and their assembly with graphene oxide for supercapacitor application. J. Alloys Compd. 2017, 711, 31–41. [Google Scholar] [CrossRef]
- Lan, Y.; Zhao, H.; Zong, Y.; Li, X.; Sun, Y.; Feng, J.; Wang, Y.; Zheng, X.; Du, Y. Phosphorization boosts the capacitance of mixed metal nanosheet arrays for high performance supercapacitor electrodes. Nanoscale 2018, 10, 11775–11781. [Google Scholar] [CrossRef]
- Sanati, S.; Rezvani, Z. g-C3N4 nanosheet@ CoAl-layered double hydroxide composites for electrochemical energy storage in supercapacitors. Chem. Eng. J. 2019, 362, 743–757. [Google Scholar] [CrossRef]
- Song, J.; Guo, X.; Zhang, J.; Chen, Y.; Zhang, C.; Luo, L.; Wang, F.; Wang, G. Rational design of free-standing 3D porous MXene/rGO hybrid aerogels as polysulfide reservoirs for high-energy lithium–sulfur batteries. J. Mater. Chem. A 2019, 7, 6507–6513. [Google Scholar] [CrossRef]
- Li, M.; Cheng, J.; Wang, J.; Liu, F.; Zhang, X. The growth of nickel-manganese and cobalt-manganese layered double hydroxides on reduced graphene oxide for supercapacitor. Electrochim. Acta 2016, 206, 108–115. [Google Scholar] [CrossRef]
- Li, L.; San Hui, K.; Hui, K.N.; Zhang, T.; Fu, J.; Cho, Y.R. High-performance solid-state flexible supercapacitor based on reduced graphene oxide/hierarchical core-shell Ag nanowire@ NiAl layered double hydroxide film electrode. Chem. Eng. J. 2018, 348, 338–349. [Google Scholar] [CrossRef]
- Liang, H.; Lin, J.; Jia, H.; Chen, S.; Qi, J.; Cao, J.; Lin, T.; Fei, W.; Feng, J. Hierarchical NiCo-LDH@ NiOOH core-shell heterostructure on carbon fiber cloth as battery-like electrode for supercapacitor. J. Power Sources 2018, 378, 248–254. [Google Scholar] [CrossRef]
- Lin, J.; Jia, H.; Liang, H.; Chen, S.; Cai, Y.; Qi, J.; Qu, C.; Cao, J.; Fei, W.; Feng, J. Hierarchical CuCo2S4@ NiMn-layered double hydroxide core-shell hybrid arrays as electrodes for supercapacitors. Chem. Eng. J. 2018, 336, 562–569. [Google Scholar] [CrossRef]
- Zhao, Y.; He, X.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Li, J.; Zhang, H.; Dong, H.; Zhang, M.; et al. A flexible all-solid-state asymmetric supercapacitors based on hierarchical carbon cloth@ CoMoO4@ NiCo layered double hydroxide core-shell heterostructures. Chem. Eng. J. 2018, 352, 29–38. [Google Scholar] [CrossRef]
- Zhang, H.; Tahir, M.U.; Yan, X.; Liu, X.; Su, X.; Zhang, L. Ni-Al layered double hydroxide with regulated interlayer spacing as electrode for aqueous asymmetric supercapacitor. Chem. Eng. J. 2019, 368, 905–913. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Yang, Y.; Luo, B.; Zhang, X.; Fong, E.; Chu, W.; Huang, K. Unique 3D flower-on-sheet nanostructure of NiCo LDHs: Controllable microwave-assisted synthesis and its application for advanced supercapacitors. J. Alloys Compd. 2019, 788, 1029–1036. [Google Scholar] [CrossRef]
- Xiao, Z.; Mei, Y.; Yuan, S.; Mei, H.; Xu, B.; Bao, Y.; Fan, L.; Kang, W.; Dai, F.; Wang, R.; et al. Controlled hydrolysis of metal–organic frameworks: Hierarchical Ni/Co-layered double hydroxide microspheres for high-performance supercapacitors. ACS Nano 2019, 13, 7024–7030. [Google Scholar] [CrossRef]
- Li, P.; Jiao, Y.; Yao, S.; Wang, L.; Chen, G. Dual role of nickel foam in NiCoAl-LDH ensuring high-performance for asymmetric supercapacitors. New J. Chem. 2019, 43, 3139–3145. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y.; Su, Y.; Zhang, B.; Li, C.; Wang, H.; Wang, L. Design and synthesis of ternary-component layered double hydroxides for high-performance supercapacitors: Understanding the role of trivalent metal ions. Electrochim. Acta 2017, 225, 263–271. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, S. Mg-Co-Al-LDH nanoparticles with attractive electrochemical performance for supercapacitor. J. Nanoparticle Res. 2019, 21, 1–11. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, H.J. Confinement of Zn-Mg-Al-layered double hydroxide and α-Fe2O3 nanorods on hollow porous carbon nanofibers: A free-standing electrode for solid-state symmetric supercapacitors. Chem. Eng. J. 2022, 429, 132345. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, A.A.; Lohani, P.C.; Yoo, D.J.; Kim, H.J. Assembling zinc cobalt hydroxide/ternary sulfides heterostructure and iron oxide nanorods on three-dimensional hollow porous carbon nanofiber as high energy density hybrid supercapacitor. J. Energy Storage 2023, 60, 106713. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, R.; Osada, M.; Takada, K.; Sasaki, T. Selective and controlled synthesis of α-and β-cobalt hydroxides in highly developed hexagonal platelets. J. Am. Chem. Soc. 2005, 127, 13869–13874. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, C.; Fan, X.; Qiu, J. 3D architecture materials made of NiCoAl-LDH Nanoplates coupled with NiCo-carbonate hydroxide nanowires grown on flexible graphite paper for asymmetric supercapacitors. Adv. Energy Mater. 2014, 4, 1400761. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, M.; Li, Y.; Wang, S. Tuning surface electronic configuration of NiFe LDHs nanosheets by introducing cation vacancies (Fe or Ni) as highly efficient electrocatalysts for oxygen evolution reaction. Small 2018, 14, 1800136. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xuan, H.; Xu, Y.; Liang, T.; Han, X.; Yang, J.; Han, P.; Wang, D.; Du, Y. Interconnected network of zinc-cobalt layered double hydroxide stick onto rGO/nickel foam for high performance asymmetric supercapacitors. Electrochim. Acta 2018, 286, 92–102. [Google Scholar] [CrossRef]
- Hou, L.; Du, Q.; Su, L.; Di, S.; Ma, Z.; Chen, L.; Shao, G. Ni-Co layered double hydroxide with self-assembled urchin like morphology for asymmetric supercapacitors. Mater. Lett. 2019, 237, 262–265. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, R.; Hui, K.N.; San Hui, K.; Lee, H. Hierarchical ultrathin NiAl layered double hydroxide nanosheet arrays on carbon nanotube paper as advanced hybrid electrode for high performance hybrid capacitors. Chem. Eng. J. 2017, 325, 554–563. [Google Scholar] [CrossRef]
- Wang, L.; Qin, K.; Li, J.; Zhao, N.; Shi, C.; Ma, L.; He, C.; He, F.; Liu, E. Nanotubular Ni-supported graphene@ hierarchical NiCo-LDH with ultrahigh volumetric capacitance for supercapacitors. Appl. Surf. Sci. 2018, 453, 230–237. [Google Scholar] [CrossRef]
- Wu, H.; Shi, P.; Gan, Y.; Wang, C.; Li, H.; Zheng, Z.; Wan, J.; Li, J.; Lv, L.; Tao, L.; et al. Mn-dopant induced Octahedral Configuration Strongly Stabilizes Ni12P5 Nanowires for Battery-Supercapacitor Hybrid Devices. J. Alloys Compd. 2022, 903, 163897. [Google Scholar] [CrossRef]
- Su, D.; Tang, Z.; Xie, J.; Bian, Z.; Zhang, J.; Yang, D.; Zhang, D.; Wang, J.; Liu, Y.; Yuan, A.; et al. Co, Mn-LDH nanoneedle arrays grown on Ni foam for high performance supercapacitors. Appl. Surf. Sci. 2019, 469, 487–494. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, J.; Rafiq, M.I.; Dai, S.; Tang, J.; Tang, W. Growth of NiMn layered double hydroxide and polypyrrole on bacterial cellulose nanofibers for efficient supercapacitors. Electrochim. Acta 2019, 295, 82–91. [Google Scholar] [CrossRef]
- Long, D.; Liu, H.; Yuan, Y.; Li, J.; Li, Z.; Zhu, J. A facile and large-scale synthesis of NiCo-LDHs@ rGO composite for high performance asymmetric supercapacitors. J. Alloys Compd. 2019, 805, 1096–1105. [Google Scholar] [CrossRef]
- Zou, J.; Xie, D.; Xu, J.; Song, X.; Zeng, X.; Wang, H.; Zhao, F. Rational design of honeycomb Ni-Co LDH/graphene composite for remarkable supercapacitor via ultrafast microwave synthesis. Appl. Surf. Sci. 2022, 571, 151322. [Google Scholar] [CrossRef]
- Zheng, K.; Liao, L.; Zhang, Y.; Tan, H.; Liu, J.; Li, C.; Jia, D. Hierarchical NiCo-LDH core/shell homostructural electrodes with MOF-derived shell for electrochemical energy storage. J. Colloid Interface Sci. 2022, 619, 75–83. [Google Scholar] [CrossRef]
- Li, S.; Luo, Y.; Wang, C.; Wu, M.; Xue, Y.; Yang, J.; Li, L. A novel hierarchical core-shell structure of NiCo2O4@ NiCo-LDH nanoarrays for higher-performance flexible all-solid-state supercapacitor electrode materials. J. Alloys Compd. 2022, 920, 165986. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Zhou, X.; Kong, L.; Ma, Z.; Su, L.; Liu, Z.; Shao, G. Alkali-Etched NiCoAl-LDH with Improved Electrochemical Performance for Asymmetric Supercapacitors. Nanomaterials 2023, 13, 1192. https://doi.org/10.3390/nano13071192
Hou L, Zhou X, Kong L, Ma Z, Su L, Liu Z, Shao G. Alkali-Etched NiCoAl-LDH with Improved Electrochemical Performance for Asymmetric Supercapacitors. Nanomaterials. 2023; 13(7):1192. https://doi.org/10.3390/nano13071192
Chicago/Turabian StyleHou, Liyin, Xufeng Zhou, Lina Kong, Zhipeng Ma, Li Su, Zhaoping Liu, and Guangjie Shao. 2023. "Alkali-Etched NiCoAl-LDH with Improved Electrochemical Performance for Asymmetric Supercapacitors" Nanomaterials 13, no. 7: 1192. https://doi.org/10.3390/nano13071192
APA StyleHou, L., Zhou, X., Kong, L., Ma, Z., Su, L., Liu, Z., & Shao, G. (2023). Alkali-Etched NiCoAl-LDH with Improved Electrochemical Performance for Asymmetric Supercapacitors. Nanomaterials, 13(7), 1192. https://doi.org/10.3390/nano13071192






