Silver Nanoclusters Tunable Visible Emission and Energy Transfer to Yb3+ Ions in Co-Doped GeO2-PbO Glasses for Photonic Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuznetsov, A.S.; Tikhomirov, V.K.; Shestakov, M.V.; Moshchalkov, V.V. Ag nanocluster functionalized glasses for efficient photonic conversion in light sources, solar cells and flexible screen monitors. Nanoscale 2013, 5, 10065–10075. [Google Scholar] [CrossRef]
- Díez, I.; Ras, R. Fluorescent silver nanoclusters. Nanoscale 2011, 3, 1963–1970. [Google Scholar] [CrossRef]
- Tikhomirov, V.K.; Rodríguez, V.D.; Kuznetsov, A.; Kirilenko, D.; Van Tendeloo, G.; Moshchalkov, V.V. Preparation and luminescence of bulk oxyfluoride glasses doped with Ag nanoclusters. Opt. Express 2010, 18, 22032–22040. [Google Scholar] [CrossRef]
- Kuznetsov, A.S.; Cuong, N.T.; Tikhomirov, V.K.; Jivanescu, M.; Stesmans, A.; Chibotaru, L.F.; Velázquez, J.J.; Rodríguez, V.D.; Kirilenko, D.; Van Tendeloo, G.; et al. Effect of heat-treatment on luminescence and structure of Ag nanoclusters doped oxyfluoride glasses and implication for fiber drawing. Opt. Mater. 2012, 34, 616–621. [Google Scholar] [CrossRef]
- Fares, H.; Santos, S.N.C.; Santos, M.V.; Franco, D.F.; Souza, A.E.; Manzani, D.; Mendonça, C.R.; Nalin, M. Higly luminescent silver nanocluster-doped fluorophosphate glasses for microfabrication of 3D waveguides. RSC Adv. 2017, 7, 55935–55944. [Google Scholar] [CrossRef]
- Fares, H.; Castro, T.; Orives, J.R.; Franco, D.F.; Nalin, M. White light and multicolor emission tuning in Ag nanocluster doped fluorophosphate glasses. RSC Adv. 2017, 7, 44356–44365. [Google Scholar] [CrossRef]
- Ennouri, M.; Gelloz, B.; Elhouichet, H. Impact of Ag species on luminescence and spectroscopic properties of Eu3+ doped fluoro-phosphate glasses. J. Non-Cryst. Solids 2021, 570, 120938. [Google Scholar] [CrossRef]
- Liu, X.Y.; Guo, H.; Ye, S.; Peng, M.Y.; Zhang, Q.Y. Enhanced tunable color emission in transparent Ag/Mn2+ codoped zinc borate glasses for broad band light source. J. Mater. Chem. C 2015, 3, 5183–5191. [Google Scholar] [CrossRef]
- Sandrini, M.; Muniz, R.F.; Zanuto, V.S.; Pedrochi, F.; Guyot, Y.; Bento, A.C.; Baesso, M.L.; Steimacher, A.; Neto, A.M. Enhanced and tunable white light emission from Ag nanoclusters and Eu3+-co-doped CaBAl glasses. RSC Adv. 2018, 8, 35263–35270. [Google Scholar] [CrossRef]
- Shestakov, M.; Chen, X.; Kaydashev, V.; Baeckelant, W.; Tikhomirov, V.; Vanacken, J.; Hofkens, J.; Moshchalkov, V. Oxyfluoride glass (SiO2-PbF2) co-doped with Ag nanoclusters and Tm3+ ions for UV-driven, Hg-free, white light generation with a tuneable tint. Opt. Mater. Express 2014, 4, 1227–1235. [Google Scholar] [CrossRef]
- Kuznetsov, A.S.; Velázquez, J.J.; Tikhomirov, V.K.; Mendez-Ramos, J.; Moshchalkov, V.V. Quantum yield of luminescence of Ag nanoclusters dispersed within transparent bulk glass vs. glass composition and temperature. Appl. Phys. Lett. 2012, 101, 251106. [Google Scholar] [CrossRef]
- Liao, H.; Ye, S.; Shen, R.; Li, X.; Wang, D. Effective formation of Ag nanoclusters and efficient energy transfer to Yb3+ ions in borosilicate glasses for photovoltaic application. Mater. Res. Bull. 2019, 111, 113–117. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, S.; Yu, J.; Liao, H.; Liu, J.; Wang, D. Simultaneous energy transfer from molecular-like silver nanoclusters to Sm3+/Ln3+ (Ln = Eu or Tb) in glass under UV excitation. Optics Express 2019, 27, 38159–38167. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P. Nanophotonics; Wiley: New York, NY, USA, 2004. [Google Scholar]
- Velázquez, J.J.; Tikhomirov, V.K.; Chibotaru, L.F.; Cuong, N.T.; Kuznetsov, A.S.; Rodríguez, V.D.; Nguyen, M.T.; Moshchalkov, V.V. Energy level diagram and kinetics of luminescence of Ag nanoclusters dispersed in a glass host. Opt. Express 2012, 20, 13582–13591. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Stambouli, W.; Mohamed, S.A.; Elhouichet, H. Ag nanoparticles induced luminescence enhancement of Eu3+ doped phosphate glasses. J. Alloys Compd. 2017, 705, 550–558. [Google Scholar] [CrossRef]
- Trave, E.; Back, M.; Cattaruzza, E.; Gonella, F.; Enrichi, F.; Cesca, T.; Kalinic, B.; Scian, C.; Bello, V.; Maurizio, C.; et al. Control of silver clustering for broadband Er3+ luminescence sensitization in Er and Ag co-implanted silica. J. Lumin. 2018, 197, 104–111. [Google Scholar] [CrossRef]
- Xu, B.; Chen, P.; Zhou, S.; Hong, Z.; Hao, J.; Qiu, J. Enhanced broadband near-infrared luminescence in Bi-doped glasses by co-doping with Ag. J. Appl. Phys. 2013, 113, 183506. [Google Scholar] [CrossRef]
- Li, J.J.; Chen, J.D.; Wei, R.F.; Guo, H. Combined White Luminescence from Eu3+, ML–Ag Particles and Ag+ in Ag–Eu3+ Co-Doped H3BO3–BaF2 Glasses. J. Am. Ceram. Soc. 2012, 95, 1208–1211. [Google Scholar] [CrossRef]
- Castro, T.; Jubera, V.; Fares, H.; Petit, Y.; Fargues, A.; Cardinal, T.; Nalin, M.; Ribeiro, S. Photoluminescence of Ag+ and Agmn+ in co-doped Pr3+/Yb3+ fuorophosphate glasses: Tuning visible emission and energy transfer to Pr3+/Yb3+ ions through excitation in diferent silver species. J. Mater. Sci. Mater 2019, 30, 16878–16885. [Google Scholar] [CrossRef]
- Fares, H.; Castro, T.; Franco, D.F.; Fucikova, A.; da Silva, R.R.; Valenta, J.; Ribeiro, S.J.L.; Nalin, M. Tuning multicolor emission in AgNCs/Tm3+/Mn2+—doped fluorophosphate glasses. J. Non. Cryst. Solids 2020, 535, 119968. [Google Scholar] [CrossRef]
- Lin, H.; Chen, D.; Yu, Y.; Zhang, R.; Wang, Y. Molecular-like Ag clusters sensitized near-infrared down-conversion luminescence in oxyfluoride glasses for broadband spectral modification. Appl. Phys. Lett. 2013, 103, 091902–091905. [Google Scholar] [CrossRef]
- Bordon, C.; Dipold, J.; Freitas, A.; Wetter, N.; de Rossi, W.; Kassab, L. A new double-line waveguide architecture for photonic applications using fs laser writing in Nd3+ doped GeO2-PbO glasses. Opt. Mater. 2022, 129, 112495. [Google Scholar] [CrossRef]
- Carvalho, D.; Kassab, L.; Del Cacho, V.; da Silva, D.; Alayo, M. A review on pedestal waveguides for low loss optical guiding, optical amplifiers and nonlinear optics applications. J. Lumin. 2018, 203, 135–144. [Google Scholar] [CrossRef]
- Gunji, R.; Santos, E.; Bordon, C.; Garcia, J.; Gómez-Malagón, L.; Kassab, L. Germanate glass layer containing Eu3+ ions and gold nanoparticles for enhanced silicon solar cell performance. J. Lumin. 2020, 226, 117497. [Google Scholar] [CrossRef]
- Camilo, M.; Silva, E.; Kassab, L.; Garcia, J.; de Araújo, C. White light generation controlled by changing the concentration of silver nanoparticles hosted by Ho3+/Tm3+/Yb3+ doped GeO2–PbO glasses. J. Alloys Compd. 2015, 644, 155–158. [Google Scholar] [CrossRef]
- Nishimura, M.V.M.; Bordon, C.D.S.; Miretzcky, L.M.; Kassab, L.R.P. Broadband visible light emission by GeO2-PbO glasses doped with Ag nanoclusters. In Proceedings of the 2021 SBMO/IEEE MTT-S International Microwave and Optoelectronics Conference (IMOC), Fortaleza, Brazil, 24–27 October 2021. [Google Scholar]
- Ma, R.; Qian, J.; Cui, S.; Qiao, X.; Wang, F.; Fan, X. Enhancing NIR emission of Yb3+ by silver nanoclusters in oxyfluoride glass. J. Lumin. 2014, 152, 222–225. [Google Scholar] [CrossRef]
- Tikhomirov, V.K.; Vosch, T.; Fron, E.; Rodríguez, V.D.; Velázquez, J.J.; Kirilenko, D.; Tendeloo, G.V.; Hofkens, J.; Van der Auweraer, M.; Moshchalkov, V.V. Luminescence of oxyfluoride glasses co-doped with Ag nanoclusters and Yb3+ ions. RSC Adv. 2012, 2, 1496–1501. [Google Scholar] [CrossRef]
- Hull, S. Superionics: Crystal structures and conduction processes. Rep. Prog. Phys. 2004, 67, 1233–1314. [Google Scholar] [CrossRef]
- Pontuschka, W.; Giehl, J.; Miranda, A.; Da Costa, Z.; Alencar, A.M. Effect of the Al2O3 addition on the formation of silver nanoparticles in heat treated soda-lime silicate glasses. J. Non-Cryst. Solids 2016, 453, 74–83. [Google Scholar] [CrossRef]
- Giehl, J.; Pontuschka, W.; Barbosa, L.; Chillcce, E.; Da Costa, Z.; Alves, S. Thermal precipitation of silver nanoparticles and thermoluminescence in tellurite glasses. Opt. Mater. 2011, 33, 1884–1891. [Google Scholar] [CrossRef]
- McQuarrie, D.A.; Simon, J.A. Physical Chemistry a Molecular Approach; University science books: Sausalito, CA, USA, 1997. [Google Scholar]
- Colvin, M.T.; Smeigh, A.L.; Giacobbe, E.M.; Conron, S.M.M.; Ricks, A.B.; Wasielewski, M.R. Ultrafast intersystem crossing and spin dynamics of zinc meso-tetraphenylporphyrin covalently bound to stable radicals. J. Phys. Chem. A 2011, 115, 7538–7549. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Su, Q. Luminescence of Pb2+ and Energy Transfer from Pb2+ to Rare Earth Ions in Silicate Oxyapatites. Phys. Solid State 1996, 196, 261–267. [Google Scholar] [CrossRef]

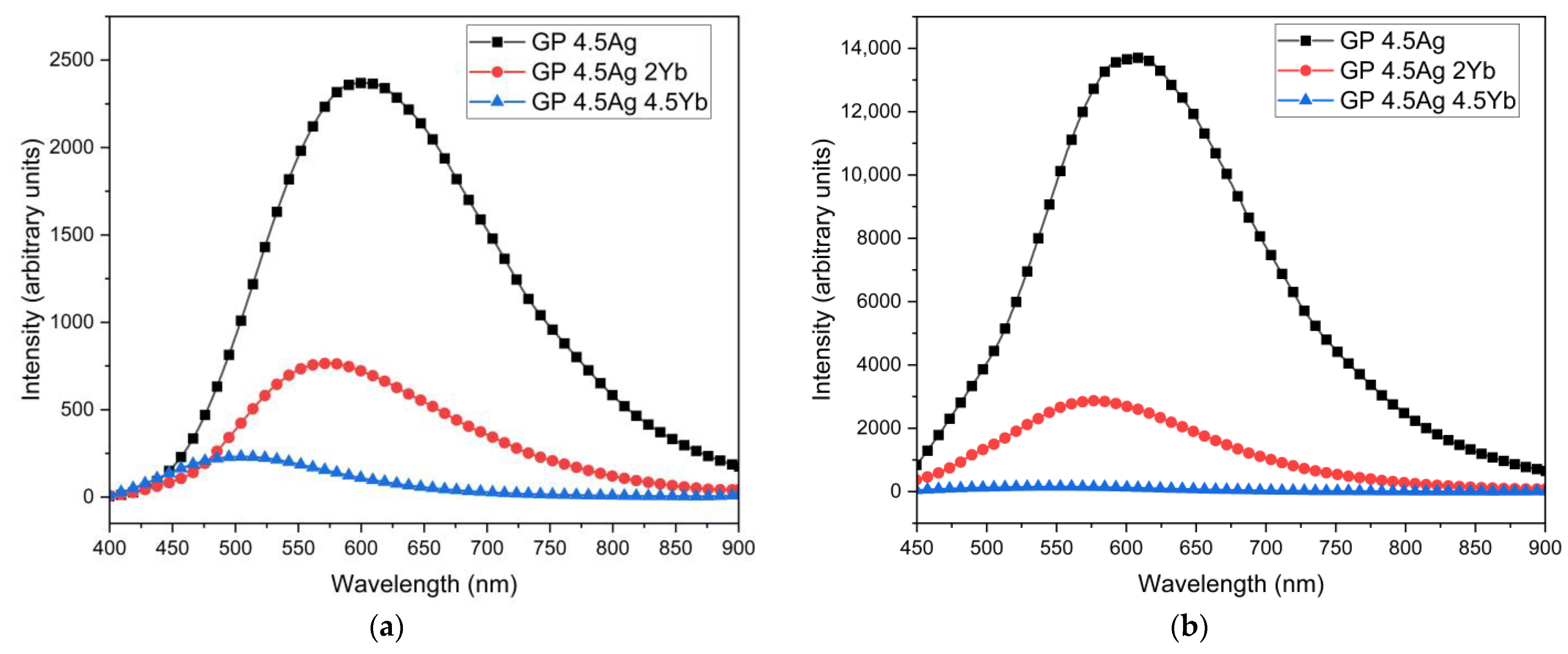

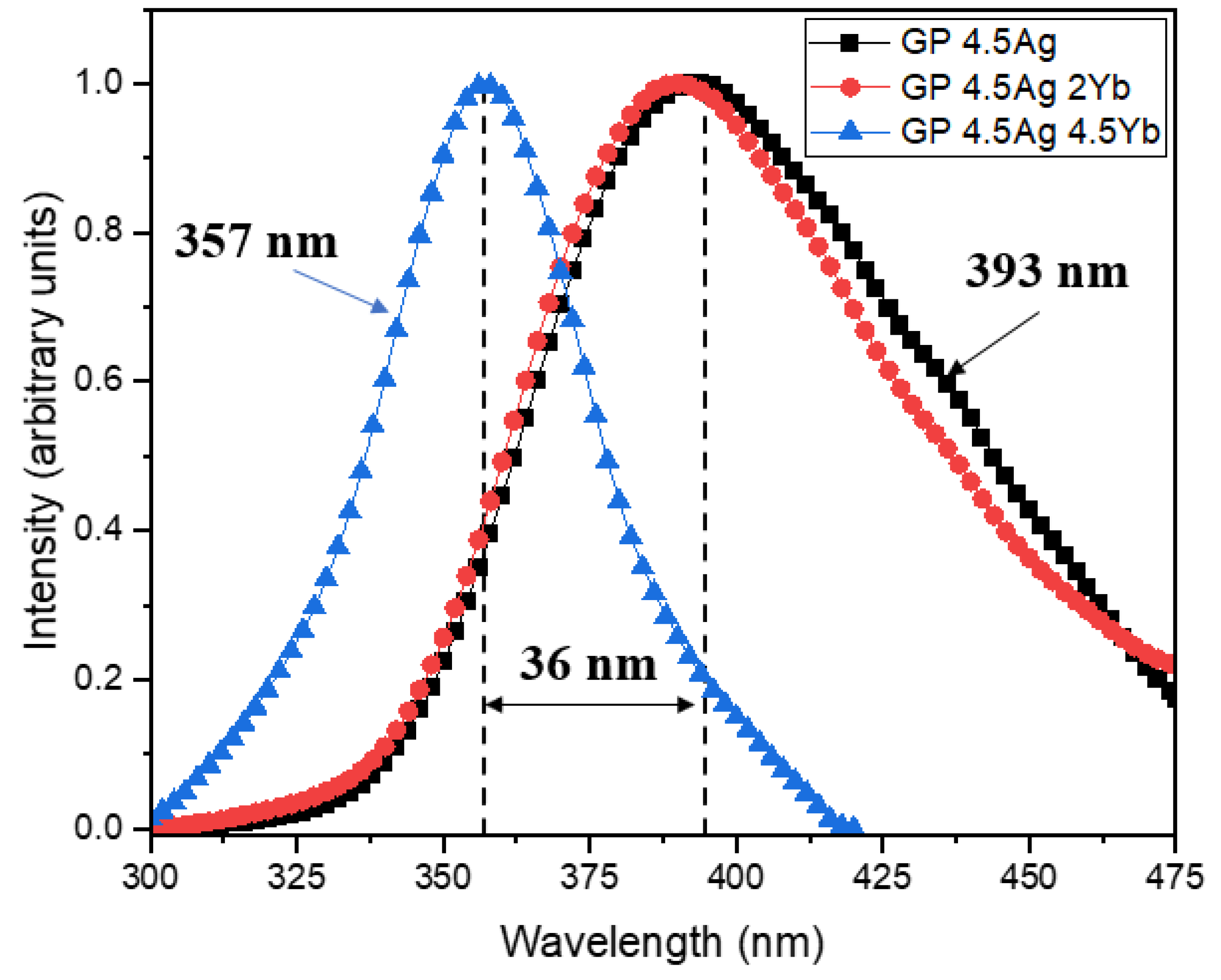
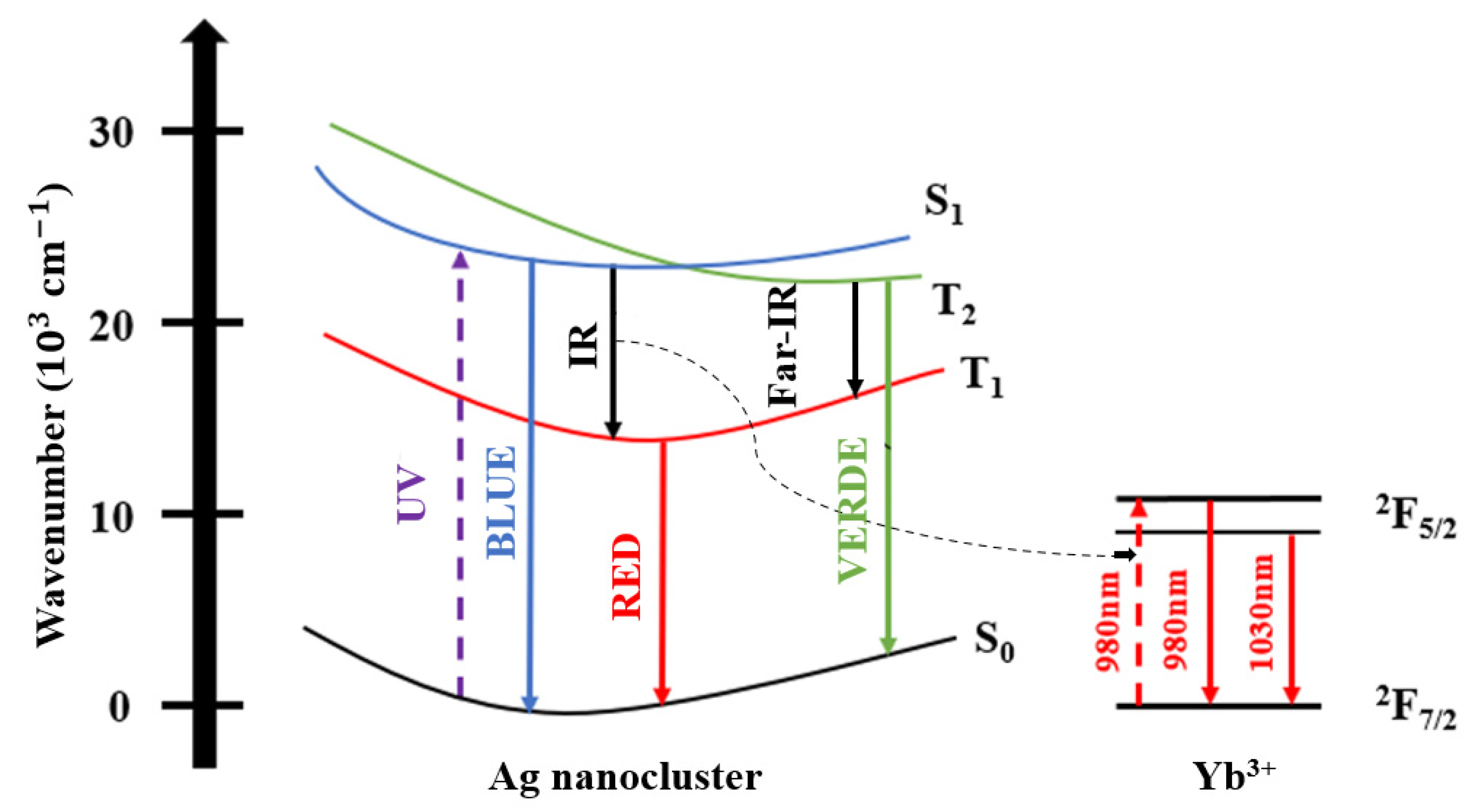

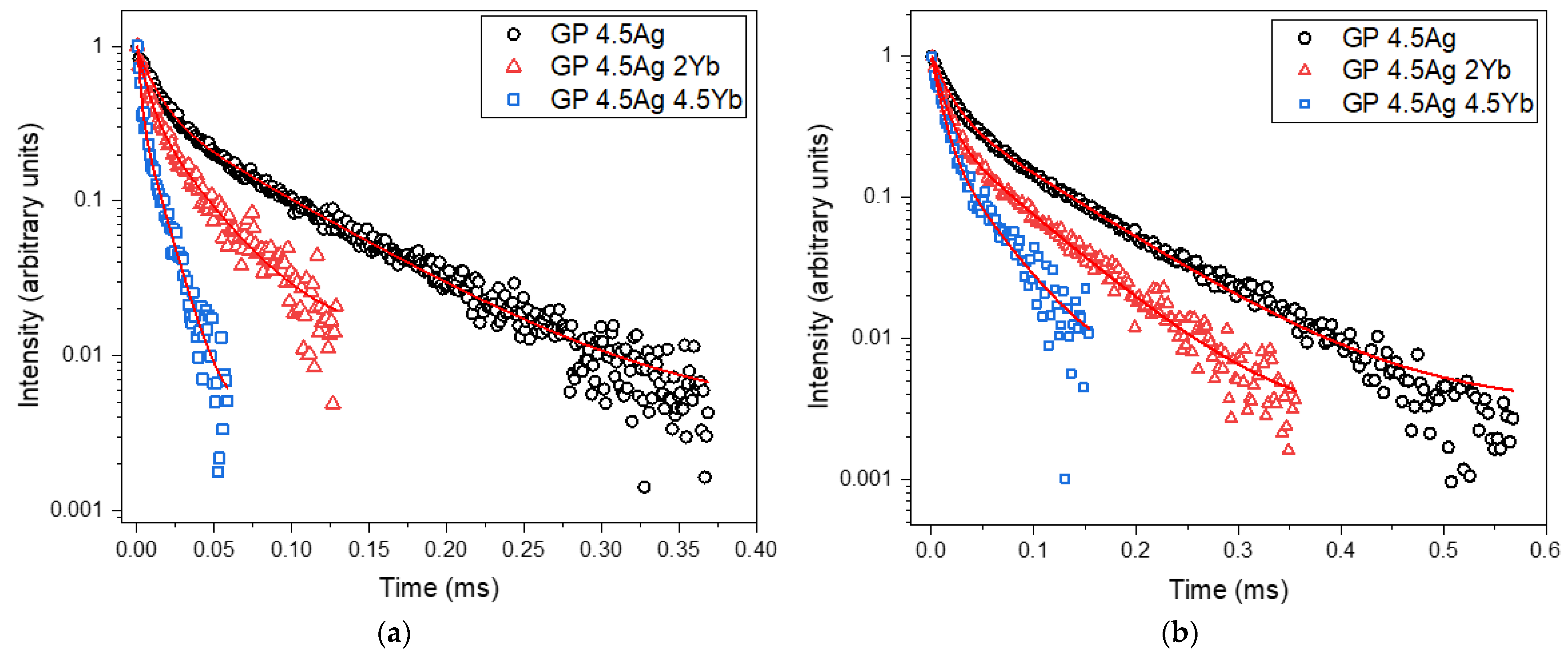



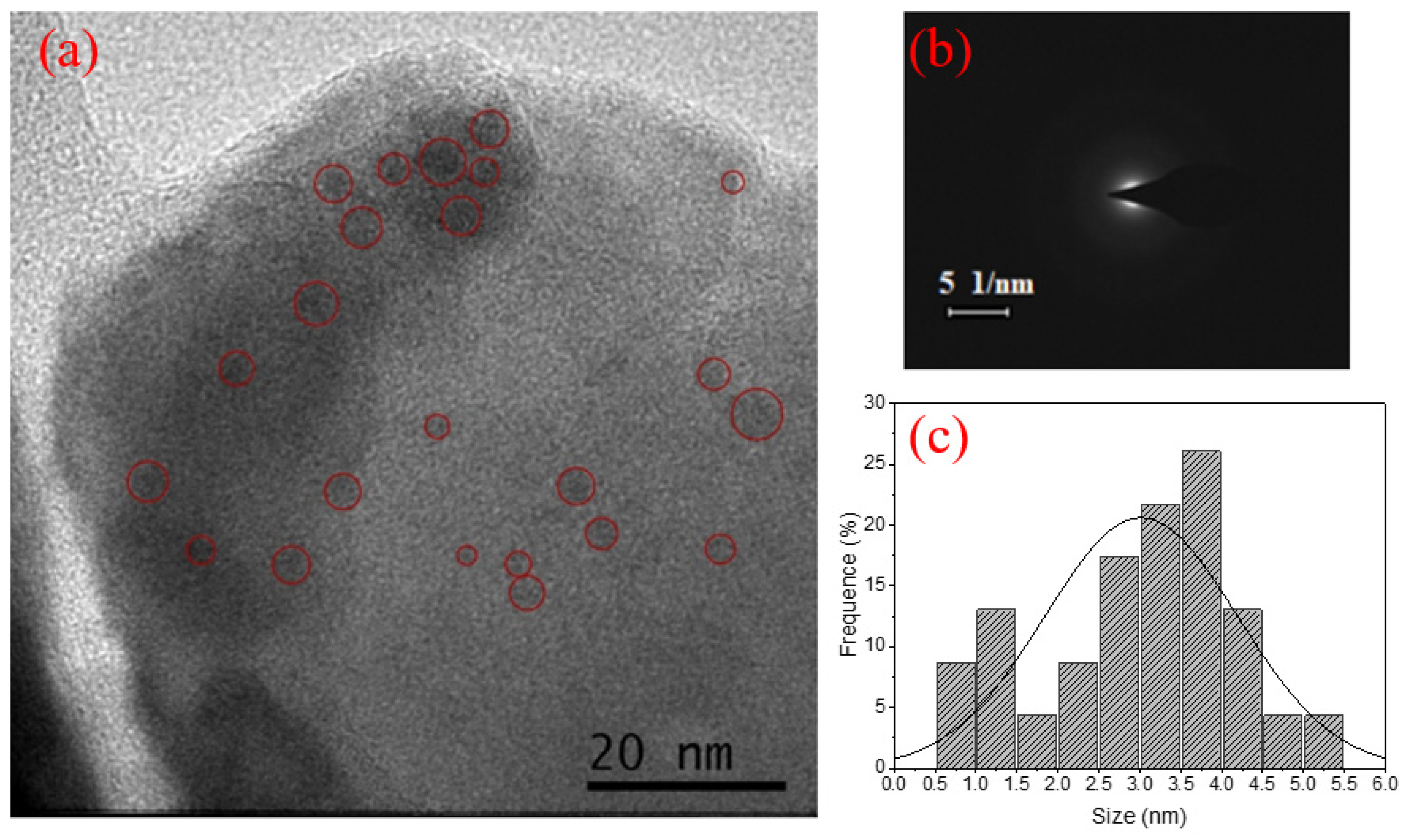
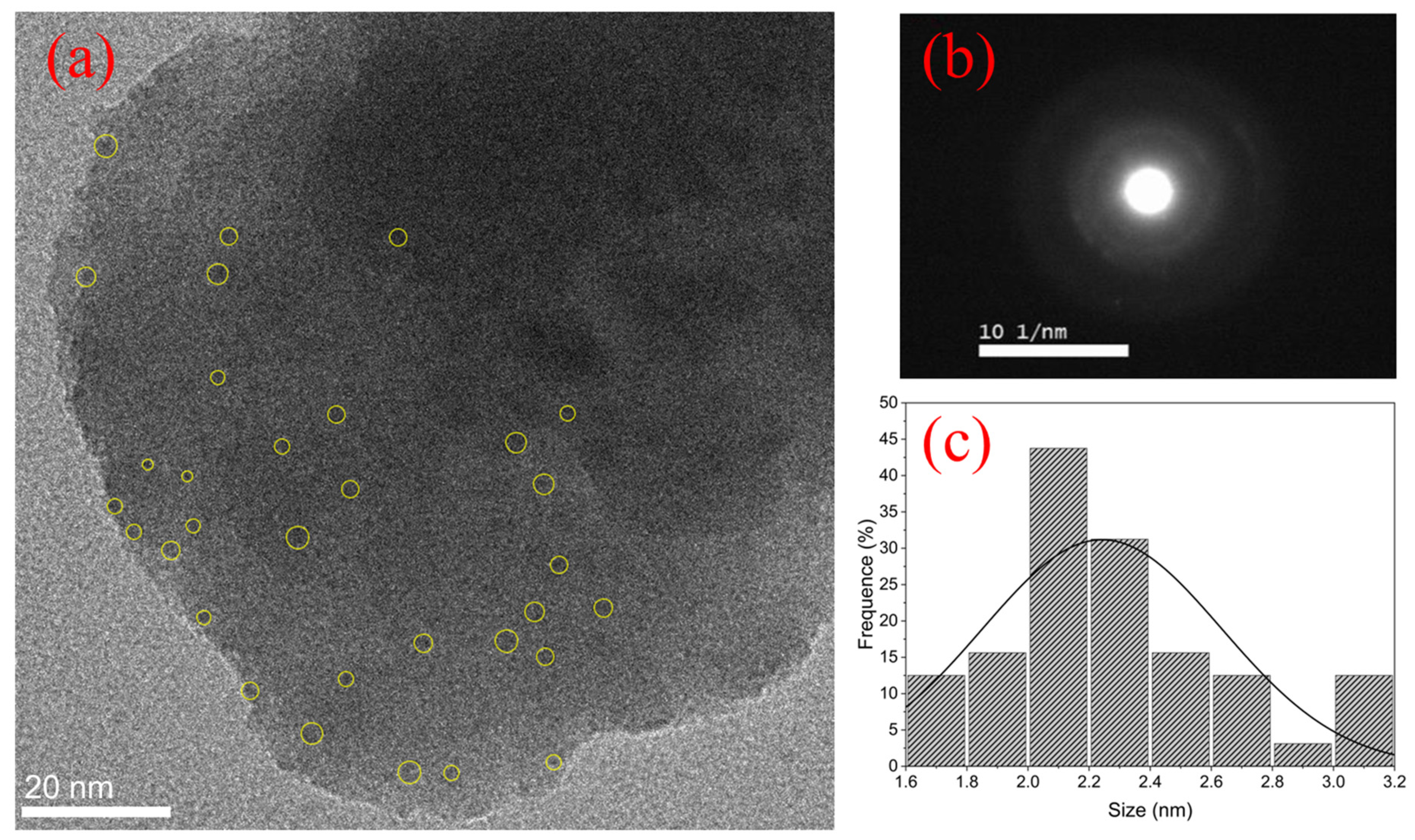
| Signal Wavelength (nm) | τfast (μs) | τslow (μs) |
|---|---|---|
| 500 | 9.2 | 33 |
| 550 | 16.1 | 93.4 |
| 600 | 18.0 | 113.4 |
| Sample | τfast (μs) | τslow (μs) |
|---|---|---|
| GP 4.5Ag | 12.3 | 75 |
| GP 4.5Ag2Yb | 7.0 | 31 |
| GP 4.5Ag4.5Yb | 2.0 | 12 |
| Sample | τfast (μs) | τslow (μs) |
|---|---|---|
| GP 4.5Ag | 16.1 | 93.4 |
| GP 4.5Ag2Yb | 11.4 | 70 |
| GP 4.5Ag4.5Yb | 9.4 | 42 |
| Sample | Time (ms) |
|---|---|
| GP 2Yb | 0.79 |
| GP 4.5Yb | 0.83 |
| GP 4.5Ag2Yb | 0.94 |
| GP 4.5Ag4.5Yb | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaro, A.A.; Mattos, G.R.d.S.; Nishimura, M.V.d.M.; Dipold, J.; Wetter, N.U.; Kassab, L.R.P. Silver Nanoclusters Tunable Visible Emission and Energy Transfer to Yb3+ Ions in Co-Doped GeO2-PbO Glasses for Photonic Applications. Nanomaterials 2023, 13, 1177. https://doi.org/10.3390/nano13071177
Amaro AA, Mattos GRdS, Nishimura MVdM, Dipold J, Wetter NU, Kassab LRP. Silver Nanoclusters Tunable Visible Emission and Energy Transfer to Yb3+ Ions in Co-Doped GeO2-PbO Glasses for Photonic Applications. Nanomaterials. 2023; 13(7):1177. https://doi.org/10.3390/nano13071177
Chicago/Turabian StyleAmaro, Augusto Anselmo, Guilherme Rodrigues da Silva Mattos, Marcos Vinicius de Morais Nishimura, Jessica Dipold, Niklaus Ursus Wetter, and Luciana Reyes Pires Kassab. 2023. "Silver Nanoclusters Tunable Visible Emission and Energy Transfer to Yb3+ Ions in Co-Doped GeO2-PbO Glasses for Photonic Applications" Nanomaterials 13, no. 7: 1177. https://doi.org/10.3390/nano13071177
APA StyleAmaro, A. A., Mattos, G. R. d. S., Nishimura, M. V. d. M., Dipold, J., Wetter, N. U., & Kassab, L. R. P. (2023). Silver Nanoclusters Tunable Visible Emission and Energy Transfer to Yb3+ Ions in Co-Doped GeO2-PbO Glasses for Photonic Applications. Nanomaterials, 13(7), 1177. https://doi.org/10.3390/nano13071177







