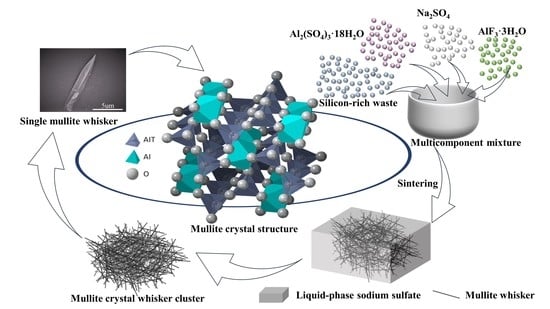
Analysis of Influencing Factors in the Preparation of Mullite Whiskers from Recovering Silicon-Rich Waste under Low-Temperature Conditions
Abstract
1. Introduction
2. Experimental Raw Materials and Preparation Methods
2.1. Experimental Materials
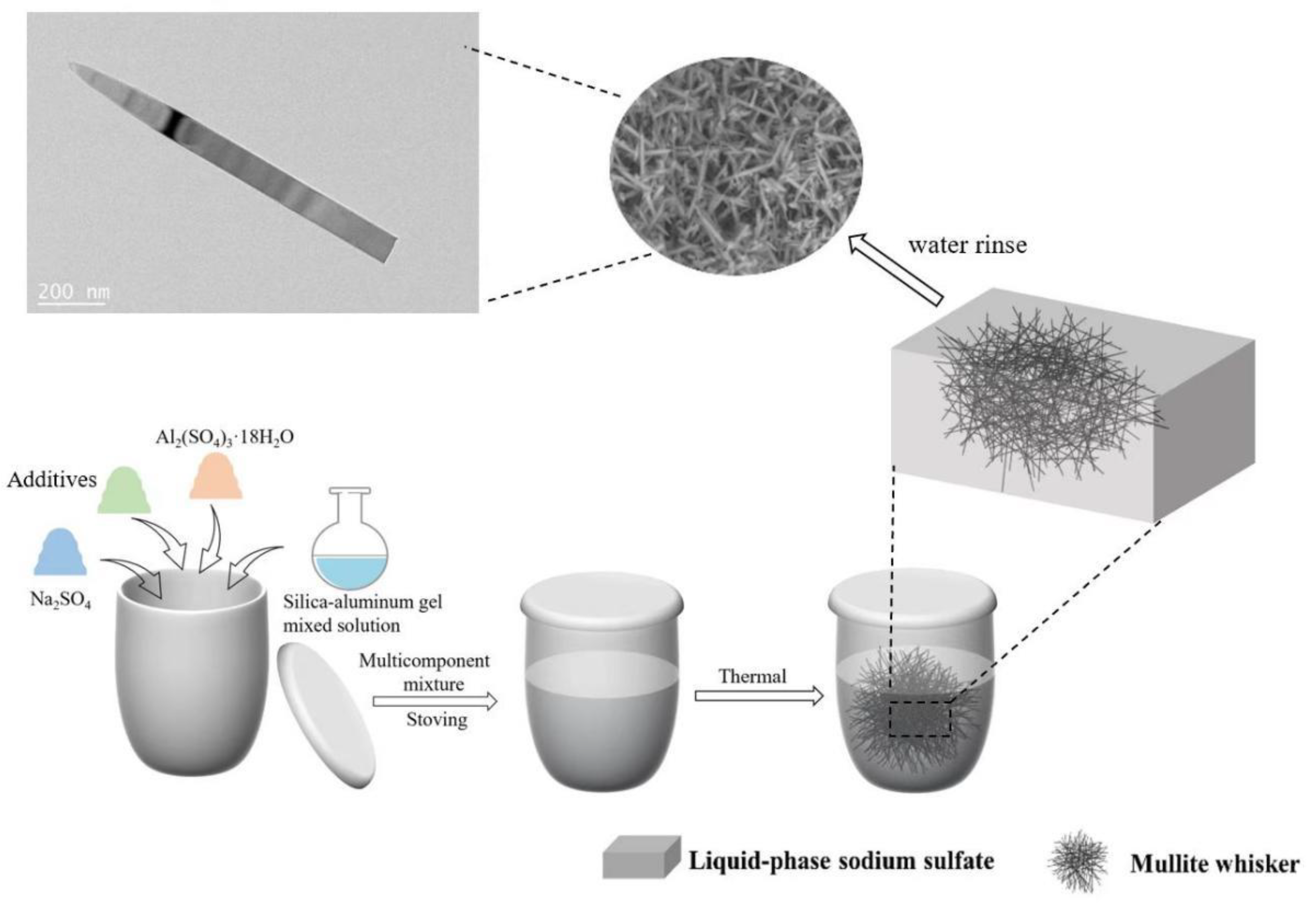
2.2. Synthesis Steps of Mullite Whiskers
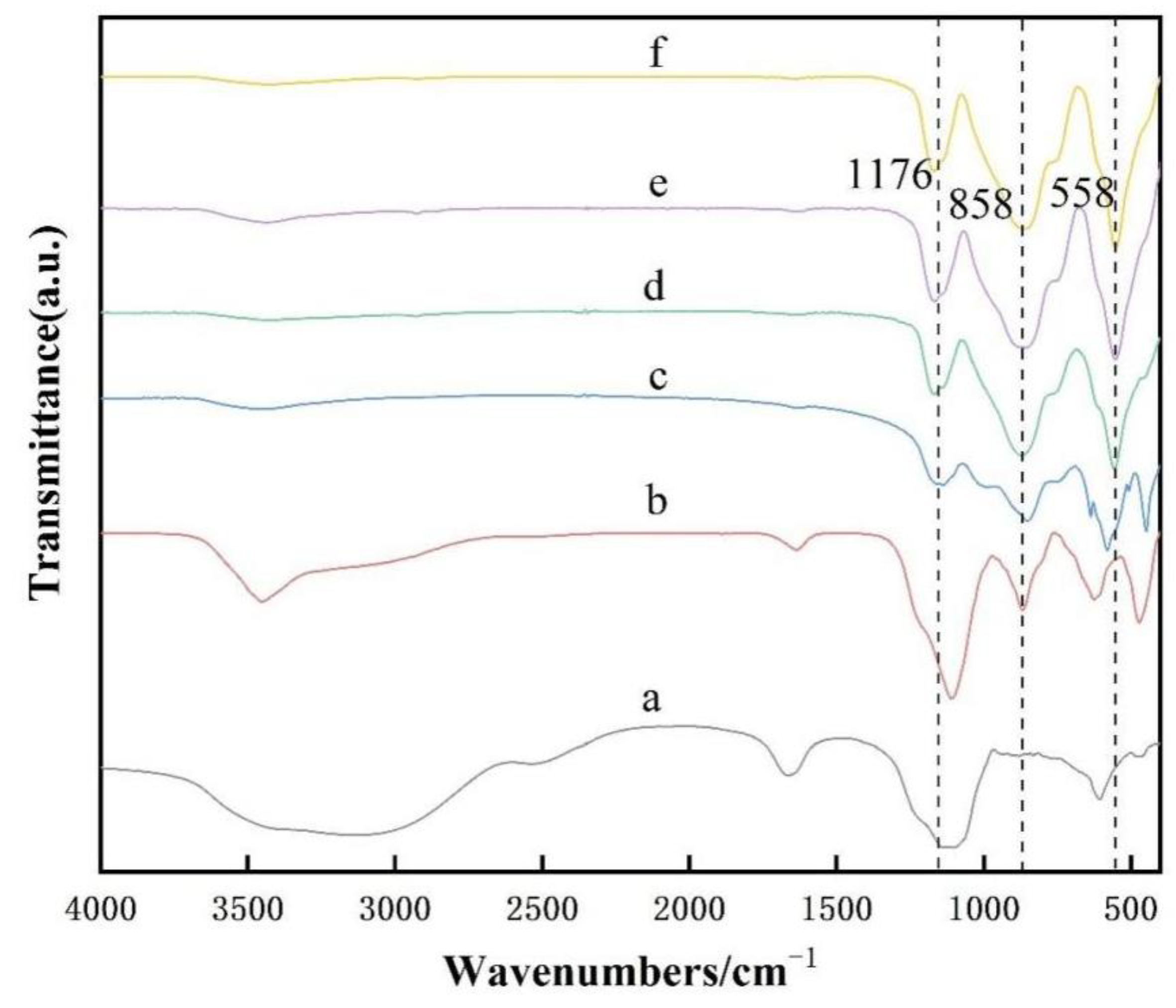
2.3. Characterization Methods
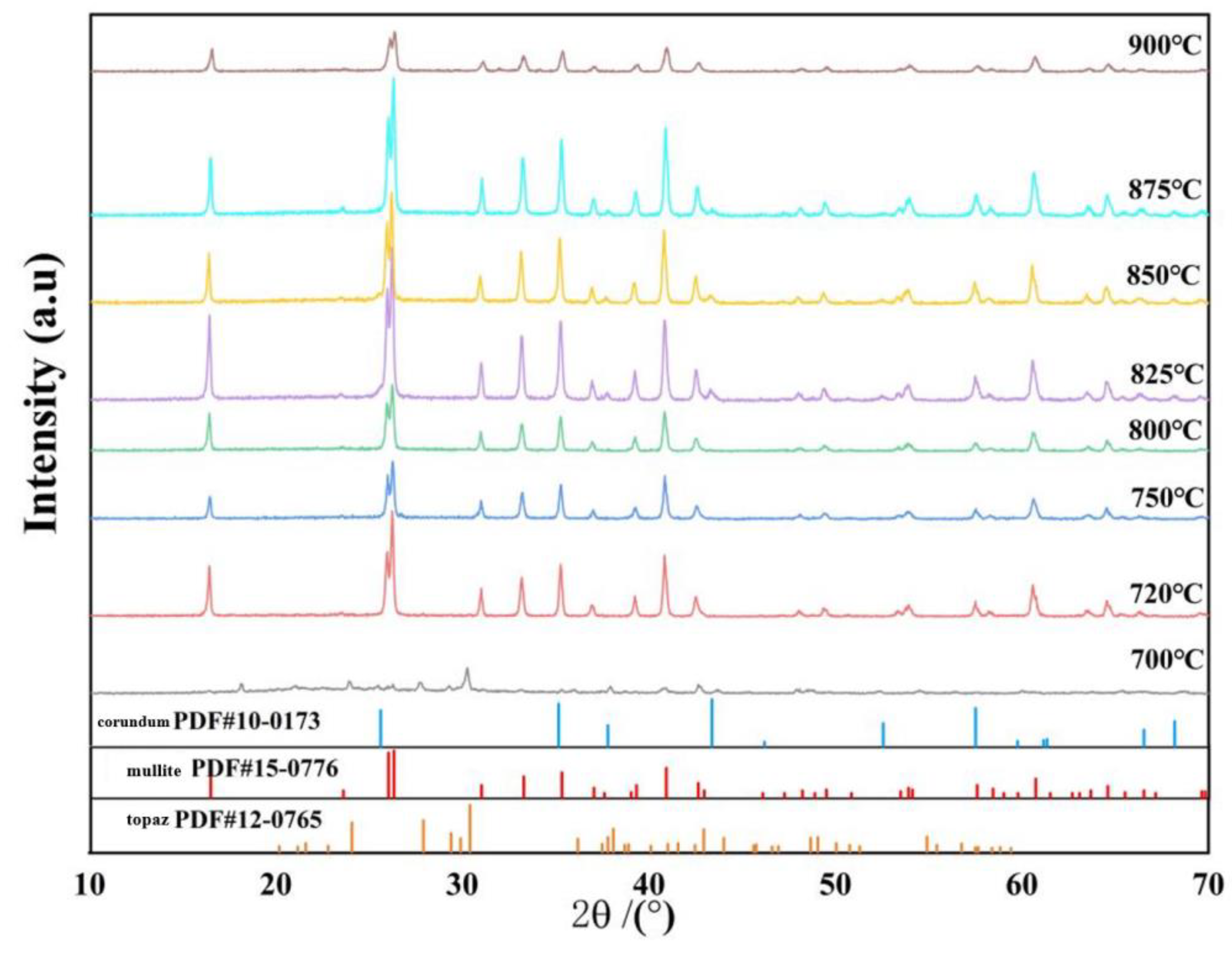
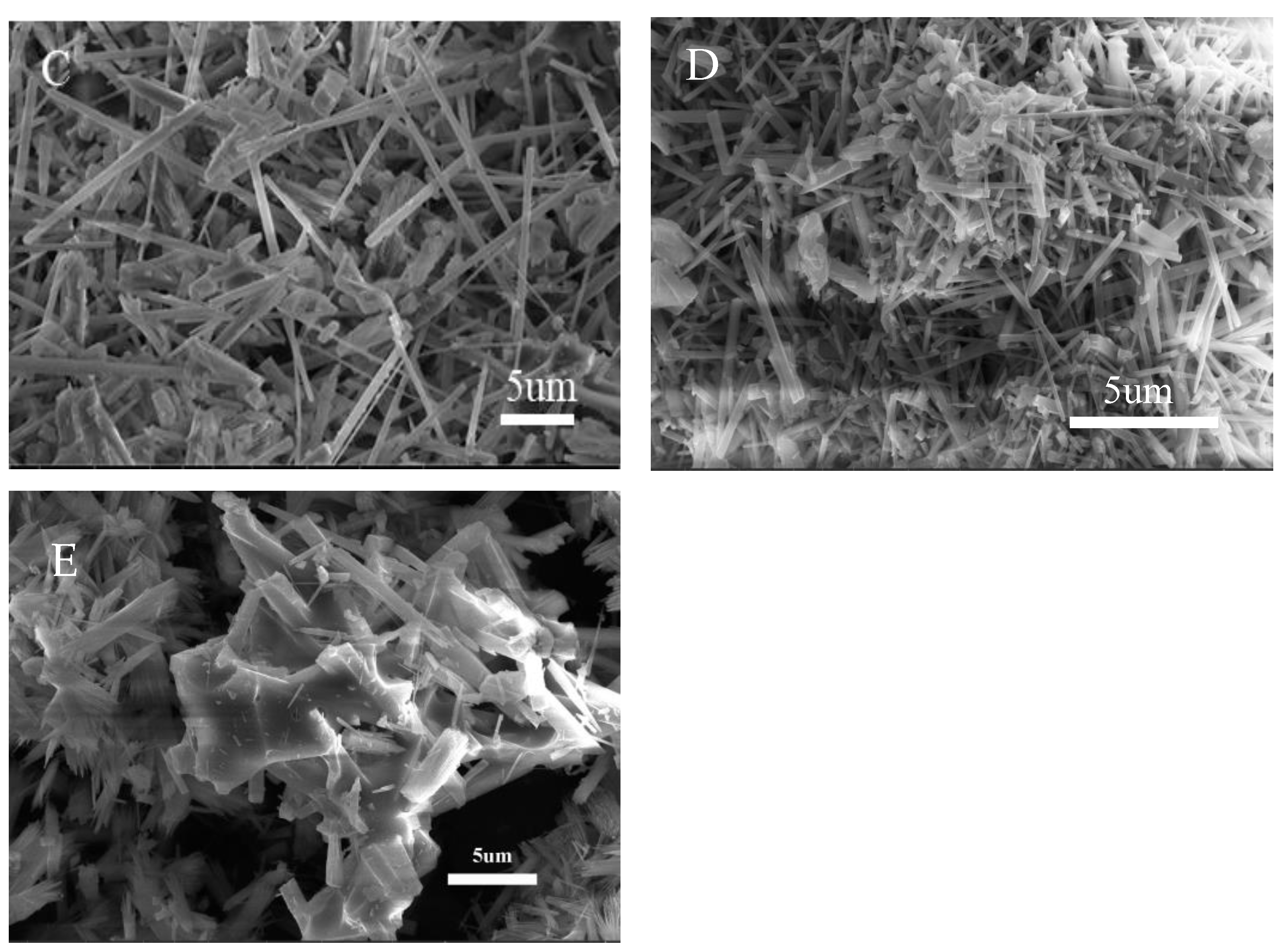
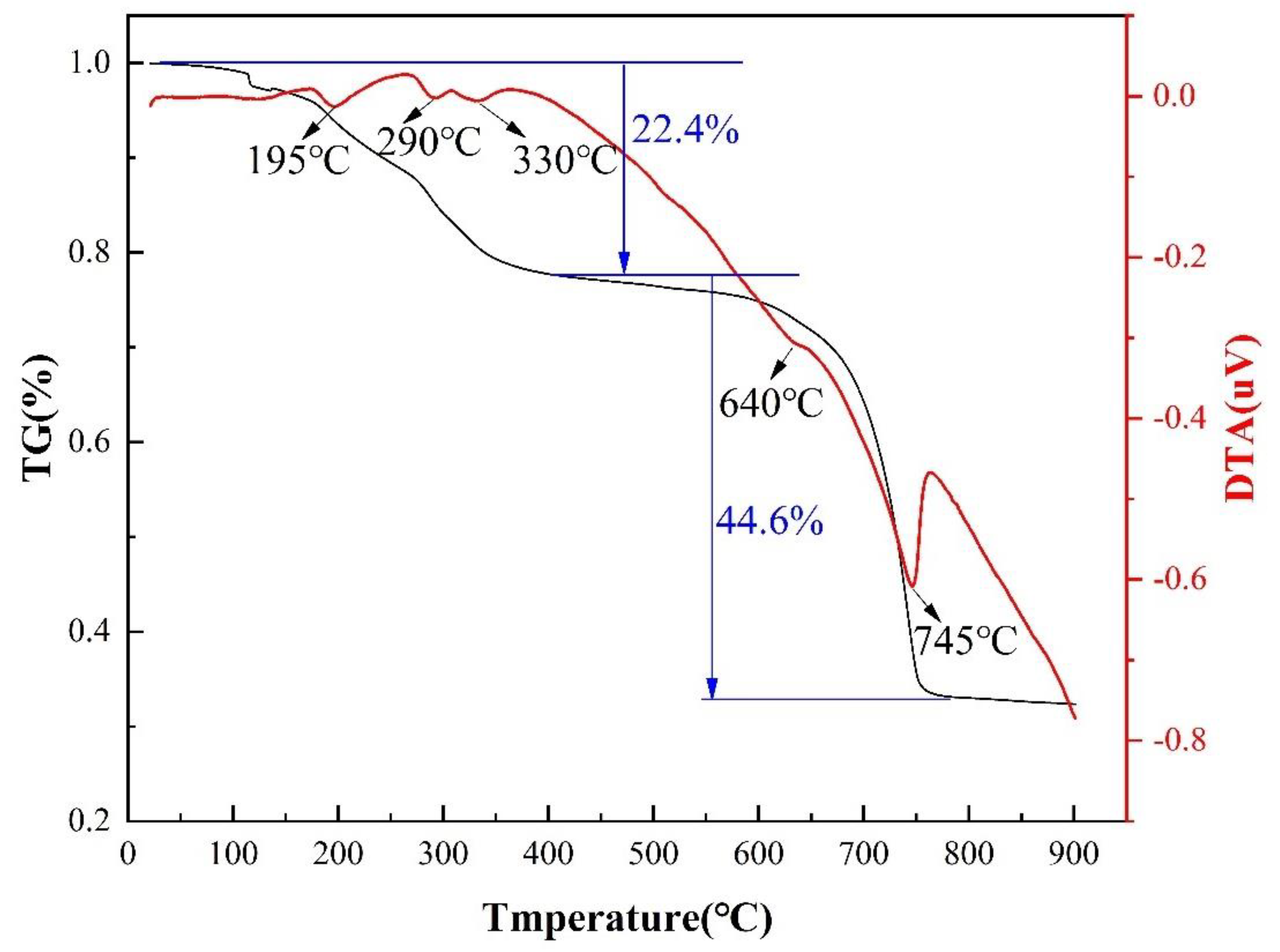
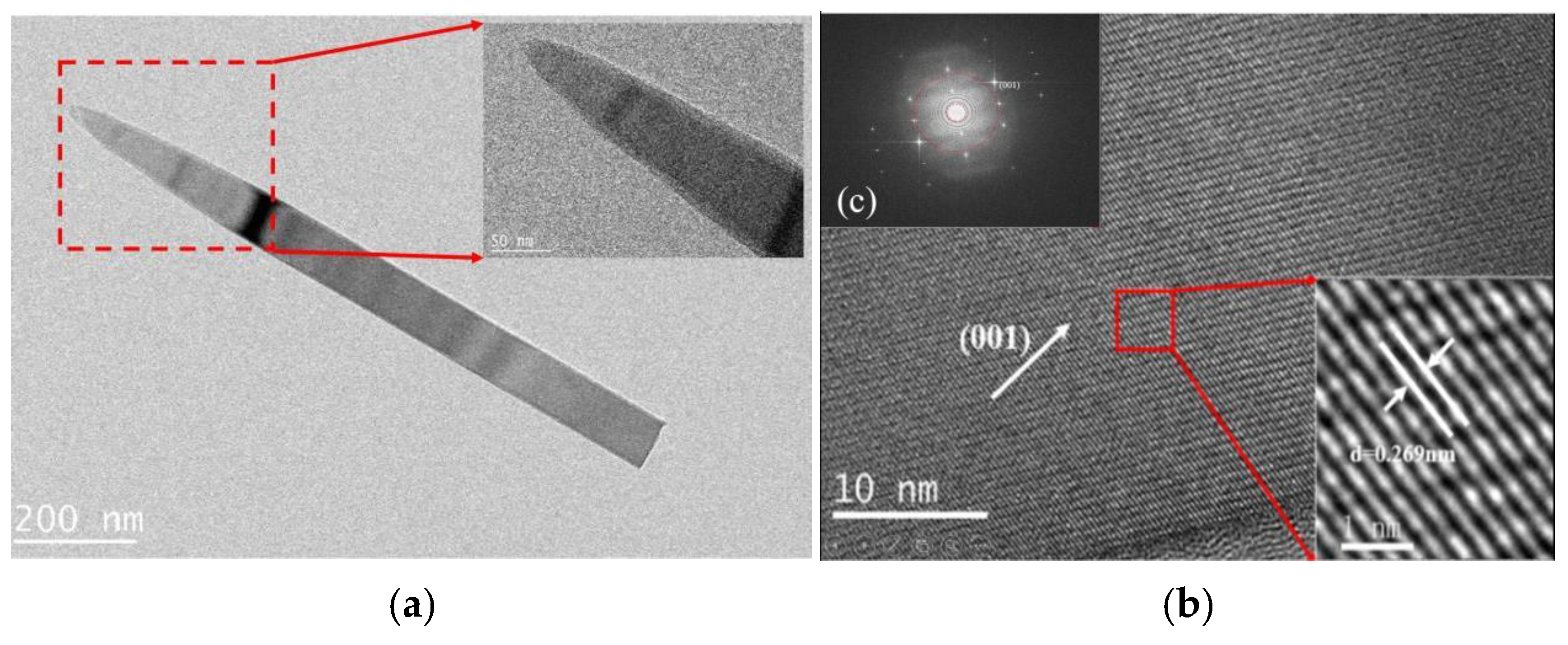
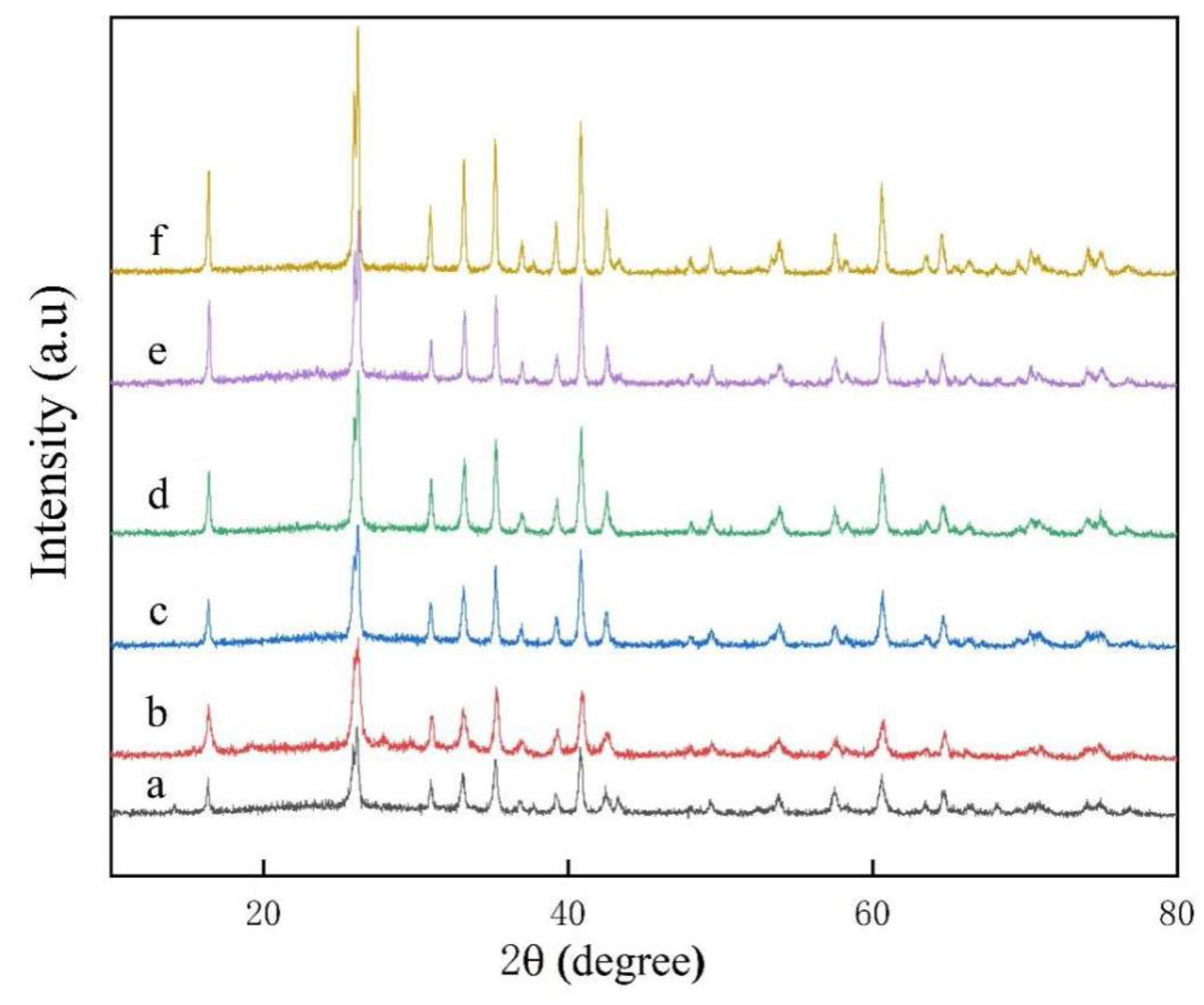
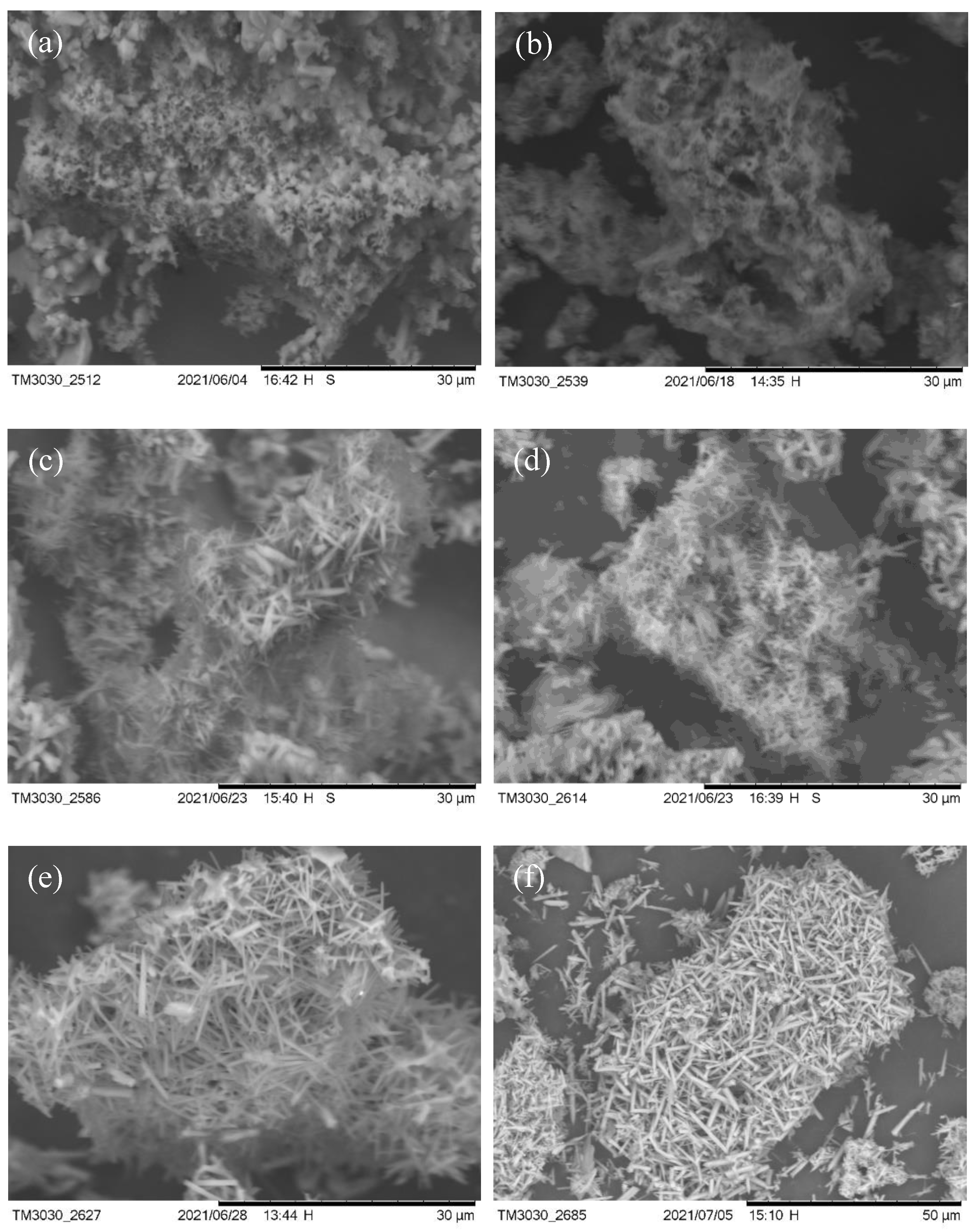
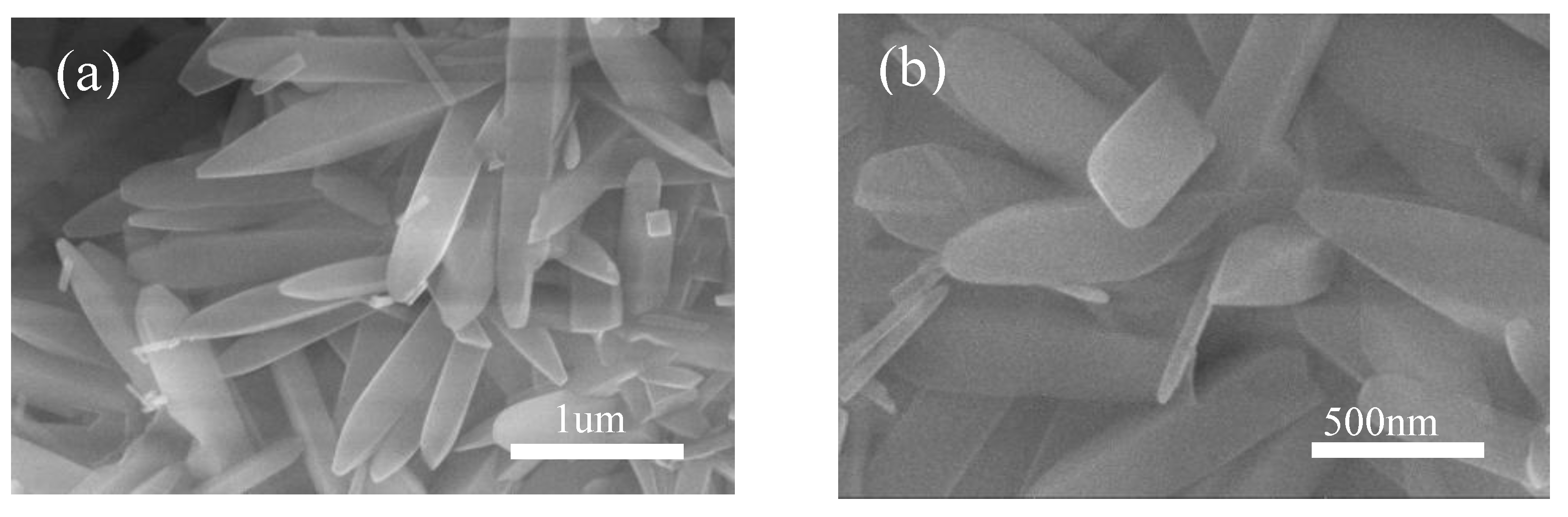
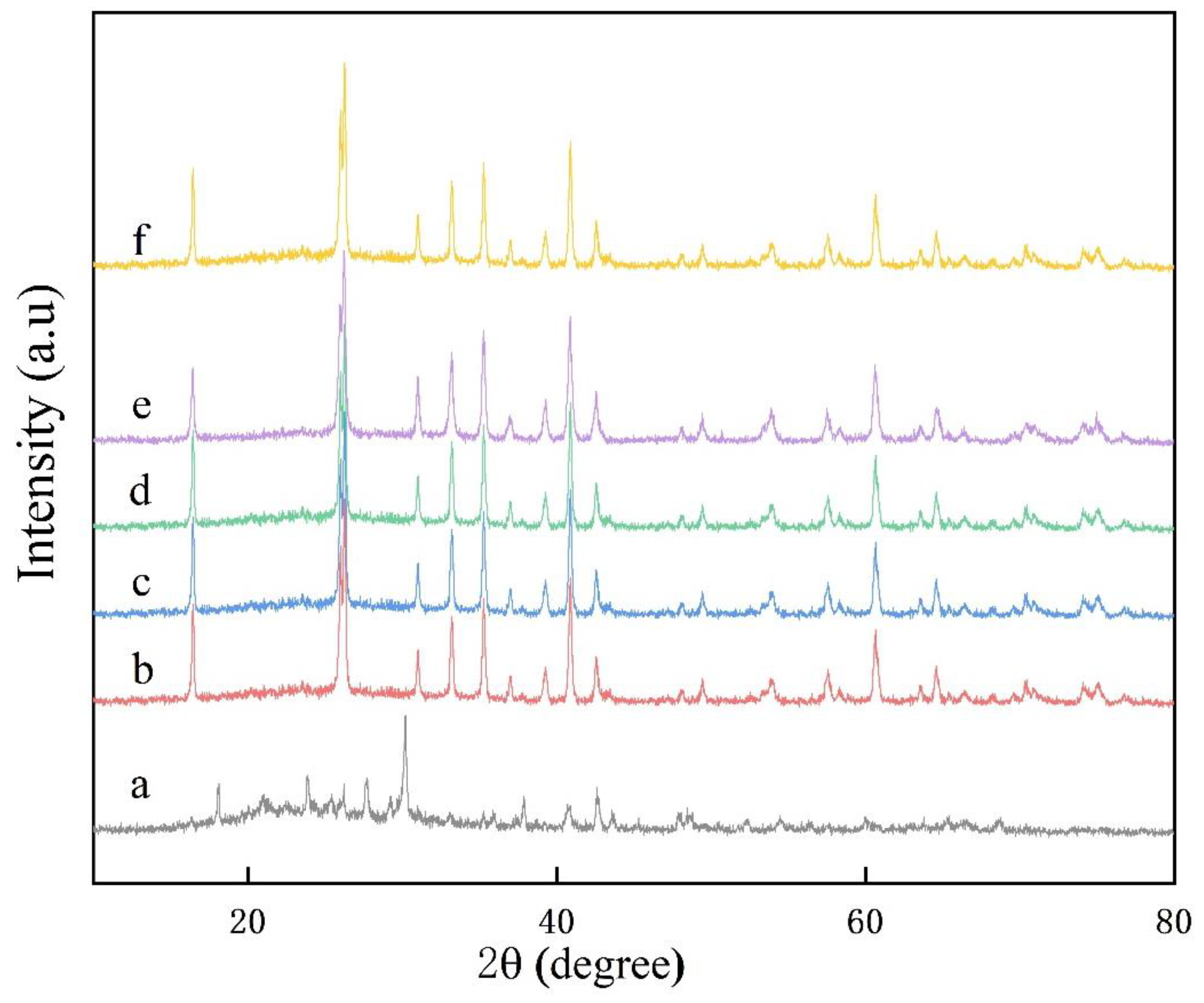
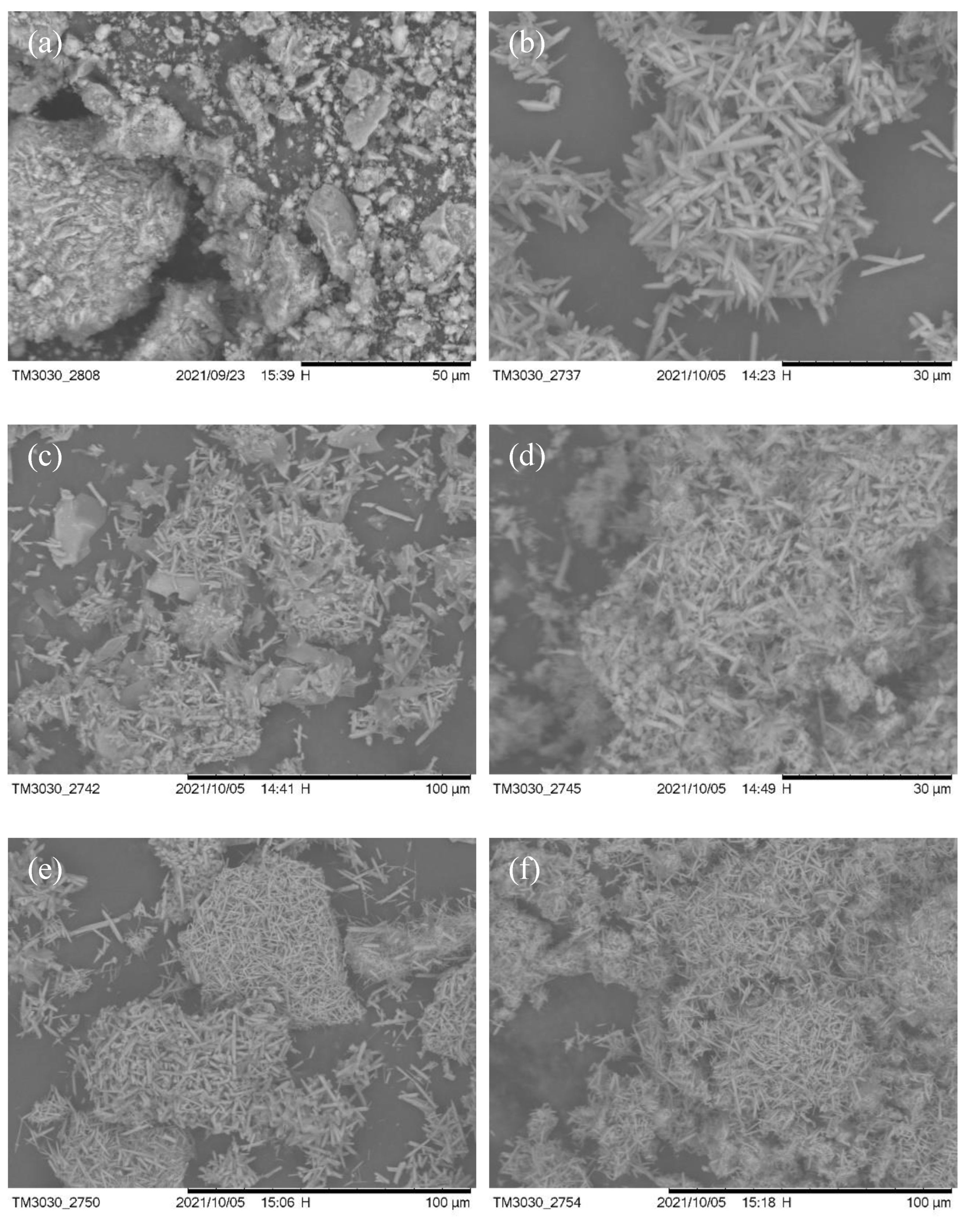
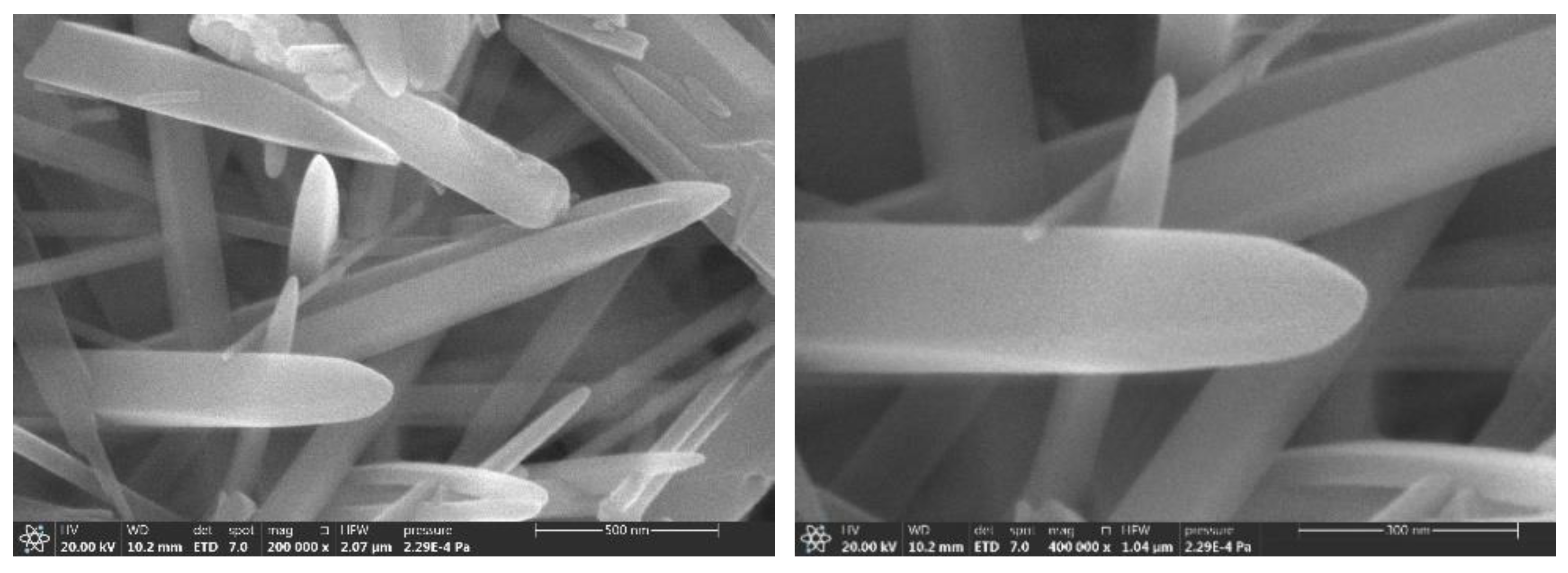
3. Results and Discussion
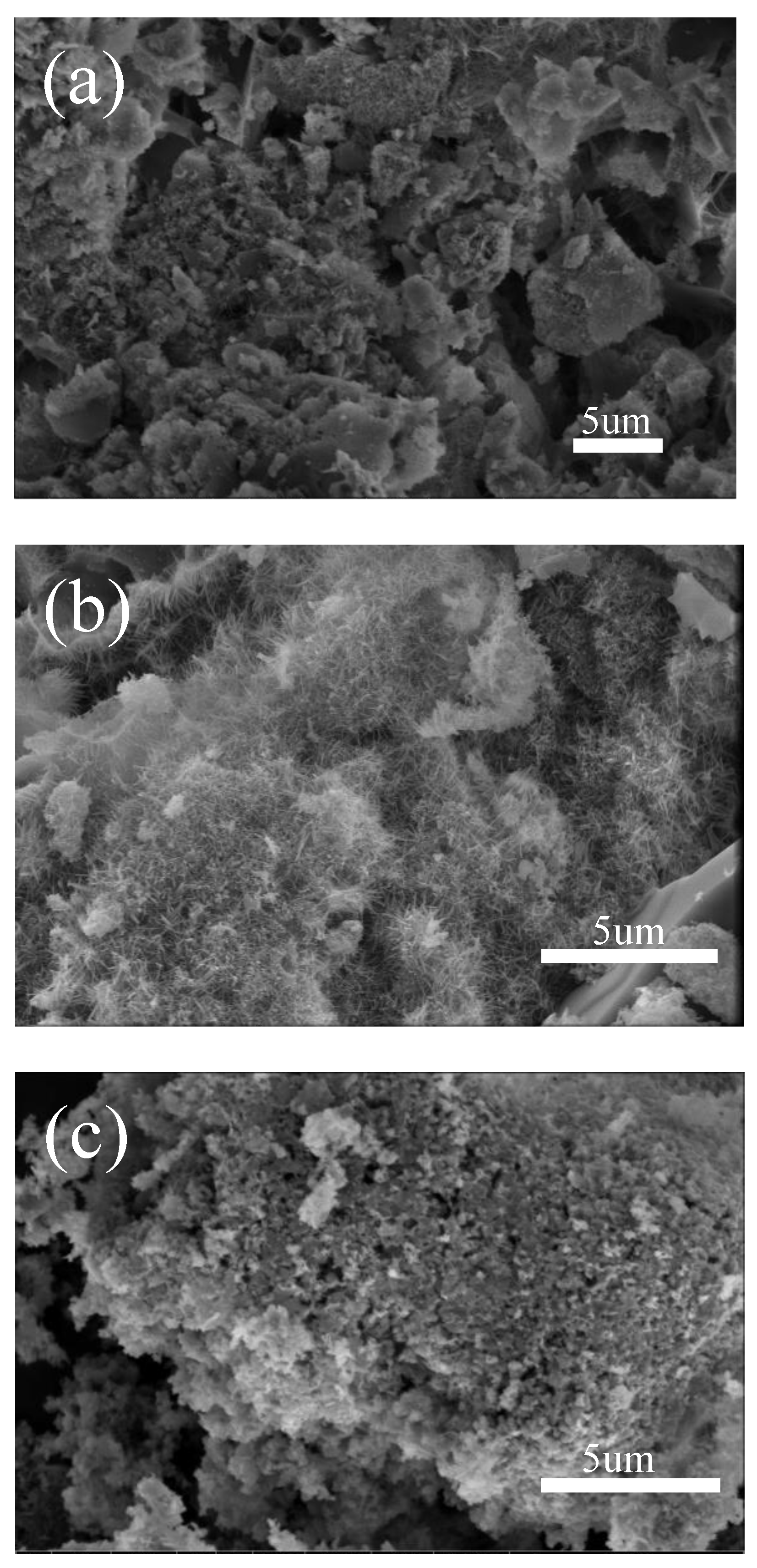
3.1. Rare Earth Oxides in Raw Materials
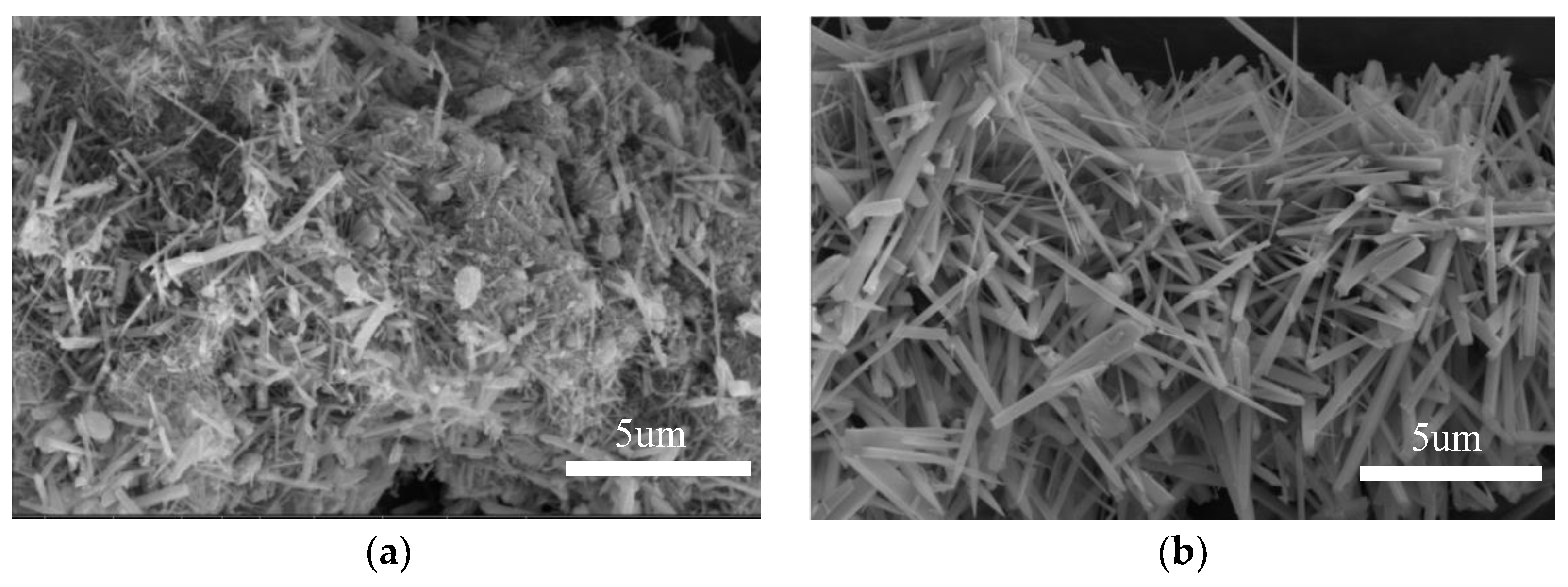
3.2. V–L–S Growth Atmosphere Influence
3.3. Effect of Flux Na2SO4
3.4. Sintering Temperature
3.5. Aluminum Fluoride Addition
3.6. Keep-Warm Time
4. Conclusions
- The rare earth elements in the raw material and the use of 10% Na2SO4 as flux promote the growth of nuclei and accelerate the crystallization rate.
- The addition of 5% aluminum fluoride resulted in stronger crystallinity and more homogeneous morphology of mullite.
- The optimal growth temperature of mullite whiskers is 825 °C.
- The optimal holding time after calcination is 5 h.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, H.; Fischer, R.X.; Schreuer, J. Mullite: Crystal Structure and Related Properties. J. Am. Ceram. Soc. 2015, 98, 2948–2967. [Google Scholar] [CrossRef]
- Zhu, L.; Li, S.; Gao, Z.; Zhang, X.; Zhang, L.; Li, H.; Liu, G. Effect of in situ formed acicular mullite whiskers on thermal shock resistance of alumina-mullite refractories. J. Aust. Ceram. Soc. 2023, 59, 259–266. [Google Scholar] [CrossRef]
- Deng, X.; Ji, P.; Yin, J.; Wang, Y.; Song, C.; Li, S.; Zhang, Y.; Ding, X.; Ran, S.; Zhang, H.; et al. Fabrication and characterization of mullite-whisker-reinforced lightweight porous materials with AlF3·3H2O. Ceram. Int. 2022, 48, 14891–14898. [Google Scholar] [CrossRef]
- Zhao, P.; Ma, S.; Wang, X.; Wu, W.; Ou, Y. Properties and mechanism of mullite whisker toughened ceramics. Ceram. Int. 2023, 49, 10238–10248. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, J.; Wang, X.; Wang, J.; Zhang, L.; Wen, T.; Jia, D.; Yan, Z.; Yuan, L.; Ma, B. Using Si/SiC solid waste to design urchin-like mullite whiskers for oil-water separation. Int. J. Appl. Ceram. Technol. 2022, 19, 1405–1414. [Google Scholar] [CrossRef]
- Dong, X.; Zheng, Y.; Xie, D. Multi-functional mullite fiber-based porous ceramics with a multilevel pore structure assembled by alumina platelets and mullite whiskers. Ceram. Int. 2023, 49, 847–854. [Google Scholar] [CrossRef]
- Yang, P.; Liu, S.; Mao, Z.; Wang, D. Preparation and properties of hierarchical structural alumina/mullite composites. J. Eur. Ceram. Soc. 2023, 43, 468–477. [Google Scholar] [CrossRef]
- Schneider, H.; Schreuer, J.; Hildmann, B. Structure and properties of mullite—A review. J. Eur. Ceram. Soc. 2008, 28, 329–344. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, S. Fibrous porous mullite ceramics modified by mullite whiskers for thermal insulation and sound absorption. J. Eur. Ceram. Soc. 2023, 43, 521–529. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, F.; Zhao, S. In-situ growth of mullite whiskers and their effect on the microstructure and properties of porous mullite ceramics with an open/closed pore structure. J. Eur. Ceram. Soc. 2021, 41, 299–308. [Google Scholar] [CrossRef]
- Mahnicka-Goremikina, L.; Svinka, R.; Svinka, V. Thermal Properties of Porous Mullite Ceramics Modified with Microsized ZrO2 and WO3. J. Mater. 2022, 15, 7935. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Oh, S.-H.; Choi, J.-H. Synthesis of porous ceramic with well-developed mullite whiskers in system of Al2O3-Kaolin-MoO3. J. Mater. Res. Technol. 2021, 15, 1457–1466. [Google Scholar] [CrossRef]
- Li, K.; Ge, S.; Yuan, G.; Zhang, H.; Zhang, J.; He, J.; Jia, Q.; Zhang, S. Effects of V2O5 addition on the synthesis of columnar self-reinforced mullite porous ceramics. Ceram. Int. 2020, 47, 11240–11248. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, S.; Zhang, Z.; Zhong, M.; Li, Y.; Huang, X.; Xu, W.; Wang, D.; Wang, L. The effect of phosphorus on the formation of mullite whiskers from citric acid activated kaolin. Ceram. Int. 2018, 44, 18796–18802. [Google Scholar] [CrossRef]
- Song, X.; Liu, W.; Xu, S. Microstructure and elastic modulus of electrospun Al2O3-SiO2-B2O3 composite nanofibers with mullite-type structure prepared at elevated temperatures. J. Eur. Ceram. Soc. 2018, 38, 201–210. [Google Scholar] [CrossRef]
- Kim, B.; Cho, Y.; Yoon, S.; Stevens, R.; Park, H. Mullite whiskers derived from kaolin. Ceram. Int. 2009, 35, 579–583. [Google Scholar] [CrossRef]
- Alves, H.P.; Silva, J.B.; Campos, L.F.; Torres, S.M.; Dutra, R.P.; Macedo, D.A. Preparation of mullite based ceramics from clay–kaolin waste mixtures. Ceram. Int. 2016, 42, 19086–19090. [Google Scholar] [CrossRef]
- Bella, M.; Hamidouche, M.; Gremillard, L. Preparation of mullite-alumina composite by reaction sintering between Algerian kaolin and amorphous aluminum hydroxide. Ceram. Int. 2021, 47, 16208–16220. [Google Scholar] [CrossRef]
- Hua, K.; Shui, A.; Xu, L.; Zhao, K.; Zhou, Q.; Xi, X. Fabrication and characterization of anorthite–mullite–corundum porous ceramics from construction waste. Ceram. Int. 2016, 42, 6080–6087. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Wu, J.; Zhang, C.; Zhang, Q.; Tian, K. Preparation of mullite whisker reinforced SiC membrane supports with high gas permeability. Ceram. Int. 2020, 47, 8150–8160. [Google Scholar] [CrossRef]
- Li, L.; Cao, G.; Zhao, R.; Wang, L.; Wu, S. High-porosity whisker-mullite/corundum membrane support prepared from recycled industrial waste coal cinder. Ceram. Int. 2019, 46, 4086–4094. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Wassel, A.R. Recycling of aluminum dross and silica fume wastes for production of mullite-containing ceramics: Powder preparation, sinterability and properties. Ceram. Int. 2022, 48, 31661–31671. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Zhang, Y.; Su, Z.; Xu, C.; Jiang, T. Co-utilization of secondary aluminum dross and ferronickel slag for preparation of cordierite–mullite insulating ceramic. J. Am. Ceram. Soc. 2022, 106, 2049–2060. [Google Scholar] [CrossRef]
- Fu, M.; Liu, J.; Dong, X.; Zhu, L.; Dong, Y.; Hampshire, S. Waste recycling of coal fly ash for design of highly porous whisker-structured mullite ceramic membranes. J. Eur. Ceram. Soc. 2019, 39, 5320–5331. [Google Scholar] [CrossRef]
- Das, D.; Nijhuma, K.; Gabriel, A.M.; Daniel, G.P.F.; Murilo, D.D.M.I. Recycling of coal fly ash for fabrication of elongated mullite rod bonded porous SiC ceramic membrane and its application in filtration. J. Eur. Ceram. Soc. 2020, 40, 2163–2172. [Google Scholar] [CrossRef]
- Yang, H.-L.; Li, Z.-S.; Ding, Y.-D.; Ge, Q.-Q.; Shi, Y.-J.; Jiang, L. Effect of Silicon Source (Fly Ash, Silica Dust, Gangue) on the Preparation of Porous Mullite Ceramics from Aluminum Dross. Materials 2022, 15, 7212. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhu, Z.; Zhang, Z.; Chu, Y.; Yuan, B.; Wei, Z. Development of highly porous mullite whisker ceramic membranes for oil-in-water separation and resource utilization of coal gangue. Sep. Purif. Technol. 2019, 237, 116483. [Google Scholar] [CrossRef]
- Liu, R.; Xiang, D. Recycling photovoltaic silicon waste for fabricating porous mullite ceramics by low-temperature reaction sintering. J. Eur. Ceram. Soc. 2021, 41, 5957–5966. [Google Scholar] [CrossRef]
- Xing, Z.; Hu, Y.; Xiang, D. Porous SiC-mullite ceramics with high flexural strength and gas permeability prepared from photovoltaic silicon waste. Ceram. Int. 2020, 46, 1236–1242. [Google Scholar] [CrossRef]
- Li, S.; Du, H.; Guo, A.; Xu, H.; Yang, D. Preparation of self-reinforcement of porous mullite ceramics through in situ synthesis of mullite whisker in fly ash body. Ceram. Int. 2012, 38, 1027–1032. [Google Scholar] [CrossRef]
- Garai, M.; Karmakar, B. Rare earth ion controlled crystallization of mica glass-ceramics. J. Alloy Compd. 2016, 678, 360–369. [Google Scholar] [CrossRef]
- Liu, H.; Zou, X.; Wang, X.; Lu, X.; Ding, W. Effect of CeO2 addition on Ni/Al2O3 catalysts for methanation of carbon dioxide with hydrogen. J. Nat. Gas Chem. 2012, 21, 703–707. [Google Scholar] [CrossRef]
- Luo, S.; Shi, Z.; Li, N.; Lin, Y.; Liang, Y.; Zeng, Y. Crystallization inhibition and microstructure refinement of Al-5Fe alloys by addition of rare earth elements. J. Alloy Compd. 2019, 789, 90–99. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, M.; Lu, R.; Xu, W.; Zhang, T.; Wei, T.; Zhang, J.; Liao, Y. A composite structural high-temperature-resistant adhesive based on in-situ grown mullite whiskers. Mater. Today Commun. 2020, 23, 100944. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, D.; Ma, L.; Deng, C.; Li, L.; Chen, K.; Yang, X. Effect of La2O3 on crystallization of Glass-ceramics. J. Non-Cryst. Solids 2020, 536, 120007. [Google Scholar] [CrossRef]
- Wang, M.; Fang, L.; Li, M. Phase separation and crystallization of La2O3 doped ZnO-B2O3-SiO2 glass. J. Rare Earths 2019, 37, 767–772. [Google Scholar] [CrossRef]
- Qi, J.; Liu, C.; Liu, H.; Li, C.; Jiang, M. Effect of rare earth oxide on the crystallization behavior of CaO-Al2O3-based mold flux for rare earth heat-resistant steel continuous casting. J. Non-Cryst. Solids 2021, 559, 120681. [Google Scholar] [CrossRef]
- Abdullayev, A.; Klimm, D.; Kamutzki, F.; Gurlo, A.; Bekheet, M.F. AlF3-assisted flux growth of mullite whiskers and their application in fabrication of porous mullite-alumina monoliths. Open Ceram. 2021, 7, 100145. [Google Scholar] [CrossRef]
- Chen, P.; Gu, X.; Liu, S.; Zhu, S.; Wang, T.; Zhu, Y.; Fan, A.; Liu, Y. Industrial waste silica-alumina gel recycling: Low-temperature synthesis of mullite whiskers for mass production. Ceram. Int. 2023, 49, 9442–9451. [Google Scholar] [CrossRef]
- Baccour, A.; Sahnoun, R.D.; Bouaziz, J. Effects of mechanochemical treatment on the properties of kaolin and phosphate–kaolin materials. Powder Technol. 2014, 264, 477–483. [Google Scholar] [CrossRef]
- Sembiring, S.; Simanjuntak, W.; Manurung, P. Synthesis and characterisation of gel-derived mullite precursors from rice husk silica. J. Ceram. Int. 2014, 40, 7067–7072. [Google Scholar] [CrossRef]
- Feng, G.; Jiang, F.; Jiang, W. Novel facile nonaqueous precipitation in-situ synthesis of mullite whisker skeleton porous materials. Ceram. Int. 2018, 44, 22904–22910. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Ji, H.; Yu, H.; Yu, B.; Qi, T. Constructing secondary-pore structure by in-situ synthesized mullite whiskers to prepare whiskers aerogels with ultralow thermal conductivity. J. Eur. Ceram. Soc. 2018, 39, 1344–1351. [Google Scholar] [CrossRef]
- Yang, T.; Peng-long, Q.; Mei, Z. Molten salt synthesis of mullite nanowhiskers using different silica sources. J. Int. Miner. Metall. Mater. 2015, 22, 884–891. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Du, H.; Hou, F.; Guo, A. Microstructure of mullite fiber-based hierarchical structures adjusted by Al/Si mole ratio of the raw material powders. Ceram. Int. 2014, 40, 11405–11410. [Google Scholar] [CrossRef]
- Zheng, G.; Xia, J.; Chen, Z. Thermodynamics and kinetics of the carbothermal reduction of aluminum sulfate. Phosphorus Sulfur Silicon Relat. Elem. 2020, 196, 71–78. [Google Scholar] [CrossRef]
- Wu, L.; Li, C.; Chen, Y. Seed assisted in-situ synthesis of porous anorthite/mullite whisker ceramics by foam-freeze casting. J. Ceram. Int. 2021, 47, 11193–11201. [Google Scholar] [CrossRef]






| Al2O3 wt% | SiO2 wt% | Na2O wt% | SO3 wt% | La2O3 wt% | CeO2 wt% | MgO wt% |
| 26.2 | 48.7 | 5.8 | 3.9 | 5.6 | 6.4 | 3.4 |
| Al2O3 wt% | SiO2 wt% | Na2O wt% | SO3 wt% | La2O3 wt% | CeO2 wt% | MgO wt% |
| 28.9 | 56.7 | 6.3 | 4.4 | 0.05 | 0.05 | 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Chen, P.; Wang, T.; Liu, S.; Zhu, S.; Zhu, Y.; Liu, Y. Analysis of Influencing Factors in the Preparation of Mullite Whiskers from Recovering Silicon-Rich Waste under Low-Temperature Conditions. Nanomaterials 2023, 13, 1143. https://doi.org/10.3390/nano13071143
Gu X, Chen P, Wang T, Liu S, Zhu S, Zhu Y, Liu Y. Analysis of Influencing Factors in the Preparation of Mullite Whiskers from Recovering Silicon-Rich Waste under Low-Temperature Conditions. Nanomaterials. 2023; 13(7):1143. https://doi.org/10.3390/nano13071143
Chicago/Turabian StyleGu, Xiaohua, Peiquan Chen, Tong Wang, Siwen Liu, Shangwen Zhu, Yanwei Zhu, and Yan Liu. 2023. "Analysis of Influencing Factors in the Preparation of Mullite Whiskers from Recovering Silicon-Rich Waste under Low-Temperature Conditions" Nanomaterials 13, no. 7: 1143. https://doi.org/10.3390/nano13071143
APA StyleGu, X., Chen, P., Wang, T., Liu, S., Zhu, S., Zhu, Y., & Liu, Y. (2023). Analysis of Influencing Factors in the Preparation of Mullite Whiskers from Recovering Silicon-Rich Waste under Low-Temperature Conditions. Nanomaterials, 13(7), 1143. https://doi.org/10.3390/nano13071143