Review of NiS-Based Electrode Nanomaterials for Supercapacitors
Abstract
1. Introduction
2. Pure NiS Nanomaterials
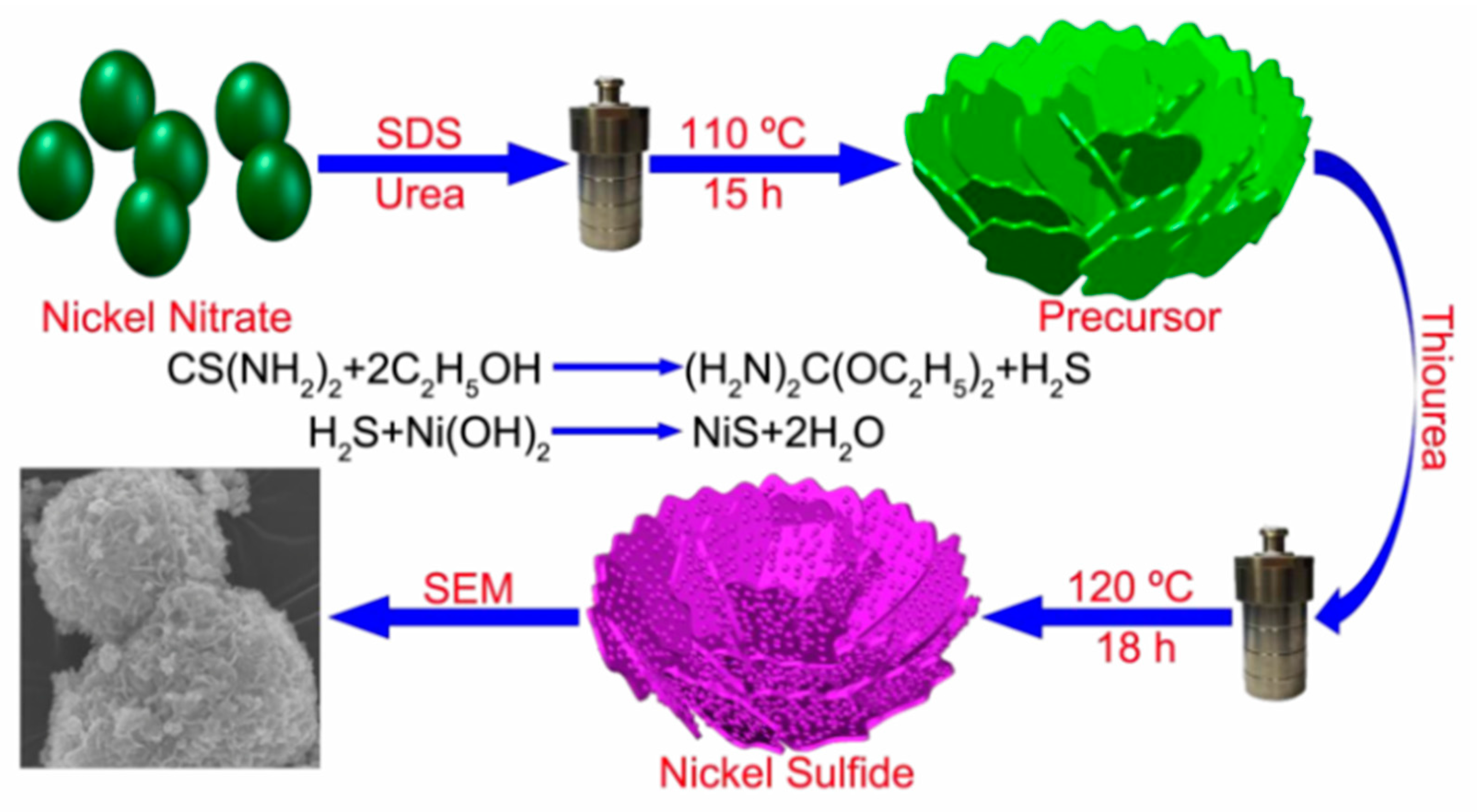
2.1. NiS Nanolayered Structure
2.2. NiS Nanosphere Structure
2.3. Other Nanoshapes of NiS
3. NiS-Based Electrode Nanomaterials
3.1. NiS/Sulfide Nanocomposites
3.2. NiS/Carbon Nanocomposites
3.2.1. NiS/Carbon Nanotube Composites
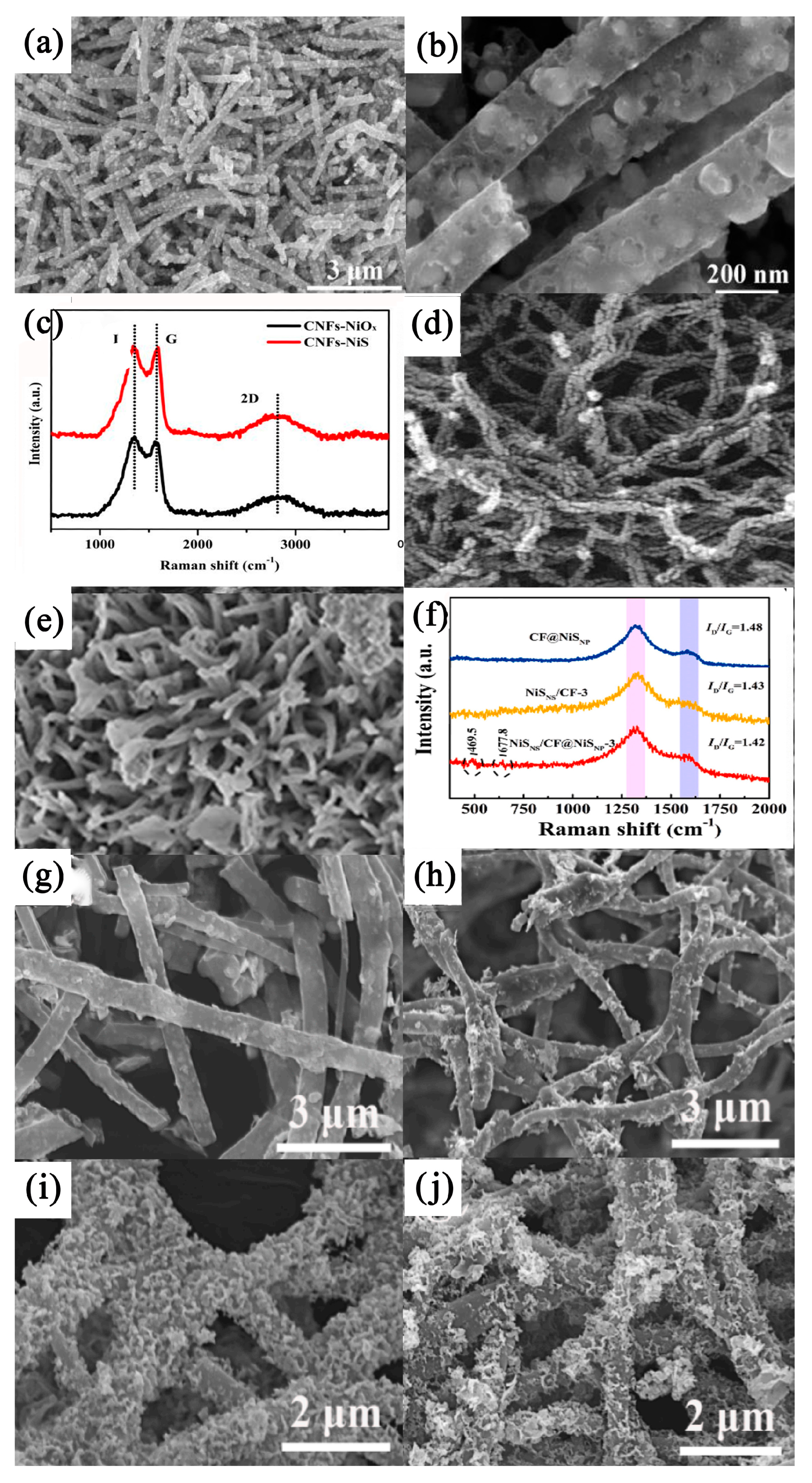
3.2.2. NiS/Carbon Nanofiber Composites
3.2.3. NiS/Carbon–Graphene and Biomass-Derived Carbon Composites

3.2.4. NiS Composites with Other Carbon Nanomaterials
3.3. NiS/Transition Metal Oxide Nanocomposites

4. Conclusions
- (1)
- Studies on the cycle stability of nickel–sulfur materials without any carbon material doping have not achieved ideal results. Therefore, the cycle stability of nickel–sulfur materials can be improved by composites with carbon materials or structural designs.
- (2)
- It is well known that the specific capacitance of oxides is relatively low. The properties of nickel–sulfur and oxide composite materials, such as nickel–sulfur and molybdenum oxide composite materials, may change when metal elements are added (e.g., doping with a small amount of nickel). In the future, this kind of material may be a valuable research direction.
- (3)
- Elemental doping is an effective method by which to improve the electrochemical stability of battery electrodes. In particular, the codoping of two or more elements will produce synergistic effects and stabilize the bulk-phase chemistry and surface chemistry of electrode materials at the same time. In the research on carbon material doping, if the size of the carbon material is large, it is not easy to dope with nickel–sulfur materials. In addition, there are still some problems to be solved with respect to doping—for example, how to ensure a high loading rate and how to accurately control the load. Whether the nickel–sulfur material is petal shaped, granular, or flake like, it is involved in uniform distribution during the process of its dispersion to the carbon material. Further, how the morphology of the interface between nickel–sulfur materials and carbon materials changes is still a topic with research value.
- (4)
- Research on nickel–sulfur and its composites still faces great challenges, which requires researchers to further explore easier synthesis methods considering large-scale manufacturing, cost effectiveness, and green performance.
- (5)
- The morphology of NiS is important for its electrochemical performance. Synthesis mainly includes hydrothermal methods and sulfidation precursors. The sulfur source and reaction time should show a great influence on the phase composition and morphology. Moreover, the vulcanizing atmosphere and flow velocity are important factors. Therefore, how to precisely control these factors is also a promising direction for future studies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Zhang, L.; Lv, X.; Lu, J.; Wang, H. Using Hetero-Structured Co(OH)2/NiS Sheets to Assemble An Array Electrode with High Charge Store Capacity. J. Nanoparticle Res. 2021, 23, 38. [Google Scholar] [CrossRef]
- Sun, P.; Li, N.; Wang, C.; Yin, J.; Zhao, G.; Hou, P.; Xu, X. Nickel-Cobalt Based Aqueous Flexible Solid State Supercapacitors with High Energy Density by Controllable Surface Modification. J. Power Sources 2019, 427, 56–61. [Google Scholar] [CrossRef]
- Yan, L.; Shen, H.; Yan, F.; Han, Y.; Huang, H.; Wei, G.; Liang, X.; Zhou, W.; Guo, J. One-step Synthesis of Co9S8/NiS Composite with Enhanced Charge Storage Performance for Supercapacitors Application. Ionics 2021, 27, 3143–3152. [Google Scholar] [CrossRef]
- Peng, S.; Fan, L.; Wei, C.; Bao, H.; Zhang, H.; Xu, W.; Xu, J. Polypyrrole/Nickel Sulfide/Bacterial Cellulose Nanofibrous Composite Membranes for Flexible Supercapacitor Electrodes. Cellulose 2016, 23, 2639–2651. [Google Scholar] [CrossRef]
- Rao, S.S.; Punnoose, D.; Bae, J.-H.; Durga, I.K.; Thulasi-Varma, C.V.; Naresh, B.; Subramanian, A.; Raman, V.; Kim, H.-J. Preparation and Electrochemical Performances of NiS with PEDOT:PSS Chrysanthemum Petal Like Nanostructure for High Performance Supercapacitors. Electrochim. Acta 2017, 254, 269–279. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, C.; Zhang, D.; Zhao, J.; Zheng, S.; Su, H.; Wei, F.; Yuan, B.; Fernandez, C. Facile Synthesis of a Nickel Sulfide (NiS) Hierarchical Flower for the Electrochemical Oxidation of H2O2 and the Methanol Oxidation Reaction (MOR). J. Electrochem. Soc. 2017, 164, B92–B96. [Google Scholar] [CrossRef]
- Zhu, M.; Li, X.; Tu, C.; Luo, Q.; Nie, Y.; Pan, J.; Li, S. Ethylene Glycol Assisted Self-Template Conversion Approach to Synthesize Hollow NiS Microspheres for a High Performance All-Solid-State Supercapacitor. Mater. Chem. Front. 2022, 6, 203–212. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, C.; Tian, Y.; Tang, Y.; Yin, X.; Que, W. A Long Cycle Life Asymmetric Supercapacitor Based on Advanced Nickel-Sulfide/Titanium Carbide (MXene) Nanohybrid and MXene Electrodes. J. Power Sources 2020, 450, 227694. [Google Scholar] [CrossRef]
- Gou, J. Ni2P/NiS2 Composite with Phase Boundaries as High-Performance Electrode Material for Supercapacitor. J. Electrochem. Soc. 2017, 164, A2956–A2961. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kim, Y.R.; Jeong, H.T. All-solid-state Supercapacitor Composed of Reduced Graphene Oxide (rGO)/Activated Carbon (AC) Composite and Polymer Electrolyte. Carbon Lett. 2020, 30, 107–113. [Google Scholar] [CrossRef]
- Hasa, I.; Adelhelm, P.; Cao, G.Z.; Mai, L.Q. Batteries & Supercaps: Beyond Lithium-Ion Batteries. Batter. Supercaps 2021, 4, 1036–1038. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.X. Environmental Decentralization, Environmental Protection Investment, and Green Technology Innovation. Environ. Sci. Pollut. R 2022, 29, 12740–12755. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Wu, X.L.; Han, Y.Q.; Li, T.X. Flexible Supercapacitor: Overview and Outlooks. J. Energy Storage 2021, 42, 103053. [Google Scholar] [CrossRef]
- Jain, D.; Kanungo, J.; Tripathi, S.K. Enhancement in Performance of Supercapacitor Using Eucalyptus Leaves Derived Activated Carbon Electrode with CH3COONa and HQ Electrolytes: A Step Towards Environment Benign Supercapacitor. J. Alloy. Compd. 2020, 832, 154956. [Google Scholar] [CrossRef]
- Lee, K.S.; Seo, Y.J.; Jeong, H.T. Enhanced Electrochemical Performances of Activated Carbon (AC)-Nickel-Metal Organic Framework (SIFSIX-3-Ni) Composite and Ion-Gel Electrolyte Based Supercapacitor. Carbon Lett. 2021, 31, 635–642. [Google Scholar] [CrossRef]
- Dam, D.H.; Lee, H.H. Battery-Inductor-Supercapacitor Hybrid Energy Storage System for DC Microgrids. J. Power Electron. 2020, 20, 308–318. [Google Scholar] [CrossRef]
- Mondal, M.; Goswami, D.K.; Bhattacharyya, T.K. High Performing Asymmetric Supercapacitor Fabricated by Defect Induced Cathodic MnV2O7 and Biowaste Derive Anodic Activated Carbon. J. Energy Storage 2023, 57, 106177. [Google Scholar] [CrossRef]
- Nath, A.R.; Sandhyarani, N. Silar Deposited Nickel Sulphide-Nickel Hydroxide Nanocomposite for High Performance Asymmetric Supercapacitor. Electrochim. Acta 2020, 356, 136844. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Ji, S.; Wang, X.; Wang, R. Porous-Sheet-Assembled Ni(OH)2/NiS Arrays with Vertical In-Plane Edge Structure for Supercapacitors with High Stability. Dalton T 2019, 48, 17364–17370. [Google Scholar] [CrossRef]
- Song, K.; Li, W.; Yang, R.; Zheng, Y.; Chen, X.; Wang, X.; Chen, G.; Lv, W. Controlled Preparation of Ni(OH)2/NiS Nanosheet Heterostructure As Hybrid Supercapacitor Electrodes for High Electrochemical Performance. Electrochim. Acta 2021, 388, 138663. [Google Scholar] [CrossRef]
- Shinde, P.A.; Olabi, A.G.; Chodankar, N.R.; Patil, S.J.; Hwang, S.-K.; Abdelkareem, M.A. Realizing Superior Redox Kinetics of Metal-Metal Carbides/Carbon Coordination Supported Heterointerface for Stable Solid-State Hybrid Supercapacitor. Chem. Eng. J. 2023, 454, 140246. [Google Scholar] [CrossRef]
- Li, Y.; Huang, B.; Zhao, X.; Luo, Z.; Liang, S.; Qin, H.; Chen, L. Zeolitic Imidazolate Framework-l-Assisted Synthesis of Inorganic and Organic Anion-Intercalated Hetero-Trimetallic Layered Double Hydroxide Sheets as Advanced Electrode Materials for Aqueous Asymmetric Super-Capacitor Battery. J. Power Sources 2022, 527, 231149. [Google Scholar] [CrossRef]
- Lee, J.W.; Hall, A.S.; Kim, J.-D.; Mallouk, T.E. A Facile and Template-Free Hydrothermal Synthesis of Mn3O4 Nanorods on Graphene Sheets for Supercapacitor Electrodes with Long Cycle Stability. Chem. Mater. 2012, 24, 1158–1164. [Google Scholar] [CrossRef]
- Liu, X.Y.; Gao, Y.Q.; Yang, G.W. A Flexible, Transparent and Super-Long-Life Supercapacitor Based on Ultrafine Co3O4 Nanocrystal Electrodes. Nanoscale 2016, 8, 4227–4235. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Li, L.; Liu, M.; Jiang, C.; Du, C.; Zhao, Z.; Hu, W.; Wang, Z.L. Wearable Self-Charging Power Textile Based on Flexible Yarn Supercapacitors and Fabric Nanogenerators. Adv. Mater. 2016, 8, 4227–4235. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huo, W.C.; Yuan, X.Y.; Zhang, Y.X. Composite of Manganese Dioxide and Two-Dimensional Materials Applied to Supercapacitors. Acta Phys. Chim. Sin. 2020, 36, 19040007. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.Y.; Tian, D.; Li, Q.; Zhong, M.; Chen, S.Y.; Hu, C.L.; Ji, H.B. In Situ Growth of Oriented Polyaniline Nanorod Arrays on the Graphite Flake for High-Performance Supercapacitors. ACS Omega 2020, 5, 32395–32402. [Google Scholar] [CrossRef]
- Bao, C.Y.; Han, J.J.; Cheng, J.N.; Zhang, R.T. Electrode Materials Blended with Graphene/Polyaniline for Supercapacitor. Prog. Chem. 2018, 30, 1349–1363. [Google Scholar] [CrossRef]
- Wang, J.; Wu, N.S.; Liu, T.; Cao, S.W.; Yu, J.G. MnCo Oxides Supported on Carbon Fibers for High-Performance Supercapacitors. Acta Phys. Chim Sin. 2020, 36, 1907072. [Google Scholar] [CrossRef]
- Fan, J.M.; Gong, Q.J.; Gong, P.N.; Zhao, X.Y. Preparation and Properties of CoNi2S4 for Supercapacitor Electrode Material. Chin. J. Anal. Chem. 2022, 50, 119–126. [Google Scholar] [CrossRef]
- Liu, T.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical NiS/N-Doped Carbon Composite Hollow Spheres with Excellent Supercapacitor Performance. J. Mater. Chem A 2017, 5, 21257–21265. [Google Scholar] [CrossRef]
- Huang, C.; Gao, A.; Yi, F.; Wang, Y.; Shu, D.; Liang, Y.; Zhu, Z.; Ling, J.; Hao, J. Metal Organic Framework Derived Hollow NiS@C with S-vacancies to Boost High-Performance Supercapacitors. Chem. Eng. J. 2021, 419, 129643. [Google Scholar] [CrossRef]
- Yuan, F.; Gao, G.; Jiang, X.; Bi, W.; Su, Y.; Guo, J.; Bao, Z.; Shen, J.; Wu, G. Suppressing the Metal-Metal Interaction by CoZn0.5V1.5O4 Derived from Two-Dimensional Metal-Organic Frameworks for Supercapacitors. Sci. China Mater. 2022, 65, 105–114. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, X.-P.; Guan, X.-H.; Wang, G.-S. Synthesis of Nickel Chalcogenide Hollow Spheres Using An l-Cysteine-Assisted Hydrothermal Process for Efficient Supercapacitor Electrodes. J. Mater. Chem A 2017, 5, 3621–3627. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, W.; Wang, N.; Jiang, Z.; Wang, X.; Zou, P.; Lin, Z.; Fan, H.J.; Kang, F.; Wong, C.-P.; et al. A Reduced Graphene Oxide/Mixed-Valence Manganese Oxide Composite Electrode for Tailorable and Surface Mountable Supercapacitors with High Capacitance And Super-Long Life. Energy Environ. Sci. 2017, 10, 941–949. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, F.; Zhang, H.; Qin, J.; Wu, Z.-S. Controllable Synthesis of 2d Mesoporous Nitrogen-Doped Carbon/Graphene Nanosheets for High-Performance Micro-Supercapacitors. New Carbon Mater. 2022, 37, 936–943. [Google Scholar] [CrossRef]
- Zheng, S.; Xue, H.; Pang, H. Supercapacitors Based on Metal Coordination Materials. Coord. Chem. Rev. 2018, 373, 2–21. [Google Scholar] [CrossRef]
- Patil, A.M.; Lokhande, A.C.; Shinde, P.A.; Kim, J.H.; Lokhande, C.D. Vertically Aligned NiS Nano-Flakes Derived From Hydrothermally Prepared Ni(OH)2 For High Performance Supercapacitor. J. Energy Chem. 2018, 27, 791–800. [Google Scholar] [CrossRef]
- Lu, Q.; Lattanzi, M.W.; Chen, Y.; Kou, X.; Li, W.; Fan, X.; Unruh, K.M.; Chen, J.G.; Xiao, J.Q. Supercapacitor Electrodes with High-Energy and Power Densities Prepared from Monolithic NiO/Ni Nanocomposites. Angew. Chem. 2011, 50, 6847–6850. [Google Scholar] [CrossRef]
- Cheng, W.X.; Jiang, T.T.; Hu, H.B. Flexible Planar Micro-Supercapacitors Based on Carbon Nanotubes. Chin. J. Chem. Phys. 2022, 35, 562–569. [Google Scholar] [CrossRef]
- Wei, W.T.; Mi, L.W.; Gao, Y.; Zheng, Z.; Chen, W.H.; Guan, X.X. Partial Ion-Exchange of Nickel-Sulfide-Derived Electrodes for High Performance Supercapacitors. Chem. Mater. 2014, 26, 3418–3426. [Google Scholar] [CrossRef]
- Manalu, A.; Tarigan, K.; Humaidi, S.; Ginting, M.; Sebayang, K.; Rianna, M.; Hamid, M.; Subhan, A.; Sebayang, P.; Manalu, I.P. Synthesis, Microstructure and Electrical Properties of NiCo2O4/rGO Composites as Pseudocapacitive Electrode for Supercapacitors. Int. J. Electrochem. Sci. 2022, 22036. [Google Scholar] [CrossRef]
- Liu, L.; Niu, Z.; Zhang, L.; Zhou, W.; Chen, X.; Xie, S. Nanostructured Graphene Composite Papers for Highly Flexible and Foldable Supercapacitors. Adv. Mater. 2014, 26, 4855–4862. [Google Scholar] [CrossRef]
- Song, J.; Du, L.; Wang, J.; Zhang, H.; Zhang, Y.; Li, B.; Zhang, Z.; Li, T.; Luo, J. NiS Nanosheets Synthesized by One-Step Microwave for High-Performance Supercapacitor. Adv. Funct. Mater. 2021, 14, 2150035. [Google Scholar] [CrossRef]
- Pan, H.; Hu, Y.S.; Chen, L. Room-Temperature Stationary Sodium-Ion batteries for Large-Scale Electric Energy Storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Yao, Z.; Quan, B.; Yang, T.; Li, J.; Gu, C. Flexible Supercapacitors Based on Vertical Graphene/Carbon Fabric with High Rate Performance. Appl. Surf. Sci. 2023, 610, 155535. [Google Scholar] [CrossRef]
- Yi, J.X.; Huo, Z.P.; Asiri, A.M.; Alamry, K.A.; Li, J.X. Development and Application of Electrolytes in Supercapacitors. Prog. Chem. 2018, 30, 1624–1633. [Google Scholar] [CrossRef]
- Sun, X.Z.; Zhang, X.; Zhang, D.C.; Ma, Y.W. Activated Carbon-Based Supercapacitors Using Li2SO4 Aqueous Electrolyte. Acta Phys. Chim. Sin. 2012, 28, 367–372. [Google Scholar] [CrossRef]
- Long, Y.; An, X.; Zhang, H.; Yang, J.; Liu, L.; Tian, Z.; Yang, G.; Cheng, Z.; Cao, H.; Liu, H.; et al. Highly Graphitized Lignin-Derived Porous Carbon with Hierarchical n/o Co-Doping “Core-Shell” Superstructure Supported by Metal-Organic Frameworks for Advanced Supercapacitor Performance. Chem. Eng. J. 2023, 451, 138877. [Google Scholar] [CrossRef]
- Wiston, B.R.; Dhivyaprasath, K.; Tewatia, S.; Ashok, M. Optimization of Nickel Manganese Ratio to Attain Highly Efficient Electroactive Composite for Supercapacitors. J. Alloy. Compd. 2023, 935, 167982. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Q.; Zhao, Y.; Zhang, R.; Liu, L.; Yu, J. Robust n-Doping Porous Carbon Nanofiber Membranes with Inter-Fiber Cross-Linked Structures for Supercapacitors. Carbon 2023, 202, 13–25. [Google Scholar] [CrossRef]
- Xiang, M.; He, L.; Wang, N.; Chen, J.; Hu, W. Hydrothermally Etching Commercial Carbon Cloth to Form a Porous Structure for Flexible Zinc-Ion Hybrid Supercapacitors. Appl. Surf. Sci. 2023, 613, 156093. [Google Scholar] [CrossRef]
- Liu, Y.P.; Wen, Y.X.; Zhang, Y.N.; Wu, X.G.; Li, H.Q.; Chen, H.D.; Huang, J.J.; Liu, G.H.; Peng, S.L. Reduced CoNi2S4 Nanosheets Decorated by Sulfur Vacancies with Enhanced Electrochemical Performance for Asymmetric Supercapacitors. Sci. China Mater. 2020, 63, 1216–1226. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Gan, H.; Jiang, Y.; Feng, J.; Liu, J.; Shi, X. Designing NiS/CoS Decorated NiCo2S4 Nanoflakes Towards High Performance Binder-Free Supercapacitors. J. Energy Storage 2022, 47, 103625. [Google Scholar] [CrossRef]
- Xie, L.X.; Li, M.J.; Zhu, P.; Xiao, X.B.; Jia, Z.F.; Wei, Z.Q.; Jiang, J.L. Novel Combination of Nickel-Cobalt Sulfide and Oxide Derived from Ni2CoS4@ZIF-67 for High Performance Supercapacitor. J. Alloy. Compd. 2022, 898, 162861. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, G.Z. New Precursors Derived Activated Carbon and Graphene for Aqueous Supercapacitors with Unequal Electrode Acta Phys. Chim. Sin. 2020, 36, 1904025. [Google Scholar] [CrossRef]
- Guo, N.N.; Zhang, S.; Wang, L.X.; Jia, D.Z. Application of Plant-Based Porous Carbon for Supercapacitors. Acta Phys. Chim. Sin. 2020, 36, 1903055. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Dong, Y.; Zhang, S.; Fan, Z.J. Application of Carbon-/Graphene Quantum Dots for Supercapacitors. Acta Phys. Chim. Sin. 2020, 36, 1903052. [Google Scholar] [CrossRef]
- Liu, R.Y.; Wu, H.R.; Wang, Z.; Wei, H.; Mai, Y.Y. Bowl-Shaped NiCo2O4 Nanosheet Clusters as Electrode Materials for High-Performance Asymmetric Supercapacitors. Sci. China Mater. 2020, 63, 2456–2464. [Google Scholar] [CrossRef]
- Zheng, M.B.; Xiao, X.; Li, L.L.; Gu, P.; Dai, X.; Tang, H.; Hu, Q.; Xue, H.G.; Pang, H. Hierarchically Nanostructured Transition Metal Oxides for Supercapacitors. Sci. China Mater. 2018, 61, 185–209. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Wang, X.; Fan, J.; Su, H.; Yang, D.; Wei, Y.; Du, F.; Dall’Agnese, Y.; Gao, Y. Flexible Solid-State Supercapacitor Integrated by Methanesulfonic Acid/Polyvinyl Acetate Hydrogel and Ti3C2Tx. Nergy Storage Mater. 2023, 54, 164–171. [Google Scholar] [CrossRef]
- Ma, C.; Shi, J.L.; Li, Y.J.; Song, Y.; Liu, L. Preparation of Nitrogen-Enriched Porous Carbon Nanofibers and Their Electrochemical Performance as Electrode Materials of Supercapacitors. New Carbon Mater. 2015, 30, 295–301. [Google Scholar] [CrossRef]
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Dong, Y.; Ding, Y.; Yang, W.; Han, J.; Jiang, S.; He, S. Nitrogen-Doped Carbon Layer on Cellulose Derived Free-Standing Carbon Paper for High-Rate Supercapacitors. Appl. Surf. Sci. 2023, 608, 155144. [Google Scholar] [CrossRef]
- Amarnath, C.A.; Chang, J.H.; Kim, D.; Mane, R.S.; Han, S.-H.; Sohn, D. Electrochemical Supercapacitor Application of Electroless Surface Polymerization of Polyaniline Nanostructures. Mater. Chem Phys. 2009, 113, 14–17. [Google Scholar] [CrossRef]
- Cao, D.; Zheng, L.; Li, Q.; Zhang, J.; Dong, Y.; Yue, J.; Wu, C. Crystal Phase-Controlled Modulation of Binary Transition Metal Oxides for Highly Reversible Li–O2 Batteries. Nano Lett. 2021, 21, 5225–5232. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; da Silva, M.I.; Toma, H.E.; Angnes, L.; Martins, P.R.; Araki, K. Trimetallic Oxides/hydroxides as hybrid supercapacitor electrode materials: A review. J. Mater. Chem. A 2020, 8, 10534–10570. [Google Scholar] [CrossRef]
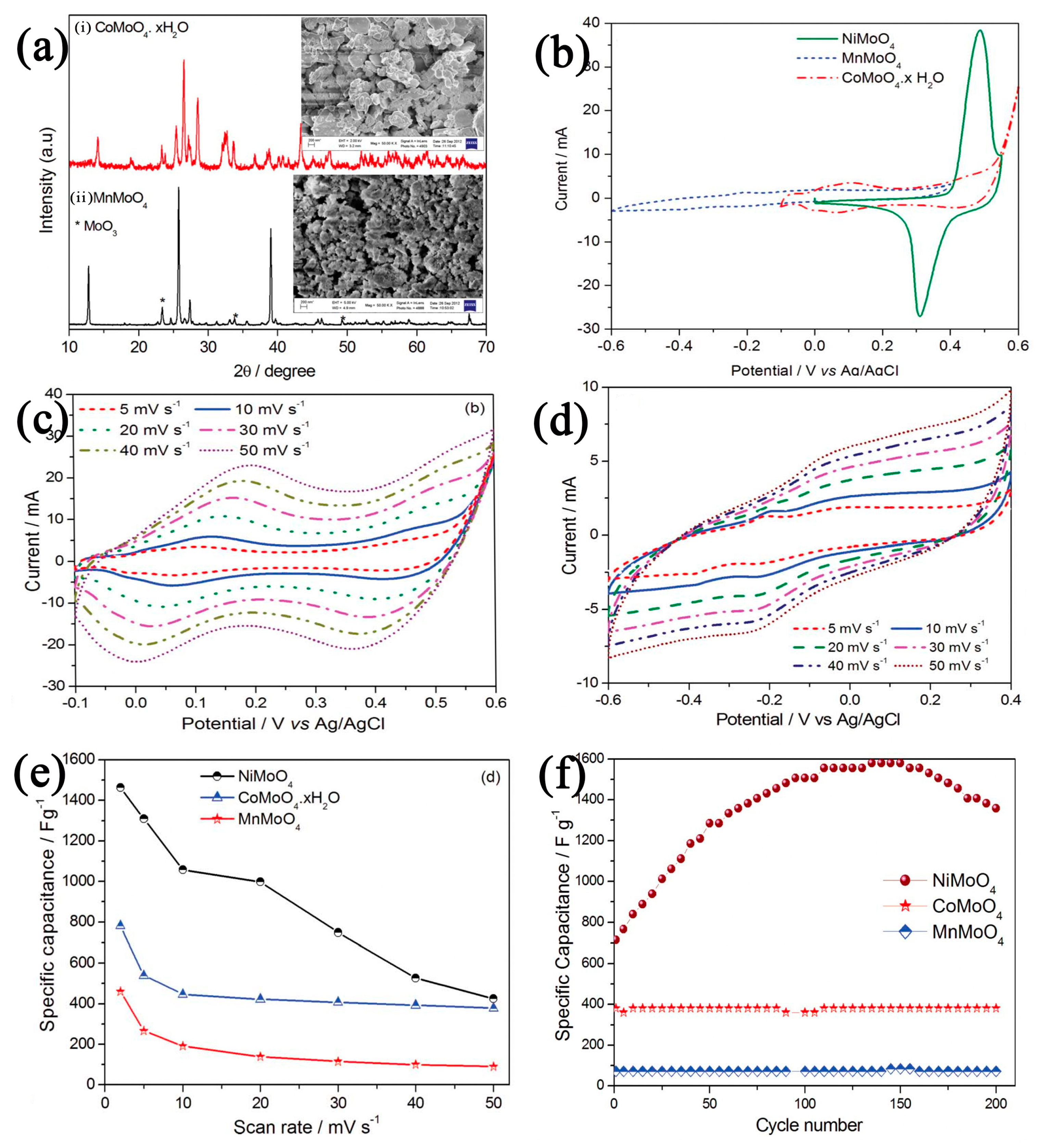
- Sharma, P.; Minakshi Sundaram, M.; Watcharatharapong, T.; Laird, D.; Euchner, H.; Ahuja, R. Zn Metal Atom Doping on the Surface Plane of One-Dimesional NiMoO4 Nanorods with Improved Redox Chemistry. ACS Appl. Mater. Interfaces 2020, 12, 44815–44829. [Google Scholar] [CrossRef]
- Sharma, P.; Minakshi Sundaram, M.; Watcharatharapong, T.; Jungthawan, S.; Ahuja, R. Tuning the Nanoparticle Interfacial Properties and Stability of the Core–Shell Structure in Zn-Doped NiMoO4@AWO4. ACS Appl. Mater. Interfaces 2021, 13, 56116–56130. [Google Scholar] [CrossRef]
- Senthilkumar, B.; Sankar, K.V.; Selvan, R.K.; Danielle, M.; Manickam, M. Nano α-NiMoO4 as a New Electrode for Electrochemical Supercapacitors. RSC Adv. 2013, 3, 352–357. [Google Scholar] [CrossRef]
- Yan, L.; Huang, H.; Yan, F.; Liang, X.; Xu, S.; Guo, J. The Hetero-Structured Nanoarray Construction of Co3O4 Nanowires Anchored on Nanoflakes as a High-Performance Electrode for Supercapacitors. Appl. Surf. Sci. 2021, 538, 147932. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, G.; Du, K.; Zhao, X.; Liu, M.; Wang, S.; Zhang, J. Ni Foam-Supported Carbon-Sheathed NiMoO4 Nanowires as Integrated Electrode for High-performance Hybrid Supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 5964–5971. [Google Scholar] [CrossRef]
- Li, X.-Q.; Chang, L.; Zhao, S.-L.; Hao, C.-L.; Lu, C.-G.; Zhu, Y.-H.; Tang, Z.-Y. Research on Carbon-Based Electrode Materials for Supercapacitors. Acta Phys. Chim Sin. 2017, 33, 130–148. [Google Scholar] [CrossRef]
- Bi, W.; Deng, S.; Tang, H.; Liu, Y.; Shen, J.; Gao, G.; Wu, G.; Atif, M.; AlSalhi, M.S.; Gao, G. Coherent V4+-Rich V2O5/Carbon Aerogel Nanocomposites for High Performance Supercapacitors. Sci. China Mater. 2022, 65, 1797–1804. [Google Scholar] [CrossRef]
- Sukhmani, S.K.; Raut, S.D.; Siddiqui, T.; Shaikh, S.F.; Shinde, N.M.; Mane, R.S. Self-promoted Nickel-chalcogenide Nanostructures: A Novel Electrochemical Supercapacitor Device-Design Strategy. Mater. Res. Bull. 2022, 156, 111975. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, W.; Zhao, K.; Zhang, M.; Guo, H.; Liu, J. Solvothermal Synthesis of CoCeSx Quantum Dots Based on Bi-Pyrene- Terminated Molecular Wires Modified Porous Graphene for High Performance Supercapacitors. J. Alloy. Compd. 2023, 932, 167614. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Prabhu, S.; Nallapureddy, R.R.; Kumar, A.S.; Banerjee, A.N.; Joo, S.W. Bio-derived Graphitic Carbon Quantum Dot Encapsulated S- and N-Doped Graphene Sheets with Unusual Battery-Type Behavior for High-Performance Supercapacitor. Carbon 2023, 202, 93–102. [Google Scholar] [CrossRef]
- Gaikar, P.; Pawar, S.P.; Mane, R.S.; Nuashad, M.; Shinde, D.V. Synthesis of Nickel Sulfide as a Promising Electrode Material for Pseudocapacitor Application. RSC Adv. 2016, 6, 112589–112593. [Google Scholar] [CrossRef]
- Yan, L.; Deng, X.; Huang, H.; Han, Y.; Yan, F.; Xu, S.; Liang, X.; Zhou, W.; Guo, J. Needle-Like NiSx@MnCoOx Core-Shell Nanoarrays with High Charge Storage Performance for Application in Hybrid Supercapacitors. J. Energy Storage 2022, 47, 103563. [Google Scholar] [CrossRef]
- Qi, M.; Zhu, W.; Lu, Z.; Zhang, H.; Ling, Y.; Ou, X. Synthesis of Nickel Sulfide-Graphene Oxide Composite Microflower Structures to Enhance Supercapacitor Performance. J. Mater. Sci. Mater. Electron. 2020, 31, 12536–12545. [Google Scholar] [CrossRef]
- Shao, W.; Wang, Q.; Zhang, D. Defect engineering of P doped Fe7S8 Porous Nanoparticles for High-Performance Asymmetric Supercapacitor and Oxygen Evolution Electrocatalyst. J. Colloid Interface Sci. 2022, 617, 84–93. [Google Scholar] [CrossRef]
- Wu, D.; Xie, X.; Ma, Y.; Zhang, J.; Hou, C.; Sun, X.; Yang, X.; Zhang, Y.; Kimura, H.; Du, W. Morphology Controlled Hierarchical NiS/Carbon Hexahedrons Derived from Nitrilotriacetic Acid-Assembly Strategy for High-Performance Hybrid Supercapacitors. Chem. Eng. J. 2022, 433, 133673. [Google Scholar] [CrossRef]
- Pan, Y.; Tian, L.; Wang, W.; Zhao, J.; Li, Y.; Xi, N.; Jian, L.; Han, S.; Zhang, L. Ameliorating Discharge Capability of Co-Free Flower-Like Spherical a-Ni (OH)2 by NiS Coating. Electrochim. Acta 2022, 430, 141074. [Google Scholar] [CrossRef]
- Guan, B.; Li, Y.; Yin, B.; Liu, K.; Wang, D.; Zhang, H.; Cheng, C. Synthesis of Hierarchical NiS Microflowers for High Performance Asymmetric Supercapacitor. Chem. Eng. J. 2017, 308, 1165–1173. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, S.A.; Ansari, S.G.; Fouad, H.; Abd El-Salam, N.M.; Cho, M.H. Solid-State Symmetrical Supercapacitor Based on Hierarchical Flower-Like Nickel Sulfide with Shape-Controlled Morphological Evolution. Electrochim. Acta 2018, 268, 82–93. [Google Scholar] [CrossRef]
- Li, Z.; Yu, X.; Gu, A.; Tang, H.; Wang, L.; Lou, Z. Anion Exchange Strategy to Synthesis of Porous NiS Hexagonal Nanoplates for Supercapacitors. Nanotechnology 2017, 28, 065406. [Google Scholar] [CrossRef]
- Yu, L.; Yang, B.; Liu, Q.; Liu, J.; Wang, X.; Song, D.; Wang, J.; Jing, X. Interconnected NiS Nanosheets Supported by Nickel Foam: Soaking Fabrication and Supercapacitors Application. J. Electroanal. Chem. 2015, 739, 156–163. [Google Scholar] [CrossRef]
- Kang, J.; Ryu, I.; Choe, G.; Kim, G.; Yim, S. Simple Fabrication of Nickel Sulfide Nanostructured Electrode Using Alternate Dip-Coating Method and Its Supercapacitive Properties. Int. J. Electrochem. Sci. 2017, 12, 9588–9600. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, K.; Liu, X.; Wang, Y.; Wang, Y.; Zhang, D.; Lu, Y.; Peng, T.; Liu, Y.; Luo, Y. Ultra-Thin NiS Nanosheets as Advanced Electrode for High Energy Density Supercapacitors. RSC Adv. 2020, 10, 8760–8765. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, W.; Li, D.; Wei, C.; Fan, H.; Wu, L.; Zheng, W. Synthesis and Electrochemical Performances of Novel Hierarchical Flower-Like Nickel Sulfide with Tunable Number of Composed Nanoplates. J. Power Sources 2014, 268, 113–120. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, X.; Tong, X.; Yang, J.; Miao, L.; Sun, Y.; Peng, L. Synthesis of Nickel Sulfide Monolayer Hollow Spheres Arrays as Cathode Materials for Alkaline Batteries. Mater. Lett. 2016, 178, 120–123. [Google Scholar] [CrossRef]
- Du, N.; Zheng, W.; Li, X.; He, G.; Wang, L.; Shi, J. Nanosheet-assembled NiS Hollow Structures with Double Shells and Controlled Shapes for High-Performance Supercapacitors. Chem. Eng. J. 2017, 323, 415–424. [Google Scholar] [CrossRef]
- Zhao, J.; Guan, B.; Hu, B.; Xu, Z.; Wang, D.; Zhang, H. Vulcanizing Time Controlled Synthesis of NiS Microflowers and Its Application in Asymmetric Supercapacitors. Electrochim. Acta 2017, 230, 428–437. [Google Scholar] [CrossRef]
- Tran, V.C.; Sahoo, S.; Shim, J.-J. Room-Temperature Synthesis of NiS Hollow Spheres on Nickel Foam for High-Performance Supercapacitor Electrodes. Mater. Lett. 2018, 210, 105–108. [Google Scholar] [CrossRef]
- Harish, S.; Naveen, A.N.; Abinaya, R.; Archana, J.; Ramesh, R.; Navaneethan, M.; Shimomura, M.; Hayakawa, Y. Enhanced Performance on Capacity Retention of Hierarchical NiS Hexagonal Nanoplate for Highly Stable Asymmetric Supercapacitor. Electrochim. Acta 2018, 283, 1053–1062. [Google Scholar] [CrossRef]
- Nandhini, S.; Mary, A.J.C.; Muralidharan, G. Facile Microwave-Hydrothermal Synthesis of NiS Nanostructures for Supercapacitor Applications. Appl. Surf. 2018, 449, 485–491. [Google Scholar] [CrossRef]
- Liu, H.; Hu, R.; Qi, J.; Sui, Y.; He, Y.; Meng, Q.; Wei, F.; Ren, Y.; Zhao, Y. A Facile Method for Synthesizing NiS Nanoflower Grown on MXene (Ti3C2Tx) as Positive Electrodes for “Supercapattery”. Electrochim. Acta 2020, 353, 136526. [Google Scholar] [CrossRef]
- Kumar, N.; Mishra, D.; Kim, S.Y.; Jin, S.H. Self-assembled NiS-SNS Heterostructure Via Facile Successive Adsorption and Reaction Method for High-Performance Solid-State Asymmetric Supercapacitors. Thin Solid Film. 2020, 709, 138138. [Google Scholar] [CrossRef]
- Raju, G.S.R.; Pavitra, E.; Nagaraju, G.; Sekhar, S.C.; Ghoreishian, S.M.; Kwak, C.H.; Yu, J.S.; Huh, Y.S.; Han, Y.-K. Rational Design of Forest-Like Nickel Sulfide Hierarchical Architectures with Ultrahigh Areal Capacity as a Binder-Free Cathode Material for Hybrid Supercapacitors. J. Mater. Chem A 2018, 6, 13178–13190. [Google Scholar] [CrossRef]
- Patil, A.M.; Lokhande, V.C.; Lokhande, A.C.; Chodankar, N.R.; Ji, T.; Kim, J.H.; Lokhande, C.D. Ultrathin Nickel Sulfide Nano-Flames as an Electrode for High Performance Supercapacitor; Comparison of Symmetric FSS-SCs and Electrochemical SCs Device. RSC Adv. 2016, 6, 68388–68401. [Google Scholar] [CrossRef]
- Gou, J.; Xie, S.; Yang, Z.; Liu, Y.; Chen, Y.; Liu, Y.; Liu, C. A High-Performance Supercapacitor Electrode Material Based on NiS/Ni3S4 Composite. Electrochim. Acta 2017, 229, 299–305. [Google Scholar] [CrossRef]
- Wu, S.; Yang, X.; Cui, T.; Feng, Q.; Zhou, S.; Xu, X.; Zhao, H.; Wu, L.; He, Y.; Yang, Q. Tubular-Like NiS/Mo2S3 Microspheres as Electrode Material for High-Energy and Long-Life Asymmetric Supercapacitors. Colloid Surf. A 2021, 628, 127332. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, X.; Zhan, J.; Sui, Y.; Qi, J.; Wei, F.; Meng, Q.; He, Y.; Ren, Y.; Zhan, Z.; et al. Hierarchical NiS@CoS with Controllable Core-Shell Structure by Two-Step Strategy for Supercapacitor Electrodes. Adv. Mater. Interfaces 2020, 121, 800–801. [Google Scholar] [CrossRef]
- Xiong, C.; Yang, Q.; Li, B.; Nie, S.; Qin, C.; Dai, L.; Khan, M.; Xu, Y.; Ni, Y. Carbonized Porous Wood as an Effective Scaffold for Loading Flower-Like CoS, NiS nanofibers with Co, Ni Nanoparticles Served As Electrode Material for High-Performance Supercapacitors. Ind. Crop. Prod. 2021, 167, 113545. [Google Scholar] [CrossRef]
- Hu, Q.; Li, W.; Xiang, B.; Zou, X.; Hao, J.; Deng, M.; Wu, Q.; Wang, Y. Sulfur Source-Inspired Synthesis of Beta-NiS with High Specific Capacity and Tunable Morphologies for Hybrid Supercapacitor. Electrochim. Acta 2020, 337, 135826. [Google Scholar] [CrossRef]
- Qin, Q.; Chen, L.; Wei, T.; Liu, X. MoS2/NiS Yolk-Shell Microsphere-Based Electrodes for Overall Water Splitting and Asymmetric Supercapacitor. Small 2019, 15, 1803639. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, S.; Chen, F.; Li, W.; Hao, J.; Liang, X.; Xiang, B.; Chen, C.; Zou, X. Controlled Synthesis of a High-Performance Alpha-NiS/Ni3S4 Hybrid by a Binary Synergy of Sulfur Sources for Supercapacitor. J. Colloid Interface Sci. 2021, 581, 56–65. [Google Scholar] [CrossRef]
- Patil, A.M.; Lokhande, A.C.; Chodankar, N.R.; Kumbhar, V.S.; Lokhande, C.D. Engineered Morphologies of Beta-NiS Thin Films Via Anionic Exchange Process and Their Supercapacitive Performance. Mater. Design 2016, 97, 407–416. [Google Scholar] [CrossRef]
- Ikkurthi, K.D.; Rao, S.S.; Ahn, J.-W.; Sunesh, C.D.; Kim, H.-J. A Cabbage Leaf Like Nanostructure of a NiS@ZnS Composite on Ni Foam with Excellent Electrochemical Performance for Supercapacitors. Dalton Trans. 2019, 48, 578–586. [Google Scholar] [CrossRef]
- Nandhini, S.; Muralidharan, G. Graphene encapsulated NiS/Ni3S4 mesoporous nanostructure: A Superlative High Energy Supercapacitor Device with Excellent Cycling Performance. Electrochim. Acta 2021, 365, 137367. [Google Scholar] [CrossRef]
- Subramanian, A.; Punnoose, D.; Raman, V.; Gopi, C.V.V.M.; Rao, S.S.; Khan, M.A.; Kim, H.-J. Layer by Layer Approach to Enhance Capacitance Using Metal Sulfides for Supercapacitor Applications. Mater. Lett 2018, 231, 64–67. [Google Scholar] [CrossRef]
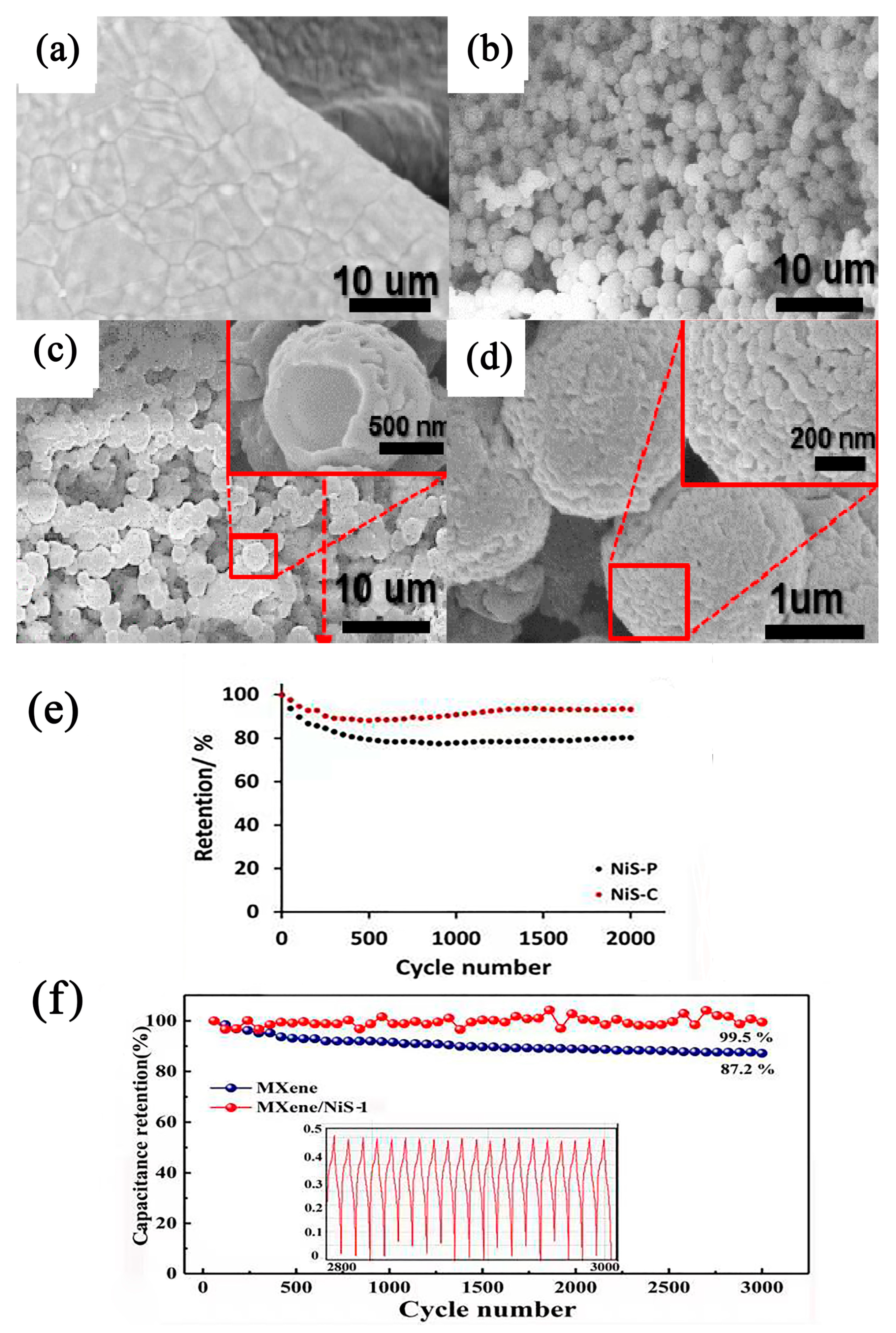
- Xu, X.; Zhong, W.; Zhang, X.; Dou, J.; Xiong, Z.; Sun, Y.; Wang, T.; Du, Y. Flexible Symmetric Supercapacitor with Ultrahigh Energy Density Based on NiS/MoS2@N-rGO Hybrids Electrode. J. Colloid. Interf. Sci. 2019, 543, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zou, X.; Huang, Y.; Wei, Y.; Wang, Y.; Chen, F.; Xiang, B.; Wu, Q.; Li, W. Graphene Oxide-Drove Transformation of NiS/Ni3S4 Microbars Towards Ni3S4 Polyhedrons for Supercapacitor. J. Colloid. Interf. Sci. 2020, 559, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Gopi, C.V.V.M.; Reddy, A.E.; Kim, H.-J. Facile Synthesis of a NiO/NiS hybrid and Its Use as an Efficient Electrode Material for Supercapacitor Applications. New J. Chem. 2018, 42, 5309–5313. [Google Scholar] [CrossRef]
- Guan, S.; Fu, X.; Zhang, B.; Lei, M.; Peng, Z. Cation-Exchange-Assisted Formation of NiS/SnS2 Porous Nanowalls with Ultrahigh Energy Density for Battery-Supercapacitor Hybrid Devices. J. Mater. Chem. A 2020, 8, 3300–3310. [Google Scholar] [CrossRef]
- Sun, C.; Ma, M.; Yang, J.; Zhang, Y.; Chen, P.; Huang, W.; Dong, X. Phase-Controlled Synthesis of Alpha-NiS Nanoparticles Confined in Carbon Nanorods for High Performance Supercapacitors. Sci. Rep. 2014, 4, 7054. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Lin, J.; Wu, J.; Huang, M.; Fan, L.; He, X.; Wang, Y.; Xu, Z. A Two-Step Hydrothermal Synthesis Approach to Synthesize NiCo2S4/NiS Hollow Nanospheres for High-Performance Asymmetric Supercapacitors. Appl. Surf. Sci. 2017, 422, 597–606. [Google Scholar] [CrossRef]
- Asghar, A.; Yousaf, M.I.; Shad, N.A.; Sajid, M.M.; Afzal, A.M.; Javed, Y.; Razzaq, A.; Shariq, M.; Gulfam, Q.U.; Sarwar, M.; et al. Enhanced Electrochemical Performance of Hydrothermally Synthesized NiS/ZnS Composites as an Electrode for Super-Capacitors. J. Clust. Sci. 2022, 33, 2325–2335. [Google Scholar] [CrossRef]
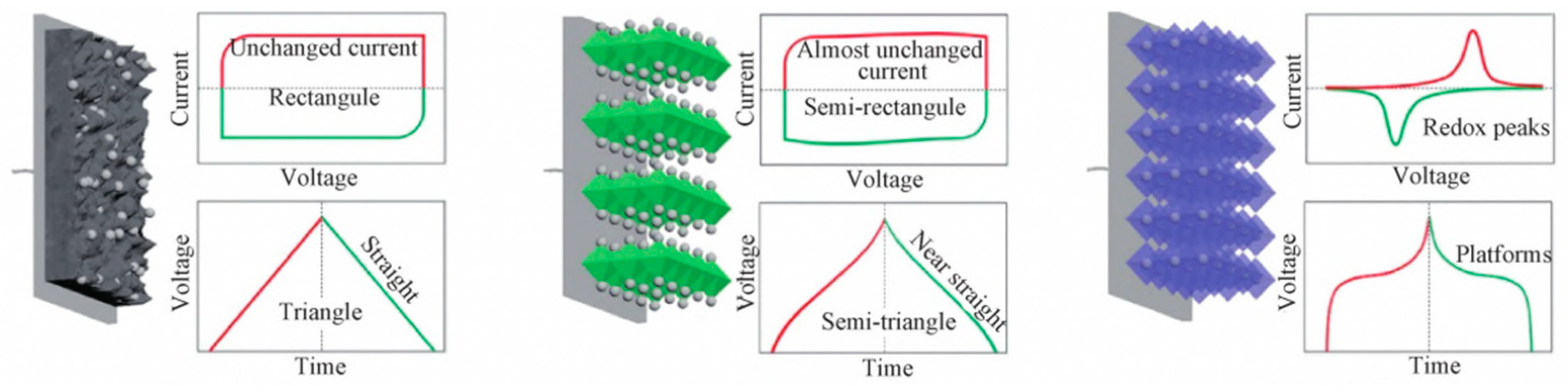
- Liang, J.; Wen, L.; Cheng, H.; Li, F. Applications of Carbon Materials in Electrochemical Energy Storage. J. Electrochem. 2015, 21, 505. [Google Scholar] [CrossRef]
- Sabeeh, H.; Aadil, M.; Zulfiqar, S.; Ayeman, I.; Shakir, I.; Agboola, P.O.; Haider, S.; Warsi, M.F. Self-Supporting Design of NiS/CNTs Nanohybrid for Advanced Electrochemical Energy Storage Applications. J. Clust. Sci. 2022, 33, 2113–2121. [Google Scholar] [CrossRef]
- Ouyang, Y.; Chen, Y.; Peng, J.; Yang, J.; Wu, C.; Chang, B.; Guo, X.; Chen, G.; Luo, Z.; Wang, X. Nickel Sulfide/Activated Carbon Nanotubes Nanocomposites as Advanced Electrode of High-Performance Aqueous Asymmetric Supercapacitors. J. Alloy. Compd. 2021, 885, 160979. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, L.; Lian, J. Arrays of hierarchical nickel sulfides/MoS2 Nanosheets Supported on Carbon Nanotubes Backbone as Advanced Anode Materials for Asymmetric Supercapacitor. J. Power Sources 2017, 343, 373–382. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, L.; Xu, G.; Sun, Z.; Zhang, C.; Ma, X.; Qi, C.; Zhang, L.; Jia, D. Facile Synthesis of NiS Anchored Carbon Nanofibers for High-Performance Supercapacitors. Appl. Surf. Sci. 2018, 434, 112–119. [Google Scholar] [CrossRef]
- Xavier, J.R.; Vinodhini, S.P.; Chandraraj, S.S. Synthesis and Electrochemical Characterization of CNTs-Based Multi Metal Sulphide Nanocomposite for Supercapacitor Applications. J. Clust. Sci. 2022, 1–13. [Google Scholar] [CrossRef]
- Wu, D.; Xie, X.; Zhang, J.; Ma, Y.; Hou, C.; Sun, X.; Yang, X.; Zhang, Y.; Kimura, H.; Du, W. Embedding NiS Nanoflakes in Electrospun Carbon Fibers Containing NiS Nanoparticles for Hybrid Supercapacitors. Chem. Eng. J. 2022, 446, 137262. [Google Scholar] [CrossRef]
- Zhu, H.; Sun, X.; Yang, H.; Pang, Y.; Ta, S.; Wang, L.; Zhu, H.; Zhang, Q. Polydopamine-Derived Nitrogen-Doped Carbon-Coated NiS Nanoparticles as a Battery-Type Electrode for High-Performance Supercapacitors. Ceram. Int. 2021, 47, 9332–9341. [Google Scholar] [CrossRef]
- Reddy, B.J.; Vickraman, P.; Justin, A.S. Asymmetric supercapacitor device Performance Based on Microwave Synthesis of N-Doped Graphene/Nickel Sulfide Nanocomposite. J. Mater. Sci. 2019, 54, 6361–6373. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Guo, X.; Liu, Y.; Zheng, Y.; Zhang, M.; Li, R.; Peng, Z.; Zhang, Y.; Zhang, T. One-Step Microwave-Hydrothermal Preparation of NiS/rGO Hybrid for High-Performance Symmetric Solid-State Supercapacitor. Appl. Surf. Sci. 2020, 514, 146080. [Google Scholar] [CrossRef]
- Li, Y.; Ye, K.; Cheng, K.; Yin, J.; Cao, D.; Wang, G. Electrodeposition of Nickel Sulfide on Graphene-Covered Make-Up Cotton as a Flexible Electrode Material for High-Performance Supercapacitors. J. Power Sources 2015, 274, 943–950. [Google Scholar] [CrossRef]
- AbdelHamid, A.A.; Yang, X.; Yang, J.; Chen, X.; Ying, J.Y. Graphene-Wrapped Nickel Sulfide Nanoprisms with Improved Performance for Li-Ion Battery Anodes and Supercapacitors. Nano Energy 2016, 26, 425–437. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, K.; Dai, W.; Ma, D.; Zhang, H.; Shen, X.; Zhu, G.; Wu, S. High Energy Density Hybrid Supercapacitor Based on Cobalt-Doped Nickel Sulfide Flower-Like Hierarchitectures Deposited with Nitrogen-Doped Carbon Dots. Nanoscale 2021, 13, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, H.; Zhu, H.; Wang, L.; Fu, Z.; Zhang, Q.; Zhu, H. Synthesis and Enhanced Supercapacitor Performance of Carbon Self-Doping Graphitic Carbon Nitride/NiS Electrode Material. J. Am. Ceram. Soc. 2021, 104, 1554–1567. [Google Scholar] [CrossRef]
- Baldinelli, A.; Dou, X.; Buchholz, D.; Marinaro, M.; Passerini, S.; Barelli, L. Addressing the Energy Sustainability of Biowaste-Derived Hard Carbon Materials for Battery Electrodes. Green Chem. 2018, 20, 1527–1537. [Google Scholar] [CrossRef]
- Wickramaarachchi, W.K.P.; Minakshi, M.; Gao, X.; Dabare, R.; Wong, K.W. Hierarchical Porous Carbon from Mango Seed Husk for Electro-Chemical Energy Storage. Chem. Eng. J. Adv. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.; Jones, R.T.; Fichtner, M. Calcined Chicken Eggshell Electrode for Battery and Supercapacitor Applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef] [PubMed]
- Wickramaarachchi, K.; Minakshi, M.; Aravindh, S.A.; Dabare, R.; Gao, X.; Jiang, Z.T.; Wong, K.W. Repurposing N-doped Grape Marc for the Fabrication of Supercapacitors with Theoretical and Machine Learning Models. Nanomaterials 2022, 12, 1847. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Q.; Liu, H.; Li, K.; Wei, M. Nature-Inspired Design of NiS/Carbon Microspheres for High-Performance Hybrid Supercapacitors. Appl. Surf. Sci. 2020, 528, 146976. [Google Scholar] [CrossRef]
- Wu, D.; Yu, H.; Hou, C.; Du, W.; Song, X.; Shi, T.; Sun, X.; Wang, B. NiS Nanoparticles Assembled on Biological Cell Walls-Derived Porous Hollow Carbon Spheres as a Novel Battery-Type Electrode for Hybrid Supercapacitor. J. Mater. Sci. 2020, 55, 14431–14446. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, C.; Shi, Z.; Liu, T.; Zhai, T.; Zhou, W. Hexagonal Phase NiS Octahedrons Co-Modified by 0D-, 1D-, and 2D Carbon Materials for High-Performance Supercapacitor. Electrochim. Acta 2019, 311, 83–91. [Google Scholar] [CrossRef]
- Ke, T.-C.; Vedhanarayanan, B.; Shao, L.-D.; Lin, T.-W. Porous and Hierarchically Structured Ammonium Nickel Molybdate/Nickel Sulfide/Reduced Graphene Oxide Ternary Composite as High Performance Electrode for Supercapacitors. ChemElectroChem 2019, 6, 3806–3814. [Google Scholar] [CrossRef]
- Guo, M.; Sun, J.; Liu, Y.; Huangfu, C.; Wang, R.; Han, C.; Qu, Z.; Wang, N.; Zhao, L.; Zheng, Q. Optimizing Fe2O3-Based Supercapacitor Cathode with Tunable Surface Pseudocapacitance Via Facile in Situ Vulcanization Process. J. Electroanal. Chem. 2021, 901, 115785. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, Y.; Liu, S.; Tan, X.; Wang, S.; Guo, Q.; Luo, J.; Li, Z. One-Step Microwave Synthesis of NiO/NiS@CNT Nanocomposites for High-Cycling-Stability Supercapacitors. J. Alloy. Compd. 2019, 806, 170–179. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, A.; Zhang, Y.; Shi, J.; Lin, J.; Liang, S.; Cao, G. Heterogeneous NiS/NiO Multi-Shelled Hollow Microspheres with Enhanced Electrochemical Performances for Hybrid-Type Asymmetric Supercapacitors. J. Mater. Chem. A 2018, 6, 9153–9160. [Google Scholar] [CrossRef]
- Yan, X.; Tong, X.; Ma, L.; Tian, Y.; Cai, Y.; Gong, C.; Zhang, M.; Liang, L. Synthesis of Porous NiS Nanoflake Arrays by Ion Exchange Reaction from NiO and Their High Performance Supercapacitor Properties. Mater. Lett. 2014, 124, 133–136. [Google Scholar] [CrossRef]
- Wu, J.; Ouyang, C.; Dou, S.; Wang, S. Hybrid NiS/CoO Mesoporous Nanosheet Arrays on Ni Foam for High-Rate Supercapacitors. Nanotechnology 2015, 26, 325401. [Google Scholar] [CrossRef]
- Mallick, S.; Mondal, A.; Raj, C.R. Rationally Designed Mesoporous Carbon-Supported Ni-NiWO4@NiS Nanostructure for the Fabrication of Hybrid Supercapacitor of Long-Term Cycling Stability. J. Power Sources 2020, 477, 229038. [Google Scholar] [CrossRef]
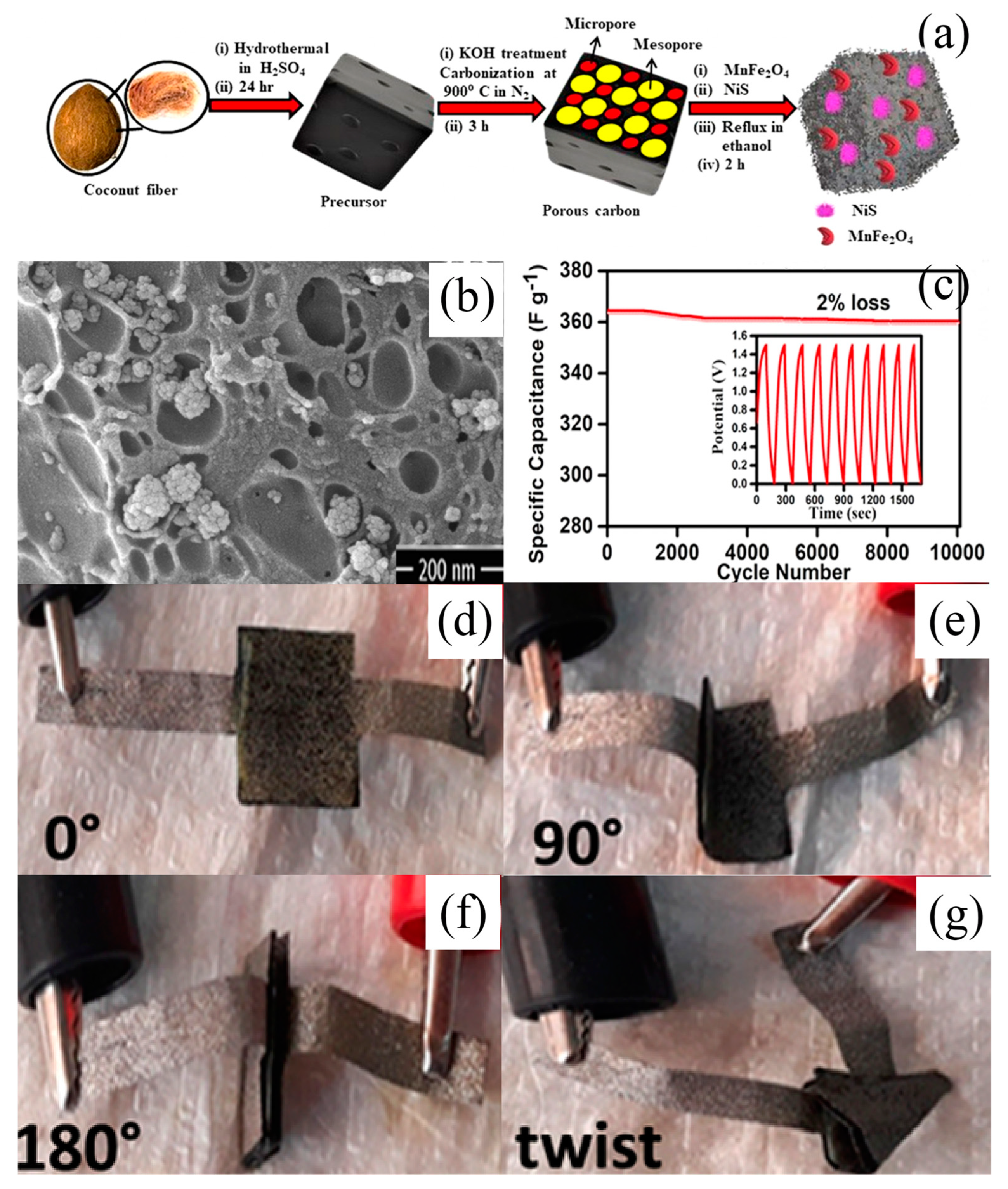
- Makkar, P.; Malik, A.; Ghosh, N.N. Biomass-Derived Porous Carbon-Anchoring MnFe2O4 Hollow Sphere and Needle-Like NiS for a Flexible All-Solid-State Asymmetric Supercapacitor. ACS Appl. Energy Mater. 2021, 4, 6015–6024. [Google Scholar] [CrossRef]
- Meng, S.; Wang, Y.; He, J.; Quan, W.; Gao, M.; Jiang, D.; Chen, M. Nanowire-assembled Co3O4@NiS Core-Shell Hierarchical with Enhanced Electrochemical Performance for Asymmetric Supercapacitors. Nanotechnology 2020, 31, 295403. [Google Scholar] [CrossRef]
- Ahmad, S.; Yang, C.; Xie, W.; Deng, Z.; Zhang, H.; Zhao, Y.; Su, X. Molten Salt-Templated Synthesis of Ternary NiS-NiCo2O4@C Composites as High Performance Catalysts for 4-Nitro Phenol Reduction and Supercapacitor. Carbon 2020, 158, 912–921. [Google Scholar] [CrossRef]

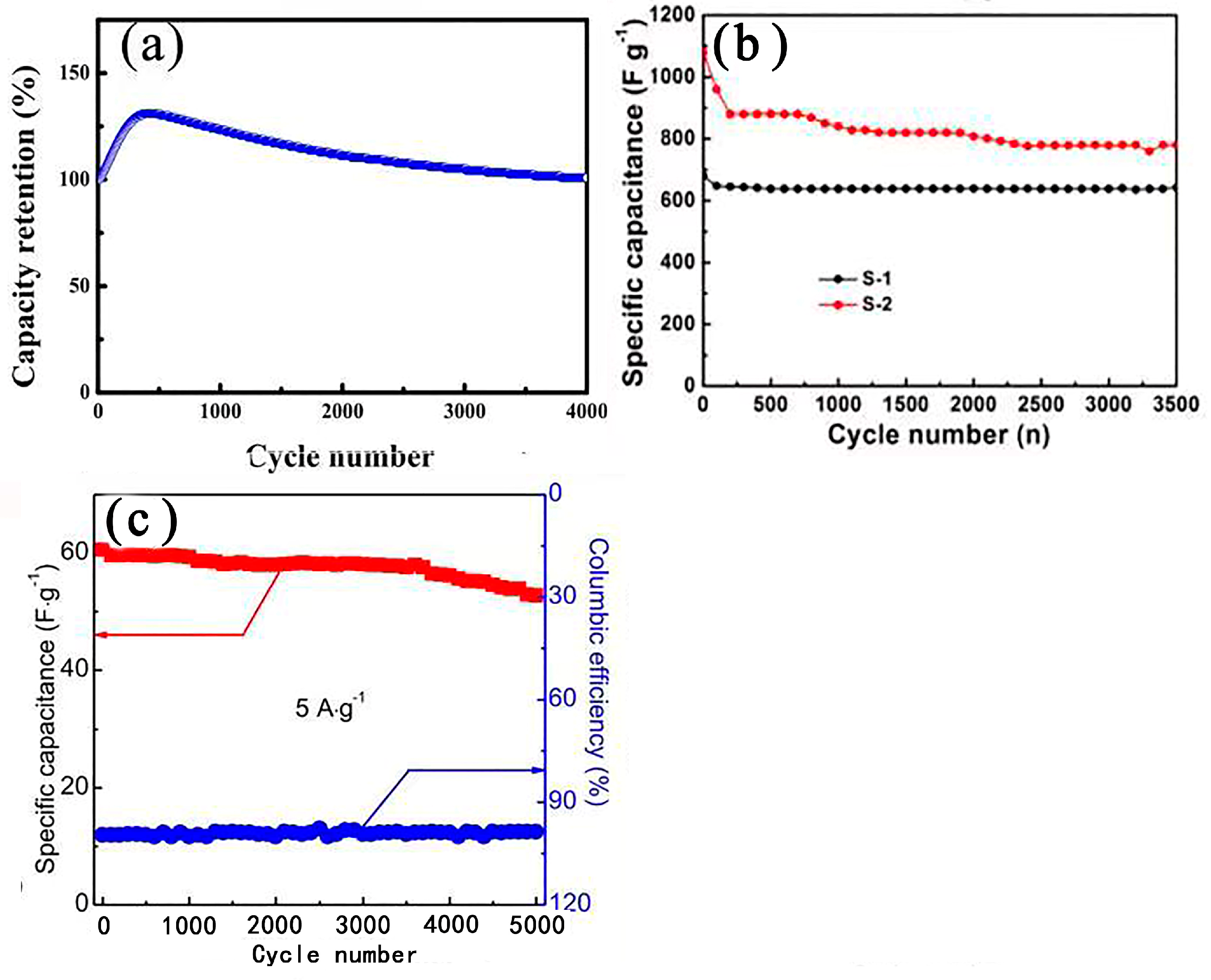
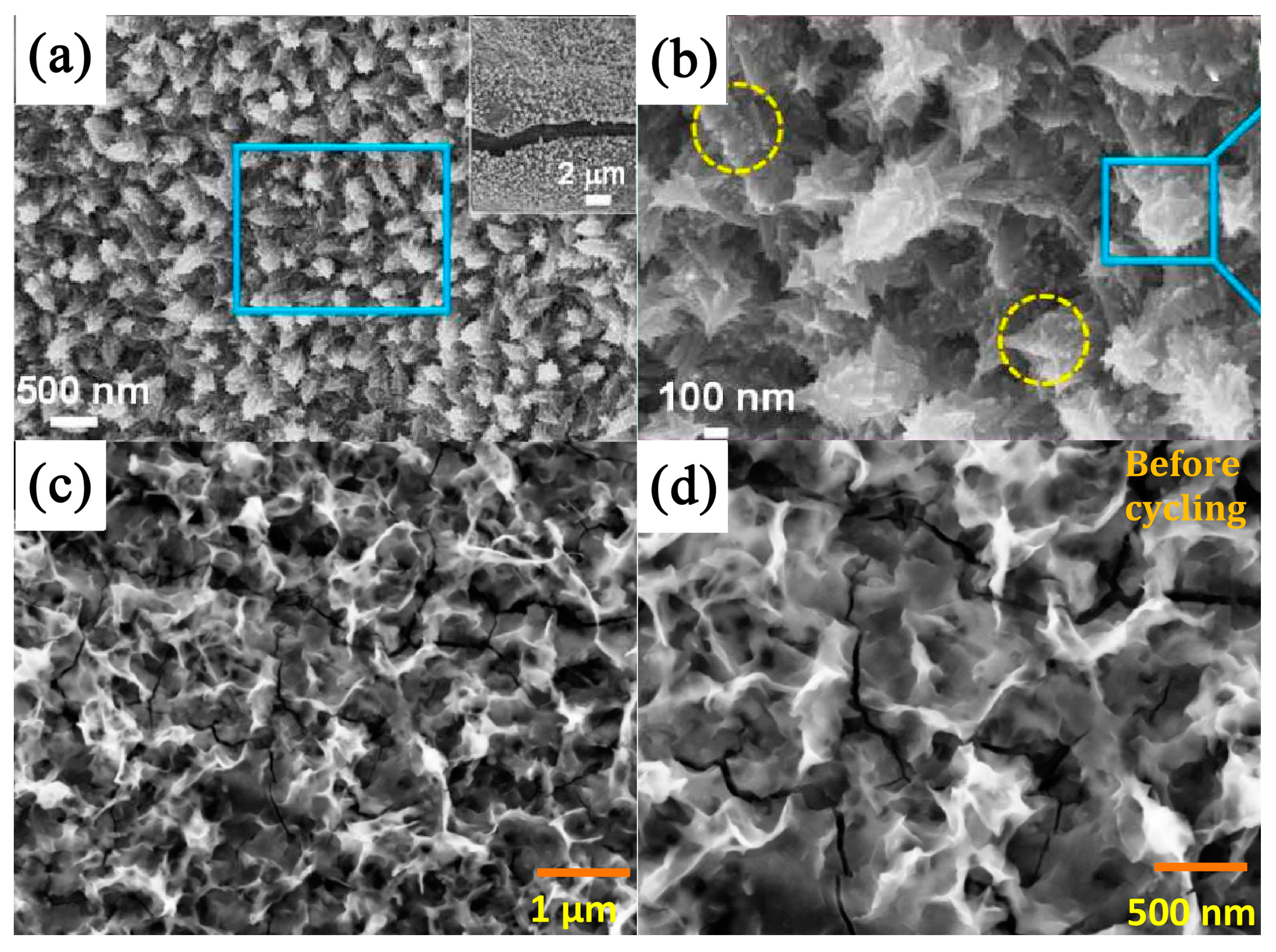
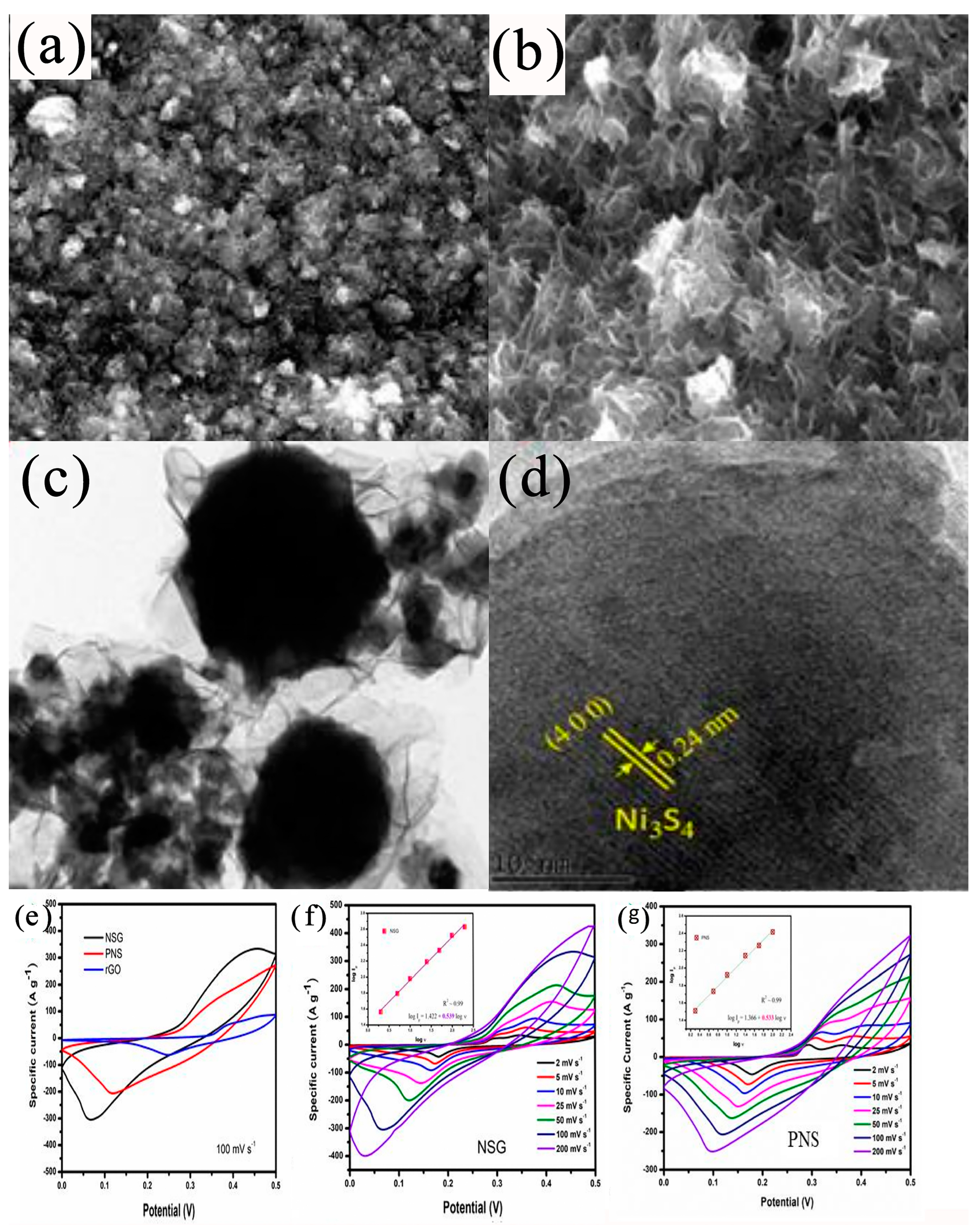
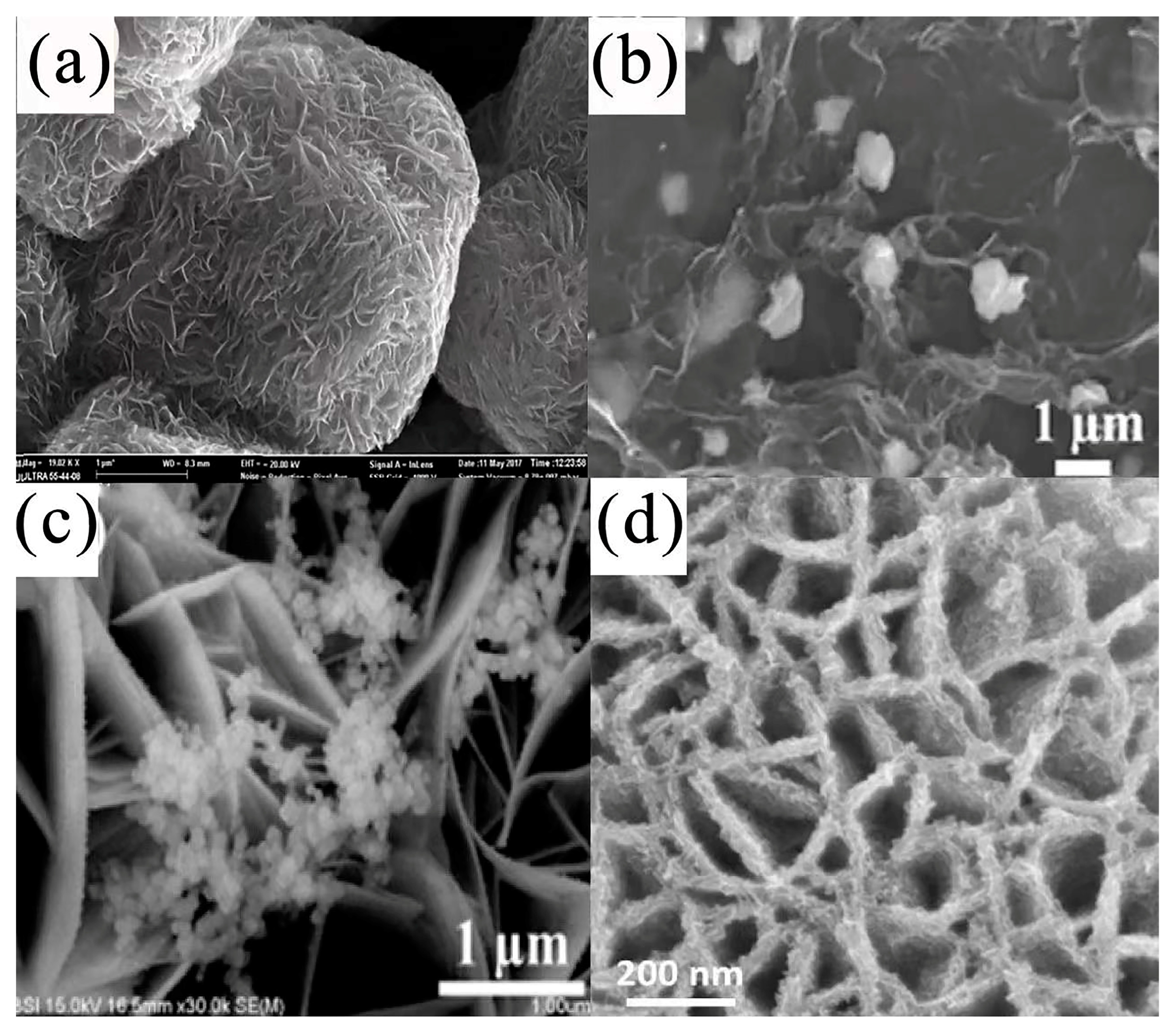


| Electrode Materials | Prepare Methods | Electrolyte | Specific Capacitance | Cyclic Stability | Ref. | |
|---|---|---|---|---|---|---|
| Pure NiS nanomaterials | Porous NiS hexagonal nanoplates | Ion exchange method | 2 mol L−1 KOH | 1897 F g−1 (1 A g−1) | 100% 4000 th (3 A g−1) | [85] |
| Ultrathin NiS nanosheets | Hydrothermal | 0.15 mol KOH | 2587 F g−1 (0.2 A g−1) | 95.8% 4000 th | [89] | |
| Nickel sulfide monolayer hollow spheres arrays | Ion exchange | 6 M KOH | 68.5 mAh g−1 (2 Ag−1) | 94.8% 3000 th | [91] | |
| NiS hollow spheres | Electrodeposition | 3 M KOH | 1553 F g−1 (2.35 A g−1) | 95.7% 2000 th | [94] | |
| Hierarchical NiS Hexagonal nanoplate | Hydrothermal | 3 M KOH | 606 C g−1 (0.5 A g−1) | 93% 2000 th | [95] | |
| NiS thin film | CBD | 2 M KOH | 104 F g−1 (5 mVs−1) | 85.3% over 3000 th | [97] | |
| NiS/sulfide nanocomposite | Nickel sulfides | Hydrothermal | 2 M KOH | 194.4 mAh g−1 (2 A g−1) | 89.5 mAh g−1 5000 th (10 A g−1) | [101] |
| MoS2/NiS | Hydrothermal | 6 M KOH | 1493 F g−1 (0.2 A g−1) | 100% 10,000 th (0.5 A g−1) | [106] | |
| MoS2/NiS | Hydrothermal | 6 M KOH | 2225 F g−1 (1 A g−1) | 94.5% 50,000 th (10 A g−1) | [112] | |
| NiO/NiS | Hydrothermal | 3 M KOH | 386.7 F g−1 (1 A g−1) | 97.6% 3000 th (5 A g−1) | [114] | |
| NiS/SnS2 | Cation exchange | 6 mol·L−1 KOH | 430.38 mAh g−1 (1.16 A g−1) | 100% 1000 th (15 mA h−1) | [115] | |
| NiCo2S4/NiS | Hydrothermal | 3 M KOH | 1947.5 F g−1(3 mA cm−2) | 90.3% 1000 th | [117] | |
| Nickel sulfides/MoS2 | Hydrothermal | 3 M KOH | 676.4 F g−1 (1 A g−1) | 100% 2000 th | [122] | |
| NiS/carbon nanocomposite | A composite material combining carbon nanotubes on CoS, NiS, and nickel foam | Hydrothermal | 3 M KOH | 1249.88 mAh g−1 (1 A g−1) | 97.17% 8000 th (1 A g−1) | [125] |
| Electrospun carbon fibers containing NiS | Embedding NiS nanoflakes in electrospun carbon fibers | 6 M KOH | 1691.1 F g−1 (1 A g−1) | 87.8% 5000 th (5 A g−1) | [126] | |
| Nitrogen-doped carbon-coated NiS nanoparticles | N-doped carbon-coated NiS core-shell composites | 6 M KOH | 1330 F g−1 (0.5 A g−1) | 92.3% 3000 th (10 A g−1) | [127] | |
| NiS/reduced graphene (rGO) hybrid | Hydrothermal | 2 M KOH | 1745.67 F g−1 (1 A g−1) | 54.2% 3000 th (10 A g−1) | [128] | |
| Graphene nanosheets (GNS)/nickel sulfide (NiS)-based material | “Dip and Dry” and Electrodeposition | 6 mol L−1 KOH | 755 F g−1 (0.5 A g−1) | 88.1% 1000 th (2 A g−1) | [129] | |
| Nickel sulfide (NiS) and nitrogen-doped graphene/NiS nanocomposite | Microwave | 6 M KOH | 1467.8 F g−1 (1 A g−1) | 86.6% 5000 th | [130] | |
| NiS/carbon microspheres | Hydrothermal | 6 M KOH | 1594 F g−1 (1 A g−1) | 82.5% 4000 th | [138] | |
| NiS/transition metal oxide nanocomposite | Nickel sulfide and reduced graphene oxide | Hydrothermal | 3 M KOH | 150 mAh (1 A g−1) | 60% 3000 th (2 A g−1) | [141] |
| NiS/Co3S4 nanosheets grown on Fe2O3 substrate | Situ solution vulcanization process | 3 mol L−1 KOH | 1213.7 F g−1 (1 A g−1) | 85.2% 5000 th (5 A g−1) | [142] | |
| NiO/NiS@CNT | Microwave | 6 M KOH | 809.7 F g−1 (1 A g−1) | 100% 20,000 th (5 A g−1) | [143] | |
| MnFe2O4−NiS−porous carbon nanocomposite | Hydrothermal | 3 M KOH + 0.1 M K4[Fe(CN)6] | 364 F g−1 (2 A g−1) | 98% 10,000 th | [148] | |
| Nickel sulfide (NiS) thin film | CBD | 2 M KOH | 750.6 F g−1 (5 mV s−1) | 85.3% over 3000 th | [149] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Hu, K.; Su, N.; Zhang, G.; Han, Y.; An, M. Review of NiS-Based Electrode Nanomaterials for Supercapacitors. Nanomaterials 2023, 13, 979. https://doi.org/10.3390/nano13060979
Guan Y, Hu K, Su N, Zhang G, Han Y, An M. Review of NiS-Based Electrode Nanomaterials for Supercapacitors. Nanomaterials. 2023; 13(6):979. https://doi.org/10.3390/nano13060979
Chicago/Turabian StyleGuan, Yuhao, Kexie Hu, Nan Su, Gaohe Zhang, Yujia Han, and Minrong An. 2023. "Review of NiS-Based Electrode Nanomaterials for Supercapacitors" Nanomaterials 13, no. 6: 979. https://doi.org/10.3390/nano13060979
APA StyleGuan, Y., Hu, K., Su, N., Zhang, G., Han, Y., & An, M. (2023). Review of NiS-Based Electrode Nanomaterials for Supercapacitors. Nanomaterials, 13(6), 979. https://doi.org/10.3390/nano13060979