Plasma-Assisted Atomic Layer Deposition of IrO2 for Neuroelectronics
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
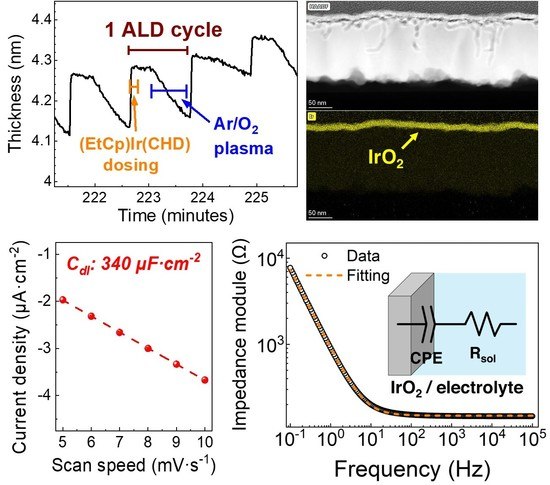
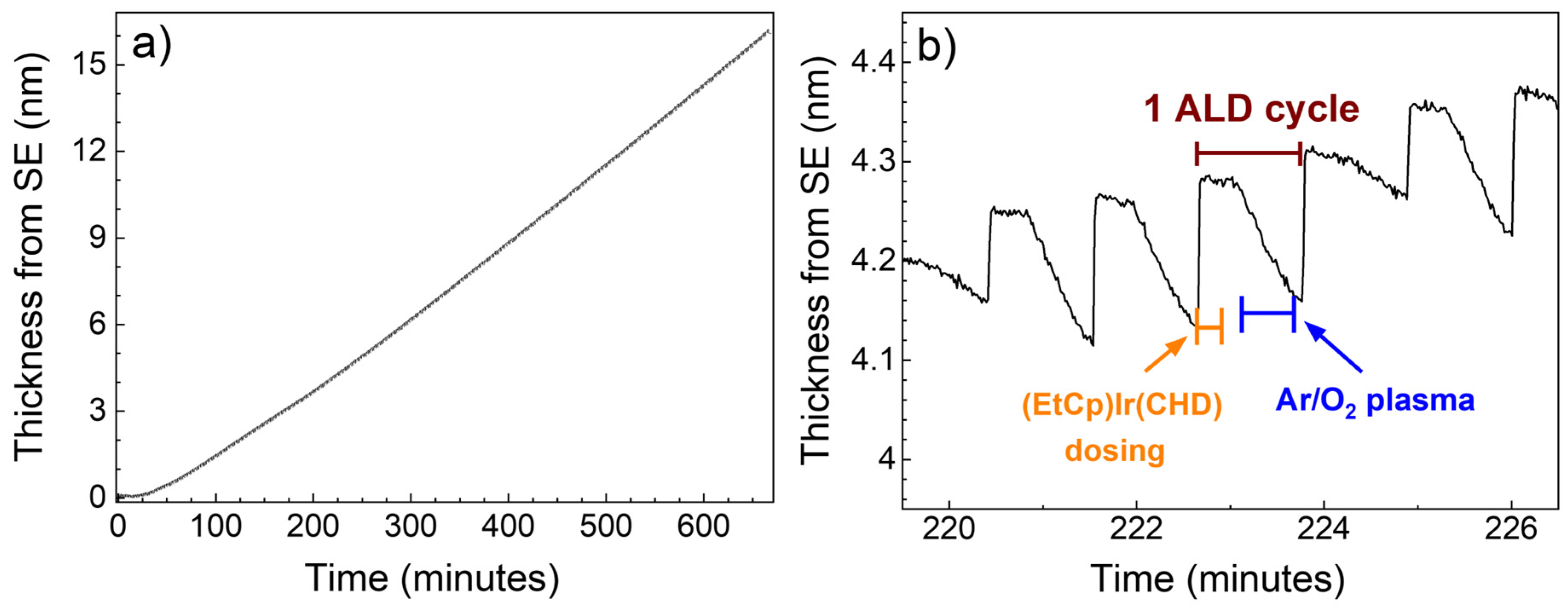
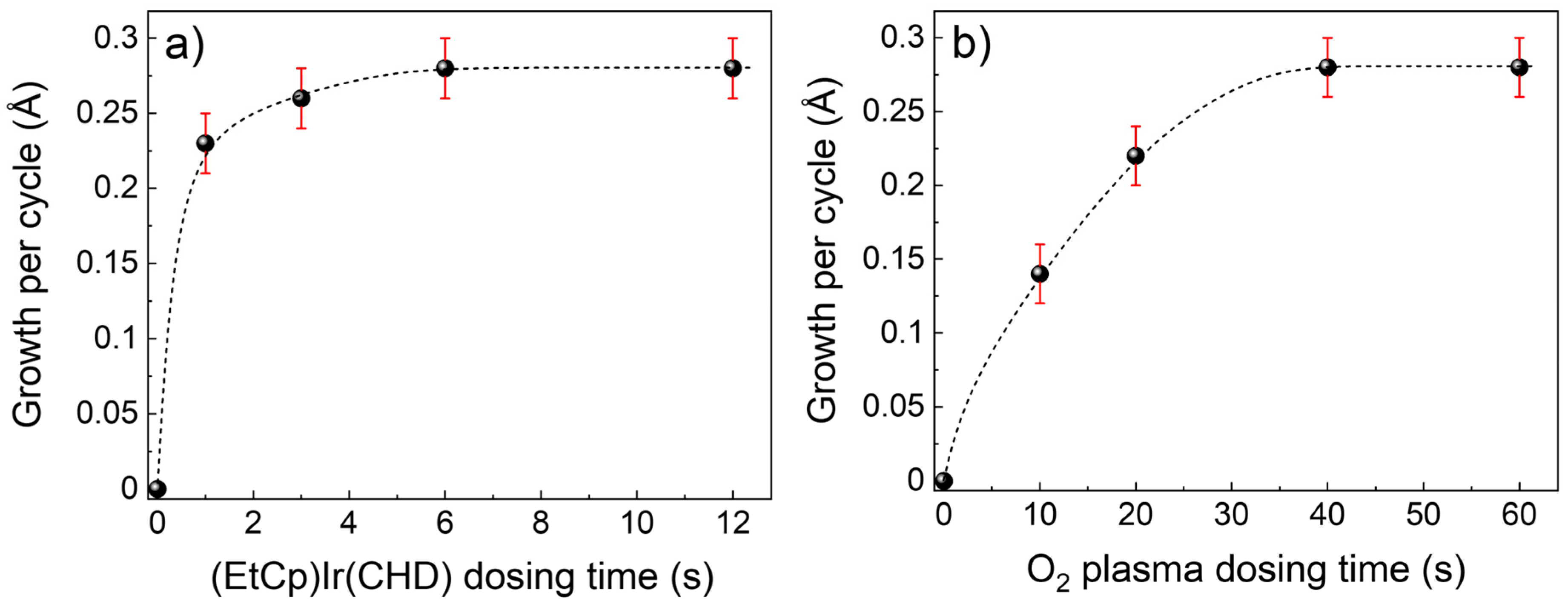
3.1. Plasma-ALD of IrO2
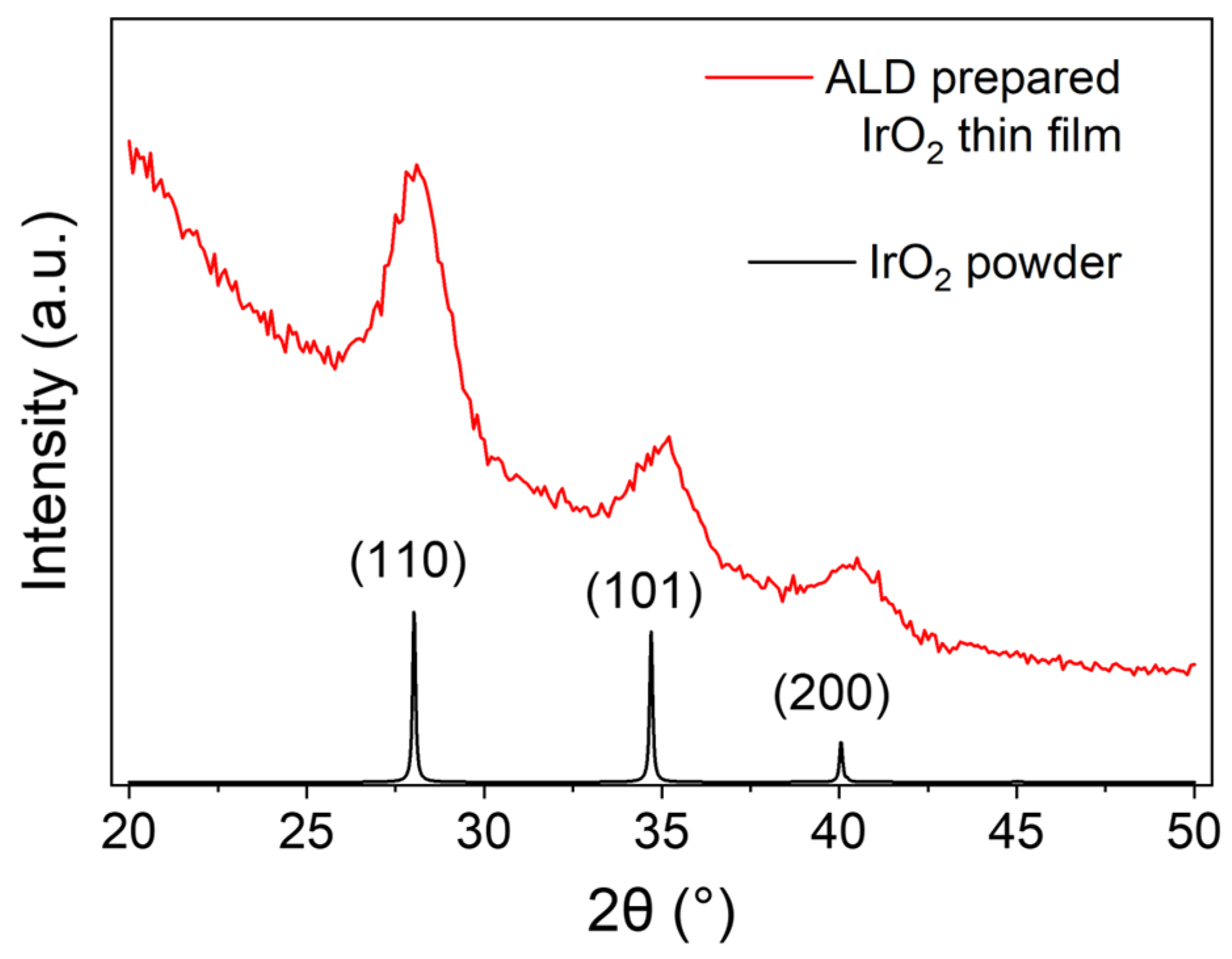
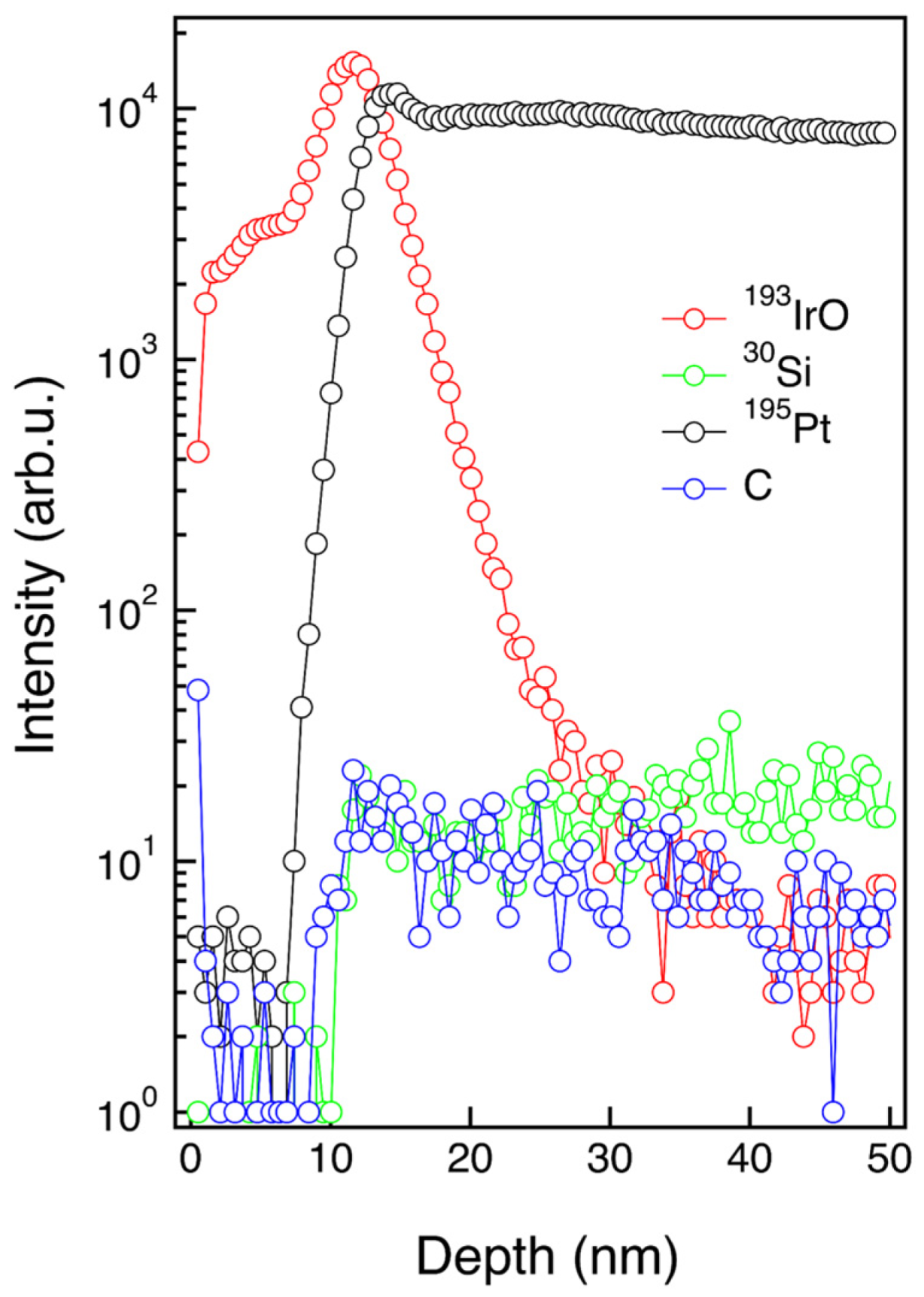
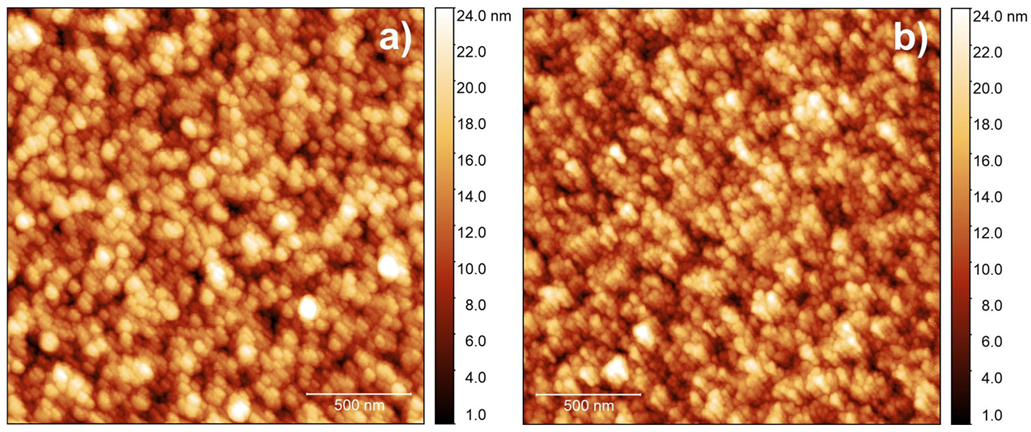
3.2. Physical and Chemical Characterization of IrO2
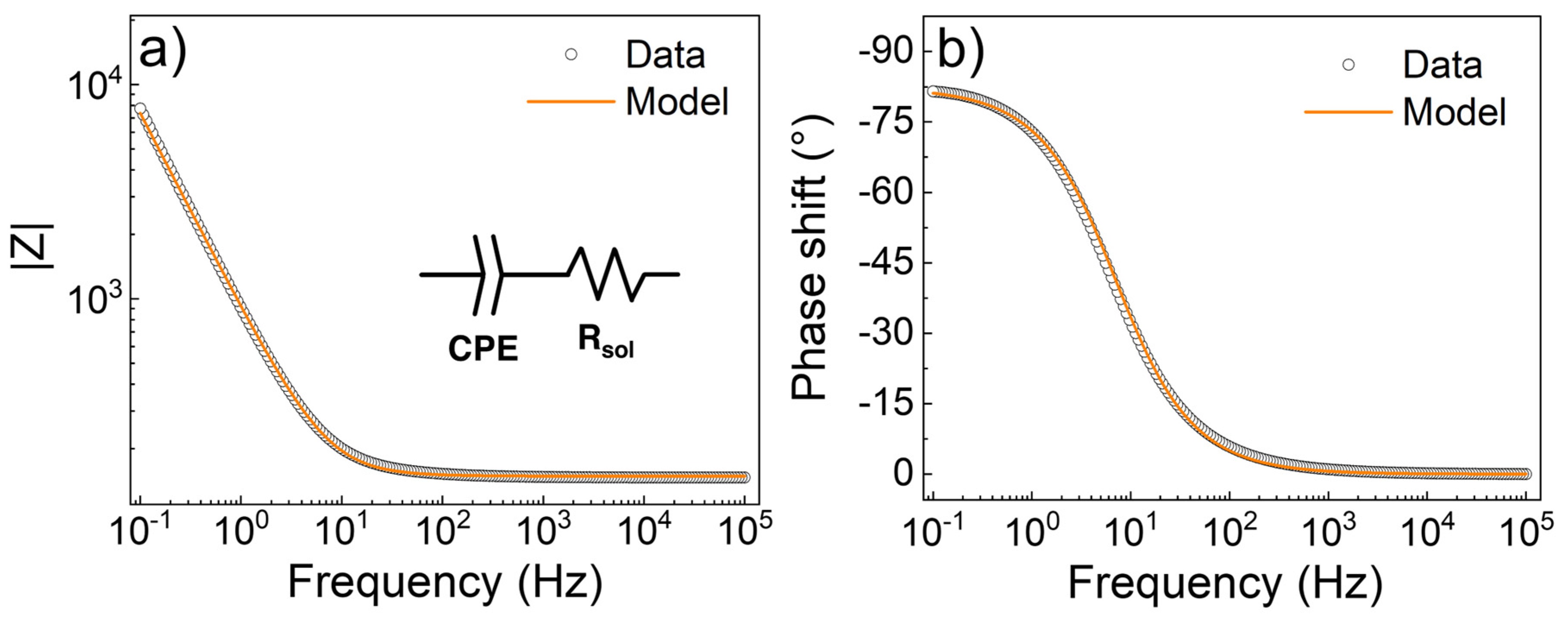
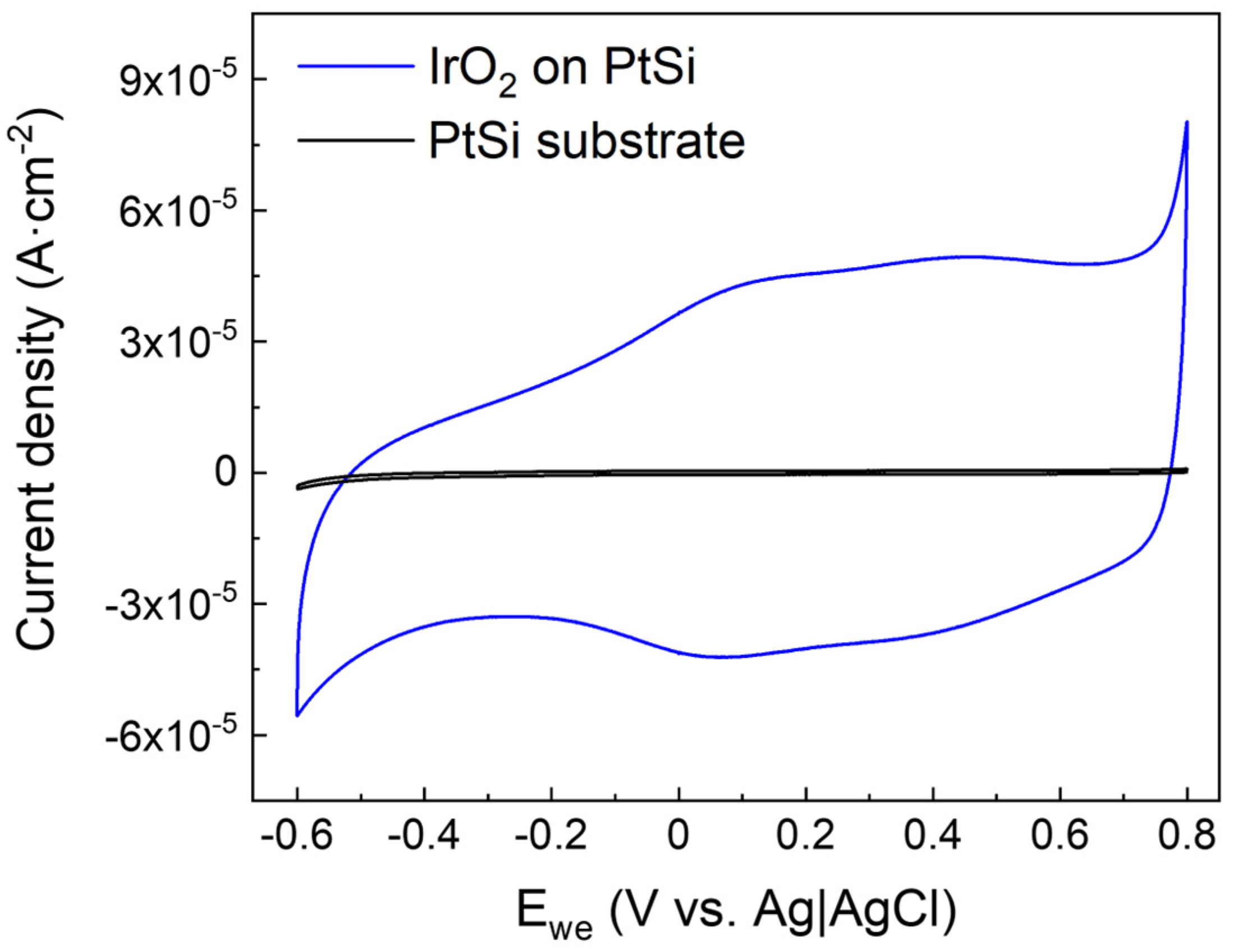
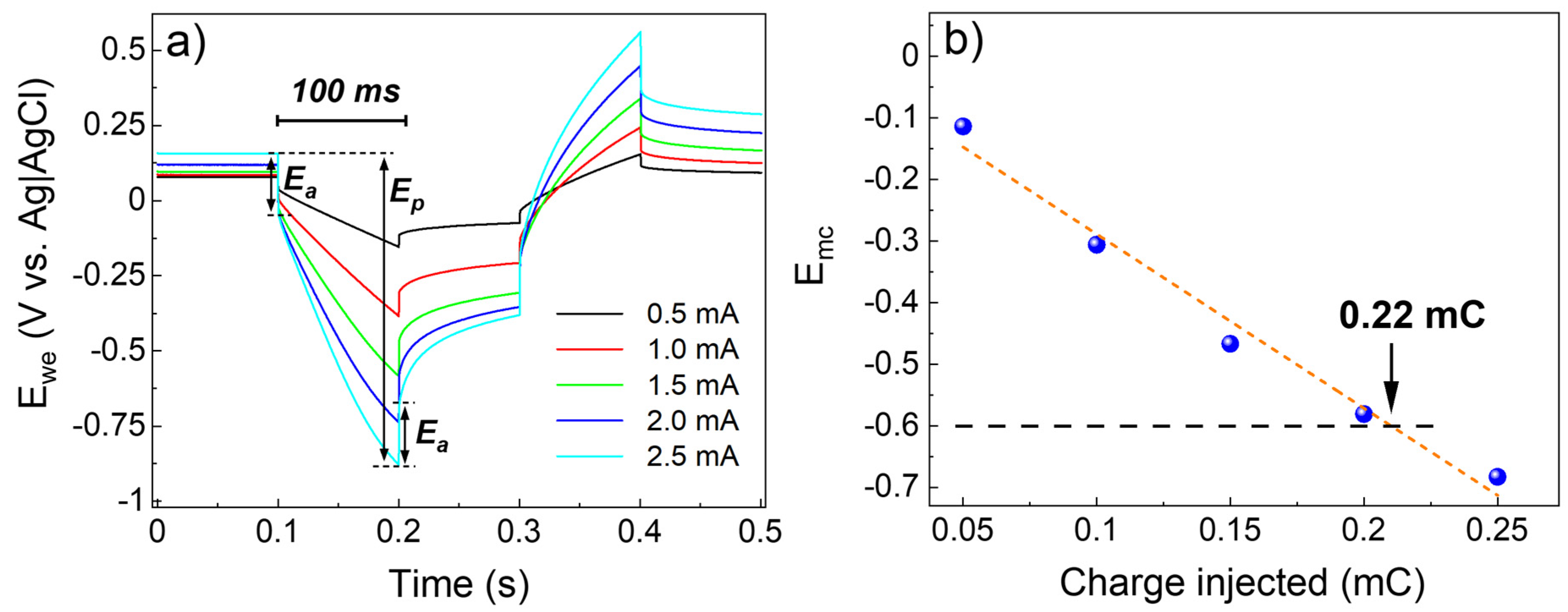
3.3. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obien, M.E.J.; Deligkaris, K.; Bullmann, T.; Bakkum, D.J.; Frey, U. Revealing Neuronal Function through Microelectrode Array Recordings. Front. Neurosci. 2015, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Alba, N.A.; Zhang, H.; Kotov, N.A.; Gaunt, R.A.; Cui, X.T. Nanostructured Coatings for Improved Charge Delivery to Neurons. In Nanotechnology and Neuroscience: Nano-Electronic, Photonic and Mechanical Neuronal Interfacing; Springer: New York, NY, USA, 2014; pp. 71–134. ISBN 9781489980380. [Google Scholar]
- Cogan, S.F. Neural Stimulation and Recording Electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Bhandari, R.; Rieth, L.; Solzbacher, F. In Vitro Comparison of Sputtered Iridium Oxide and Platinum-Coated Neural Implantable Microelectrode Arrays. Biomed. Mater. 2010, 5, 15007. [Google Scholar] [CrossRef]
- Ryynänen, T.; Ylä-Outinen, L.; Narkilahti, S.; Tanskanen, J.M.A.; Hyttinen, J.; Hämäläinen, J.; Leskelä, M.; Lekkala, J. Atomic Layer Deposited Iridium Oxide Thin Film as Microelectrode Coating in Stem Cell Applications. J. Vac. Sci. Technol. A Vac. Surf. Film. 2012, 30, 041501. [Google Scholar] [CrossRef]
- Kang, X.; Liu, J.; Tian, H.; Zhang, C.; Yang, B.; Nuli, Y.; Zhu, H.; Yang, C. Controlled Activation of Iridium Film for AIROF Microelectrodes. Sens. Actuators B Chem. 2014, 190, 601–611. [Google Scholar] [CrossRef]
- Frederick, R.A.; Meliane, I.Y.; Joshi-Imre, A.; Troyk, P.R.; Cogan, S.F. Activated Iridium Oxide Film (AIROF) Electrodes for Neural Tissue Stimulation. J. Neural Eng. 2020, 17, 056001. [Google Scholar] [CrossRef]
- Taylor, G.; Paladines, R.; Marti, A.; Jacobs, D.; Tint, S.; Fones, A.; Hamilton, H.; Yu, L.; Amini, S.; Hettinger, J. Electrochemical Enhancement of Reactively Sputtered Rhodium, Ruthenium, and Iridium Oxide Thin Films for Neural Modulation, Sensing, and Recording Applications. Electrochim. Acta 2021, 394, 139118. [Google Scholar] [CrossRef]
- Slavcheva, E.; Vitushinsky, R.; Mokwa, W.; Schnakenberg, U. Sputtered Iridium Oxide Films as Charge Injection Material for Functional Electrostimulation. J. Electrochem. Soc. 2004, 151, E226. [Google Scholar] [CrossRef]
- Knoops, H.C.M.; Potts, S.E.; Bol, A.A.; Kessels, W.M.M. Atomic Layer Deposition. In Handbook of Crystal Growth: Thin Films and Epitaxy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 3, pp. 1101–1134. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A Brief Review of Atomic Layer Deposition: From Fundamentals to Applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Simon, N.; Asplund, M.; Stieglitz, T.; Bucher, V. Plasma Enhanced Atomic Layer Deposition of Iridium Oxide for Application in Miniaturized Neural Implants. Curr. Dir. Biomed. Eng. 2021, 7, 539–542. [Google Scholar] [CrossRef]
- Kim, S.W.; Kwon, S.H.; Kwak, D.K.; Kang, S.W. Phase Control of Iridium and Iridium Oxide Thin Films in Atomic Layer Deposition. J. Appl. Phys. 2008, 103, 023517. [Google Scholar] [CrossRef]
- Hämäläinen, J.; Hatanpää, T.; Puukilainen, E.; Sajavaara, T.; Ritala, M.; Leskelä, M. Iridium Metal and Iridium Oxide Thin Films Grown by Atomic Layer Deposition at Low Temperatures. J. Mater. Chem. 2011, 21, 16488–16493. [Google Scholar] [CrossRef]
- Matienzo, D.J.D.; Settipani, D.; Instuli, E.; Kallio, T. Active IrO2 and NiO Thin Films Prepared by Atomic Layer Deposition for Oxygen Evolution Reaction. Catalysts 2020, 10, 92. [Google Scholar] [CrossRef]
- Schlicht, S.; Haschke, S.; Mikhailovskii, V.; Manshina, A.; Bachmann, J. Highly Reversible Water Oxidation at Ordered Nanoporous Iridium Electrodes Based on an Original Atomic Layer Deposition. ChemElectroChem 2018, 5, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Hendricks, O.L.; Meng, A.C.; Braun, M.R.; McGehee, M.D.; Chidsey, C.E.D.; McIntyre, P.C. Atomic Layer Deposited TiO2–IrOx Alloy as a Hole Transport Material for Perovskite Solar Cells. Adv. Mater. Interfaces 2018, 5, 1800191. [Google Scholar] [CrossRef]
- Profijt, H.B.; Potts, S.E.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-Assisted Atomic Layer Deposition: Basics, Opportunities, and Challenges. J. Vac. Sci. Technol. A Vac. Surf. Film. 2011, 29, 050801. [Google Scholar] [CrossRef]
- Park, P.K.; Roh, J.S.; Choi, B.H.; Kang, S.W. Interfacial Layer Properties of HfO2 Films Formed by Plasma-Enhanced Atomic Layer Deposition on Silicon. Electrochem. Solid-State Lett. 2006, 9, F34–F37. [Google Scholar] [CrossRef]
- Hoex, B.; Schmidt, J.; Pohl, P.; van de Sanden, M.C.M.; Kessels, W.M.M. Silicon Surface Passivation by Atomic Layer Deposited Al2O3. J. Appl. Phys. 2008, 104, 044903-1–044903-12. [Google Scholar] [CrossRef]
- Kim, W.H.; Maeng, W.J.; Moon, K.J.; Myoung, J.M.; Kim, H. Growth Characteristics and Electrical Properties of La2O3 Gate Oxides Grown by Thermal and Plasma-Enhanced Atomic Layer Deposition. Thin Solid Film 2010, 519, 362–366. [Google Scholar] [CrossRef]
- Xie, Q.; Musschoot, J.; Deduytsche, D.; van Meirhaeghe, R.L.; Detavernier, C.; van den Berghe, S.; Jiang, Y.-L.; Ru, G.-P.; Li, B.-Z.; Qu, X.-P. Growth Kinetics and Crystallization Behavior of TiO2 Films Prepared by Plasma Enhanced Atomic Layer Deposition. J. Electrochem. Soc. 2008, 155, H688. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, H.B.R.; Lansalot, C.; Dussarrat, C.; Gatineau, J.; Kim, H. Plasma-Enhanced Atomic Layer Deposition of Cobalt Using Cyclopentadienyl Isopropyl Acetamidinato-Cobalt as a Precursor. Jpn. J. Appl. Phys. 2010, 49, 05FA10-1–05FA10-5. [Google Scholar] [CrossRef]
- Potts, S.E.; Keuning, W.; Langereis, E.; Dingemans, G.; van de Sanden, M.C.M.; Kessels, W.M.M. Low Temperature Plasma-Enhanced Atomic Layer Deposition of Metal Oxide Thin Films. J. Electrochem. Soc. 2010, 157, P66. [Google Scholar] [CrossRef]
- Choi, S.; Cha, Y.K.; Seo, B.S.; Park, S.; Park, J.H.; Shin, S.; Seol, K.S.; Park, J.B.; Jung, Y.S.; Park, Y.; et al. Atomic-Layer Deposited IrO2 Nanodots for Charge-Trap Flash-Memory Devices. J. Phys. D Appl. Phys. 2007, 40, 1426–1429. [Google Scholar] [CrossRef]
- Kim, D.K.; Sung, J.H. IrO2 Nanodot Formation by Plasma Enhanced Atomic Layer Deposition as a Charge Storage Layer. J. Nanosci. Nanotechnol. 2014, 14, 5386–5389. [Google Scholar] [CrossRef]
- Kawano, K.; Takamori, M.; Yamakawa, T.; Watari, S.; Fujisawa, H.; Shimizu, M.; Niu, H.; Oshima, N. A Novel Iridium Precursor for MOCVD. Mat Res. Soc. Symp. Proc. 2004, 784, C3.30.1–C3.30.6. [Google Scholar]
- Langereis, E.; Heil, S.B.S.; Knoops, H.C.M.; Keuning, W.; van de Sanden, M.C.M.; Kessels, W.M.M. In Situ Spectroscopic Ellipsometry as a Versatile Tool for Studying Atomic Layer Deposition. J. Phys. D Appl. Phys. 2009, 42. [Google Scholar] [CrossRef]
- Lamagna, L.; Fusi, M.; Spiga, S.; Fanciulli, M.; Brammertz, G.; Merckling, C.; Meuris, M.; Molle, A. Effects of Surface Passivation during Atomic Layer Deposition of Al2O3 on In0.53Ga0.47As Substrates. Microelectron. Eng. 2011, 88, 431–434. [Google Scholar] [CrossRef]
- Lamagna, L.; Molle, A.; Wiemer, C.; Spiga, S.; Grazianetti, C.; Congedo, G.; Fanciulli, M. Atomic Layer Deposition of Al-Doped ZrO2 Thin Films as Gate Dielectric for In0.53Ga0.47As. J. Electrochem. Soc. 2012, 159, H220–H224. [Google Scholar] [CrossRef]
- Leick, N.; Weber, J.W.; Mackus, A.J.M.; Weber, M.J.; van de Sanden, M.C.M.; Kessels, W.M.M. In Situ Spectroscopic Ellipsometry during Atomic Layer Deposition of Pt, Ru and Pd. J. Phys. D Appl. Phys. 2016, 49, 115504. [Google Scholar] [CrossRef]
- Casanova, A.; Bettamin, L.; Blatche, M.C.; Mathieu, F.; Martin, H.; Gonzalez-Dunia, D.; Nicu, L.; Larrieu, G. Nanowire Based Bioprobes for Electrical Monitoring of Electrogenic Cells. J. Phys. Condens. Matter 2018, 30, 464001. [Google Scholar] [CrossRef]
- Pfeifer, V.; Jones, T.E.; Velasco Vélez, J.J.; Massué, C.; Arrigo, R.; Teschner, D.; Girgsdies, F.; Scherzer, M.; Greiner, M.T.; Allan, J.; et al. The Electronic Structure of Iridium and Its Oxides. Surf. Interface Anal. 2016, 48, 261–273. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1974; ISBN 978-0-471-49369-3. [Google Scholar]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley Publishing Company Inc.: Reading, MA, USA, 1978. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Etzi Coller Pascuzzi, M.; Hofmann, J.P.; Hensen, E.J.M. Promoting Oxygen Evolution of IrO2 in Acid Electrolyte by Mn. Electrochim. Acta 2021, 366, 137448. [Google Scholar] [CrossRef]
- Bondarenko, A.S.; Ragoisha, G.A. EIS. In Progress in Chemometrics Research; Pomerantsev, A.L., Ed.; Nova Science Publishers: New York, NY, USA, 2005; pp. 89–102. [Google Scholar]
- Orazem, M.E.; Frateur, I.; Tribollet, B.; Vivier, V.; Marcelin, S.; Pébère, N.; Bunge, A.L.; White, E.A.; Riemer, D.P.; Musiani, M. Dielectric Properties of Materials Showing Constant-Phase-Element (CPE) Impedance Response. J. Electrochem. Soc. 2013, 160, C215–C225. [Google Scholar] [CrossRef]
- Brug, G.J.; Van Den Eeden, A.L.G.; Sluyters-rehbach, M.; Sluyters, J.H. The Analysis of Electrode Impedances Complicated by the Presence of a Constant Phase Element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, J.; Park, Y.S.; Yang, J.; Jang, M.J.; Jeong, J.; Choe, S.; Lee, J.W.; Kwon, J.D.; Choi, S.M. Electrodeposition of High-Surface-Area IrO2 Films on Ti Felt as an Efficient Catalyst for the Oxygen Evolution Reaction. Front. Chem. 2020, 8, 593272. [Google Scholar] [CrossRef]
- Silva, T.M.; Simoes, A.M.P.; Ferreira, M.G.S.; Walls, M.; Da, M.; Belo, C. Electronic Structure of Iridium Oxide Films Formed in Neutral Phosphate Buffer Solution. J. Electroanal. Chem. 1998, 441, 5–12. [Google Scholar] [CrossRef]
- Grupioni, A.A.F.; Arashiro, E.; Lassali, T.A.F. Voltammetric Characterization of an Iridium Oxide-Based System: The Pseudocapacitive Nature of the Ir0.3Mn0.7O2 Electrode. Electrochim. Acta 2002, 48, 407. [Google Scholar] [CrossRef]
- Weiland, J.D.; Anderson, D.J.; Humayun, M.S. In Vitro Electrical Properties for Iridium Oxide versus Titanium Nitride Stimulating Electrodes. IEEE Trans. Biomed. Eng. 2002, 49, 1574–1579. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Palma, V.; Pianalto, A.; Perego, M.; Tallarida, G.; Codegoni, D.; Fanciulli, M. Plasma-Assisted Atomic Layer Deposition of IrO2 for Neuroelectronics. Nanomaterials 2023, 13, 976. https://doi.org/10.3390/nano13060976
Di Palma V, Pianalto A, Perego M, Tallarida G, Codegoni D, Fanciulli M. Plasma-Assisted Atomic Layer Deposition of IrO2 for Neuroelectronics. Nanomaterials. 2023; 13(6):976. https://doi.org/10.3390/nano13060976
Chicago/Turabian StyleDi Palma, Valerio, Andrea Pianalto, Michele Perego, Graziella Tallarida, Davide Codegoni, and Marco Fanciulli. 2023. "Plasma-Assisted Atomic Layer Deposition of IrO2 for Neuroelectronics" Nanomaterials 13, no. 6: 976. https://doi.org/10.3390/nano13060976
APA StyleDi Palma, V., Pianalto, A., Perego, M., Tallarida, G., Codegoni, D., & Fanciulli, M. (2023). Plasma-Assisted Atomic Layer Deposition of IrO2 for Neuroelectronics. Nanomaterials, 13(6), 976. https://doi.org/10.3390/nano13060976