Preparation and Thermal Conductivity Enhancement of Boron Nitride Nano-Material PiG Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of BN Nanoparticles and Nano-Sheets
2.2. Preparation of BN Nanotubes
2.3. Preparation of BN Nano-Material Composite Fluorescent Film
2.4. Characterization
3. Results and Discussion
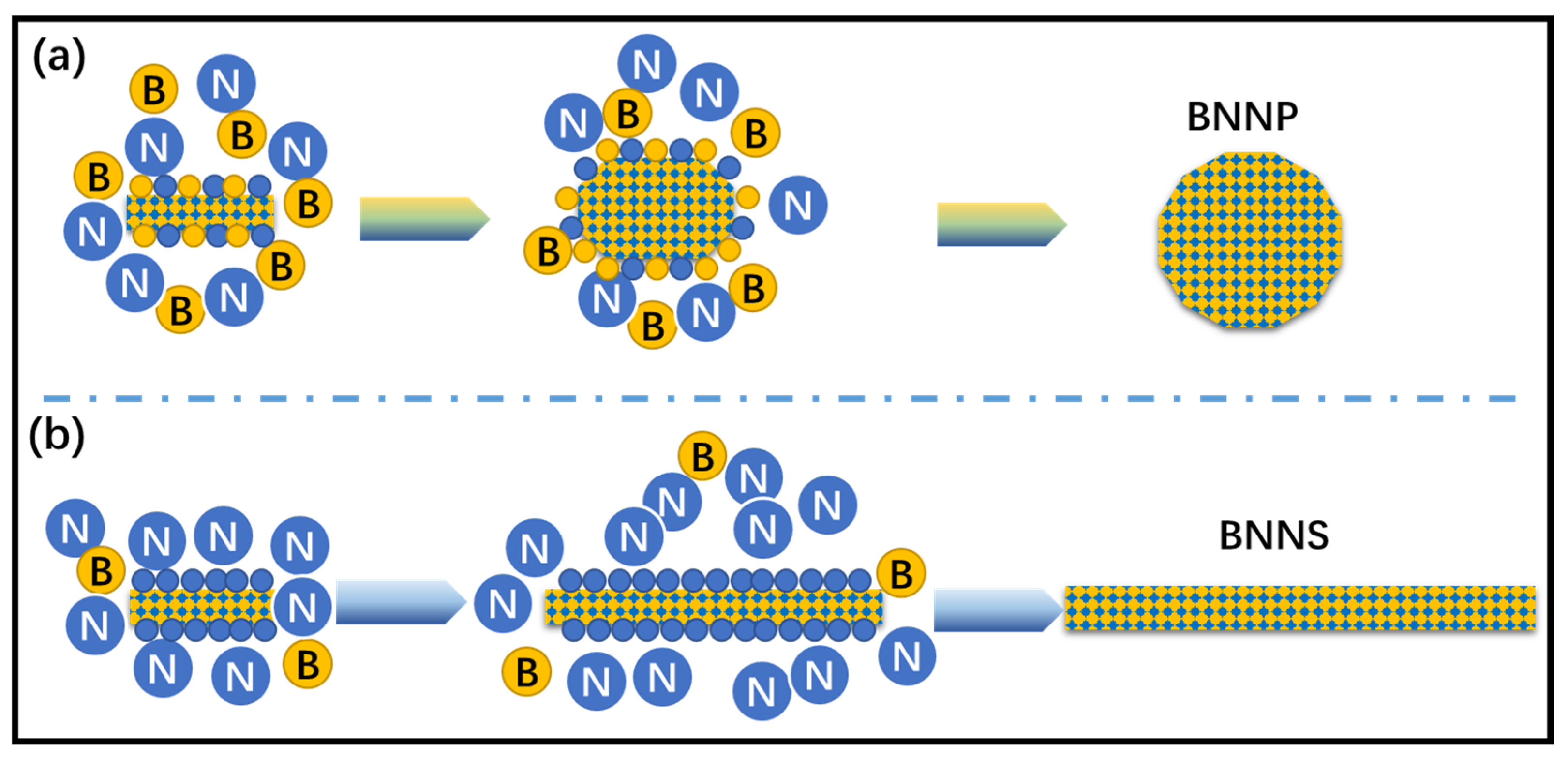
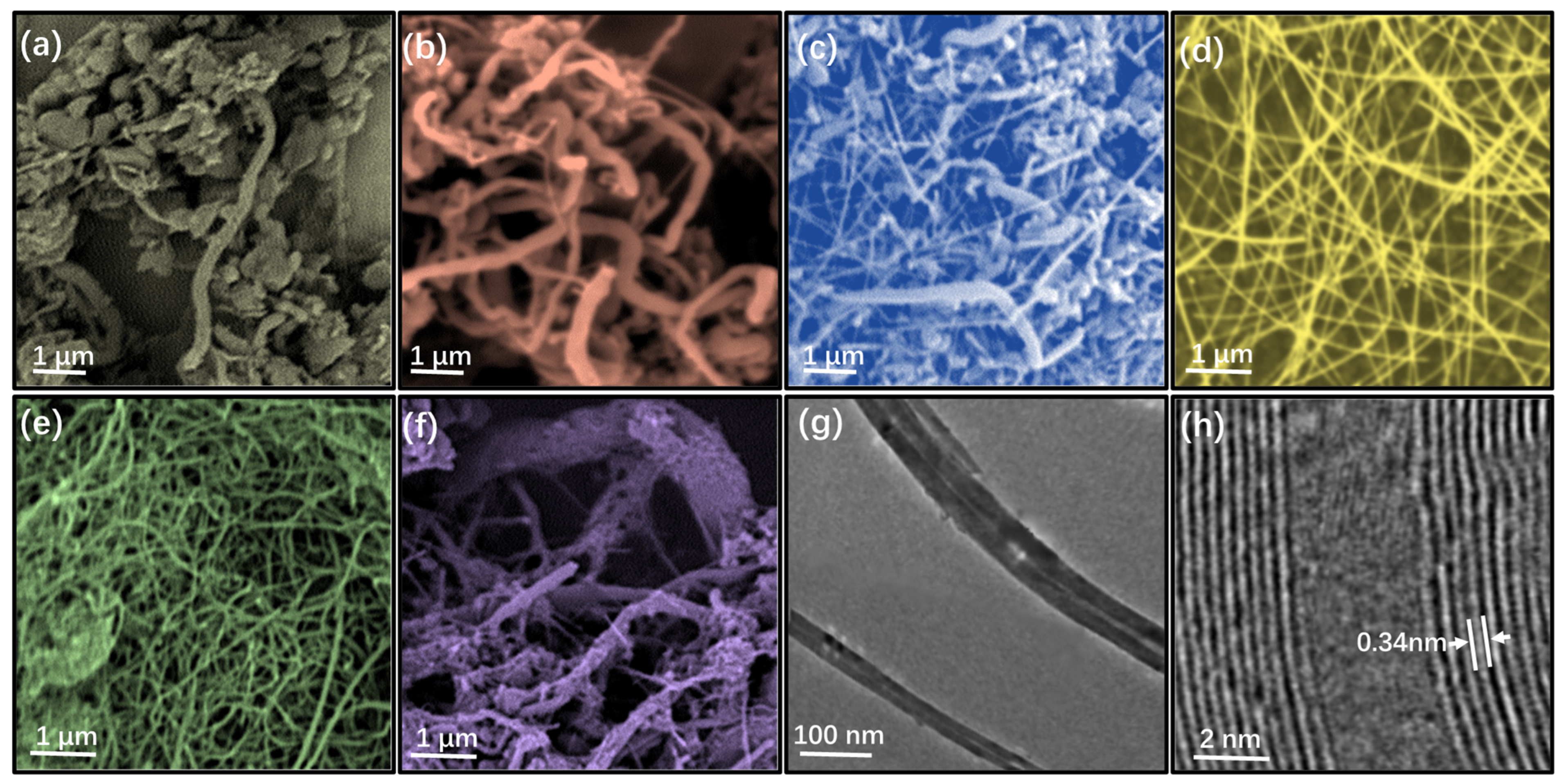
3.1. Phase Analysis and Morphology Control of BN Nanomaterials
3.1.1. Phase Analysis
3.1.2. Morphology Control
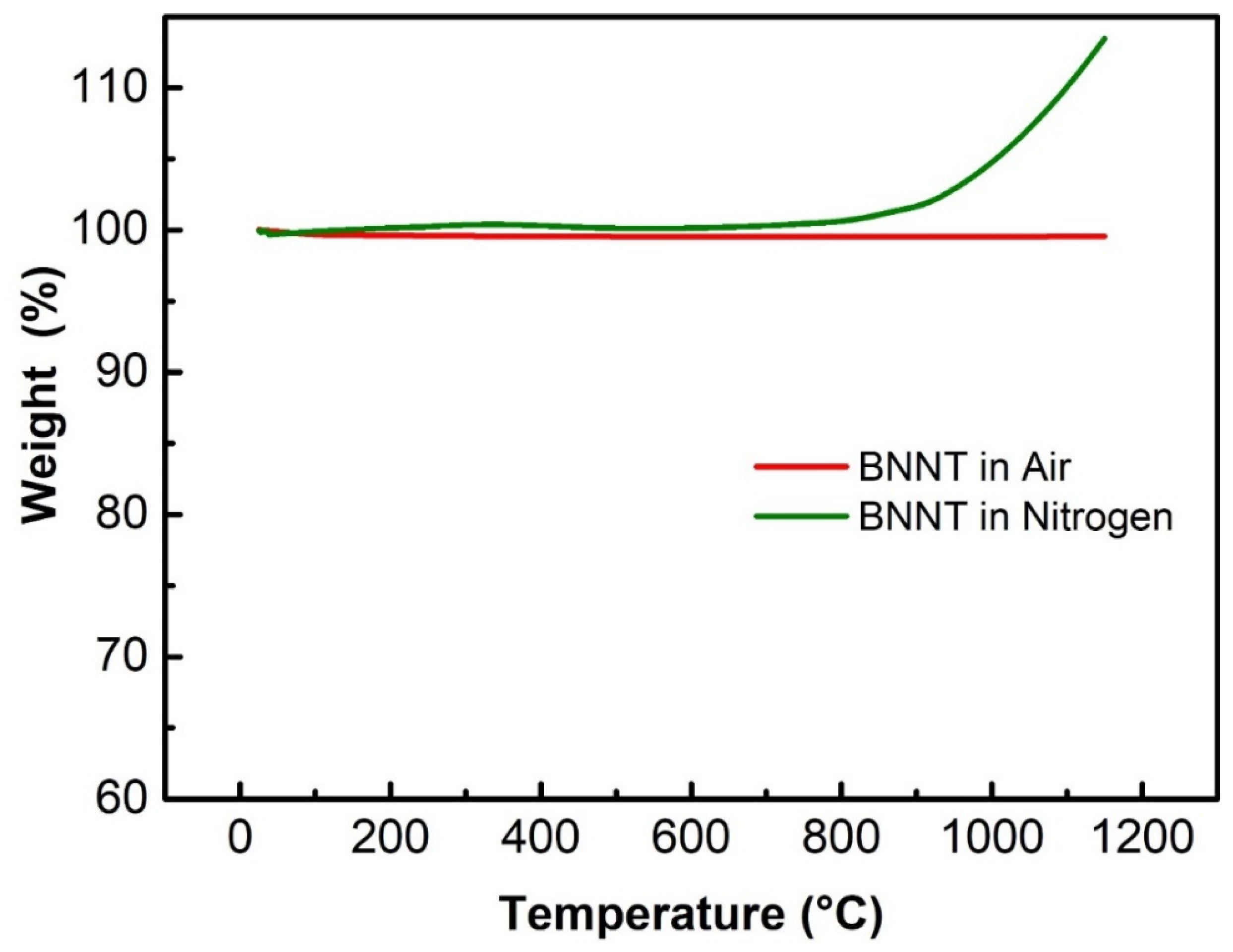
3.2. Stability and Distribution Properties of BN Nanomaterials
3.2.1. High Temperature Stability of BN
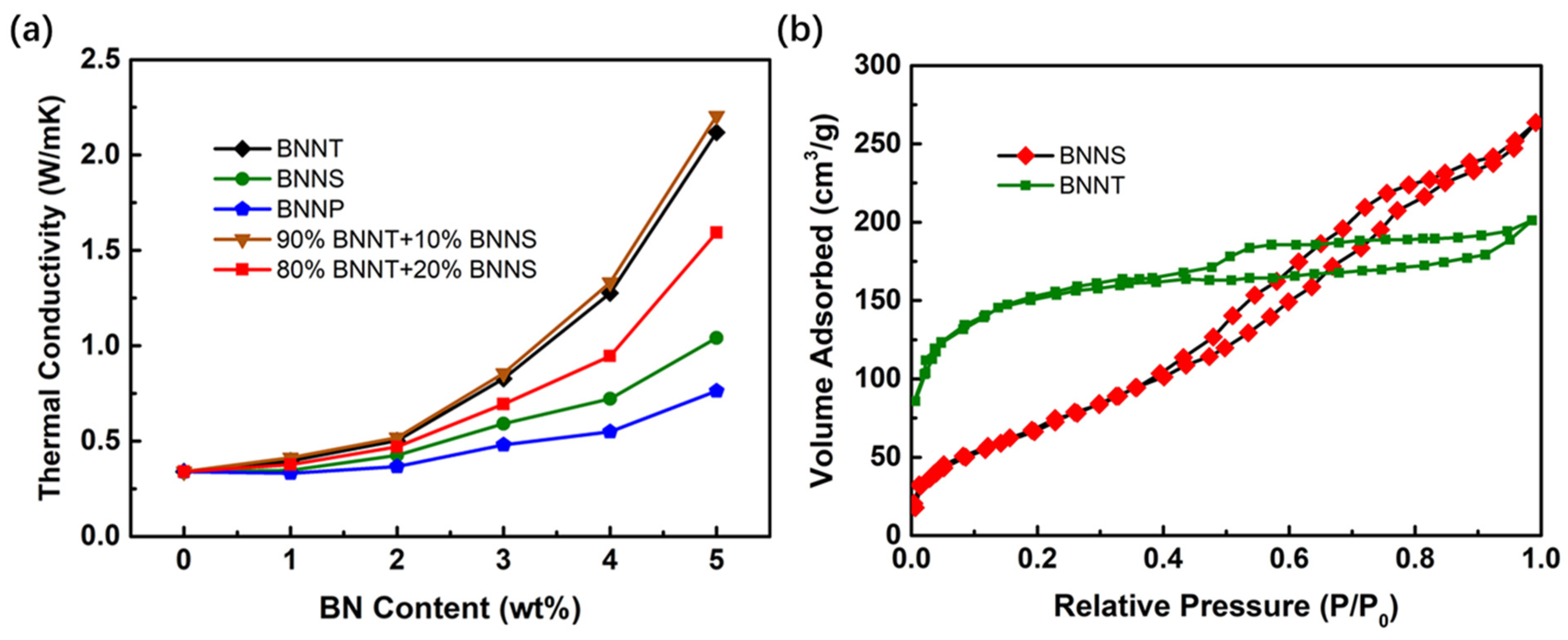
3.2.2. Distribution of BN Nanomaterials
3.3. Effect of BN Nano-Materials on the Mechanical Properties of Fluorescent Films
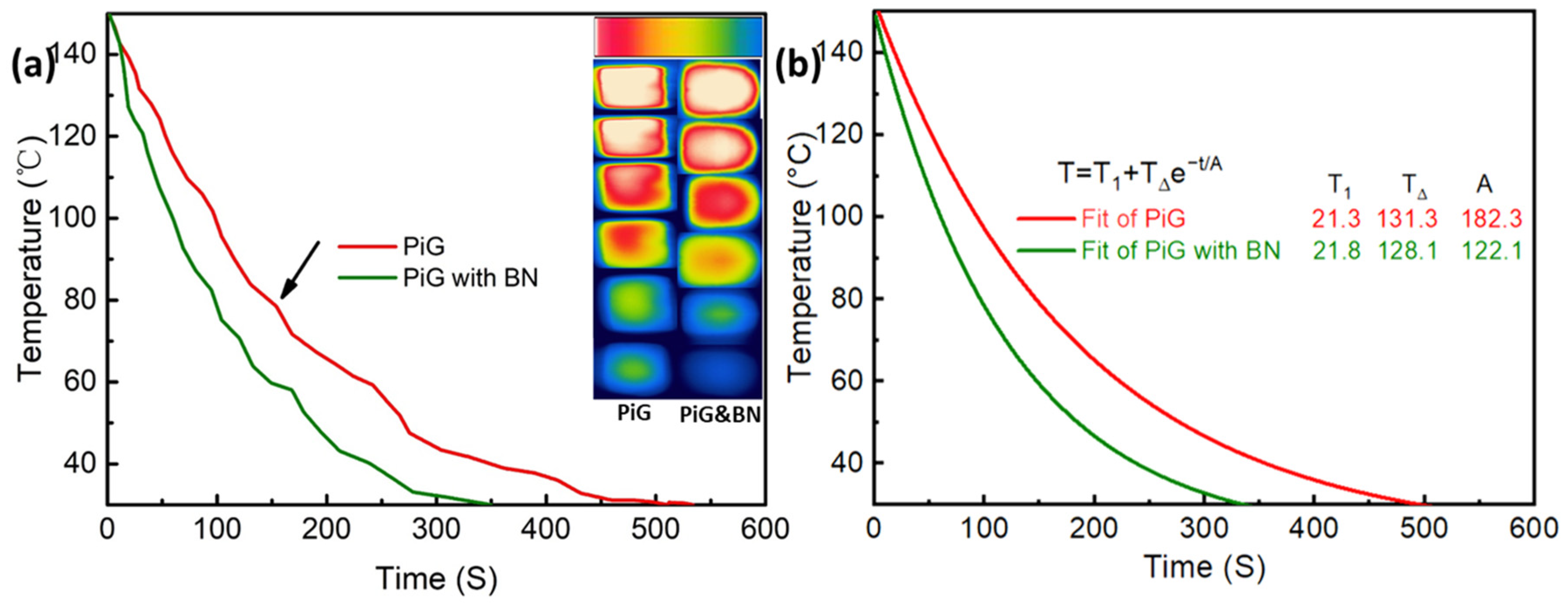
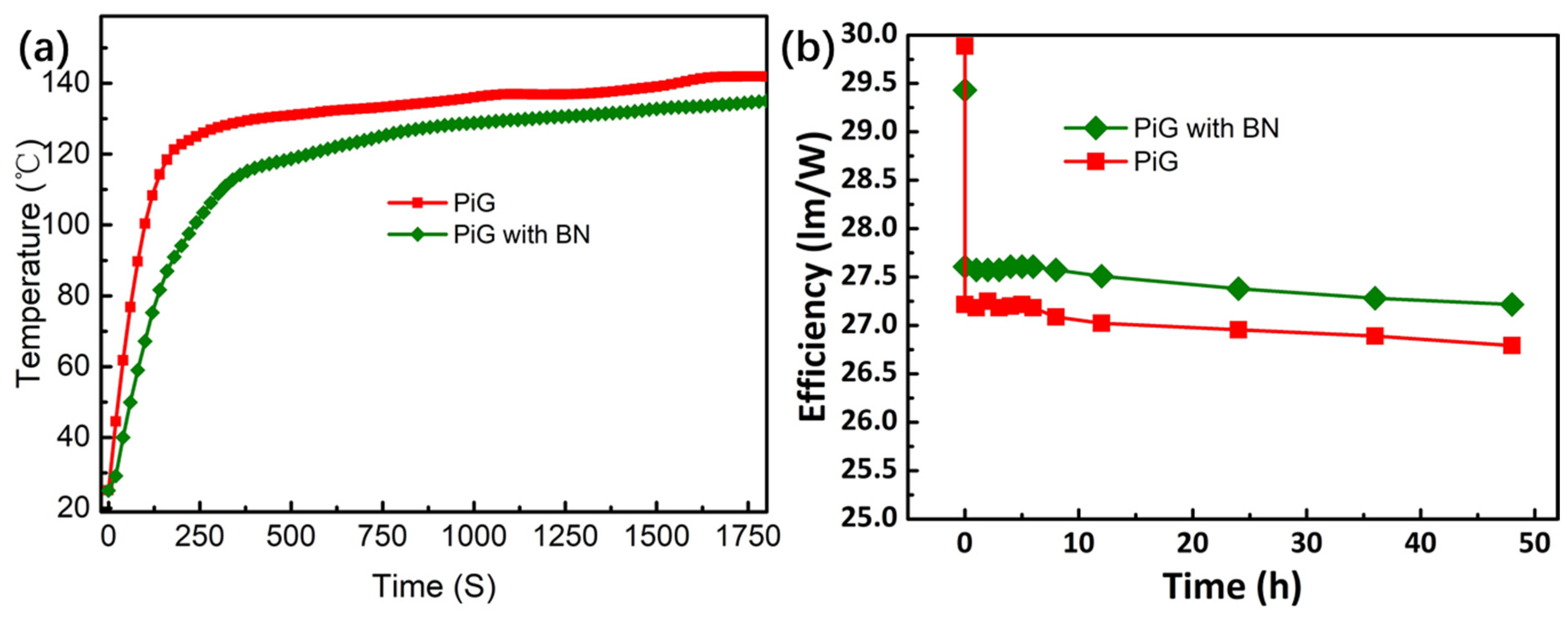
3.4. Effect of BN Nano-Materials on Heat Dissipation Performance of Fluorescent Fin
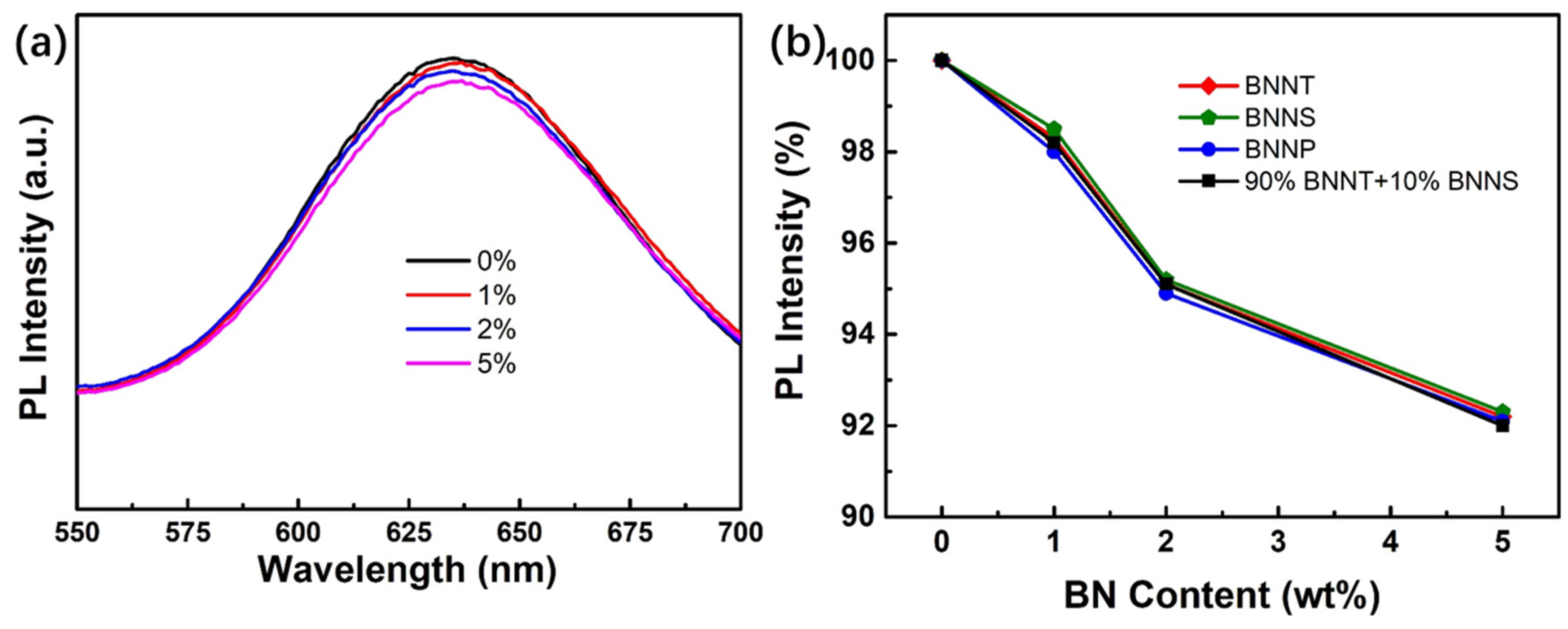
3.5. Effect of BN Nano-Materials on PiG Fluorescence and Packaging Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Z.; Wang, M.; Lyu, B.; Chen, H. Preparation and Luminescent Properties of (Gd, Lu)3Al5O12:Tb3+, Eu3+ Transparent Ceramics. J. Synth. Cryst. 2021, 50, 1984–1990. [Google Scholar]
- Xie, Y.; Tian, T.; Mao, C.; Wang, Z.; Shi, J.; Yang, L.; Wang, C. Recent Research Progress of Mn4+-Doped A2MF6 (A = Li, Na, K, Cs, or Rb; M = Si, Ti, Ge, or Sn) Red Phosphors Based on a Core–Shell Structure. Nanomaterials 2023, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-H.; Han, G.S.; Mang, S.R.; Jung, M.K.; Jung, H.S.; Yoon, D.-H. Design of a thermally stable rGO-embedded remote phosphor for applications in white LEDs. J. Mater. Chem. C 2015, 3, 235–238. [Google Scholar] [CrossRef]
- Anoop, G.; Rani, J.R.; Lim, J.; Jang, M.S.; Suh, D.W.; Kang, S.; Jun, S.C.; Yoo, J.S. Reduced graphene oxide enwrapped phosphors for long-term thermally stable phosphor converted white light emitting diodes. Sci. Rep. 2016, 6, 33993. [Google Scholar] [CrossRef]
- Li, L.H.; Cervenka, J.; Watanabe, K.; Taniguchi, T.; Chen, Y. Strong oxidation resistance of atomically thin boron nitride nanosheets. ACS Nano 2014, 8, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou, N.; Tampaxis, C.; Charalambopoulou, G.; Constantinides, G.; Ryzhkov, V.; Doumanidis, C.; Matovic, B.; Mitterer, C.; Rebholz, C. Boron Nitride Nanotubes Versus Carbon Nanotubes: A Thermal Stability and Oxidation Behavior Study. Nanomaterials 2020, 10, 2435. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tang, C.; Golberg, D. Boron nitride nanotubes. Mater. Sci. Eng. R-Rep. 2010, 70, 92–111. [Google Scholar] [CrossRef]
- Liu, C.; Fang, Q.; Wang, D.; Yan, C.; Liu, F.; Wang, N.; Guo, Z.; Jiang, Q. Carbon and Boron Nitride Nanotubes: Structure, Property and Fabrication. ES Mater. Manuf. 2019, 3, 2–15. [Google Scholar]
- Li, Y.; Wang, X.; Wang, J.; Wang, X.; Zeng, D. A Simple Method for the Synthesis of a Coral-like Boron Nitride Micro-/Nanostructure Catalyzed by Fe. Nanomaterials 2023, 13, 753. [Google Scholar] [CrossRef]
- Jedrzejczak-Silicka, M.; Trukawka, M.; Dudziak, M.; Piotrowska, K.; Mijowska, E. Hexagonal Boron Nitride Functionalized with Au Nanoparticles—Properties and Potential Biological Applications. Nanomaterials 2018, 8, 605. [Google Scholar] [CrossRef]
- Liu, C.; Gao, W.; Yin, H. Research Progress of Cubic Boron Nitride. J. Synth. Cryst. 2022, 51, 781–800. [Google Scholar]
- Li, T.; Chen, Y.; Li, W.; Li, J.; Luo, L.; Yang, T.; Liu, L.; Wu, G. Fabrication and mechanical properties of boron nitride nanotube reinforced silicon nitride ceramics. Ceram. Int. 2018, 44, 6456–6460. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, F.; Wang, W.; Fu, Z.; Zhang, J.; Chen, W. Effects of c-BN on Microstructure and Properties of Si3N4/BN Sintered by SPS. J. Synth. Cryst. 2019, 48, 1116–1122. [Google Scholar]
- Qing, H.; Bando, Y.; Xin, X.; Nishimura, T.; Chunyi, Z.; Chengchun, T.; Fangfang, X.; Lian, G.; Golberg, D. Enhancing superplasticity of engineering ceramics by introducing BN nanotubes. Nanotechnology 2007, 18, 485706. [Google Scholar]
- Lahiri, D.; Hadjikhani, A.; Zhang, C.; Xing, T.; Li, L.; Chen, Y.A. Agarwal. Boron nitride nanotubes reinforced aluminum composites prepared by spark plasma sintering: Microstructure, mechanical properties and deformation behavior. Mater. Sci. Eng. A 2013, 574, 149–156. [Google Scholar] [CrossRef]
- Saggar, R.; Porwal, H.; Tatarko, P.; Dlouhý, I.; Reece, M.J. Boron nitride nanosheets reinforced glass matrix composites. Adv. Appl. Ceram. 2015, 114 (Suppl. S1), S26–S33. [Google Scholar] [CrossRef]
- Bansal, N.P.; Hurst, J.B.; Choi, S.R. Boron Nitride Nanotubes-Reinforced Glass Composites. J. Am. Ceram. Soc. 2006, 89, 388–390. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Qi, S.H.; Li, H.D.; Shao, S.Y. Study on insulating thermal conductive BN/HDPE composites. Thermochim. Acta 2007, 452, 36–42. [Google Scholar] [CrossRef]
- Ishida, H.; Rimdusit, S. Very high thermal conductivity obtained by boron nitride-filled polybenzoxazine. Thermochim. Acta 1998, 320, 177–186. [Google Scholar] [CrossRef]
- Liu, G.; Tian, Z.; Chen, Z.; Wang, H.; Zhang, Q.; Li, Y. CaAlSiN3:Eu2+ phosphors bonding with bismuth borate glass for high power light excitation. Opt. Mater. 2015, 40, 63–67. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Gu, Y.; Pan, X.; Zhao, G.; Zhang, Z. A self-propagation high-temperature synthesis and annealing route to synthesis of wave-like boron nitride nanotubes. Mater. Res. Bull. 2013, 48, 943–947. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Yin, Y.; Chen, Y.; Bi, X. Water-assisted chemical vapor deposition synthesis of boron nitride nanotubes and their photoluminescence property. Nanotechnology 2013, 24, 365605. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhu, W.; Chao, Y.; Zhang, J.; Zhang, P.; Zhu, H.; Li, C.; Chen, Z.; Li, H.; Dai, S. A template-free solvent-mediated synthesis of high surface area boron nitride nanosheets for aerobic oxidative desulfurization. Chem. Commun. 2016, 52, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Gu, Y.; Qian, Q.; Wang, J.; Zhao, G.; Pan, X. High-yield Synthesis of Boron Nitride Nanotubes by Annealing Fe3BO6. Chem. Lett. 2011, 40, 540–541. [Google Scholar] [CrossRef]
- Ozmen, D.; Sezgi, N.A.; Balci, S. Synthesis of boron nitride nanotubes from ammonia and a powder mixture of boron and iron oxide. Chem. Eng. J. 2013, 219, 28–36. [Google Scholar] [CrossRef]
- Hong, J.P.; Yoon, S.W.; Hwang, T.; Oh, J.S.; Hong, S.C.; Lee, Y.; Nam, J.D. High thermal conductivity epoxy composites with bimodal distribution of aluminum nitride and boron nitride fillers. Thermochim. Acta 2012, 537, 70–75. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Wei, Q.; Tang, G.; Shi, H.; Qin, L. Preparation and Thermal Conductivity Enhancement of Boron Nitride Nano-Material PiG Composite. Nanomaterials 2023, 13, 1106. https://doi.org/10.3390/nano13061106
Chen Z, Wei Q, Tang G, Shi H, Qin L. Preparation and Thermal Conductivity Enhancement of Boron Nitride Nano-Material PiG Composite. Nanomaterials. 2023; 13(6):1106. https://doi.org/10.3390/nano13061106
Chicago/Turabian StyleChen, Zhenhua, Qinhua Wei, Gao Tang, Hongsheng Shi, and Laishun Qin. 2023. "Preparation and Thermal Conductivity Enhancement of Boron Nitride Nano-Material PiG Composite" Nanomaterials 13, no. 6: 1106. https://doi.org/10.3390/nano13061106
APA StyleChen, Z., Wei, Q., Tang, G., Shi, H., & Qin, L. (2023). Preparation and Thermal Conductivity Enhancement of Boron Nitride Nano-Material PiG Composite. Nanomaterials, 13(6), 1106. https://doi.org/10.3390/nano13061106





