A Simple Method for the Synthesis of a Coral-like Boron Nitride Micro-/Nanostructure Catalyzed by Fe
Abstract
1. Introduction
2. Materials and Methods
3. Results
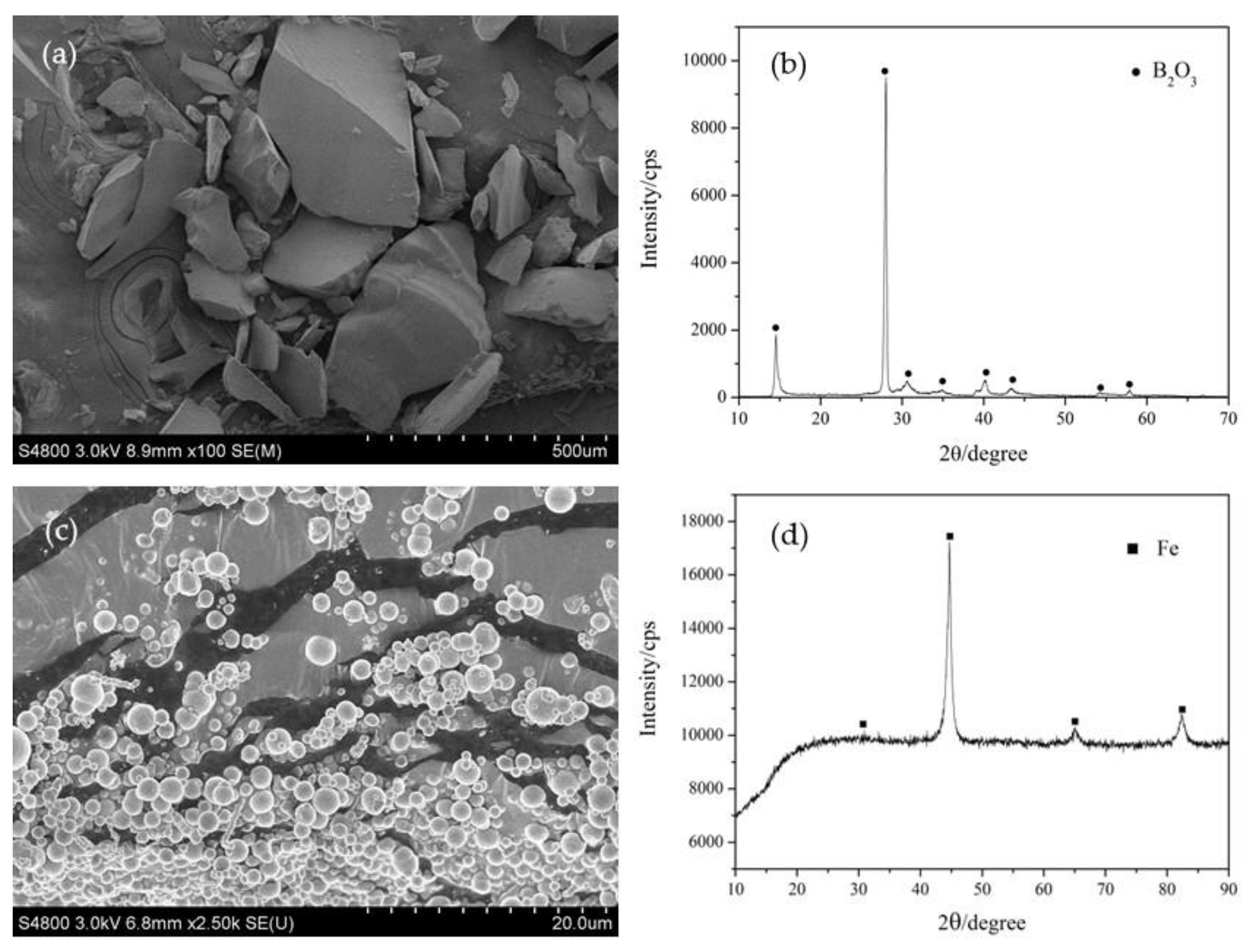
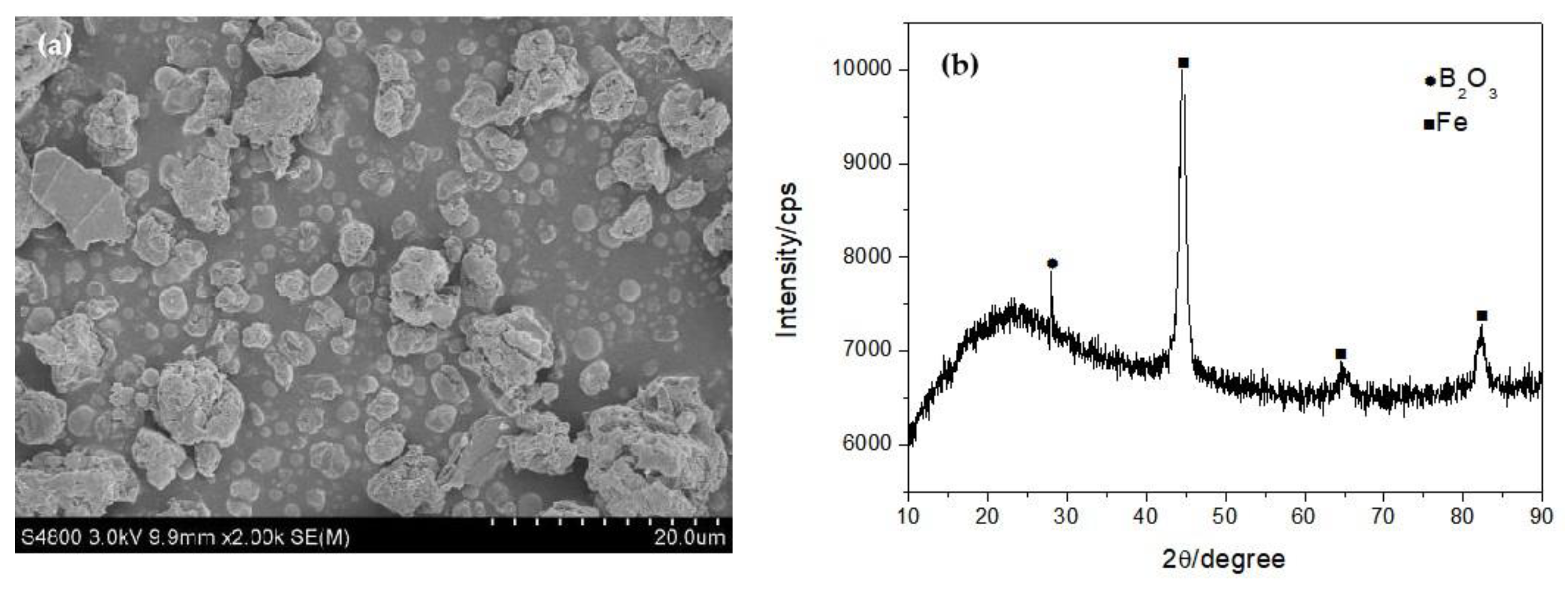
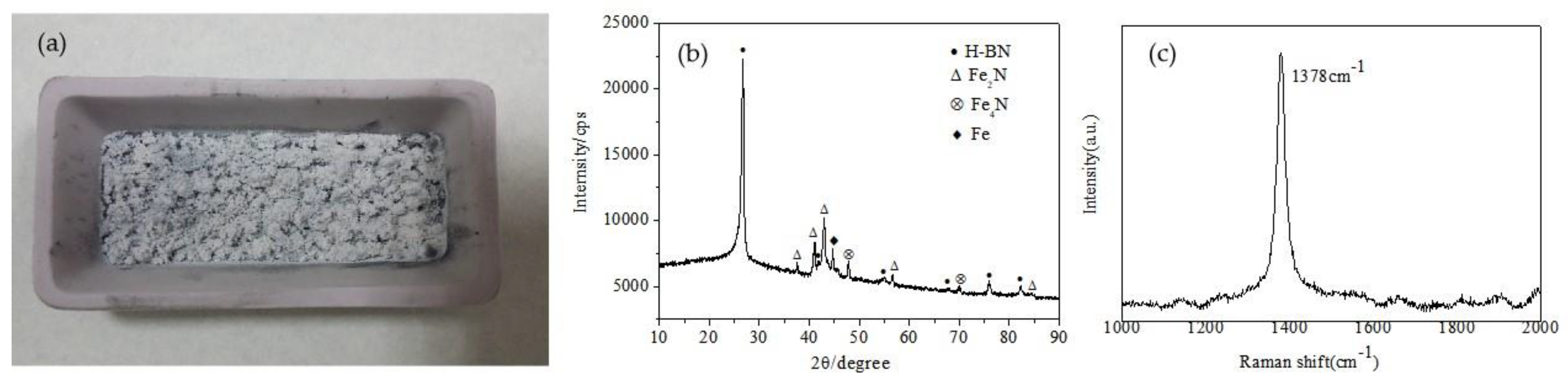
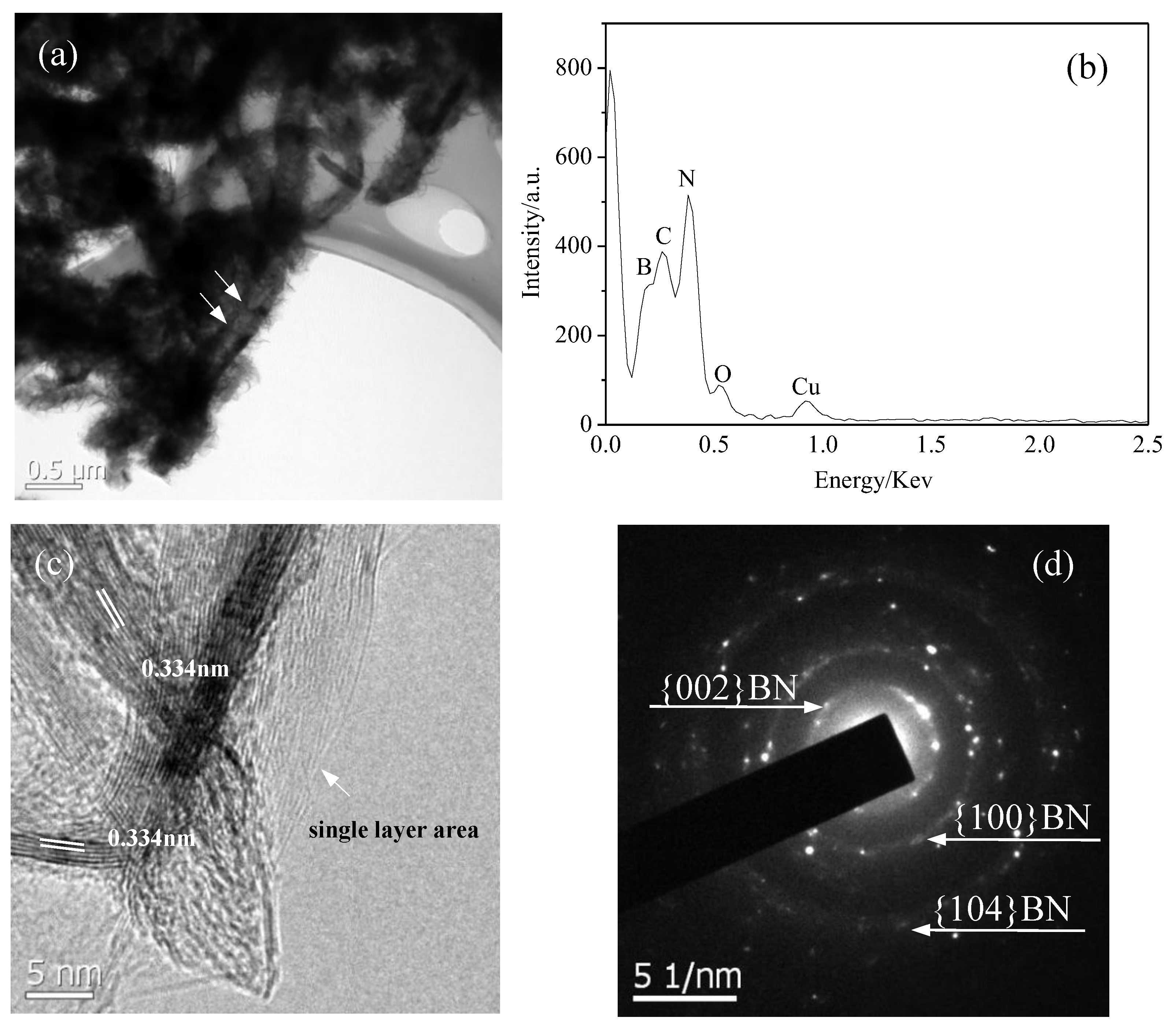
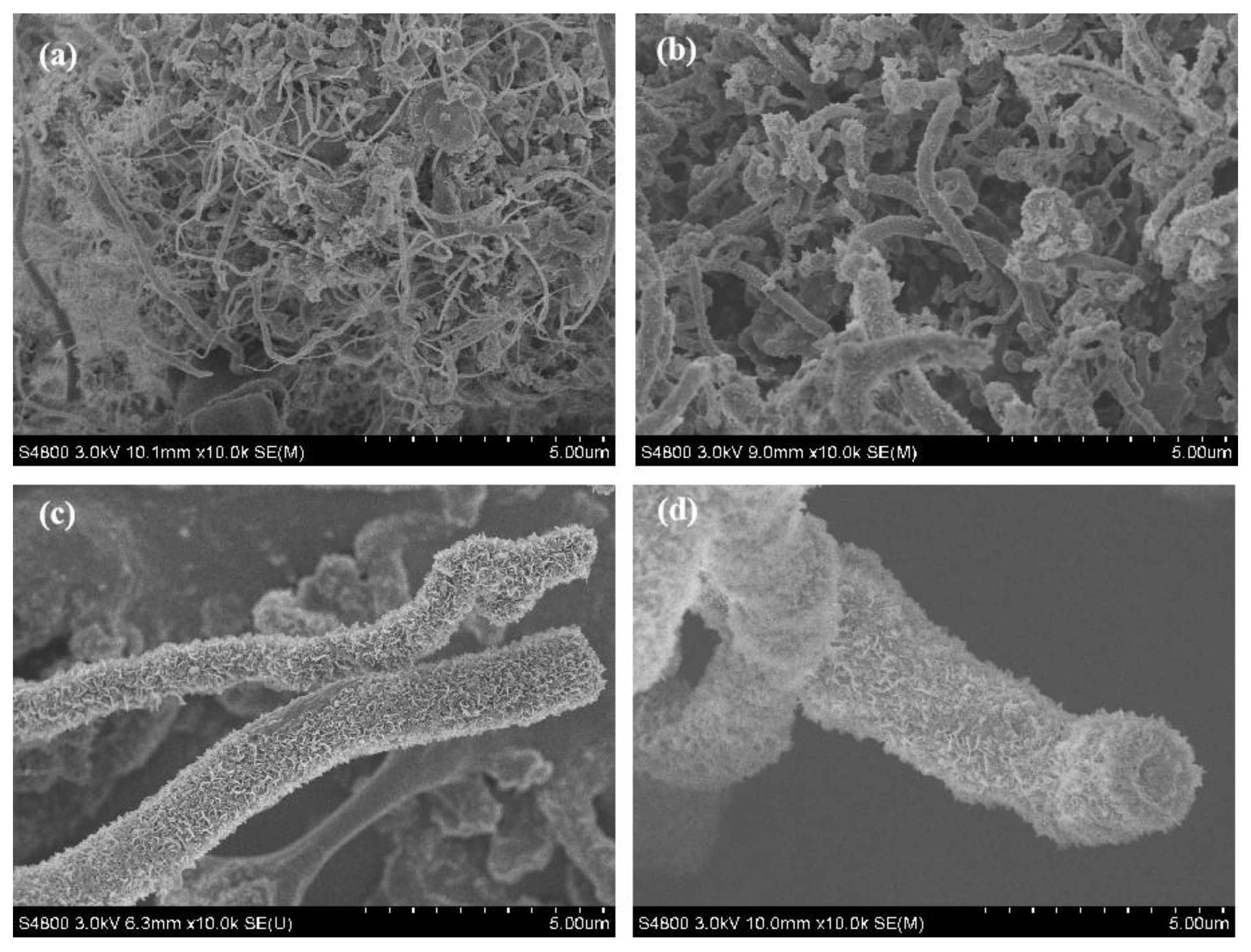
3.1. Synthesis and Characterization
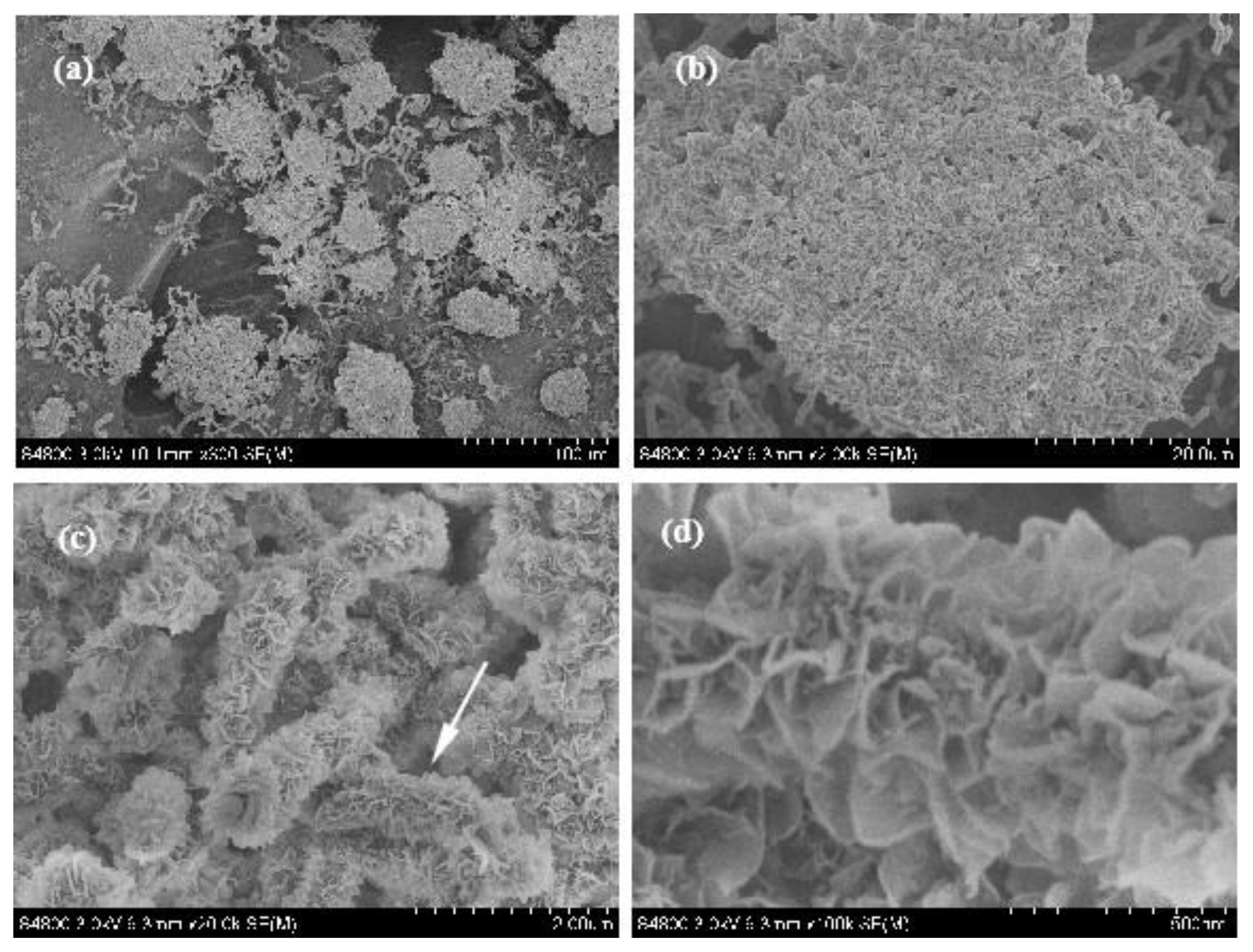
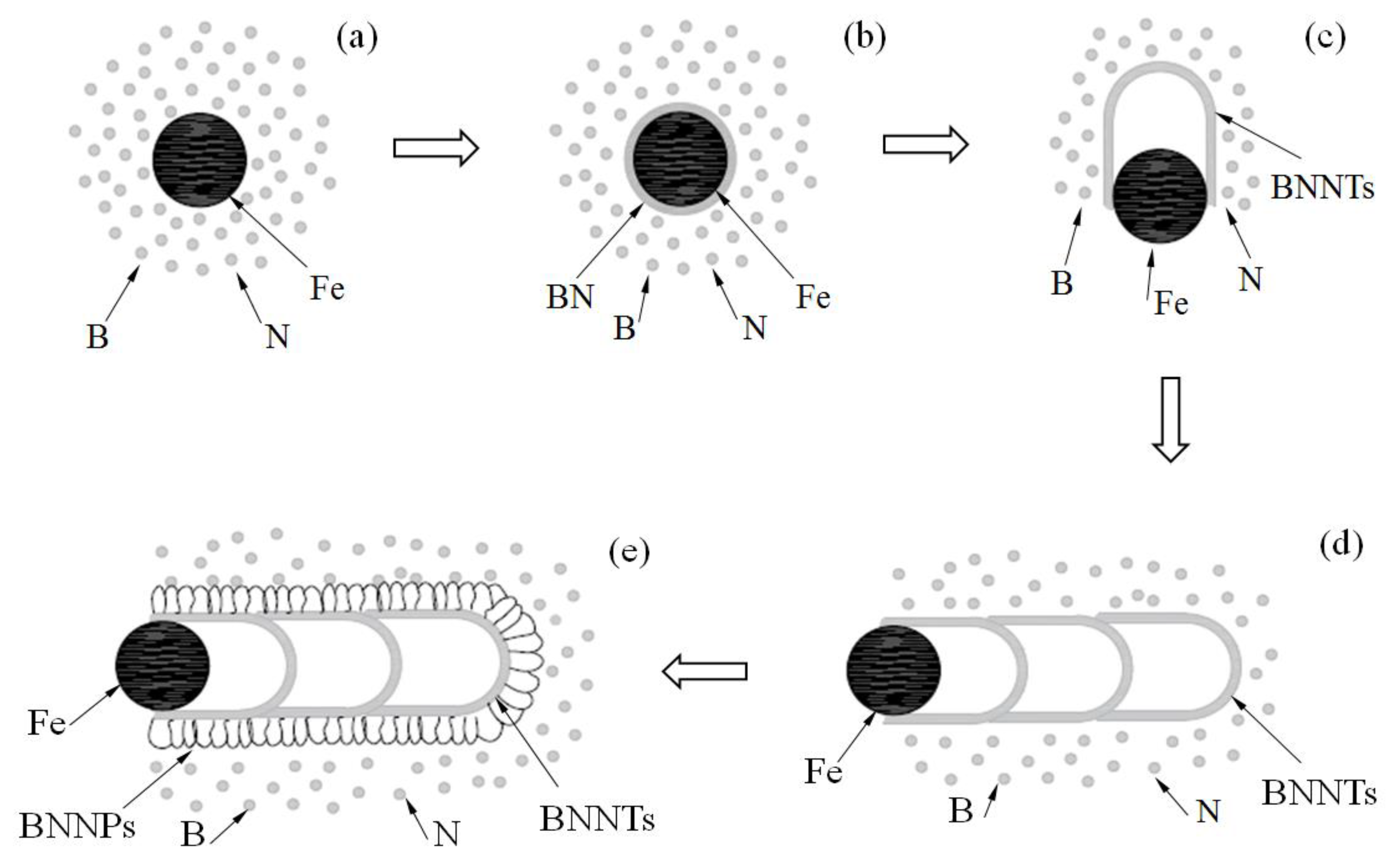
3.2. Formation Mechanism
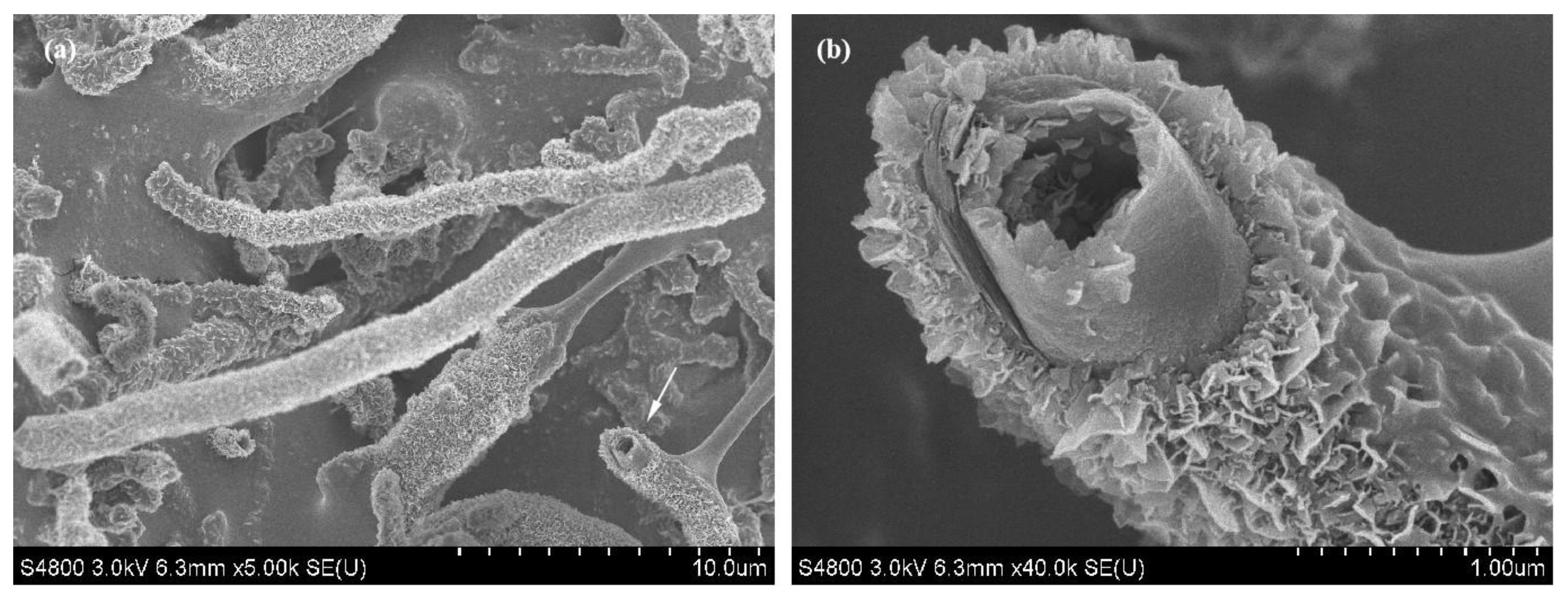
3.3. Purification Technology
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Manikandan, N.; Suresh, K.; Siva, S.; Rathis, G.; Vishnu, S.; Shabariganesh, T. Carbon nanotubes and their properties-The review. Mater. Today Proc. 2021, 47, 4682–4685. [Google Scholar]
- Blase, X.; Rubio, A.; Louie, S.G.; Cohen, M.L. Stability and band gap constancy of boron nitride nanotubes. Europhys. Lett. 1994, 5, 335–340. [Google Scholar] [CrossRef]
- Chopra, N.G.; Luyken, R.J.; Cherrey, K.; Crespi, V.H.; Cohen, M.L.; Louie, S.G.; Zettl, A. Boron nitride nanotubes. Science 1995, 269, 966–967. [Google Scholar] [CrossRef]
- Saito, Y.; Maida, M.; Matsumoto, T. Structures of BNNTs with single layer and multilayers produced by arc discharge. Jpn. J. Appl. Phys. 1999, 38, 159–163. [Google Scholar] [CrossRef]
- Yeh, Y.-W.; Raitses, Y.; Koel, B.E.; Yao, N. Stable synthesis of few-layered boron nitride nanotubes by anodic arc discharge. Sci. Rep. 2017, 7, 3075. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Eremets, M.; Takemura, K.; Kurashima, K.; Yusa, H. Nanotubes in boron nitride laser heated at high pressure. Appl. Phys. Lett. 1996, 69, 2045–2047. [Google Scholar] [CrossRef]
- Gnofo, P.; Fay, C. Laser vaporization and plume chemistry in a boron nitride nanotube production rig. J. Thermophys. Heat Transf. 2013, 27, 369–381. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, H.; Pham, T.V.; Hwang, J.H.; Ahn, S.; Jang, S.G.; Lee, H.; Park, C.; Kim, C.S.; Kim, M.J. Dual growth mode of boron nitride nanotubes in high temperature pressure laser ablation. Sci. Rep. 2019, 9, 15674. [Google Scholar] [CrossRef]
- Bae, D.; Kim, C.; Lee, H.; Khater, O.; Kim, K.; Shin, H.; Lee, K.; Kim, M. Spontaneous formation of boron nitride nanotube fibers by boron impurity reduction in laser ablation of ammonia borane. Nano Converg. 2022, 9, 20. [Google Scholar] [CrossRef]
- Lourie, O.R.; Jones, C.R.; Bartlett, B.M.; Gibbons, P.C.; Ruoff, R.S.; Buhro, W.E. CVD growth of boron nitride nanotubes. Chem. Mater. 2000, 12, 1808–1810. [Google Scholar] [CrossRef]
- Kim, M.J.; Chatterjee, S.; Kim, S.M.; Stach, E.A.; Bradley, M.G.; Pender, M.J.; Sneddon, L.G.; Maruyama, B. Double-walled boron nitride nanotubes grown by floating catalyst chemical vapor deposition. Nano Lett. 2008, 8, 3298–3302. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Khandaker, M.U.; Khan, Z.R.; Amin, Y.M. Synthesis of boron nitride nanotubes via chemical vapour deposition: A comprehensive review. RSC Adv. 2015, 5, 35116–35137. [Google Scholar] [CrossRef]
- Li, C.; Long, X.; Songfeng, E.; Zhang, Q.; Li, T.; Wu, J.; Yao, Y. Magnesium-induced preparation of boron nitride nanotubes and their application in thermal interface materials. Nanoscale 2019, 11, 11457. [Google Scholar] [CrossRef]
- Chen, Y.; Fitz Gerald, J.; Williams, J.; Bulcock, S. Synthesis of boron nitride nanotubes at low temperatures using reactive ball milling. Chem. Phys. Lett. 1999, 299, 260–264. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Zhao, K.; Tung, S.; Schneider, E. Synthesis of boron nitride nanotubes from boron oxide by ball milling and annealing process. Mater. Lett. 2009, 63, 1733–1736. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Uhm, Y.R.; Jun, J.; Rhee, C.K.; Kim, G.M. Synthesis and growth of boron nitride nanotubes by a ball milling-annealing process. Acta Mater. 2011, 59, 2807–2813. [Google Scholar] [CrossRef]
- Zhuang, C.; Xu, H.; Li, L.; Liu, Y.; Ban, C.; Liu, X. Systematic investigation of the ball milling–annealing growth and electrical properties of boron nitride nanotubes. RSC Adv. 2016, 6, 113415–113423. [Google Scholar] [CrossRef]
- Shimizu, Y.; Moriyoshi, Y.; Tanaka, H.; Komatsu, S. Boron nitride nanotubes, webs, and coexisting amorphous phase formed by the plasma jet method. Appl. Phys. Lett. 1999, 75, 929–931. [Google Scholar] [CrossRef]
- Lee, C.; Choi, S.; Hong, S. Synthesis of boron nitride nanotubes by arc-jet plasma. Curr. Appl. Phys. 2006, 6, 166–170. [Google Scholar] [CrossRef]
- Kim, K.S.; Kingston, C.T.; Hrdina, A.; Jakubinek, M.B.; Guan, J.; Plunkett, M.; Simard, B. Hydrogen-catalyzed, pilot-scale production of small-diameter boron nitride nanotubes and their macroscopic assemblies. ACS Nano 2014, 8, 6211–6220. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Couillard, M.; Shin, H.; Plunkett, M.; Ruth, D.; Kingston, C.T.; Simard, B. Role of hydrogen in high-yield growth of boron nitride nanotubes at atmospheric pressure by induction thermal plasma. ACS Nano 2018, 12, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Fathalizadeh, A.; Pham, T.; Mickelson, W.; Zettl, A. Scaled synthesis of boron nitride nanotubes, nanoribbons, and nanococoons using direct feedstock injection into an extended-pressure, inductively-coupled thermal plasma. Nano Lett. 2014, 14, 4881–4886. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Iatsunskyi, I.; Coy, E.; Miele, P.; Cornu, D.; Bechelany, M. Novel and facile route for the synthesis of tunable boron nitride nanotubes combining atomic layer deposition and annealing processes for water purification. Adv. Mater. 2018, 5, 1800056. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Zheng, Y.; Miyuki, T.; Taiki, I.; Xiang, R.; Maruyama, S. Synthesis of vertically aligned boron nitride nanotubes with a template of single-walled carbon nanotubes. J. Marer. Res. 2022, 37, 4428–4438. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Zhao, L.; He, X.; Fang, W.; Li, W.; Du, X. Synthesis of boron nitride nanotubes by catalytic pyrolysis of organic-inorganic hybrid precursor. Ceram. Int. 2017, 43, 5145–5149. [Google Scholar] [CrossRef]
- Guo, H.; Xu, Y.; Chen, H.; Wang, Z.; Mao, X.; Zhou, G.; Zhang, J.; Wang, S. Synthesis of multiwall boron nitride (BN) nanotubes by a PVD method based on Vapor–Liquid–Solid growth. Materials 2020, 13, 915. [Google Scholar] [CrossRef]
- Chen, G.; Lu, H.; Cui, J.; Yu, H.; Wang, B.; Liu, Y.; Li, H.; Jiang, N. In situ real-time study buckling behavior of boron nitride nanotubes with axial compression by TEM. Chin. Chem. Lett. 2019, 30, 1401–1404. [Google Scholar] [CrossRef]
- Zhou, X.; Tang, D.-M.; Mitome, M.; Bando, Y.; Sasaki, T.; Golberg, D. Intrinsic and defect-related elastic moduli of boron nitride nanotubes as revealed by in situ transmission electron microscopy. Nano Lett. 2019, 19, 4974–4980. [Google Scholar] [CrossRef]
- Belkerk, B.E.; Achour, A.; Zhang, D.; Sahli, S.; Djouadi, M.-A.; Yap, Y.K. Thermal conductivity of vertically aligned boron nitride nanotubes. Appl. Phys. Express 2016, 9, 075002. [Google Scholar] [CrossRef]
- Jakubinek, M.B.; Niven, J.F.; Johnson, M.B.; Ashrafi, B.; Kim, K.S.; Simard, B.; White, M.A. Thermal conductivity of bulk boron nitride nanotube sheets and their epoxy-impregnated composites. Phys. Status Solidi A 2016, 213, 2237. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, J.; Campbell, S.J.; Le Caer, G. Boron nitride nanotubes: Pronounced resistance to oxidation. Appl. Phys. Lett. 2004, 84, 2430–2432. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Y.N.; Shin, D.H.; Yun, K.N.; Song, Y.H.; Miline, W.I.; Lee, C.J. Excellent oxidation endurance of boron nitride nanotube field electron emitters. Appl. Phys. Lett. 2014, 104, 163102. [Google Scholar] [CrossRef]
- Chen, H.; Chena, Y.; Liu, Y. Cathodoluminescence of boron nitride nanotubes doped by ytterbium. Alloy. Compd. 2010, 504S, S353–S355. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Li, C.P.; Zhang, H.; Williams, J.S.; Liu, Y.; Liu, Z.; Ringer, S.P. Eu-doped boron nitride nanotubes as a nanometer-sized visible-light source. Adv. Mater. 2007, 19, 1845–1848. [Google Scholar] [CrossRef]
- Hu, W.; Cao, X.; Zhang, Y.; Li, T.-D.; Jiang, J.; Luo, Y. Tunable single-photon emission by defectiveboron-nitride nanotubes for high-precision force detection. J. Phys. Chem. C 2019, 123, 9624–9628. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, W. Tuning the magnetic and transport properties of boron-nitride nanotubes via oxygen-doping. Solid State Commun. 2009, 149, 486–490. [Google Scholar] [CrossRef]
- Lee, C.; Drelich, J.; Yap, Y. Superhydrophobicity of boron nitride nanotubes grown on silicon substrates. Langmuir 2009, 25, 4853–4860. [Google Scholar] [CrossRef]
- Aliev, A.D.; Boinovich, L.B.; Bukhovets, V.L.; Emelyanenko, A.M.; Gorbunov, A.M.; Gorodetskii, A.E.; Pashinin, A.S. Superhydrophobic coatings based on boron nitride nanotubes: The mechanism of superhydrophobicity and self regeneration of highly hydrophobic properties. Nanotechnologies Russ. 2011, 6, 723–732. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y. Superhydrophobic properties of nonaligned boron nitride nanotube films. Langmuir 2010, 26, 5135–5140. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Liu, Y.; Craig, V.; Li, L.H.; Chen, Y. Superhydrophobic and superoleophilic boron nitride nanotube-coated stainless steel meshes for oil and water separation. Adv. Mater. Interfaces 2014, 1, 1300002. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tang, C.; Golberg, D. Specific heat capacity and density of multi-walled boron nitride nanotubes by chemical vapor deposition. Solid State Commun. 2011, 151, 183–186. [Google Scholar] [CrossRef]
- Marjan, A.N.; Philipp, U.; Herbert, M.U. Boron nitride nanotubes as containers for targeted drug delivery of doxorubicin. J. Mol. Model. 2020, 26, 54. [Google Scholar]
- Turhan, E.A.; Pazarçeviren, A.E.; Evis, Z.; Tezcaner, A.C. Properties and applications of boron nitride nanotubes. Nanotechnology 2022, 33, 242001. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, K.; Cai, Q.; Wang, N.; Wu, L.; He, Q.; Wang, H.; Zhang, Y.; Xie, Y.; Yao, Y.; et al. Advances in synthesis and applications of boron nitride nanotubes: A review. Chem. Eng. J. 2022, 431, 134118. [Google Scholar] [CrossRef]
- Mohd, Y.; Shafat, A.-K. A Review of Synthesis and applications of boron nitride nanotubes (BNNTs) with future prospects. Curr. Smart Mater. 2021, 5, 66–78. [Google Scholar]
- Lin, L.; Zheng, Y.; Li, Z.; Wei, K. Synthesis of novel acetabuliform boron nitride nanoparticles with high surface area. Scr. Mater. 2008, 59, 1151–1154. [Google Scholar] [CrossRef]
- Melis, E.-C.; Özlem, Ş.; Mustafa, Ç.-C. Hexagonal boron nitride nanoparticles for prostate cancer treatment. ACS Appl. Nano Mater. 2020, 3, 2364–2372. [Google Scholar]
- Aditi, K.; Kriti, B.; Smriti, N.; Himani, S.; Charu, D. Hydrothermal synthesis of highly stable boron nitride nanoparticles. Mater. Today Proc. 2020, 28, 138–140. [Google Scholar]
- Srikanth, M.; Cynthia, S.W.; Liu, Z.; Yang, W.R.; Li, Y.C.; Li, L.H.; Chen, Y. Biocompatibility of boron nitride nanosheets. Nano Res. 2018, 11, 334–342. [Google Scholar]
- Vishnu, S.S.B.; Bindhu, C.-A.; Indulal, C.-R.; Krishnakumar, T.-S. Surface modification of boron nitride nanosheets for effective heat transfer applications. Chem. Afr. 2022, 5, 761–770. [Google Scholar]
- Md, G.-R.; Alper, K.; Babak, A.; Reza, S.-Y. 2D boron nitride nanosheets for polymer composite materials. 2d Mater. Appl. 2021, 5, 1–18. [Google Scholar]
- Ewa, B.-P.; Rümmeli, M.-H.; Martin, K.; Günter, B.; Kati, B.; Thomas, G.; Ryszard, J.K.; Thomas, P. Bulk quantity an d physical properties of boron nitride nanocapsules with a narrow size distribution. Carbon 2005, 43, 615–621. [Google Scholar]
- Zheng, M.T.; Gu, Y.L. Synthesis and characterization of boron nitride nanoropes. Mater. Lett. 2007, 61, 1943–1945. [Google Scholar] [CrossRef]
- Hao, J.-D.; Li, L.; Gao, P.; Jiang, X.-Q.; Ban, C.-C.; Shi, N.-Q. Boron nitride nanoribbons grown by chemical vapor deposition for VUV applications. Micromachines 2022, 13, 1372. [Google Scholar] [CrossRef]
- Liu, F.-Q.; Feng, J.; Jiang, Y.-G.; Li, L. Preparation and application of boron nitride aerogels. J. Inorg. Mater. 2020, 35, 1193–1202. [Google Scholar] [CrossRef]
- Xia, D.; Yu, H.Y.; Li, Q.; Mannering, J.; Menzel, R.; Huang, P.-C.; Li, H.-C. Compressive and thermally stable boron nitride aerogels as multifunctional sorbents. Dalton Trans. 2022, 51, 836–841. [Google Scholar] [CrossRef]
- Yang, H.; Wang, L.; Gao, F.; Dai, M.; Hu, Y.; Chen, H.; Zhang, J.; Qiu, Y. Shape evolution of two dimensional hexagonal boron nitride single domains on Cu/Ni alloy and its applications in ultraviolet detection. Nanotechnology 2019, 30, 245706. [Google Scholar] [CrossRef]
- Tan, B.; Yang, H.; Hu, Y.; Gao, F.; Wang, L.; Dai, M.; Zhang, S.; Shang, H.; Chen, H.; Hu, P. Synthesis of high-quality multilayer hexagonal boron nitride films on Au foils for ultrahigh rejection ratio solar-blind photodetection. ACS Appl. Mater. Interfaces 2020, 12, 28351–28359. [Google Scholar] [CrossRef]
- Yang, H.; Wang, G.; Guod, Y.; Wang, L.; Tan, B.; Zhang, S.; Zhang, X.; Zhang, J.; Shuai, Y.; Lin, J.; et al. Growth of wafer-scale graphene–hexagonal boron nitride vertical heterostructures with clear interfaces for obtaining atomically thin electrical analogs. Nanoscale 2022, 14, 4204–4215. [Google Scholar] [CrossRef]
- Woo, S.; Seung, Y.; Jeunghee, P.; Jae-Pyoung, A. Thorn-like BN nanostructures. Solid State Commun. 2005, 133, 139–143. [Google Scholar]
- Tang, C.-C.; Bando, Y.; Shen, G.-Z. Single-source precursor for chemical vapour deposition of collapsed boron nitride nanotubes. Nanotechnology 2006, 17, 5882–5888. [Google Scholar] [CrossRef]
- Terao, T.; Bando, Y.; Mitome, M.; Kurashima, K.; Zhi, C.-Y.; Tang, C.-C.; Golberg, D. Effective synthesis of surface-modified boron nitride nanotubes and related nanostructures and their hydrogen uptake. Phys. Sect. E 2008, 40, 2551–2555. [Google Scholar] [CrossRef]
- Sunil, K.; Avanish, K.; Rakesh, B. Growth of boron nitride nanotubes having large surface area using mechanothermal process. World J. Nano Sci. Eng. 2011, 1, 119–128. [Google Scholar]
- Mikhael, B.; Arnaud, B.; Samuel, B.; Pierre, S.; David, C.; Philippe, M. Boron nitride multiwall nanotubes decorated with BN nanosheets. CrystEngComm 2011, 13, 6526–6530. [Google Scholar]
- Yu, H.-M.; Huang, X.-X.; Wen, G.-W.; Zhang, T.; Zhong, B.; Bai, H.-W. A facile method for synthesis of novel coral-like boron nitride nanostructures. Mater. Chem. Phys. 2011, 129, 30–34. [Google Scholar] [CrossRef]
- Pan, A.; Chen, Y.-J.; Li, J.-B. An effective route for the synthesis of boron nitride micro-nano structures and the growth mechanism. CrystEngComm 2015, 17, 1098–1105. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.-M.; Wang, H.; Zhang, F.; Li, Y.-W.; Fu, Z.Y. Urchin-like boron nitride hierarchical structure assembled by nanotubes nanosheets for effective removal of heavy metal ions. Ceram. Int. 2018, 44, 12216–12224. [Google Scholar] [CrossRef]
- Wang, J.; Ji, Y.; Gu, Y.; Liao, H.; Yang, L.; Long, F.; Zhou, B.; Wang, W. Effective Preparation of one-dimensional boron-nitride- nanotube-supported nanosheet hierarchical structures and their optical/adsorption properties. Chem. Sel. 2018, 3, 10832–10836. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Li, Y.; Wang, W.; Wang, H.; Fu, Z. Defect-induced formation mechanism for boron nitride nanosheets nanotubes hybrid structures. Scr. Mater. 2019, 171, 16–20. [Google Scholar] [CrossRef]
- Li, J.; Lin, H.; Chen, Y.-J.; Su, Q.-Q.; Bi, X.-F. Synthesis and anti-oxidation performance of nanoflake-decorated boron notrode hollow microspheres. J. Alloy. Compd. 2013, 550, 292–296. [Google Scholar] [CrossRef]
- Velazquez-Salazar, J.-J.; Munoz-Sandoval, E. Synthesis and state of art characterization of BN bamboo-like nanotubes: Evidence of a root growth mechanism catalyzed by Fe. Chem. Phys. Lett. 2006, 416, 342–348. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, X.; Wang, J.; Wang, X.; Zeng, D. A Simple Method for the Synthesis of a Coral-like Boron Nitride Micro-/Nanostructure Catalyzed by Fe. Nanomaterials 2023, 13, 753. https://doi.org/10.3390/nano13040753
Li Y, Wang X, Wang J, Wang X, Zeng D. A Simple Method for the Synthesis of a Coral-like Boron Nitride Micro-/Nanostructure Catalyzed by Fe. Nanomaterials. 2023; 13(4):753. https://doi.org/10.3390/nano13040753
Chicago/Turabian StyleLi, Yanjiao, Xueren Wang, Jian Wang, Xinfeng Wang, and Dejun Zeng. 2023. "A Simple Method for the Synthesis of a Coral-like Boron Nitride Micro-/Nanostructure Catalyzed by Fe" Nanomaterials 13, no. 4: 753. https://doi.org/10.3390/nano13040753
APA StyleLi, Y., Wang, X., Wang, J., Wang, X., & Zeng, D. (2023). A Simple Method for the Synthesis of a Coral-like Boron Nitride Micro-/Nanostructure Catalyzed by Fe. Nanomaterials, 13(4), 753. https://doi.org/10.3390/nano13040753





