Microwave Synthesis of Visible-Light-Activated g-C3N4/TiO2 Photocatalysts
Abstract
1. Introduction
2. Experimental Procedure
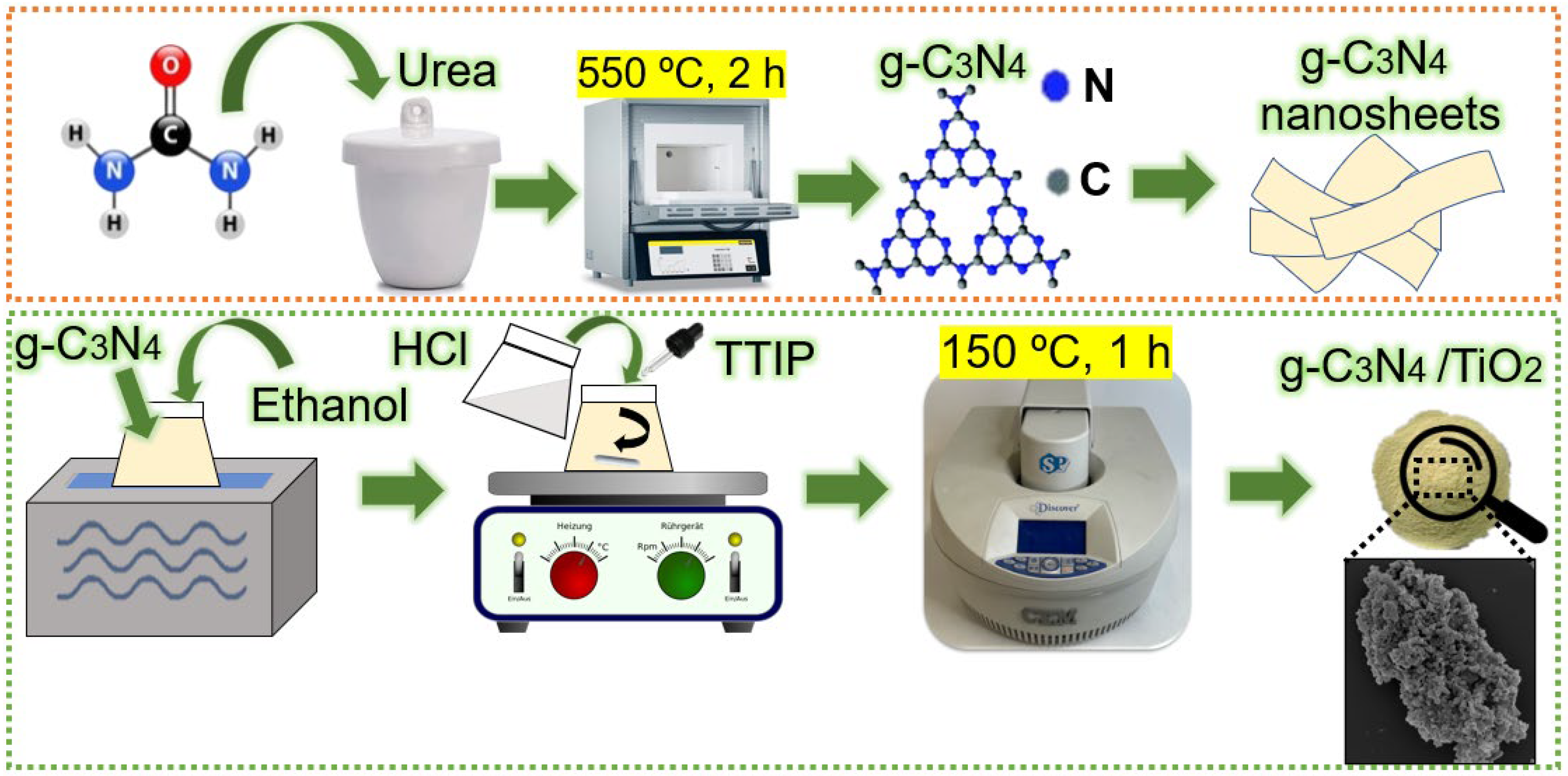
2.1. Synthesis of g-C3N4 Nanopowder
2.2. Microwave Synthesis of the g-C3N4/TiO2 Heterostructures
2.3. Structural and Optical Characterization of the Nanostructures
2.4. Characterization of the Nanostructures as Photocatalysts in the Degradation of MO under Solar Simulating Light
2.5. Electrochemical Characterization
3. Results and Discussion
3.1. Structural Characterization
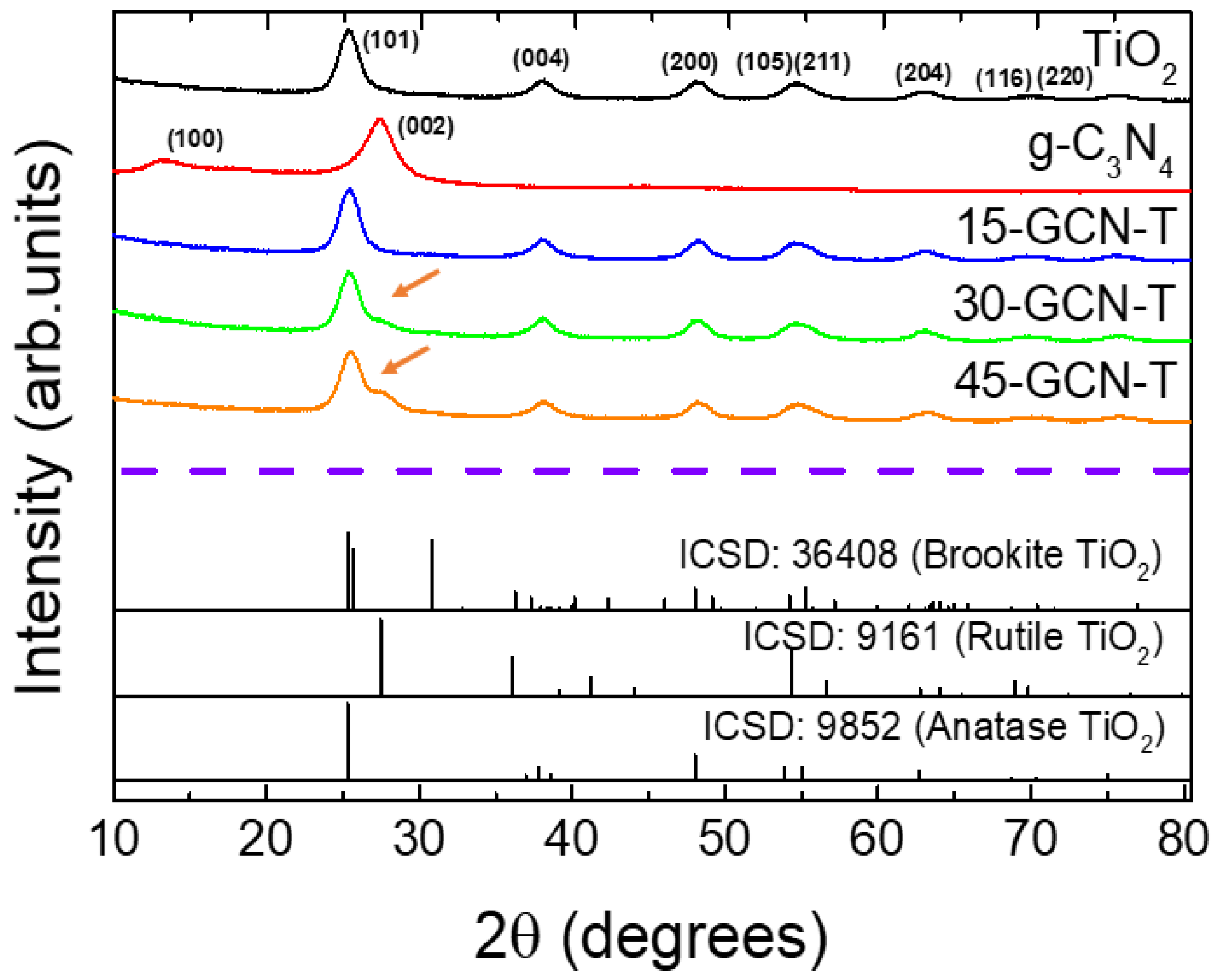
3.1.1. XRD
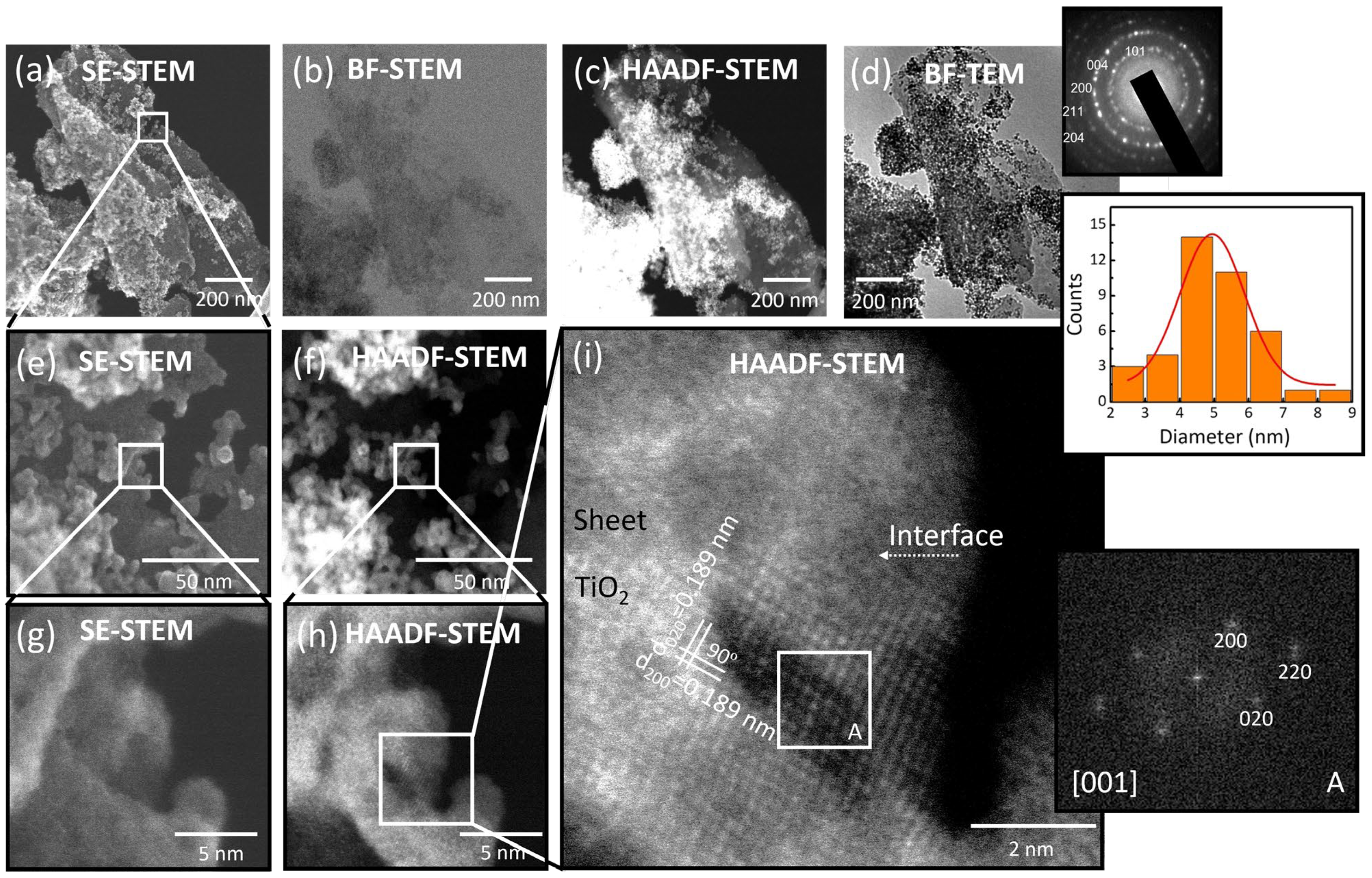
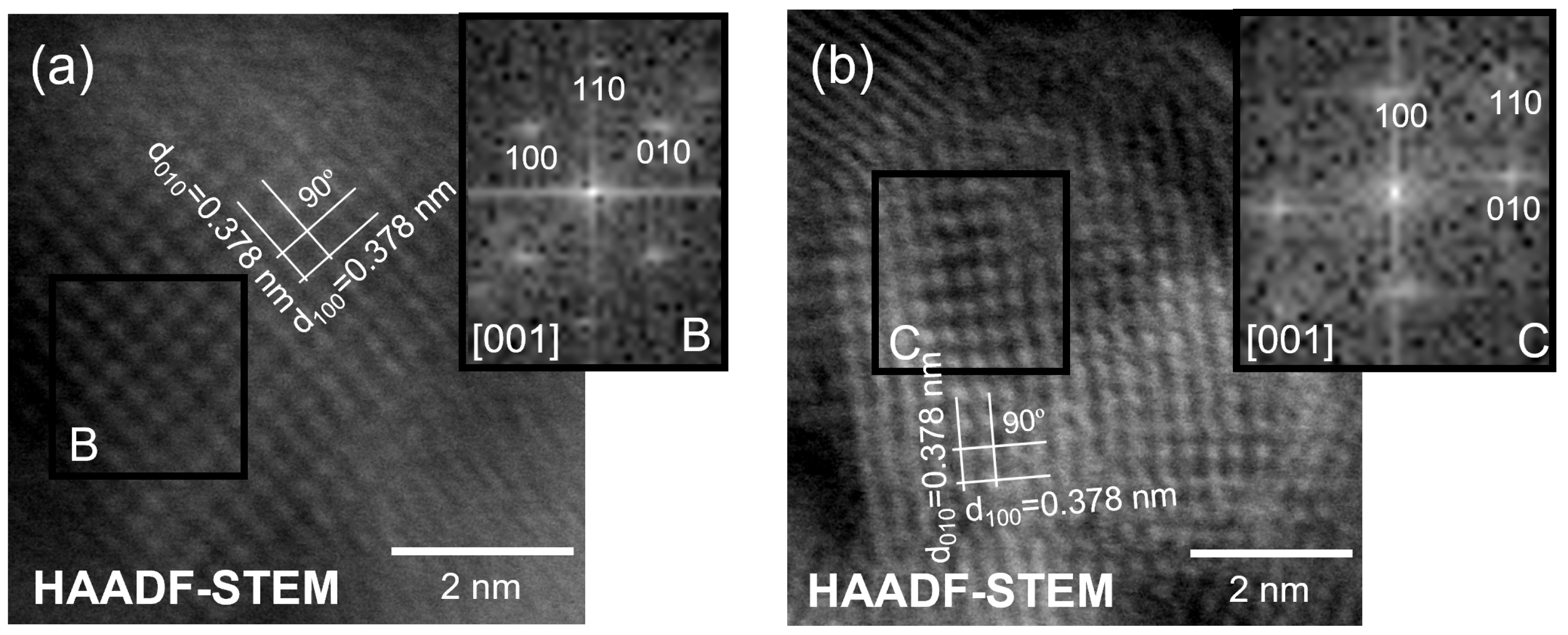
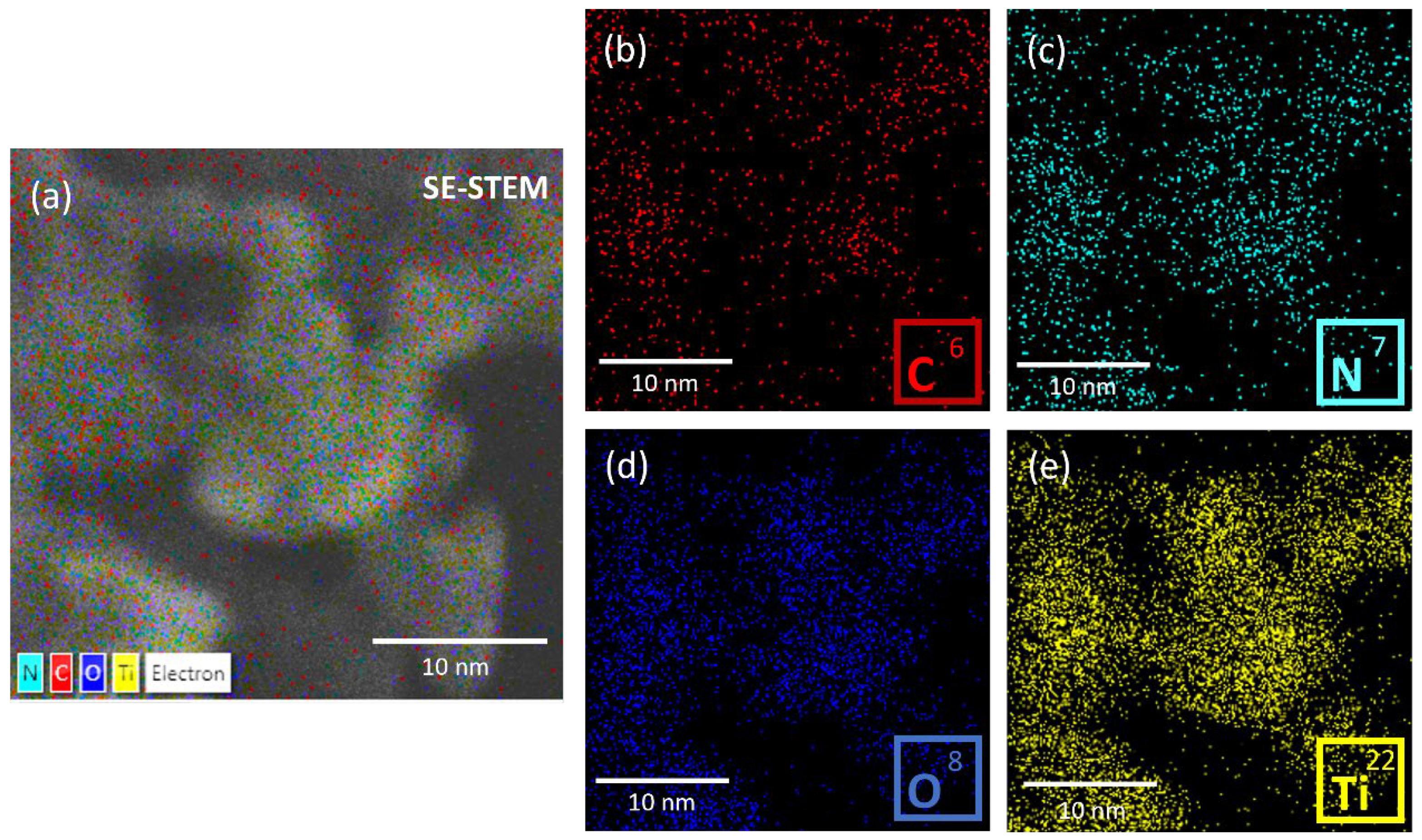
3.1.2. Electron Microscopy
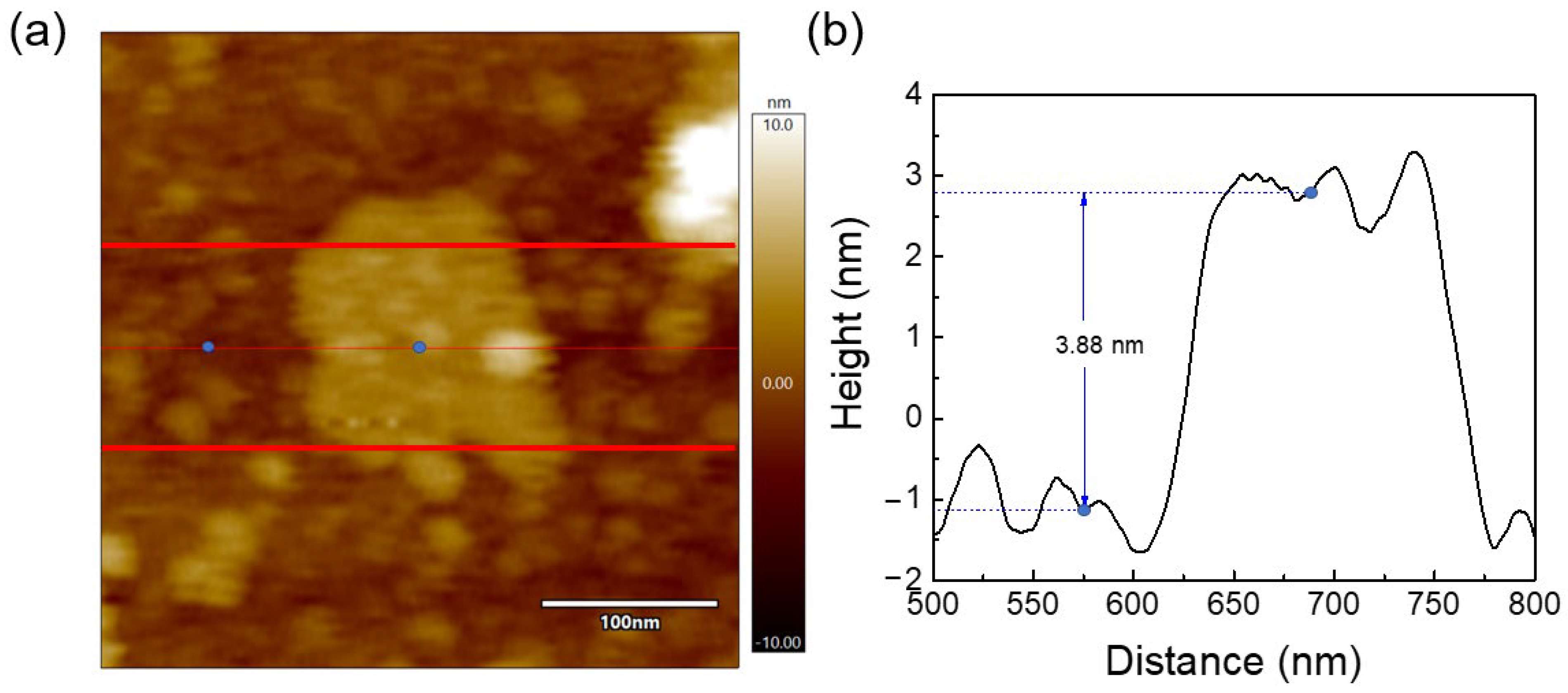
3.1.3. AFM
3.1.4. XPS
3.2. Optical Characterization
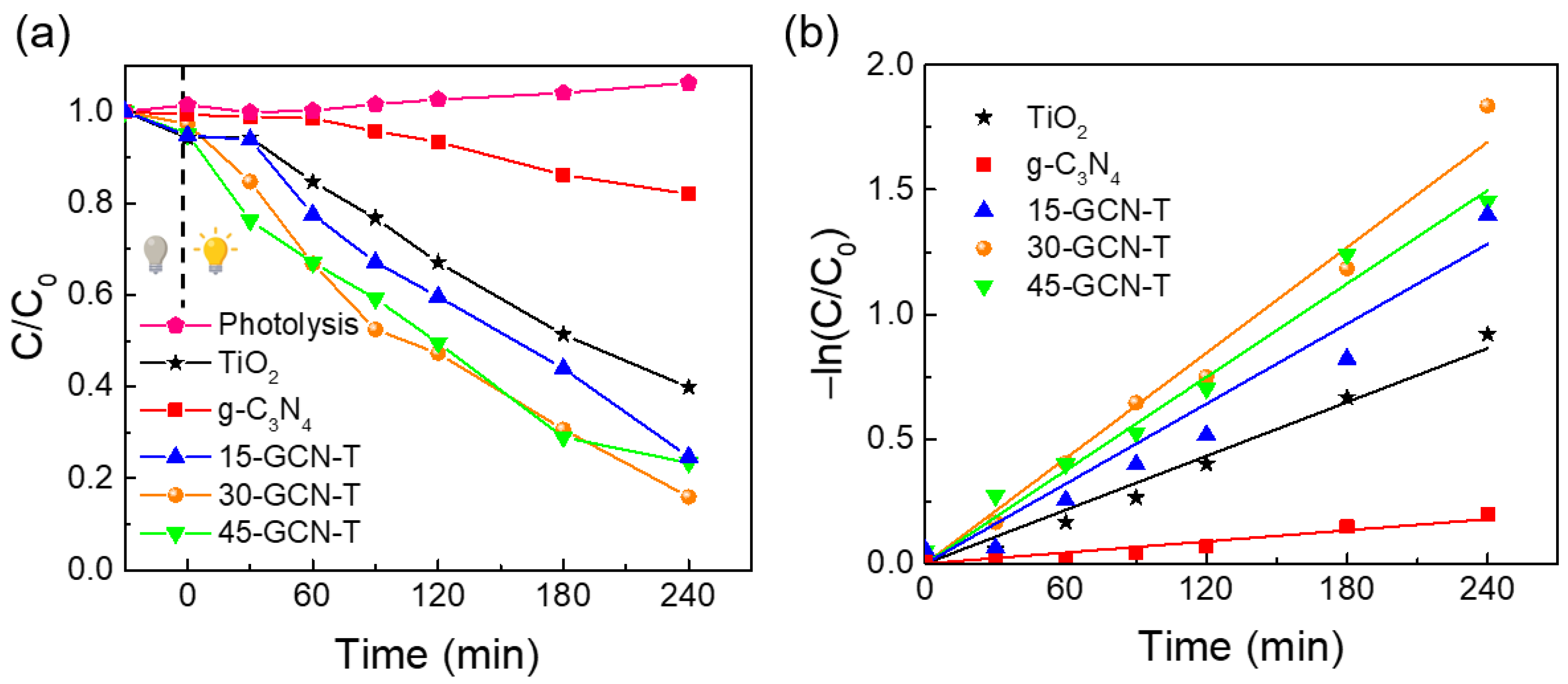
3.3. Photocatalytic Performance in the Degradation of MO under Solar Simulating Light
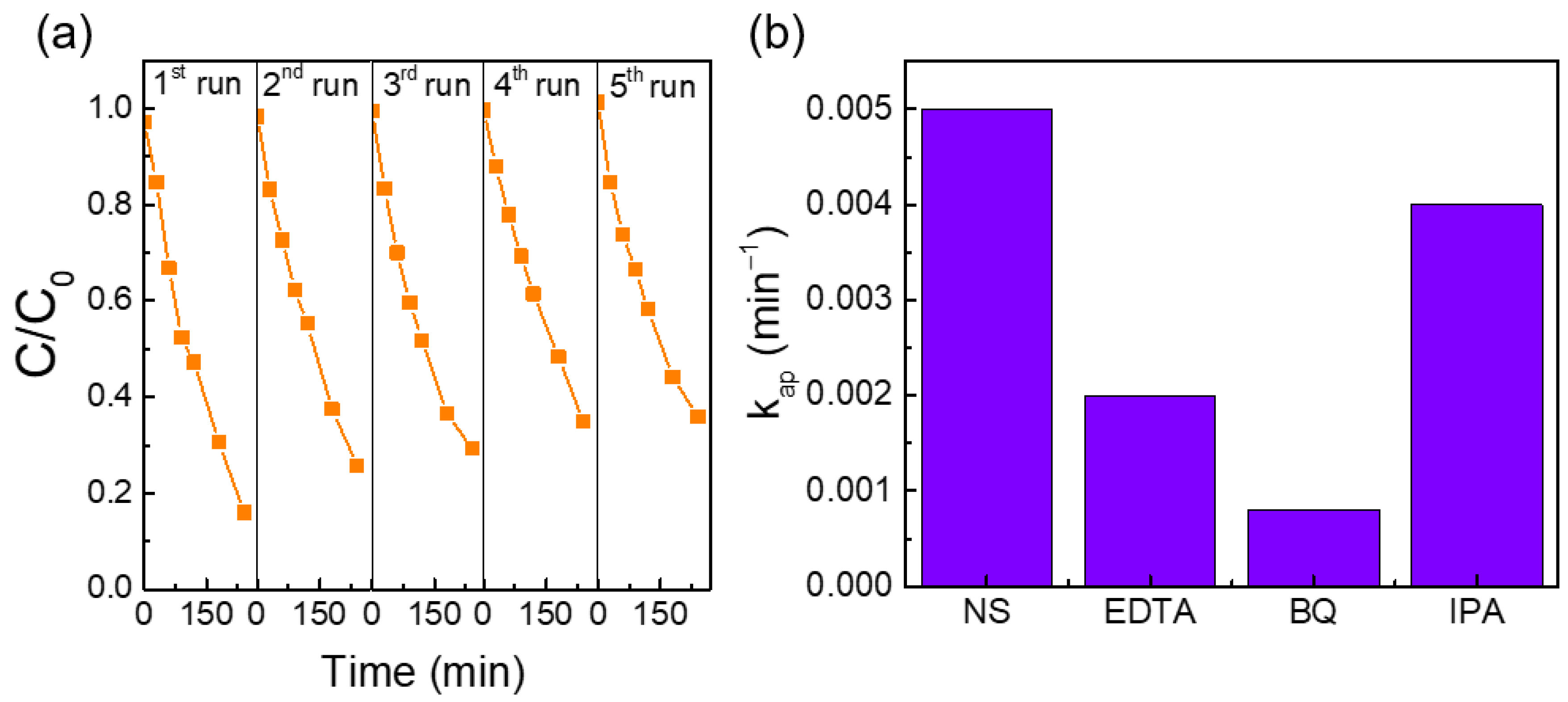
Reusability Tests and Possible Photocatalytic Degradation Mechanism
3.4. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Samsami, S.; Mohamadi, M.; Sarrafzadeh, M.H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Ju, L.; Tang, X.; Li, X.; Liu, B.; Qiao, X.; Wang, Z.; Yin, H. NO2 Physical-to-Chemical Adsorption Transition on Janus WSSe Monolayers Realized by Defect Introduction. Molecules 2023, 28, 1644. [Google Scholar] [CrossRef] [PubMed]
- Nan Chong, M.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Aich, V.; Ehlert, K. (Eds.) Drought and Water Scarcity. In Global Water Partnership and World Meteorological Organization; WMO: Geneva, Switzerland, 2022; pp. 1–24. [Google Scholar]
- World Health Organization Drinking-Water. Available online: https://www.who.int/news-room/factsheets/detail/drinking-water (accessed on 29 November 2022).
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, Z.; Xu, L.; Liu, T.; Fan, Y.; Jin, L.; Dong, R.; Yi, Y.; Li, Y. Waste Textile Reutilization Via a Scalable Dyeing Technology: A Strategy to Enhance Dyestuffs Degradation Efficiency. Adv. Fiber Mater. 2022, 4, 1595–1608. [Google Scholar] [CrossRef]
- Moussavi, G.; Mahmoudi, M. Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J. Hazard. Mater. 2009, 168, 806–812. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Joseph, C.G.; Pang, C.K.; Affandi, N.A.; Maruja, S.N.; Vijayan, V. Current Trends in the Utilization of Photolysis and Photocatalysis Treatment Processes for the Remediation of Dye Wastewater: A Short Review. ChemEngineering 2022, 6, 58. [Google Scholar] [CrossRef]
- Sheikh, M.U.D.; Naikoo, G.A.; Thomas, M.; Bano, M.; Khan, F. Solar-assisted photocatalytic reduction of methyl orange azo dye over porous TiO2 nanostructures. New J. Chem. 2016, 40, 5483–5494. [Google Scholar] [CrossRef]
- Sandoval, C.; Molina, G.; Vargas Jentzsch, P.; Pérez, J.; Muñoz, F. Photocatalytic Degradation of Azo Dyes Over Semiconductors Supported on Polyethylene Terephthalate and Polystyrene Substrates. J. Adv. Oxid. Technol. 2017, 20, 20170006. [Google Scholar] [CrossRef]
- Sha, Y.; Mathew, I.; Cui, Q.; Clay, M.; Gao, F.; Zhang, X.J.; Gu, Z. Rapid degradation of azo dye methyl orange using hollow cobalt nanoparticles. Chemosphere 2016, 144, 1530–1535. [Google Scholar] [CrossRef]
- Paz, A.; Carballo, J.; Pérez, M.J.; Domínguez, J.M. Biological treatment of model dyes and textile wastewaters. Chemosphere 2017, 181, 168–177. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Branquinho, R.; Fortunato, E.; Martins, R. Metal oxide-based photocatalytic paper: A green alternative for environmental remediation. Catalysts 2021, 11, 504. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Kumar, S.; Ahlawat, W.; Bhanjana, G.; Heydarifard, S.; Nazhad, M.M.; Dilbaghi, N. Nanotechnology-based water treatment strategies. J. Nanosci. Nanotechnol. 2014, 14, 1838–1858. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Sahu, O.; Karthikeyan, M.R. Reduction of Textile Dye by Using Heterogeneous Photocatalysis. Am. J. Environ. Prot. 2013, 2, 94. [Google Scholar] [CrossRef]
- Kuldeep, A.R.; Dhabbe, R.S.; Garadkar, K.M. Development of g-C3N4-TiO2 visible active hybrid photocatalyst for the photodegradation of methyl orange. Res. Chem. Intermed. 2021, 47, 5155–5174. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Araujo, A.; Calmeiro, T.R.; Panigrahi, S.; Pinto, J.V.; Barquinha, P.; Gama, M.; Fortunato, E.; Martins, R. Enhanced UV Flexible Photodetectors and Photocatalysts Based on TiO2 Nanoplatforms. Top. Catal. 2018, 61, 1591–1606. [Google Scholar] [CrossRef]
- Matias, M.L.; Pimentel, A.; Reis-Machado, A.S.; Rodrigues, J.; Deuermeier, J.; Fortunato, E.; Martins, R.; Nunes, D. Enhanced Fe-TiO2 Solar Photocatalysts on Porous Platforms for Water Purification. Nanomaterials 2022, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zou, C.; Yang, S.; Yang, Z.; Yang, Y. Ferroelectric polarization effect promoting the bulk charge separation for enhance the efficiency of photocatalytic degradation. Chem. Eng. J. 2021, 410, 128430. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Fortunato, E.; Martins, R. Photocatalytic TiO2 Nanorod Spheres and Arrays Compatible with Flexible Applications. Catalysts 2017, 7, 60. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Pinto, J.V.; Calmeiro, T.R.; Nandy, S.; Barquinha, P.; Pereira, L.; Carvalho, P.A.; Fortunato, E.; Martins, R. Photocatalytic behavior of TiO2 films synthesized by microwave irradiation. Catal. Today 2016, 278, 262–270. [Google Scholar] [CrossRef]
- Ratshiedana, R.; Kuvarega, A.T.; Mishra, A.K. Titanium dioxide and graphitic carbon nitride–based nanocomposites and nanofibres for the degradation of organic pollutants in water: A review. Environ. Sci. Pollut. Res. 2021, 28, 10357–10374. [Google Scholar] [CrossRef]
- Jeon, J.P.; Kweon, D.H.; Jang, B.J.; Ju, M.J.; Baek, J.B. Enhancing the Photocatalytic Activity of TiO2 Catalysts. Adv. Sustain. Syst. 2020, 4, 2000197. [Google Scholar] [CrossRef]
- Pimentel, A.; Nunes, D.; Pereira, S.; Martins, R.; Fortunato, E. Photocatalytic Activity of TiO2 Nanostructured Arrays Prepared by Microwave-Assisted Solvothermal Method. In Semiconductor Photocatalysis—Materials, Mechanisms and Applications; IntechOpen: London, UK, 2016; Chapter 3. [Google Scholar]
- Freire, T.; Fragoso, A.R.; Matias, M.; Vaz Pinto, J.; Marques, A.C.; Pimentel, A.; Barquinha, P.; Huertas, R.; Fortunato, E.; Martins, R.; et al. Enhanced solar photocatalysis of TiO2 nanoparticles and nanostructured thin films grown on paper. Nano Express 2021, 2, 040002. [Google Scholar] [CrossRef]
- Ansari, S.A.; Cho, M.H. Highly Visible Light Responsive, Narrow Band gap TiO2 Nanoparticles Modified by Elemental Red Phosphorus for Photocatalysis and Photoelectrochemical Applications. Sci. Rep. 2016, 6, 25405. [Google Scholar] [CrossRef]
- Valencia, S.; Marín, J.M.; Restrepo, G. Study of the bandgap of synthesized titanium dioxide nanoparticules using the sol-gel method and a hydrothermal treatment. Open Mater. Sci. J. 2010, 4, 9–14. [Google Scholar] [CrossRef]
- Ibrahim, N.S.; Leaw, W.L.; Mohamad, D.; Alias, S.H.; Nur, H. A critical review of metal-doped TiO2 and its structure–physical properties–photocatalytic activity relationship in hydrogen production. Int. J. Hydrog. Energy 2020, 45, 28553–28565. [Google Scholar] [CrossRef]
- Szkoda, M.; Siuzdak, K.; Lisowska-Oleksiak, A. Non-metal doped TiO2 nanotube arrays for high efficiency photocatalytic decomposition of organic species in water. Phys. E: Low-Dimens. Syst. Nanostructures 2016, 84, 141–145. [Google Scholar] [CrossRef]
- Park, K.; Meunier, V.; Pan, M.; Plummer, W. Defect-Driven Restructuring of TiO2 Surface and Modified Reactivity Toward Deposited Gold Atoms. Catalysts 2013, 3, 276–287. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Y.; Bu, X.; Wu, Q.; Hang, Z.; Dong, Z.; Wu, X. Facile one-step synthesis of TiO2/Ag/SnO2 ternary heterostructures with enhanced visible light photocatalytic activity. Sci. Rep. 2018, 8, 10532. [Google Scholar] [CrossRef]
- Angel, R.D.; Durán-Álvarez, J.C.; Zanella, R.; Angel, R.D.; Durán-Álvarez, J.C.; Zanella, R. TiO2-Low Band Gap Semiconductor Heterostructures for Water Treatment Using Sunlight-Driven Photocatalysis. In Titanium Dioxide—Material for a Sustainable Environment; IntechOpen: London, UK, 2018; ISBN 978-1-78923-327-8. [Google Scholar]
- Zhang, X.; Li, L.; Zeng, Y.; Liu, F.; Yuan, J.; Li, X.; Yu, Y.; Zhu, X.; Xiong, Z.; Yu, H.; et al. TiO2/Graphitic Carbon Nitride Nanosheets for the Photocatalytic Degradation of Rhodamine B under Simulated Sunlight. ACS Appl. Nano Mater. 2019, 2, 7255–7265. [Google Scholar] [CrossRef]
- Pham, M.T.; Luu, H.Q.; Nguyen, T.M.T.; Tran, H.H.; You, S.J.; Wang, Y.F. Rapid and Scalable Fabrication of TiO2@g-C3N4 Heterojunction for Highly Efficient Photocatalytic NO Removal under Visible Light. Aerosol Air Qual. Res. 2021, 21, 210276. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A.C. Graphitic carbon nitride: Synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef]
- Tripathi, A.; Narayanan, S. Structural modification of a 2D π-conjugated polymeric material (g-C3N4) through boron doping for extended visible light absorption. Synth. Met. 2020, 260, 116284. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Q.; Chai, G.; Liang, M.; Dong, G.; Zhang, Q.; Qiu, J. Synthesis and luminescence mechanism of multicolor-emitting g-C3N4 nanopowders by low temperature thermal condensation of melamine. Sci. Rep. 2013, 3, 1943. [Google Scholar] [CrossRef]
- Iqbal, W.; Yang, B.; Zhao, X.; Rauf, M.; Waqas, M.; Gong, Y.; Zhang, J.; Mao, Y. Controllable synthesis of graphitic carbon nitride nanomaterials for solar energy conversion and environmental remediation: The road travelled and the way forward. Catal. Sci. Technol. 2018, 8, 4576–4599. [Google Scholar] [CrossRef]
- Fu, J.; Wang, S.; Wang, Z.; Liu, K.; Li, H.; Liu, H.; Hu, J.; Xu, X.; Li, H.; Liu, M. Graphitic carbon nitride based single-atom photocatalysts. Front. Phys. 2020, 15, 33201. [Google Scholar] [CrossRef]
- Ye, S.; Wang, R.; Wu, M.Z.; Yuan, Y.P. A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar] [CrossRef]
- Xu, H.Y.; Wu, L.C.; Zhao, H.; Jin, L.G.; Qi, S.Y. Synergic Effect between Adsorption and Photocatalysis of Metal-Free g-C3N4 Derived from Different Precursors. PLoS ONE 2015, 10, e0142616. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, X.; Liu, Z.; Wang, C.; Liu, G.; Li, Q.; Feng, X. Enhanced photocatalytic activity of g-C3N4 2D nanosheets through thermal exfoliation using dicyandiamide as precursor. Ceram. Int. 2018, 44, 20613–20619. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hasan, I.M.U.; Qiao, J.; He, R.; Peng, L.; Xu, N.; Niazi, N.K.; Zhang, J.-N.; Farwa, F. Copper as a single metal atom based photo-, electro-, and photoelectrochemical catalyst decorated on carbon nitride surface for efficient CO2 reduction: A review. Nano Res. Energy 2022, 1, e9120015. [Google Scholar] [CrossRef]
- Thurston, J.H.; Hunter, N.M.; Wayment, L.J.; Cornell, K.A. Urea-derived graphitic carbon nitride (u-g-C3N4) films with highly enhanced antimicrobial and sporicidal activity. J. Colloid Interface Sci. 2017, 505, 910. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.; Wu, L.; Fu, M.; Wu, Z. Facile transformation of low cost thiourea into nitrogen-rich graphitic carbon nitride nanocatalyst with high visible light photocatalytic performance. Catal. Sci. Technol. 2012, 2, 1332–1335. [Google Scholar] [CrossRef]
- Asaithambi, S.; Sakthivel, P.; Karuppaiah, M.; Yuvakkumar, R.; Velauthapillai, D.; Ahamad, T.; Khan, M.A.M.; Mohammed, M.K.A.; Vijayaprabhu, N.; Ravi, G. The bifunctional performance analysis of synthesized Ce doped SnO2/g-C3N4 composites for asymmetric supercapacitor and visible light photocatalytic applications. J. Alloy. Compd. 2021, 866, 158807. [Google Scholar] [CrossRef]
- International Energy Agency. Final List of Critical Minerals 2022. Available online: https://www.iea.org/policies/15271-final-list-of-critical-minerals-2022 (accessed on 30 December 2022).
- Song, G.; Chu, Z.; Jin, W.; Sun, H. Enhanced performance of g-C3N4/TiO2 photocatalysts for degradation of organic pollutants under visible light. Chin. J. Chem. Eng. 2015, 23, 1326–1334. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, X.; Chen, Q.; Liu, Y.; Lou, X.; Zhang, L.; Zhang, X.; Li, Y.; Guan, J. Photocatalytic Applications of g-C3N4 Based on Bibliometric Analysis. Catalysts 2022, 12, 1017. [Google Scholar] [CrossRef]
- Le, S.; Zhu, C.; Cao, Y.; Wang, P.; Liu, Q.; Zhou, H.; Chen, C.; Wang, S.; Duan, X. V2O5 nanodot-decorated laminar C3N4 for sustainable photodegradation of amoxicillin under solar light. Appl. Catal. B Environ. 2022, 303, 120903. [Google Scholar] [CrossRef]
- Acharya, R.; Parida, K. A review on TiO2/g-C3N4 visible-light- responsive photocatalysts for sustainable energy generation and environmental remediation. J. Environ. Chem. Eng. 2020, 8, 103896. [Google Scholar] [CrossRef]
- Kobkeatthawin, T.; Chaveanghong, S.; Trakulmututa, J.; Amornsakchai, T.; Kajitvichyanukul, P.; Smith, S.M. Photocatalytic Activity of TiO2/g-C3N4 Nanocomposites for Removal of Monochlorophenols from Water. Nanomaterials 2022, 12, 2852. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, Z.; Zhang, W.; Zhang, D. High-Efficiency g-C3N4 Based Photocatalysts for CO2 Reduction: Modification Methods. Adv. Fiber Mater. 2022, 4, 342–360. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, C.; Hao, D.; Wei, W.; Wang, L.; Ni, B.J. Ultralight biodegradable 3D-g-C3N4 aerogel for advanced oxidation water treatment driven by oxygen delivery channels and triphase interfaces. J. Clean. Prod. 2021, 288, 125091. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yang, Y.; Zhang, Y.; He, D.; An, Q.; Cao, G. Seed-induced growing various TiO2 nanostructures on g-C3N4 nanosheets with much enhanced photocatalytic activity under visible light. J. Hazard. Mater. 2015, 292, 79–89. [Google Scholar] [CrossRef]
- Zang, Y.; Li, L.; Xu, Y.; Zuo, Y.; Li, G. Hybridization of brookite TiO2 with g-C3N4: A visible-light-driven photocatalyst for As3+ oxidation, MO degradation and water splitting for hydrogen evolution. J. Mater. Chem. A 2014, 2, 15774–15780. [Google Scholar] [CrossRef]
- Zeng, L.; He, Z.; Luo, Y.; Xu, J.; Chen, J.; Wu, L.; Huang, P.; Xu, S. A Simple g-C3N4/TNTs Heterojunction for Improving the Photoelectrocatalytic Degradation of Methyl Orange. J. Electrochem. Soc. 2021, 168, 116520. [Google Scholar] [CrossRef]
- Mohini, R.; Lakshminarasimhan, N. Coupled semiconductor nanocomposite g-C3N4/TiO2 with enhanced visible light photocatalytic activity. Mater. Res. Bull. 2016, 76, 370–375. [Google Scholar] [CrossRef]
- Liu, X.; Chen, N.; Li, Y.; Deng, D.; Xing, X.; Wang, Y. A general nonaqueous sol-gel route to g-C3N4-coupling photocatalysts: The case of Z-scheme g-C3N4/TiO2 with enhanced photodegradation toward RhB under visible-light. Sci. Rep. 2016, 6, 39531. [Google Scholar] [CrossRef]
- Imbar, A.; Vadivel, V.K.; Mamane, H. Solvothermal Synthesis of g-C3N4/TiO2 Hybrid Photocatalyst with a Broaden Activation Spectrum. Catalysts 2023, 13, 46. [Google Scholar] [CrossRef]
- Miranda, C.; Mansilla, H.; Yáñez, J.; Obregón, S.; Colón, G. Improved photocatalytic activity of g-C3N4/TiO2 composites prepared by a simple impregnation method. J. Photochem. Photobiol. A Chem. 2013, 253, 16–21. [Google Scholar] [CrossRef]
- Wang, X.J.; Yang, W.Y.; Li, F.T.; Xue, Y.B.; Liu, R.H.; Hao, Y.J. In situ microwave-assisted synthesis of porous N-TiO2/g-C3N4 heterojunctions with enhanced visible-light photocatalytic properties. Ind. Eng. Chem. Res. 2013, 52, 17140–17150. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, J.; Wei, L.; Wang, Q.; Wang, C.; Yang, C. A novel microwave-assisted impregnation method with water as the dispersion medium to synthesize modified g-C3N4/TiO2 heterojunction photocatalysts. Opt. Mater. 2020, 107, 110128. [Google Scholar] [CrossRef]
- Negrescu, A.M.; Killian, M.S.; Raghu, S.N.V.; Schmuki, P.; Mazare, A.; Cimpean, A. Metal Oxide Nanoparticles: Review of Synthesis, Characterization and Biological Effects. J. Funct. Biomater. 2022, 13, 274. [Google Scholar] [CrossRef]
- Raza, G. Titanium Dioxide Nanomaterials, Synthesis, Stability and Mobility in Natural and Synthetic Porous Media. Ph.D. Thesis, The University of Birmingham, Birmingham, UK, 2016. [Google Scholar]
- Pimentel, A.; Rodrigues, J.; Duarte, P.; Nunes, D.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Effect of solvents on ZnO nanostructures synthesized by solvothermal method assisted by microwave radiation: A photocatalytic study. J. Mater. Sci. 2015, 50, 5777–5787. [Google Scholar] [CrossRef]
- Tiwari, S.; Talreja, S. Green Chemistry and Microwave Irradiation Technique: A Review. J. Pharm. Res. Int. 2022, 34, 74–79. [Google Scholar] [CrossRef]
- Pimentel, A.; Samouco, A.; Nunes, D.; Araújo, A.; Martins, R.; Fortunato, E. Ultra-Fast Microwave Synthesis of ZnO Nanorods on Cellulose Substrates for UV Sensor Applications. Materials 2017, 10, 1308. [Google Scholar] [CrossRef]
- Zou, X.X.; Li, G.D.; Wang, Y.N.; Zhao, J.; Yan, C.; Guo, M.Y.; Li, L.; Chen, J.S. Direct conversion of urea into graphitic carbon nitride over mesoporous TiO2 spheres under mild condition. Chem. Commun. 2011, 47, 1066–1068. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Guan, R.; Li, J.; Zhang, J.; Zhao, Z.; Wang, D.; Zhai, H.; Sun, D. Photocatalytic Performance and Mechanistic Research of ZnO/g-C3N4 on Degradation of Methyl Orange. ACS Omega 2019, 4, 20742–20747. [Google Scholar] [CrossRef]
- Rovisco, A.; Morais, M.; Branquinho, R.; Fortunato, E.; Martins, R.; Barquinha, P. Microwave-Assisted Synthesis of Zn2SnO4 Nanostructures for Photodegradation of Rhodamine B under UV and Sunlight. Nanomaterials 2022, 12, 2119. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Z.; Jiang, X.; Sun, R.; Xie, M.; Han, W. Supporting Information: Direct Z-scheme Sn-In2O3/In2S3 heterojunction nanostructures for enhanced photocatalytic CO2 reduction activity. J. Mater. Chem. C 2021, 9, 3987–3997. [Google Scholar] [CrossRef]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T.S. Structural investigation of graphitic carbon nitride via XRD and neutron diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, F.; Wu, H.; Cao, X.; Sun, J.; Lei, W. In situ synthesis of g-C3N4/TiO2 heterostructures with enhanced photocatalytic hydrogen evolution under visible light. RSC Adv. 2017, 7, 40327–40333. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Xu, Y.; Li, P.; Guo, J. Enhanced photocatalytic degradation activity of Z-scheme heterojunction BiVO4/Cu/g-C3N4 under visible light irradiation. Water Environ. Res. 2021, 93, 2010–2024. [Google Scholar] [CrossRef]
- Mo, Z.; She, X.; Li, Y.; Liu, L.; Huang, L.; Chen, Z.; Zhang, Q.; Xu, H.; Li, H. Synthesis of g-C3N4 at different temperatures for superior visible/UV photocatalytic performance and photoelectrochemical sensing of MB solution. RSC Adv. 2015, 5, 101552–101562. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Hussain, M.I.; Zhou, W.; Chen, Y.; Wang, L.N. g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials 2022, 12, 121. [Google Scholar] [CrossRef]
- Rhimi, B.; Wang, C.; Bahnemann, D.W. Latest progress in g-C3N4 based heterojunctions for hydrogen production via photocatalytic water splitting: A mini review. J. Phys Energy 2020, 2, 042003. [Google Scholar] [CrossRef]
- Matias, M.L.; Morais, M.; Pimentel, A.; Vasconcelos, F.X.; Reis Machado, A.S.; Rodrigues, J.; Fortunato, E.; Martins, R.; Nunes, D. Floating TiO2-Cork Nano-Photocatalysts for Water Purification Using Sunlight. Sustainability 2022, 14, 9645. [Google Scholar] [CrossRef]
- Shakeelur Raheman, A.R.; Wilson, H.M.; Momin, B.M.; Annapure, U.S.; Jha, N. TiO2 nanosheet/ultra-thin layer g-C3N4 core-shell structure: Bifunctional visible-light photocatalyst for H2 evolution and removal of organic pollutants from water. Appl. Surf. Sci. 2020, 528, 146930. [Google Scholar] [CrossRef]
- DeRita, L.; Resasco, J.; Dai, S.; Boubnov, A.; Thang, H.V.; Hoffman, A.S.; Ro, I.; Graham, G.W.; Bare, S.R.; Pacchioni, G.; et al. Structural evolution of atomically dispersed Pt catalysts dictates reactivity. Nat. Mater. 2019, 18, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Sun, K.; Shen, J.; Liu, Q.; Tang, H.; Zhang, M.; Zulfiqar, S.; Lei, C. Synergistic effect of Co(II)-hole and Pt-electron cocatalysts for enhanced photocatalytic hydrogen evolution performance of P-doped g-C3N4. Chin. J. Catal. 2020, 41, 72–81. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Luo, Q.; Dong, F.; Li, H.; Ho, W.K. Mass-Controlled Direct Synthesis of Graphene-like Carbon Nitride Nanosheets with Exceptional High Visible Light Activity. Less is Better. Sci. Rep. 2015, 5, 14643. [Google Scholar] [CrossRef]
- Zhang, S.; Hang, N.T.; Zhang, Z.; Yue, H.; Yang, W. Preparation of g-C3N4/Graphene Composite for Detecting NO2 at Room Temperature. Nanomaterials 2017, 7, 12. [Google Scholar] [CrossRef]
- Li, K.; Gao, S.; Wang, Q.; Xu, H.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. In-situ-reduced synthesis of Ti3+ self-doped TiO2/g-C3N4 heterojunctions with high photocatalytic performance under LED light irradiation. ACS Appl. Mater. Interfaces 2015, 7, 9023–9030. [Google Scholar] [CrossRef]
- Alcudia-Ramos, M.A.; Fuentez-Torres, M.O.; Ortiz-Chi, F.; Espinosa-González, C.G.; Hernández-Como, N.; García-Zaleta, D.S.; Kesarla, M.K.; Torres-Torres, J.G.; Collins-Martínez, V.; Godavarthi, S. Fabrication of g-C3N4/TiO2 heterojunction composite for enhanced photocatalytic hydrogen production. Ceram. Int. 2020, 46, 38–45. [Google Scholar] [CrossRef]
- Cao, J.; Qin, C.; Wang, Y.; Zhang, H.; Sun, G.; Zhang, Z. Solid-state method synthesis of SnO2-decorated g-C3N4 nanocomposites with enhanced gas-sensing property to ethanol. Materials 2017, 10, 604. [Google Scholar] [CrossRef]
- Gong, S.; Jiang, Z.; Zhu, S.; Fan, J.; Xu, Q.; Min, Y. The synthesis of graphene-TiO2/g-C3N4 super-thin heterojunctions with enhanced visible-light photocatalytic activities. J. Nanoparticle Res. 2018, 20, 310. [Google Scholar] [CrossRef]
- Boonprakob, N.; Wetchakun, N.; Phanichphant, S.; Waxler, D.; Sherrell, P.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Enhanced visible-light photocatalytic activity of g-C3N4/TiO2 films. J. Colloid Interface Sci. 2014, 417, 402–409. [Google Scholar] [CrossRef]
- Shen, L.; Xing, Z.; Zou, J.; Li, Z.; Wu, X.; Zhang, Y.; Zhu, Q.; Yang, S.; Zhou, W. Black TiO2 nanobelts/g-C3N4 nanosheets Laminated Heterojunctions with Efficient Visible-Light-Driven Photocatalytic Performance. Sci. Rep. 2017, 7, 41978. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Davies, P.R.; Pattisson, S.; Davies, T.E.; Morgan, D.J.; Dlamini, M.W. Facile synthesis of a porous 3D g-C3N4 photocatalyst for the degradation of organics in shale gas brines. Catal. Commun. 2022, 169, 106480. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Joshi, C.; Manchanda, M.; Boukherroub, R.; Jain, S.L. Nickel decorated on phosphorous-doped carbon nitride as an efficient photocatalyst for reduction of nitrobenzenes. Nanomaterials 2016, 6, 59. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Wu, B.; Ning, Z.; Song, M.; Zhang, H.; Sun, X.; Wan, D.; Li, B. Porous g-C3N4 with defects for the efficient dye photodegradation under visible light. Water Sci. Technol. 2021, 84, 1354–1365. [Google Scholar] [CrossRef]
- Landi, S.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.; Trapalis, C. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2 composite photocatalysts for NOx removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Abdel-Moniem, S.M.; El-Liethy, M.A.; Ibrahim, H.S.; Ali, M.E.M. Innovative green/non-toxic Bi2S3@g-C3N4 nanosheets for dark antimicrobial activity and photocatalytic depollution: Turnover assessment. Ecotoxicol. Environ. Saf. 2021, 226, 112808. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.-K. In Situ Construction of g-C3N4/g-C3N4 Metal-Free Heterojunction for Enhanced Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.P.; Behera, A.; Martha, S.; Acharya, R.; Parida, K. Facile synthesis of exfoliated graphitic carbon nitride for photocatalytic degradation of ciprofloxacin under solar irradiation. J. Mater. Sci. 2019, 54, 5726–5742. [Google Scholar] [CrossRef]
- Jia, T.; Li, J.; Long, F.; Fu, F.; Zhao, J.; Deng, Z.; Wang, X.; Zhang, Y. Ultrathin g-C3N4 nanosheet-modified biocl hierarchical flower-like plate heterostructure with enhanced photostability and photocatalytic performance. Crystals 2017, 7, 266. [Google Scholar] [CrossRef]
- Das, D.; Shinde, S.L.; Nanda, K.K. Temperature-Dependent Photoluminescence of g-C3N4: Implication for Temperature Sensing. ACS Appl. Mater. Interfaces 2016, 8, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, L.; Xing, J.; Utama, M.I.B.; Lu, X.; Du, K.; Li, Y.; Hu, X.; Wang, S.; Genç, A.; et al. High-yield synthesis and optical properties of g-C3N4. Nanoscale 2015, 7, 12343–12350. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Liao, J.; Dong, F.; Li, H.; Liu, H. Growth of g-C3N4 layer on commercial TiO2 for enhanced visible light photocatalytic activity. J. Nanomater. 2014, 2014, 86909. [Google Scholar] [CrossRef]
- Lin, T.H.; Chang, Y.H.; Chiang, K.P.; Wang, J.C.; Wu, M.C. Nanoscale Multidimensional Pd/TiO2/g-C3N4 Catalyst for Efficient Solar-Driven Photocatalytic Hydrogen Production. Catalysts 2021, 11, 59. [Google Scholar] [CrossRef]
- Farahani, N.; Kelly, P.J.; West, G.; Ratova, M.; Hill, C.; Vishnyakov, V. An investigation into W or Nb or ZnFe2O4 Doped Titania nanocomposites deposited from Blended powder targets for UV/Visible photocatalysis. Coatings 2013, 3, 153–165. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, G.; Chen, T.; Fei, X.; Hu, S.; Shan, Q.; Hei, D.; Feng, H.; Jia, W. Irradiation-catalysed degradation of methyl orange using BaF2–TiO2 nanocomposite catalysts prepared by a sol–gel method. R. Soc. Open Sci. 2019, 6, 191156. [Google Scholar] [CrossRef]
- Hassan, F.; Bonnet, P.; Dangwang Dikdim, J.M.; Gatcha Bandjoun, N.; Caperaa, C.; Dalhatou, S.; Kane, A.; Zeghioud, H. Synthesis and Investigation of TiO2/g-C3N4 Performance for Photocatalytic Degradation of Bromophenol Blue and Eriochrome Black T: Experimental Design Optimization and Reactive Oxygen Species Contribution. Water 2022, 14, 3331. [Google Scholar] [CrossRef]
- Zhang, T.; Souza, I.P.A.F.; Xu, J.; Almeida, V.C.; Asefa, T. Mesoporous Graphitic Carbon Nitrides Decorated with Cu Nanoparticles: Efficient Photocatalysts for Degradation of Tartrazine Yellow Dye. Nanomaterials 2018, 8, 636. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Chen, J.; Wu, X.; Zhou, L. TiO2 mesocrystals built of nanocrystals with exposed {001} facets: Facile synthesis and superior photocatalytic ability. J. Mater. Chem. A 2014, 2, 19589–19593. [Google Scholar] [CrossRef]
- Pan, F.; Wu, K.; Li, H.; Xu, G.; Chen, W. Synthesis of {100} Facet Dominant Anatase TiO2 Nanobelts and the Origin of Facet-Dependent Photoreactivity. Chem. —A Eur. J. 2014, 20, 15095–15101. [Google Scholar] [CrossRef]
- Xu, H.; Ouyang, S.; Li, P.; Kako, T.; Ye, J. High-active anatase TiO2 nanosheets exposed with 95% {100} facets toward efficient H2 evolution and CO2 photoreduction. ACS Appl. Mater. Interfaces 2013, 5, 1348–1354. [Google Scholar] [CrossRef]
- Zerjav, G.; Zizek, K.; Zavasnik, J.; Pintar, A. Brookite vs. rutile vs. anatase: What’s behind their various photocatalytic activities? J. Environ. Chem. Eng. 2022, 10, 107722. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Lin, P.; Hu, H.; Lv, H.; Ding, Z.; Xu, L.; Qian, D.; Wang, P.; Pan, J.; Li, C.; Cui, C. Hybrid reduced graphene oxide/TiO2/graphitic carbon nitride composites with improved photocatalytic activity for organic pollutant degradation. Appl. Phys. A Mater. Sci. Process. 2018, 124, 510. [Google Scholar] [CrossRef]
- Kobkeatthawin, T.; Trakulmututa, J.; Amornsakchai, T.; Kajitvichyanukul, P.; Smith, S.M. Identification of Active Species in Photodegradation of Aqueous Imidacloprid over g-C3N4/TiO2 Nanocomposites. Catalysts 2022, 12, 120. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Lin, Y.; Wang, P.; Chen, W.; Fu, X.; Shao, Y. Evidence for the active species involved in the photodegradation process of methyl Orange on TiO2. J. Phys. Chem. C 2012, 116, 3552–3560. [Google Scholar] [CrossRef]
- Mahalakshmi, G.; Rajeswari, M.; Ponnarasi, P. Synthesis of few-layer g-C3N4 nanosheets-coated MoS2/TiO2 heterojunction photocatalysts for photo-degradation of methyl orange (MO) and 4-nitrophenol (4-NP) pollutants. Inorg. Chem. Commun. 2020, 120, 108146. [Google Scholar] [CrossRef]
- Jiang, D.; Li, J.; Xing, C.; Zhang, Z.; Meng, S.; Chen, M. Two-Dimensional CaIn2S4/g-C3N4 Heterojunction Nanocomposite with Enhanced Visible-Light Photocatalytic Activities: Interfacial Engineering and Mechanism Insight. ACS Appl. Mater. Interfaces 2015, 7, 19234–19242. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef]
- Hankin, A.; Bedoya-Lora, F.E.; Alexander, J.C.; Regoutz, A.; Kelsall, G.H. Flat band potential determination: Avoiding the pitfalls. J. Mater. Chem. A 2019, 7, 26162–26176. [Google Scholar] [CrossRef]
- Drisya, K.T.; Solís-López, M.; Ríos-Ramírez, J.J.; Durán-Álvarez, J.C.; Rousseau, A.; Velumani, S.; Asomoza, R.; Kassiba, A.; Jantrania, A.; Castaneda, H. Electronic and optical competence of TiO2/BiVO4 nanocomposites in the photocatalytic processes. Sci. Rep. 2020, 10, 13507. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Chen, Z.; Feng, C.; Sun, M.; Hou, J. Transforming g-C3N4 from amphoteric to n-type semiconductor: The important role of p/n type on photoelectrochemical cathodic protection. J. Alloy. Compd. 2021, 851, 156820. [Google Scholar] [CrossRef]
- Nunes, D.; Fragoso, A.R.; Freire, T.; Matias, M.; Marques, A.C.; Martins, R.; Fortunato, E.; Pimentel, A. Ultrafast Microwave Synthesis of WO3 Nanostructured Films for Solar Photocatalysis. Phys. Status Solidi (RRL)—Rapid Res. Lett. 2021, 15, 2100196. [Google Scholar] [CrossRef]
- Naeem, R.; Ehsan, M.A.; Rehman, A.; Yamani, Z.H.; Hakeem, A.S.; Mazhar, M. Single step aerosol assisted chemical vapor deposition of p-n Sn(II) oxide-Ti(IV) oxide nanocomposite thin film electrodes for investigation of photoelectrochemical properties. New J. Chem. 2018, 42, 5256–5266. [Google Scholar] [CrossRef]
- Ismael, M.; Wu, Y. A facile synthesis method for fabrication of LaFeO3/g-C3N4 nanocomposite as efficient visible-light-driven photocatalyst for photodegradation of RhB and 4-CP. New J. Chem. 2019, 43, 13783–13793. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, J.; Jiang, M.; Yu, J.; Wang, Q.; Zhao, J.; Li, J.; Yu, X.; Zhao, J. Photo-induced synthesis of nanostructured Pt-on-Au/g-C3N4 composites for visible light photocatalytic hydrogen production. J. Mater. Sci. 2020, 55, 15574–15587. [Google Scholar] [CrossRef]
- Beranek, R. (Photo)electrochemical methods for the determination of the band edge positions of TiO2-based nanomaterials. Adv. Phys. Chem. 2011, 2011, 786759. [Google Scholar] [CrossRef]
- Jo, W.K.; Natarajan, T.S. Influence of TiO2 morphology on the photocatalytic efficiency of direct Z-scheme g-C3N4/TiO2 photocatalysts for isoniazid degradation. Chem. Eng. J. 2015, 281, 549–565. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, J.; Zhai, X.; Guan, F.; Wang, X.; Zhang, J.; Hou, B. Photocatalytic degradation and antibacterial properties of Fe3+-doped alkalized carbon nitride. Nanomaterials 2020, 10, 1751. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef]
- Liu, M.; Wei, S.; Chen, W.; Gao, L.; Li, X.; Mao, L.; Dang, H. Construction of direct Z-scheme g-C3N4/TiO2 nanorod composites for promoting photocatalytic activity. J. Chin. Chem. Soc. 2019, 67, 246–252. [Google Scholar] [CrossRef]
- Sewnet, A.; Abebe, M.; Asaithambi, P.; Alemayehu, E. Visible-Light-Driven g-C3N4/TiO2 Based Heterojunction Nanocomposites for Photocatalytic Degradation of Organic Dyes in Wastewater: A Review. Air Soil Water Res. 2022, 15, 1–23. [Google Scholar] [CrossRef]
- Naveed, A.B.; Javaid, A.; Zia, A.; Ishaq, M.T.; Amin, M.; Farooqi, Z.U.R.; Mahmood, A. TiO2/g-C3N4 Binary Composite as an Efficient Photocatalyst for Biodiesel Production from Jatropha Oil and Dye Degradation. ACS Omega 2022, 8, 2173–2182. [Google Scholar] [CrossRef]



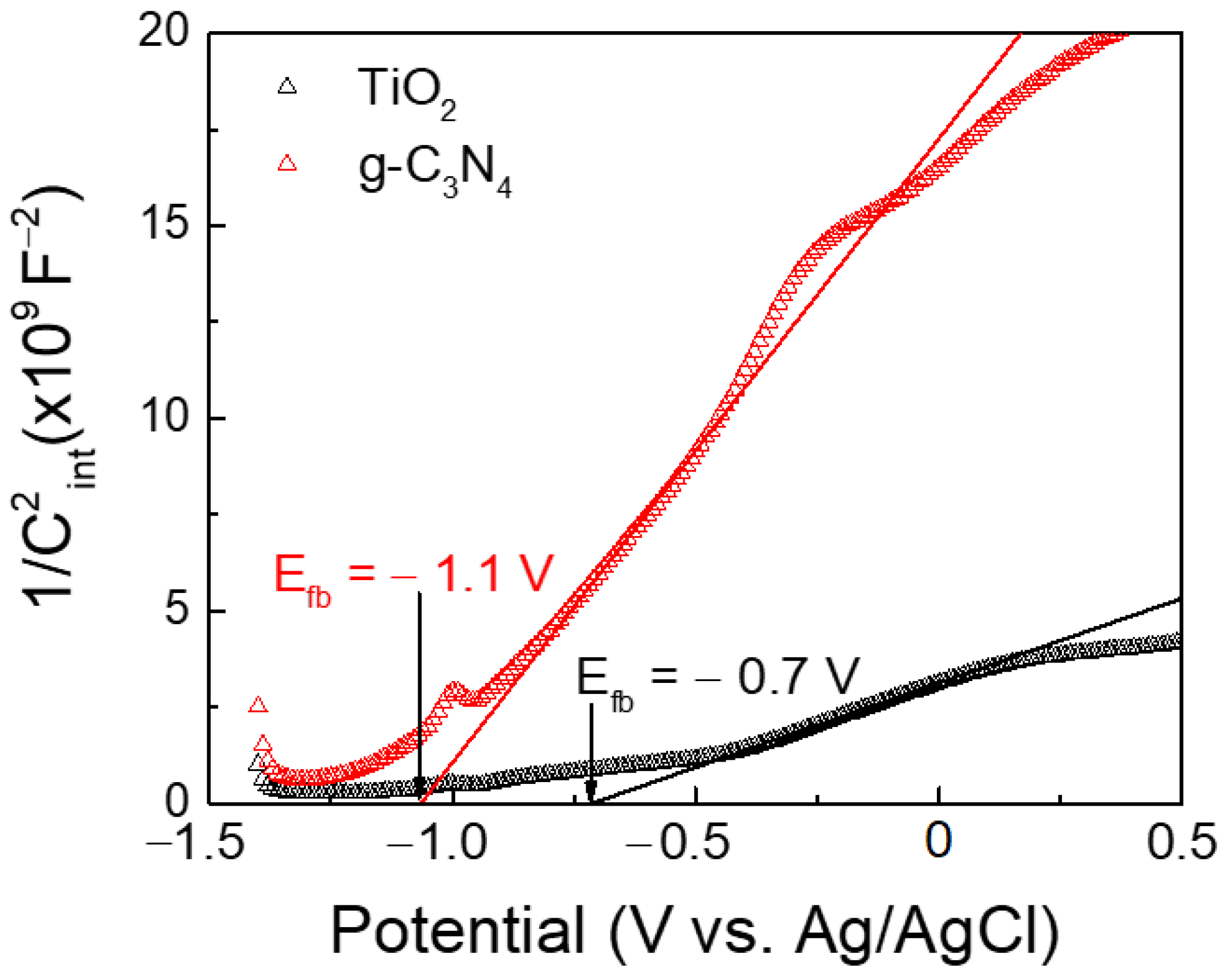
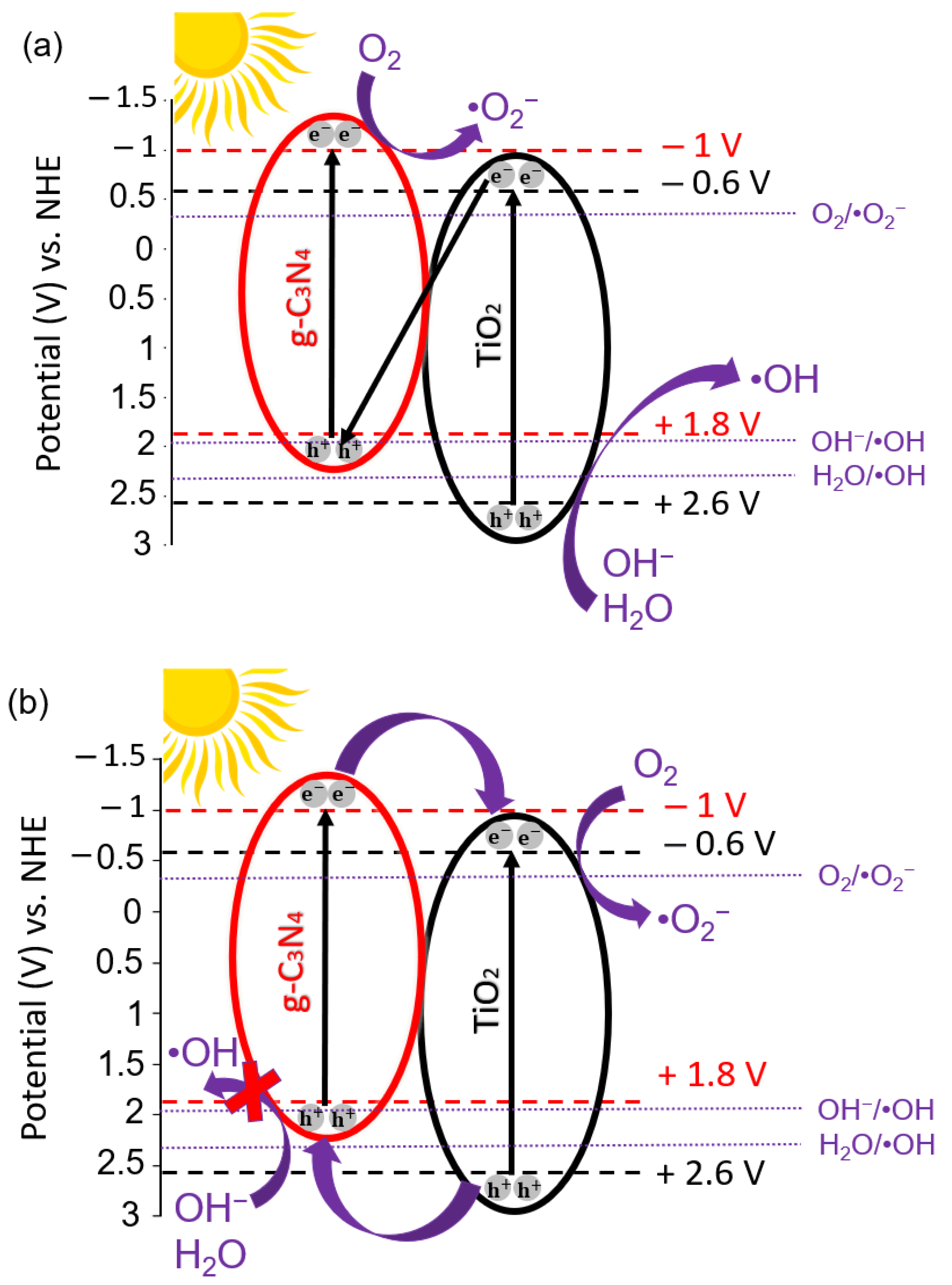
| Material | Preparation Method | Light Source | MO Concentration | Optimum Loading | Degradation Efficiency (%) | Kinetic Constant (min−1) | Reference |
|---|---|---|---|---|---|---|---|
| g-C3N4/seed grown mesoporous TiO2 | Seed induced solvothermal (MW: 105 °C/48 h) | Visible light | 10 mg/L | Ti:g-C3N4 (1 molar ratio) | Around 100% MO degradation in 60 min (pH = 3) | 0.1014 | [61] |
| g-C3N4/TiO2 (brookite) | Calcination (400 °C/1 h) | Visible light | 10 mg/L | g-C3N4:TiO2 (35% weight ratio) | 55% MO degradation in 180 min | No data | [62] |
| g-C3N4/TiO2 nanotube array | Anodic oxidation method/ultrasonic loading | Xe lamp irradiation (intensity 100 mW/cm2) | 15 mg/L | No data | 84.6% MO degradation in 120 min | No data | [63] |
| g-C3N4 nanosheets/mesoporous TiO2 | Hydrothermal synthesis (MW: 180 °C/6 h) | 300 W Xe lamp irradiation with a cut-off filter (λ > 420 nm) | 32.7 mg/L (100 μM) | g-C3N4:TiO2 (2:1 weight ratio) | Around 60% MO degradation in 300 min (pH = 3) | No data | [64] |
| g-C3N4 nanosheets/TiO2 nanoflakes | in situ sol-gel (400 °C/3 h) | UV-VIS light | 20 mg/L | g-C3N4:TiO2 (1:4 weight ratio) | 97% MO degradation in 80 min | 0.0718 | [21] |
| Nanostructures | R2 | |
|---|---|---|
| TiO2 | 0.0036 | 0.99 |
| g-C3N4 | 0.0007 | 0.95 |
| 15-GCN-T | 0.0053 | 0.97 |
| 30-GCN-T | 0.0071 | 0.99 |
| 45-GCN-T | 0.0062 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matias, M.L.; Reis-Machado, A.S.; Rodrigues, J.; Calmeiro, T.; Deuermeier, J.; Pimentel, A.; Fortunato, E.; Martins, R.; Nunes, D. Microwave Synthesis of Visible-Light-Activated g-C3N4/TiO2 Photocatalysts. Nanomaterials 2023, 13, 1090. https://doi.org/10.3390/nano13061090
Matias ML, Reis-Machado AS, Rodrigues J, Calmeiro T, Deuermeier J, Pimentel A, Fortunato E, Martins R, Nunes D. Microwave Synthesis of Visible-Light-Activated g-C3N4/TiO2 Photocatalysts. Nanomaterials. 2023; 13(6):1090. https://doi.org/10.3390/nano13061090
Chicago/Turabian StyleMatias, Maria Leonor, Ana S. Reis-Machado, Joana Rodrigues, Tomás Calmeiro, Jonas Deuermeier, Ana Pimentel, Elvira Fortunato, Rodrigo Martins, and Daniela Nunes. 2023. "Microwave Synthesis of Visible-Light-Activated g-C3N4/TiO2 Photocatalysts" Nanomaterials 13, no. 6: 1090. https://doi.org/10.3390/nano13061090
APA StyleMatias, M. L., Reis-Machado, A. S., Rodrigues, J., Calmeiro, T., Deuermeier, J., Pimentel, A., Fortunato, E., Martins, R., & Nunes, D. (2023). Microwave Synthesis of Visible-Light-Activated g-C3N4/TiO2 Photocatalysts. Nanomaterials, 13(6), 1090. https://doi.org/10.3390/nano13061090













