Chemical Vapor Transport Synthesis of Fibrous Red Phosphorus Crystal as Anodes for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of FP
2.2. Preparation of FP-C and RP-C
2.3. Measurement of Material Characteristics
2.4. Electrochemical Characterization of Materials
3. Results and Discussion
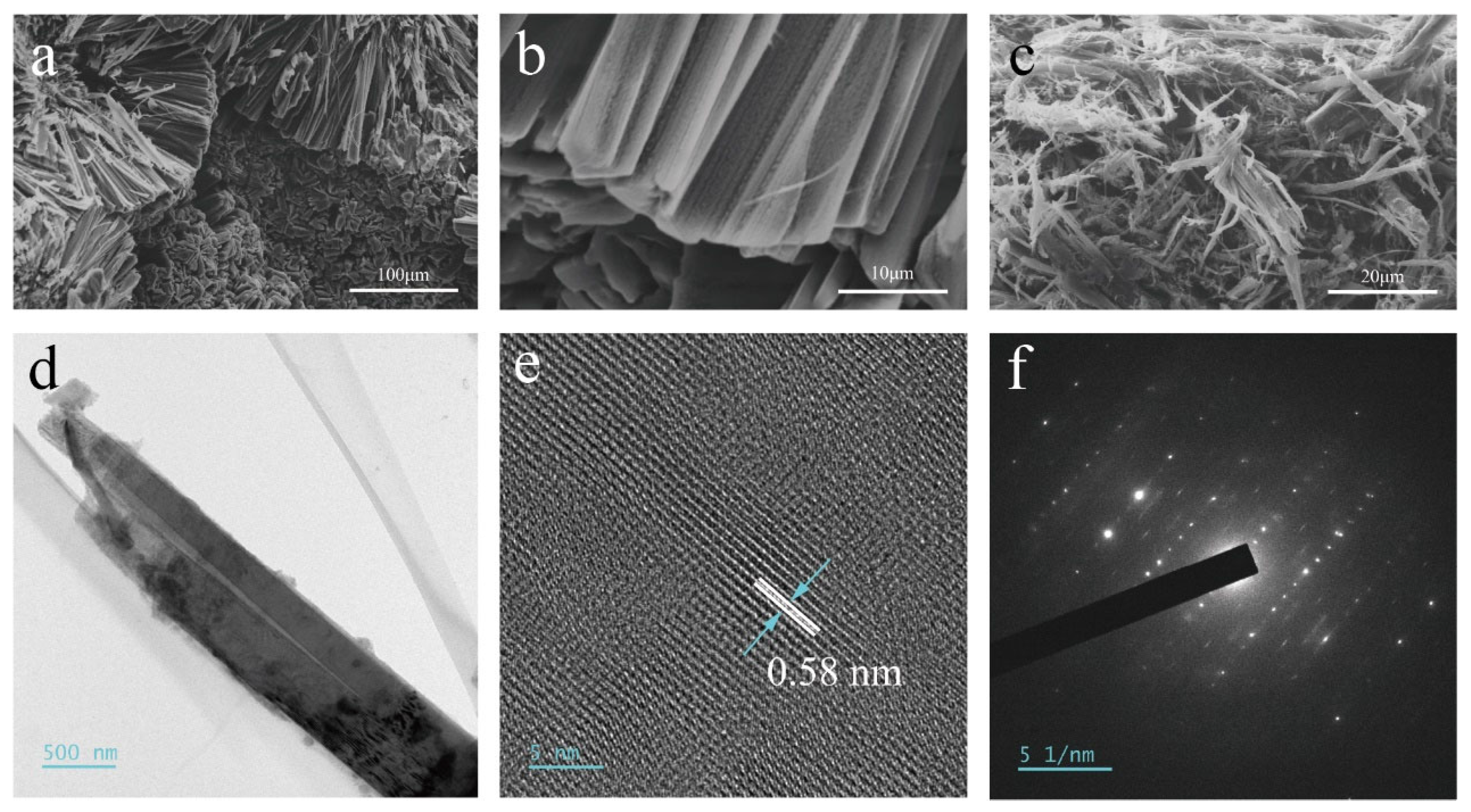
3.1. Characterization of FP
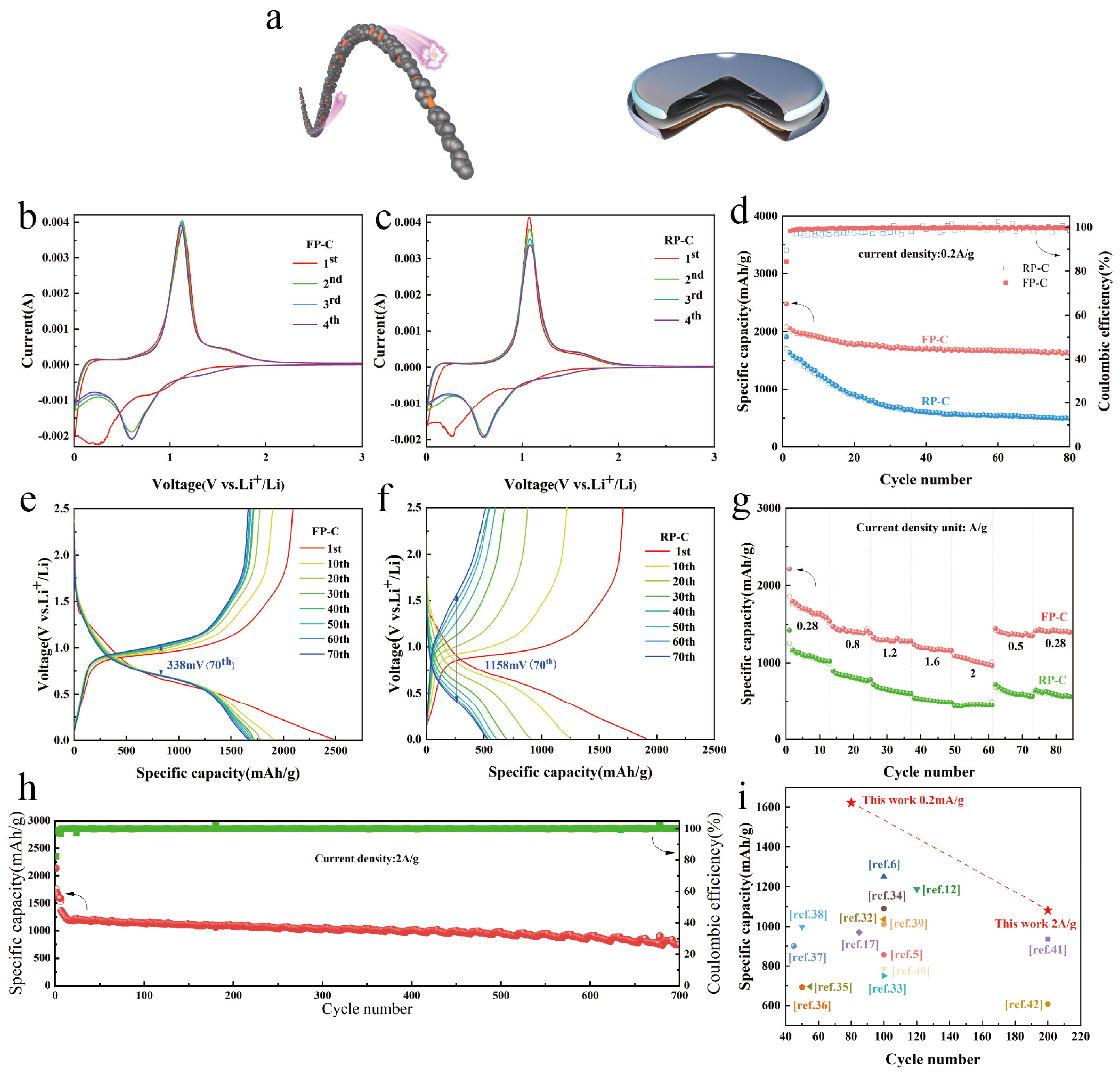
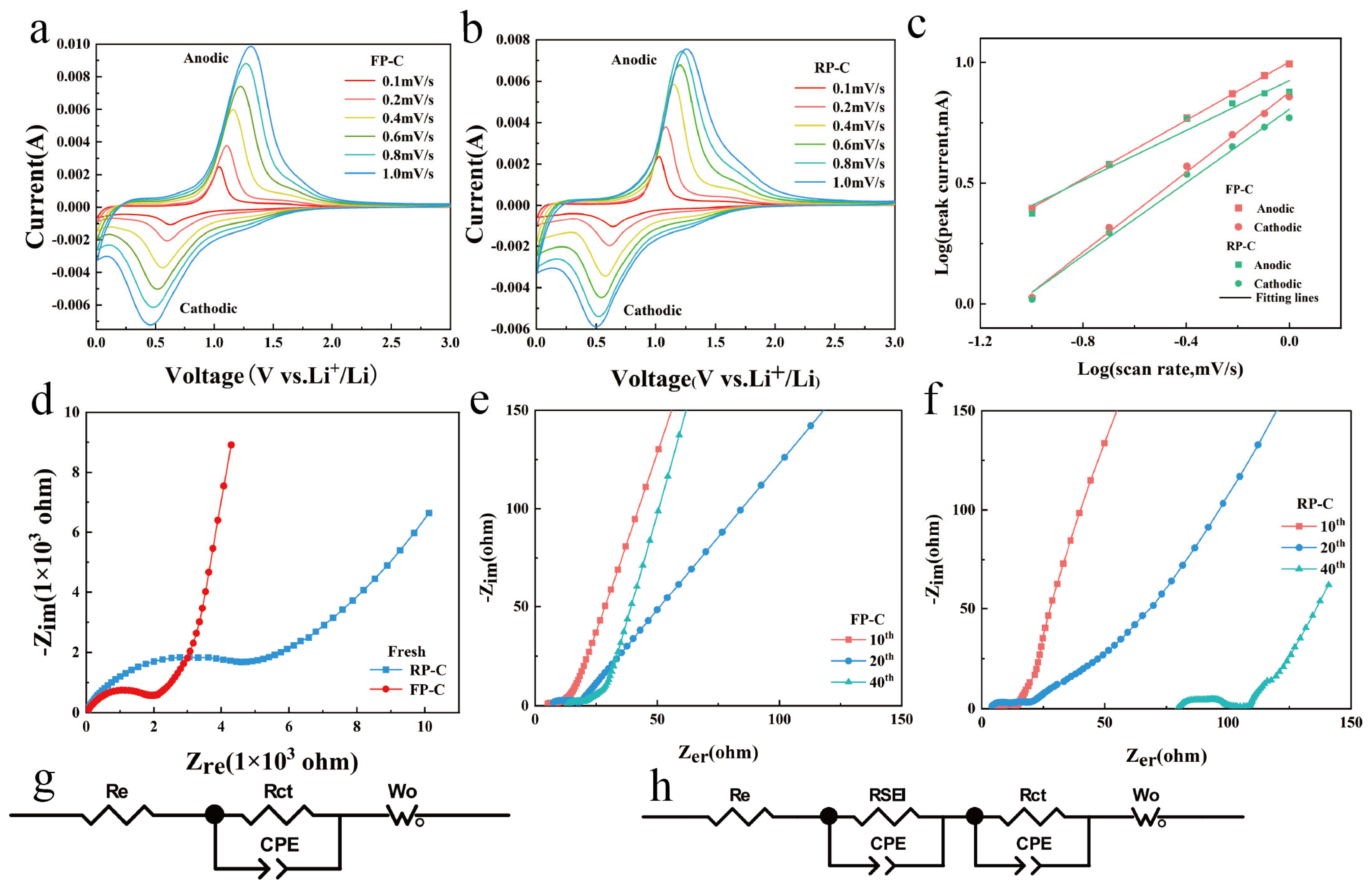
3.2. Electrochemical Properties of FP-C and RP-C
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marom, R.; Amalraj, S.F.; Leifer, N.; Jacob, D.; Aurbach, D. A review of advanced and practical lithium battery materials. J. Mater. Chem. 2011, 21, 9938–9954. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Yu, H.-C.; Ling, C.; Bhattacharya, J.; Thomas, J.C.; Thornton, K.; Van der Ven, A. Designing the next generation high capacity battery electrodes. Energy Environ. Sci. 2014, 7, 1760–1768. [Google Scholar] [CrossRef]
- Ramireddy, T.; Xing, T.; Rahman, M.M.; Chen, Y.; Dutercq, Q.; Gunzelmann, D.; Glushenkov, A.M. Phosphorus-carbon nanocomposite anodes for lithium-ion and sodium-ion batteries. J. Mater. Chem. A 2015, 3, 5572–5584. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, G.; Lee, H.-W.; Liu, N.; Wang, H.; Yao, H.; Yang, W.; Cui, Y. Formation of Stable Phosphorus-Carbon Bond for Enhanced Performance in Black Phosphorus Nanoparticle-Graphite Composite Battery Anodes. Nano Lett. 2014, 14, 4573–4580. [Google Scholar] [CrossRef]
- Jin, H.; Xin, S.; Chuang, C.; Li, W.; Wang, H.; Zhu, J.; Xie, H.; Zhang, T.; Wan, Y.; Qi, Z.; et al. Black phosphorus composites with engineered interfaces for high-rate high-capacity lithium storage. Science 2020, 370, 192–197. [Google Scholar] [CrossRef]
- Sun, J.; Lee, H.-W.; Pasta, M.; Yuan, H.; Zheng, G.; Sun, Y.; Li, Y.; Cui, Y. A phosphorene-graphene hybrid material as a high-capacity anode for sodium-ion batteries. Nat. Nanotechnol. 2015, 10, 980–985. [Google Scholar] [CrossRef]
- Zhu, Y.; Wen, Y.; Fan, X.; Gao, T.; Han, F.; Luo, C.; Liou, S.-C.; Wang, C. Red Phosphorus–Single-Walled Carbon Nanotube Composite as a Superior Anode for Sodium Ion Batteries. ACS Nano 2015, 9, 3254–3264. [Google Scholar] [CrossRef]
- Yu, Z.; Song, J.; Gordin, M.L.; Yi, R.; Tang, D.; Wang, D. Phosphorus-Graphene Nanosheet Hybrids as Lithium-Ion Anode with Exceptional High-Temperature Cycling Stability. Adv. Sci. 2015, 2, 1400020. [Google Scholar] [CrossRef]
- Park, C.-M.; Sohn, H.-J. Black Phosphorus and its Composite for Lithium Rechargeable Batteries. Adv. Mater. 2007, 19, 2465–2468. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Cai, W.; Zhu, Y.; Liang, J.; Zhang, K.; Lan, Y.; Jiang, Z.; Wang, G.; Qian, Y. Wet-Chemical Synthesis of Hollow Red-Phosphorus Nanospheres with Porous Shells as Anodes for High-Performance Lithium-Ion and Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1700214. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Li, J.; Sun, W.; Gao, J.; Guo, J.; Jiang, C. Nano-Structured Phosphorus Composite as High-Capacity Anode Materials for Lithium Batteries. Angew. Chem. 2012, 124, 9168–9171. [Google Scholar] [CrossRef]
- Hao, G.; Jiao, R.; Deng, Z.; Liu, Y.; Lan, D.; He, W.; Lang, Z.; Cui, J. Red phosphorus infiltrated into porous C/SiOx derived from rice husks to improve its initial Coulomb efficiency in lithium-ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2023, 665, 131180. [Google Scholar] [CrossRef]
- Vorfolomeeva, A.A.; Stolyarova, S.G.; Asanov, I.P.; Shlyakhova, E.V.; Plyusnin, P.E.; Maksimovskiy, E.A.; Gerasimov, E.Y.; Chuvilin, A.L.; Okotrub, A.V.; Bulusheva, L.G. Single-Walled Carbon Nanotubes with Red Phosphorus in Lithium-Ion Batteries: Effect of Surface and Encapsulated Phosphorus. Nanomaterials 2022, 13, 153. [Google Scholar] [CrossRef]
- Zhou, J.; Shi, Q.; Ullah, S.; Yang, X.; Bachmatiuk, A.; Yang, R.; Rummeli, M.H. Phosphorus-Based Composites as Anode Materials for Advanced Alkali Metal Ion Batteries. Adv. Funct. Mater. 2020, 30, 2004648. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Z.; Niu, S.; Zhu, S.; Zhou, J.; Zhu, Y.; Liang, J.; Han, D.; Xu, K.; Zhu, L. Self-standing hierarchical P/CNTs@ rGO with unprecedented capacity and stability for lithium and sodium storage. Chem 2018, 4, 372–385. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, C.; Wang, H.; Wang, H.; Sun, J.; Zhang, Y.; Han, X.; Cao, Y.; Liu, S.; Sun, J. A Covalent P–C Bond Stabilizes Red Phosphorus in an Engineered Carbon Host for High-Performance Lithium-Ion Battery Anodes. ACS Nano 2021, 15, 3365–3375. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.; Wang, Q.; Liu, W.; Cui, Y.; Tao, X.; Zhang, D. Fibrous phosphorus: A promising candidate as anode for lithium-ion batteries. Electrochim. Acta 2019, 295, 230–236. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Wang, J.; Zhang, Y.; Bian, S.; Cheng, Z.; Kang, N.; Huang, H.; Gu, S.; Wang, Y.; et al. Crystalline Red Phosphorus Nanoribbons: Large-Scale Synthesis and Electrochemical Nitrogen Fixation. Angew. Chem. 2020, 132, 14489–14493. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2007, 3, 31–35. [Google Scholar] [CrossRef]
- Wang, D.; Luo, F.; Lu, M.; Xie, X.; Huang, L.; Huang, W. Chemical Vapor Transport Reactions for Synthesizing Layered Materials and Their 2D Counterparts. Small 2019, 15, e1804404. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, B.; Zhao, Y.; Khurram, M.; Yan, Q. Synthesis, Exfoliation, and Transport Properties of Quasi-1D van der Waals Fibrous Red Phosphorus. Chem. Mater. 2021, 33, 6240–6248. [Google Scholar] [CrossRef]
- Du, L.; Zhao, Y.; Wu, L.; Hu, X.; Yao, L.; Wang, Y.; Bai, X.; Dai, Y.; Qiao, J.; Uddin, G.; et al. Giant anisotropic photonics in the 1D van der Waals semiconductor fibrous red phosphorus. Nat. Commun. 2021, 12, 4822. [Google Scholar] [CrossRef] [PubMed]
- Winchester, R.A.L.; Whitby, M.; Shaffer, M.S.P. Synthesis of Pure Phosphorus Nanostructures. Angew. Chem. 2009, 121, 3670–3675. [Google Scholar] [CrossRef]
- Fasol, G.; Cardona, M.; Hönle, W.; Von Schnering, H. Lattice dynamics of Hittorf’s phosphorus and identification of structural groups and defects in amorphous red phosphorus. Solid State Commun. 1984, 52, 307–310. [Google Scholar] [CrossRef]
- Wood, J.D.; Wells, S.A.; Jariwala, D.; Chen, K.-S.; Cho, E.; Sangwan, V.K.; Liu, X.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Effective Passivation of Exfoliated Black Phosphorus Transistors against Ambient Degradation. Nano Lett. 2014, 14, 6964–6970. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Z.; Zhang, B.; Yan, Q. Unveiling the degradation chemistry of fibrous red phosphorus under ambient conditions. ACS Appl. Mater. Interfaces 2022, 14, 9925–9932. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Du, J.; Liu, L.; Mao, C.; Sun, J.; Chen, A. Strong interaction between phosphorus and wrinkle carbon sphere promote the performance of phosphorus anode material for lithium-ion batteries. Nano Res. 2023. [Google Scholar] [CrossRef]
- Marino, C.; Boulet, L.; Gaveau, P.; Fraisse, B.; Monconduit, L. Nanoconfined phosphorus in mesoporous carbon as an electrode for Li-ion batteries: Performance and mechanism. J. Mater. Chem. 2012, 22, 22713–22720. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Ahn, H.-J.; Kim, J.-S.; Kim, I.-P.; Kweon, J.-H.; Nam, T.-H.; Kim, K.-W.; Ahn, J.-H.; Hong, S.-H. The Electrochemical Properties of Nano-Sized Cobalt Powder as an Anode Material for Lithium Batteries. Electron. Mater. Lett. 2009, 5, 183–186. [Google Scholar] [CrossRef]
- Hembram, K.P.S.S.; Jung, H.; Yeo, B.C.; Pai, S.J.; Lee, H.J.; Lee, K.-R.; Han, S.S. A comparative first-principles study of the lithiation, sodiation, and magnesiation of black phosphorus for Li-, Na-, and Mg-ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 21391–21397. [Google Scholar] [CrossRef]
- Li, W.; Yang, Z.; Li, M.; Jiang, Y.; Wei, X.; Zhong, X.; Gu, L.; Yu, Y. Amorphous Red Phosphorus Embedded in Highly Ordered Mesoporous Carbon with Superior Lithium and Sodium Storage Capacity. Nano Lett. 2016, 16, 1546–1553. [Google Scholar] [CrossRef]
- Kong, W.; Wen, Z.; Zhou, Z.; Wang, G.; Yin, J.; Cui, L.; Sun, W. A self-healing high-performance phosphorus composite anode enabled by in situ preformed intermediate lithium sulfides. J. Mater. Chem. A 2019, 7, 27048–27056. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Hu, Y.; Yakovenko, A.A.; Ren, Y.; Luo, J.; Holden, W.M.; Shakouri, M.; Xiao, Q.; Gao, X. New insights into the high-performance black phosphorus anode for lithium-ion batteries. Adv. Mater. 2021, 33, 2101259. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Xu, H.; Cao, J.; Chen, D.; Han, W. 3D Chemical Cross-Linking Structure of Black Phosphorus@CNTs Hybrid as a Promising Anode Material for Lithium Ion Batteries. Adv. Funct. Mater. 2020, 30, 1909372. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Q.; Li, L.; Jin, Z.; Wang, C.; Zhang, L.; Su, Z.-M. Encapsulating Red Phosphorus in Ultralarge Pore Volume Hierarchical Porous Carbon Nanospheres for Lithium/Sodium-Ion Half/Full Batteries. ACS Nano 2019, 13, 13513–13523. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Wang, W.; Teng, K.; Xu, Z.; Chen, C.; Li, C.; Yang, C.; Hu, C. Preparation of sandwich-like phosphorus/reduced graphene oxide composites as anode materials for lithium-ion batteries. Electrochim. Acta 2016, 211, 499–506. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Z.; Han, M.; Cui, Y.; Sun, J. Fabrication of red phosphorus anode for fast-charging lithium-ion batteries based on TiN/TiP2-enhanced interfacial kinetics. Energy Storage Mater. 2019, 26, 147–156. [Google Scholar] [CrossRef]
- Yuan, D.; Cheng, J.; Qu, G.; Li, X.; Ni, W.; Wang, B.; Liu, H. Amorphous red phosphorous embedded in carbon nano-tubes scaffold as promising anode materials for lithium-ion batteries. J. Power Sources 2016, 301, 131–137. [Google Scholar] [CrossRef]
- Li, J.; Jin, H.; Yuan, Y.; Lu, H.; Su, C.; Fan, D.; Li, Y.; Wang, J.; Lu, J.; Wang, S. Encapsulating phosphorus inside carbon nanotubes via a solution approach for advanced lithium ion host. Nano Energy 2019, 58, 23–29. [Google Scholar] [CrossRef]
- Liu, H.; Zou, Y.; Tao, L.; Ma, Z.; Liu, D.; Zhou, P.; Liu, H.; Wang, S. Sandwiched Thin-Film Anode of Chemically Bonded Black Phosphorus/Graphene Hybrid for Lithium-Ion Battery. Small 2017, 13, 1700758. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhao, H.; Li, J.; Jin, H.; Liu, L.; Wu, W.; Wang, J.; Lei, Y.; Wang, S. Mild-Temperature Solution-Assisted Encapsulation of Phosphorus into ZIF-8 Derived Porous Carbon as Lithium-Ion Battery Anode. Small 2020, 16, 1907141. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Gao, X.; Cui, X.; Wang, B.; Hu, F.; Yuan, T.; Li, J.; Zu, L.; Lian, H.; Cui, X. Chemical Vapor Transport Synthesis of Fibrous Red Phosphorus Crystal as Anodes for Lithium-Ion Batteries. Nanomaterials 2023, 13, 1060. https://doi.org/10.3390/nano13061060
Liu L, Gao X, Cui X, Wang B, Hu F, Yuan T, Li J, Zu L, Lian H, Cui X. Chemical Vapor Transport Synthesis of Fibrous Red Phosphorus Crystal as Anodes for Lithium-Ion Batteries. Nanomaterials. 2023; 13(6):1060. https://doi.org/10.3390/nano13061060
Chicago/Turabian StyleLiu, Lei, Xing Gao, Xuemei Cui, Bofeng Wang, Fangzheng Hu, Tianheng Yuan, Jianhua Li, Lei Zu, Huiqin Lian, and Xiuguo Cui. 2023. "Chemical Vapor Transport Synthesis of Fibrous Red Phosphorus Crystal as Anodes for Lithium-Ion Batteries" Nanomaterials 13, no. 6: 1060. https://doi.org/10.3390/nano13061060
APA StyleLiu, L., Gao, X., Cui, X., Wang, B., Hu, F., Yuan, T., Li, J., Zu, L., Lian, H., & Cui, X. (2023). Chemical Vapor Transport Synthesis of Fibrous Red Phosphorus Crystal as Anodes for Lithium-Ion Batteries. Nanomaterials, 13(6), 1060. https://doi.org/10.3390/nano13061060





