Thermal Diffusivity of Aqueous Dispersions of Silicon Oxide Nanoparticles by Dual-Beam Thermal Lens Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Thermal Lens Measurements
2.3. Thermal Lens Spectrometer
2.4. UV Visible Spectroscopy
2.5. Vibration Density Meter
2.6. Differential Scanning Calorimetry
2.7. Heat-Flow Method
3. Results and Discussion
3.1. Photothermal Measurements of Reference Samples
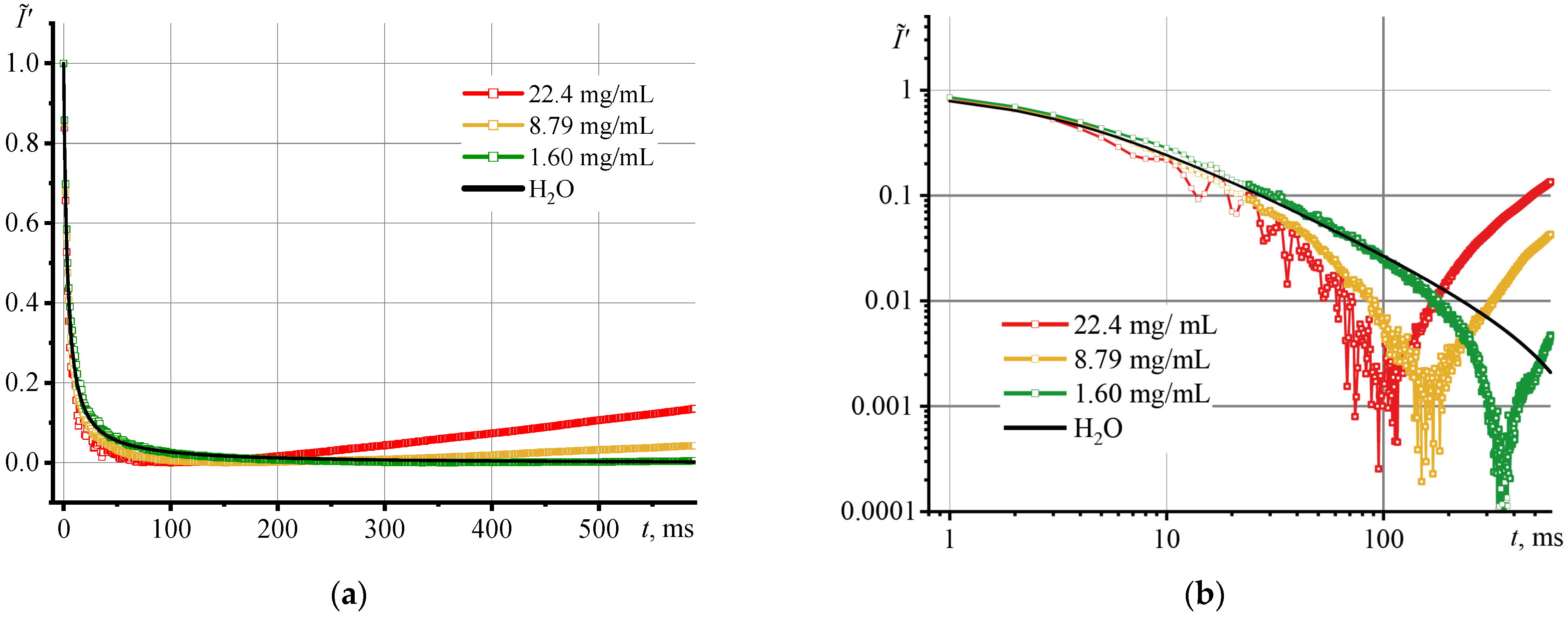
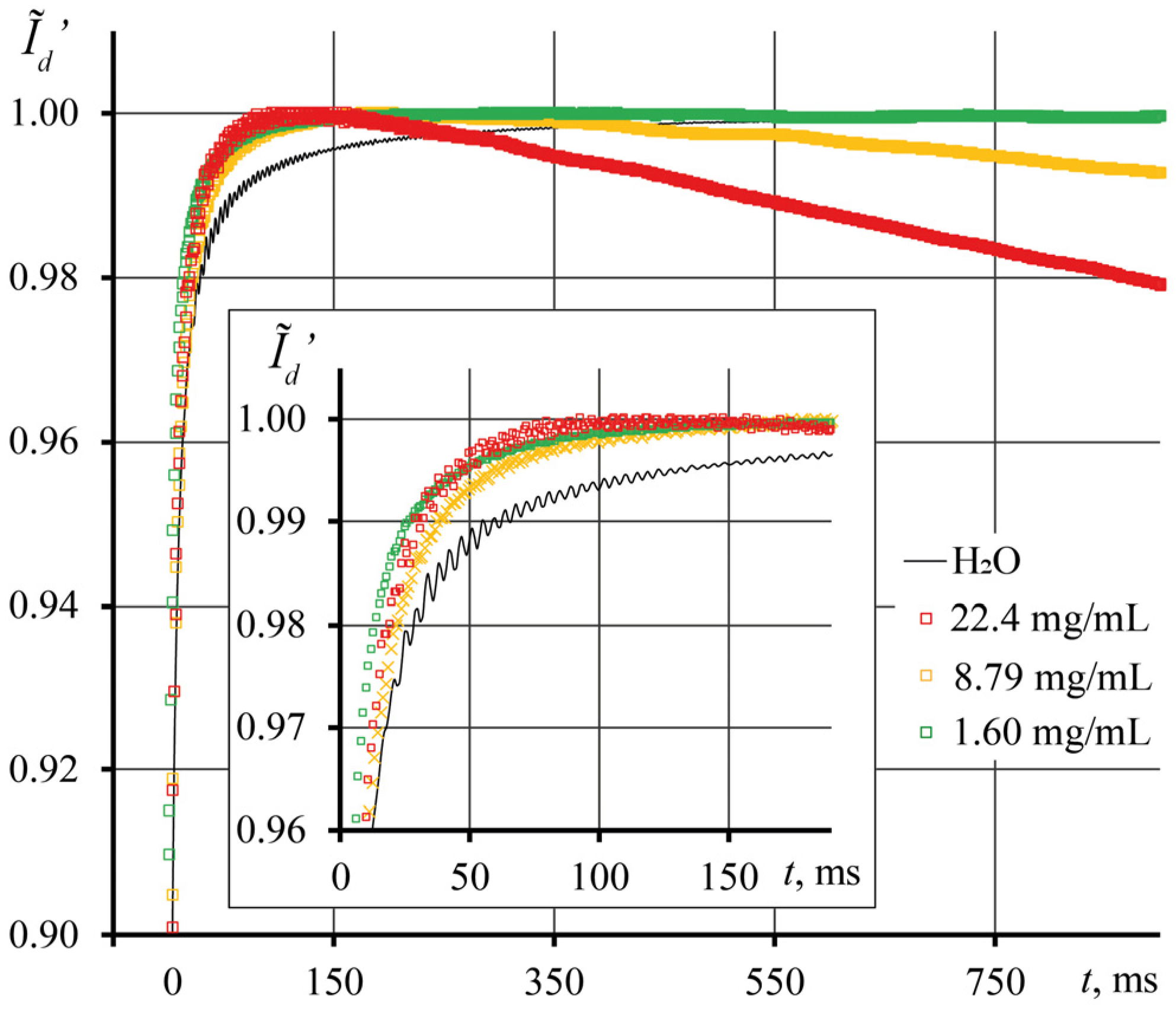
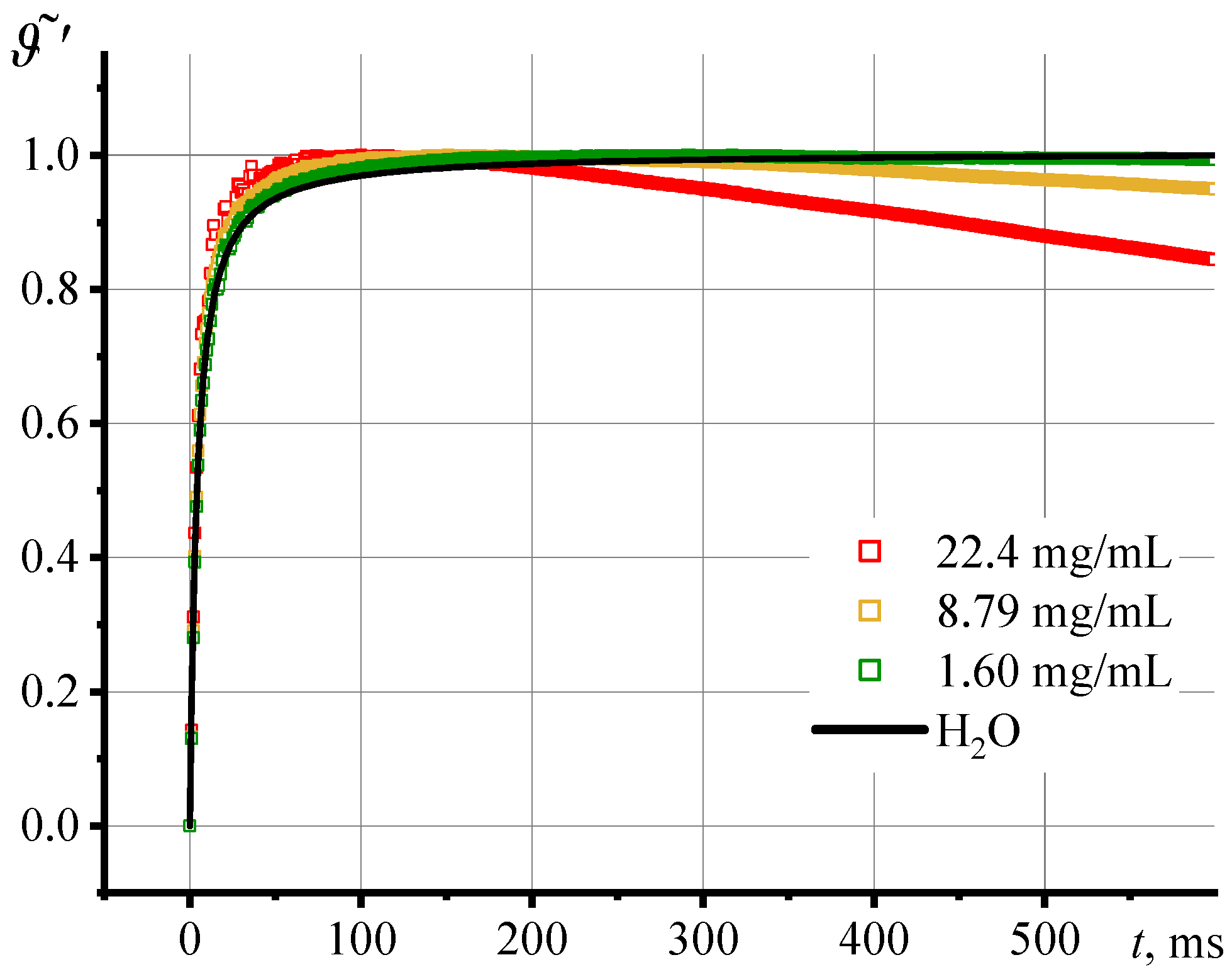
3.2. Photothermal Measurements of SiO2 Nanofluids
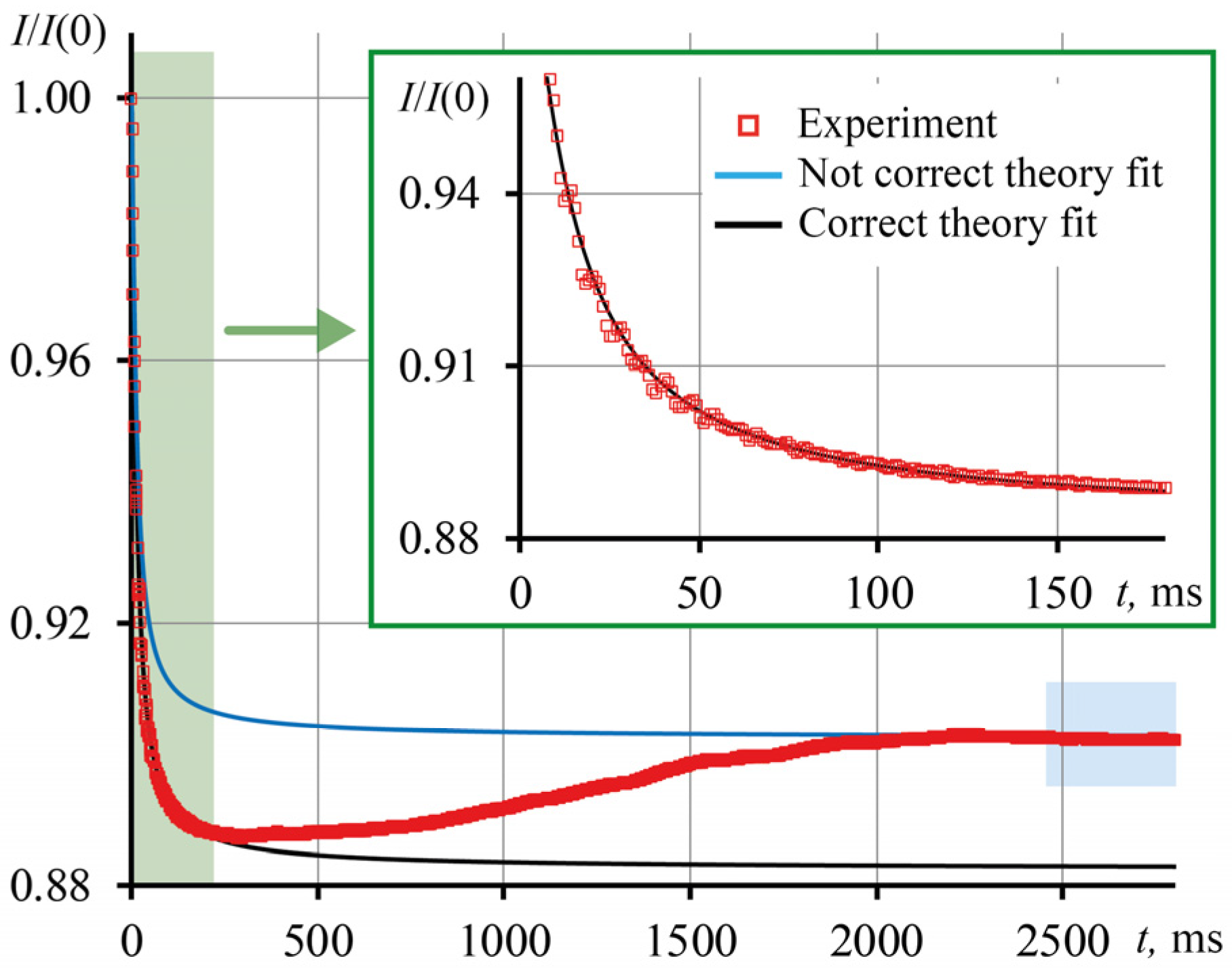
3.3. Correctness of Thermal Lens Measurements
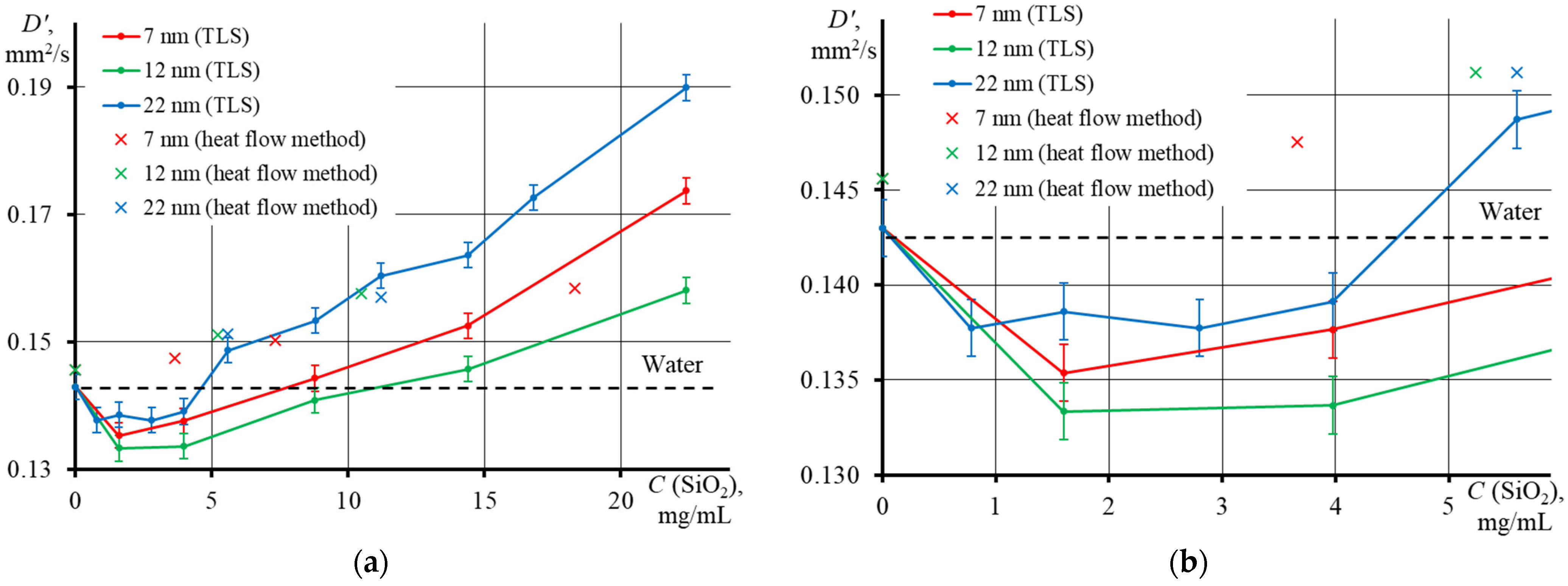
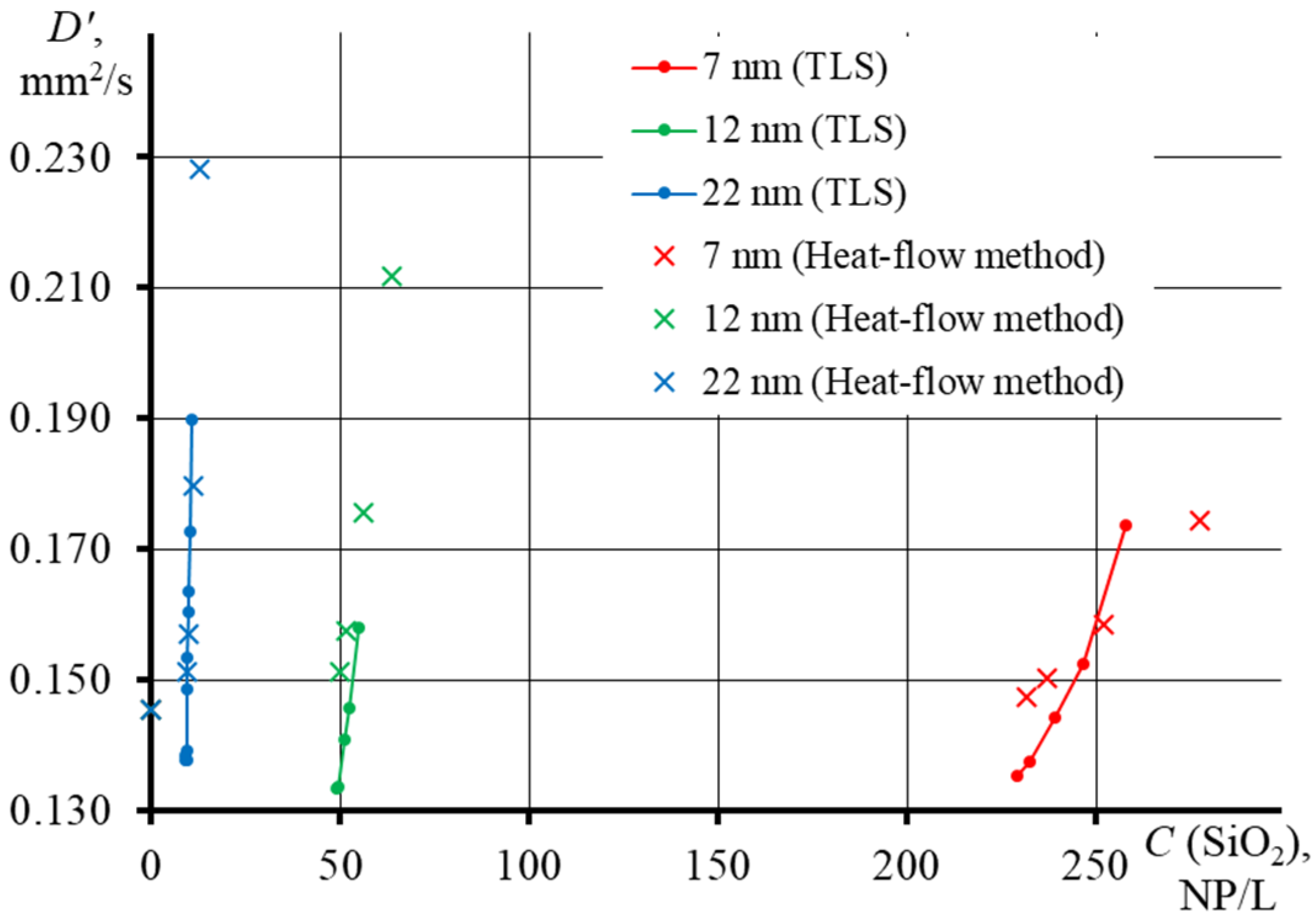
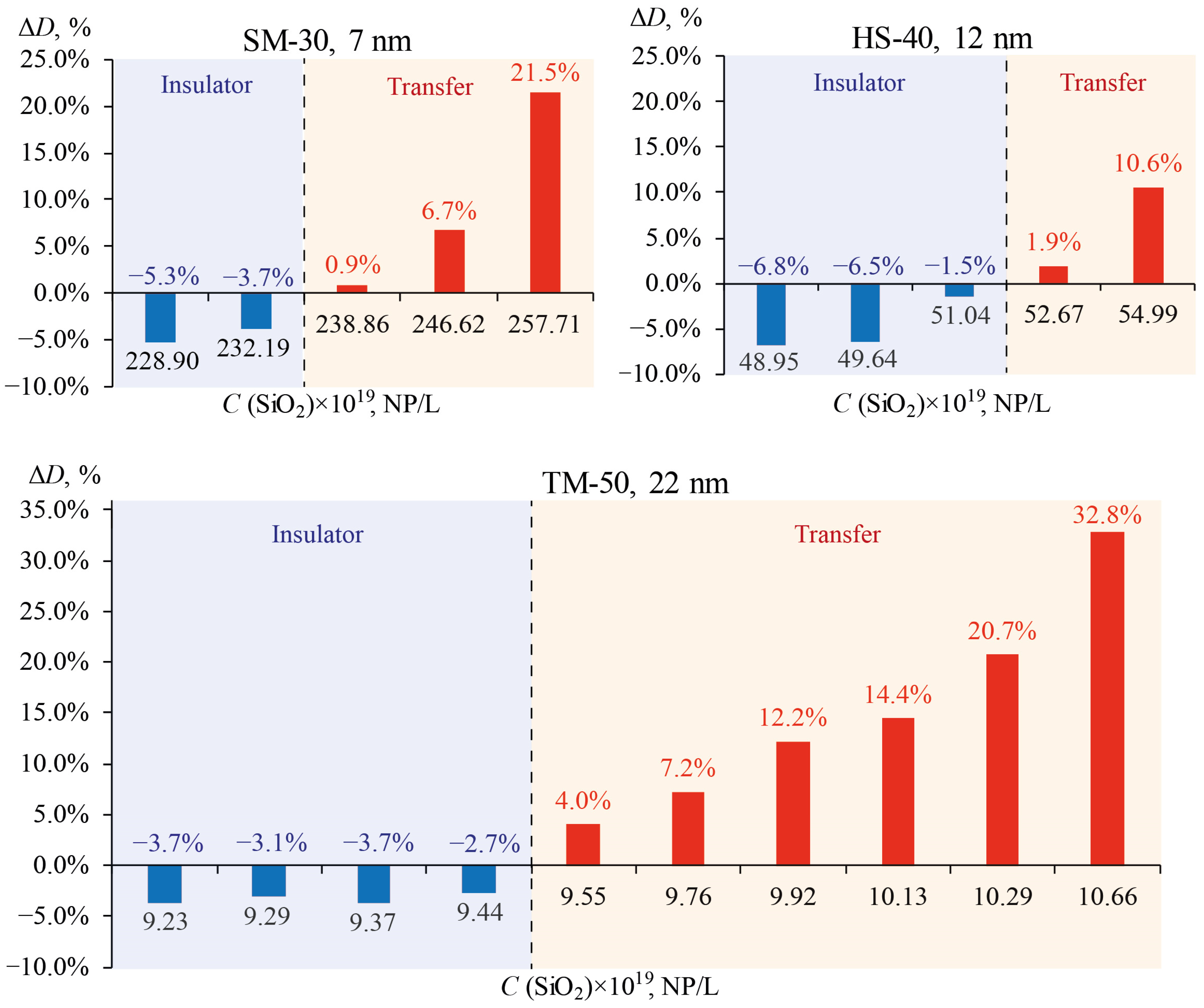
3.4. Thermal Diffusivity of SiO2 Nanofluids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamat, H.; Ramadan, M. Nanofluids: Thermal Conductivity and Applications. In Encyclopedia of Smart Materials; Olabi, A.-G., Ed.; Elsevier: Oxford, UK, 2022; pp. 288–296. [Google Scholar]
- Said, Z.; Sohail, M.; Tiwari, A.K. Chapter 33—Nanofluids as coolants. In Nanotechnology in the Automotive Industry; Song, H., Nguyen, T.A., Yasin, G., Singh, N.B., Gupta, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 713–735. [Google Scholar]
- Gupta, M.; Singh, V.; Kumar, R.; Said, Z. A review on thermophysical properties of nanofluids and heat transfer applications. Renew. Sustain. Energy Rev. 2017, 74, 638–670. [Google Scholar] [CrossRef]
- Sindhu Swapna, M.N.; Raj, V.; Cabrera, H.; Sankararaman, S.I. Thermal Lensing of Multi-walled Carbon Nanotube Solutions as Heat Transfer Nanofluids. ACS Appl. Nano Mater. 2021, 4, 3416–3425. [Google Scholar] [CrossRef]
- Ganvir, R.B.; Walke, P.V.; Kriplani, V.M. Heat transfer characteristics in nanofluid—A review. Renew. Sustain. Energy Rev. 2017, 75, 451–460. [Google Scholar] [CrossRef]
- Muneeshwaran, M.; Srinivasan, G.; Muthukumar, P.; Wang, C.-C. Role of hybrid-nanofluid in heat transfer enhancement—A review. Int. Commun. Heat Mass Transf. 2021, 125, 105341. [Google Scholar] [CrossRef]
- Prakash, A.; Pathrose, B.P.; Nampoori, V.P.N.; Radhakrishnan, P.; Mujeeb, A. Thermal diffusivity of neutral red dye using dual beam thermal lens technique: A comparison on the effects using nano pulsed laser ablated silver and gold nanoparticles. Phys. E Low-Dimens. Syst. Nanostructures 2019, 107, 203–208. [Google Scholar] [CrossRef]
- Qiu, L.; Ouyang, Y.; Li, F. Chapter 2—Experimental techniques overview. In Micro and Nano Thermal Transport; Qiu, L., Feng, Y., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 19–45. [Google Scholar]
- Franko, M.; Tran, C.D. Thermal Lens Spectroscopy. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Vijesh, K.R.; Sony, U.; Ramya, M.; Mathew, S.; Nampoori, V.P.N.; Thomas, S. Concentration dependent variation of thermal diffusivity in highly fluorescent carbon dots using dual beam thermal lens technique. Int. J. Therm. Sci. 2018, 126, 137–142. [Google Scholar] [CrossRef]
- Mathew, S.; Francis, F.; Joseph, S.A.; Kala, M.S. Enhanced thermal diffusivity of water based ZnO nanoflower/rGO nanofluid using the dual-beam thermal lens technique. Nano-Struct. Nano-Objects 2021, 28, 100784. [Google Scholar] [CrossRef]
- Netzahual-Lopantzi, Á.; Sánchez-Ramírez, J.F.; Jiménez-Pérez, J.L.; Cornejo-Monroy, D.; López-Gamboa, G.; Correa-Pacheco, Z.N. Study of the thermal diffusivity of nanofluids containing SiO2 decorated with Au nanoparticles by thermal lens spectroscopy. Appl. Phys. A 2019, 125, 588. [Google Scholar] [CrossRef]
- Lenart, V.M.; Astrath, N.G.C.; Turchiello, R.F.; Goya, G.F.; Gómez, S.L. Thermal diffusivity of ferrofluids as a function of particle size determined using the mode-mismatched dual-beam thermal lens technique. J. Appl. Phys. 2018, 123, 5017025. [Google Scholar] [CrossRef]
- Nideep, T.K.; Ramya, M.; Nampoori, V.P.N.; Kailasnath, M. The size dependent thermal diffusivity of water soluble CdTe quantum dots using dual beam thermal lens spectroscopy. Phys. E Low-Dimens. Syst. Nanostructures 2020, 116, 113724. [Google Scholar] [CrossRef]
- Simon, J.; Anugop, B.; Nampoori, V.P.N.; Kailasnath, M. Effect of pulsed laser irradiation on the thermal diffusivity of bimetallic Au/Ag nanoparticles. Opt. Laser Technol. 2021, 139, 106954. [Google Scholar] [CrossRef]
- Zamiri, R.; Azmi, B.Z.; Shahriari, E.; Naghavi, K.; Saion, E.; Rizwan, Z.; Husin, M.S. Thermal diffusivity measurement of silver nanofluid by thermal lens technique. J. Laser Appl. 2011, 23, 042002. [Google Scholar] [CrossRef]
- Ramya, M.; Nideep, T.K.; Nampoori, V.P.N.; Kailasnath, M. Particle size and concentration effect on thermal diffusivity of water-based ZnO nanofluid using the dual-beam thermal lens technique. Appl. Phys. B 2019, 125, 181. [Google Scholar] [CrossRef]
- Oliveira, G.M.; Zanuto, V.S.; Flizikowski, G.A.S.; Kimura, N.M.; Sampaio, A.R.; Novatski, A.; Baesso, M.L.; Malacarne, L.C.; Astrath, N.G.C. Soret effect in lyotropic liquid crystal in the isotropic phase revealed by time-resolved thermal lens. J. Mol. Liq. 2020, 312, 113381. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Volkov, D.S.; Gor’kova, T.A.; Bendrysheva, S.N.; Smirnova, A.P.; Nedosekin, D.A. Advances in thermal lens spectrometry. J. Anal. Chem. 2015, 70, 249–276. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Khabibullin, V.R.; Usoltseva, L.O.; Vyrko, E.A.; Mikheev, I.V.; Volkov, D.S. Photothermal and optoacoustic spectroscopy: State of the art and prospects. Phys. Uspekhi 2022, 65, 270. [Google Scholar] [CrossRef]
- Rajesh Kumar, B.; Shemeena Basheer, N.; Jacob, S.; Kurian, A.; George, S.D. Thermal-lens probing of the enhanced thermal diffusivity of gold nanofluid-ethylene glycol mixture. J. Therm. Anal. Calorim. 2015, 119, 453–460. [Google Scholar] [CrossRef]
- Shen, J.; Lowe, R.D.; Snook, R.D. A model for cw laser induced mode-mismatched dual-beam thermal lens spectrometry. Chem. Phys. 1992, 165, 385–396. [Google Scholar] [CrossRef]
- Khabibullin, V.R.; Franko, M.; Proskurnin, M.A. Accuracy of Measurements of Thermophysical Parameters by Dual-Beam Thermal-Lens Spectrometry. Nanomaterials 2023, 13, 430. [Google Scholar] [CrossRef]
- Abbasgholi-Na, B.; Nokhbeh, S.R.; Aldaghri, O.A.; Ibnaouf, K.H.; Madkhali, N.; Cabrera, H. Thermal Diffusivity and Conductivity of Polyolefins by Thermal Lens Technique. Polymers 2022, 14, 2707. [Google Scholar] [CrossRef] [PubMed]
- Luna-Sánchez, J.L.; Jiménez-Pérez, J.L.; Carbajal-Valdez, R.; Lopez-Gamboa, G.; Pérez-González, M.; Correa-Pacheco, Z.N. Green synthesis of silver nanoparticles using Jalapeño Chili extract and thermal lens study of acrylic resin nanocomposites. Thermochimica Acta 2019, 678, 178314. [Google Scholar] [CrossRef]
- John, J.; Mathew, R.M.; Rejeena, I.; Jayakrishnan, R.; Mathew, S.; Thomas, V.; Mujeeb, A. Nonlinear optical limiting and dual beam mode matched thermal lensing of nano fluids containing green synthesized copper nanoparticles. J. Mol. Liq. 2019, 279, 63–66. [Google Scholar] [CrossRef]
- Rusconi, R.; Isa, L.; Piazza, R. Thermal-lensing measurement of particle thermophoresis in aqueous dispersions. J. Opt. Soc. Am. B 2004, 21, 605–616. [Google Scholar] [CrossRef]
- Gutierrez Fuentes, R.; Pescador Rojas, J.A.; Jiménez-Pérez, J.L.; Sanchez Ramirez, J.F.; Cruz-Orea, A.; Mendoza-Alvarez, J.G. Study of thermal diffusivity of nanofluids with bimetallic nanoparticles with Au(core)/Ag(shell) structure. Appl. Surf. Sci. 2008, 255, 781–783. [Google Scholar] [CrossRef]
- Raznjevic, K.; Bošković, M.; Podhorsky, R. Handbook of Thermodynamic Tables and Charts; Hemisphere Publishing Corporation: London, UK, 1976. [Google Scholar]
- Usoltseva, L.O.; Volkov, D.S.; Karpushkin, E.A.; Korobov, M.V.; Proskurnin, M.A. Thermal Conductivity of Detonation Nanodiamond Hydrogels and Hydrosols by Direct Heat Flux Measurements. Gels 2021, 7, 248. [Google Scholar] [CrossRef]
- Numan, Y. The Review of Some Commonly Used Methods and Techniques to Measure the Thermal Conductivity of Insulation Materials. In Insulation Materials in Context of Sustainability; Amjad, A., Asaad, A., Eds.; IntechOpen: Rijeka, Croatia, 2016; p. 6. [Google Scholar]
- Dovichi, N.J.; Bialkowski, S.E. Thermo-Optical Spectrophotometries in Analytical Chemistry. CRC Crit. Rev. Anal. Chem. 1987, 17, 357–423. [Google Scholar] [CrossRef]
- Savi, E.L.; Herculano, L.S.; Lukasievicz, G.V.B.; Regatieri, H.R.; Torquato, A.S.; Malacarne, L.C.; Astrath, N.G.C. Assessing thermal and optical properties of biodiesel by thermal lens spectrometry: Theoretical and experimental aspects. Fuel 2018, 217, 404–408. [Google Scholar] [CrossRef]
- Savi, E.L.; Malacarne, L.C.; Baesso, M.L.; Pintro, P.T.M.; Croge, C.; Shen, J.; Astrath, N.G.C. Investigation into photostability of soybean oils by thermal lens spectroscopy. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2015, 145, 125–129. [Google Scholar] [CrossRef]
- Joseph, S.A.; Hari, M.; Mathew, S.; Sharma, G.; Soumya; Hadiya, V.M.; Radhakrishnan, P.; Nampoori, V.P.N. Thermal diffusivity of rhodamine 6G incorporated in silver nanofluid measured using mode-matched thermal lens technique. Opt. Commun. 2010, 283, 313–317. [Google Scholar] [CrossRef]
- Augustine, A.K.; Mathew, S.; Girijavallabhan, C.P.; Radhakrishnan, P.; Nampoori, V.P.N.; Kailasnath, M. Size dependent variation of thermal diffusivity of CdSe nanoparticles based nanofluid using laser induced mode-matched thermal lens technique. J. Opt. 2014, 44, 85–91. [Google Scholar] [CrossRef]
- Jiménez Pérez, J.L.; Gutierrez Fuentes, R.; Sanchez Ramirez, J.F.; Cruz-Orea, A. Study of gold nanoparticles effect on thermal diffusivity of nanofluids based on various solvents by using thermal lens spectroscopy. Eur. Phys. J. Spec. Top. 2008, 153, 159–161. [Google Scholar] [CrossRef]
- Sánchez-Calderón, I.; Merillas, B.; Bernardo, V.; Rodríguez-Pérez, M.Á. Methodology for measuring the thermal conductivity of insulating samples with small dimensions by heat flow meter technique. J. Therm. Anal. Calorim. 2022, 147, 12523–12533. [Google Scholar] [CrossRef]
- Thomas, L.; John, J.; Kumar, B.R.; George, N.A.; Kurian, A. Thermal Diffusivity of Gold Nanoparticle Reduced by Polyvinyl Alcohol Using Dual Beam Thermal Lens Technique. Mater. Today Proc. 2015, 2, 1017–1020. [Google Scholar] [CrossRef]
- Liu, M.; Ding, C.; Wang, J. Modeling of thermal conductivity of nanofluids considering aggregation and interfacial thermal resistance. RSC Adv. 2016, 6, 3571–3577. [Google Scholar] [CrossRef]
- Lopes, C.S.; Lenart, V.M.; Turchiello, R.F.; Gómez, S.L. Determination of the Thermal Diffusivity of Plasmonic Nanofluids Containing PVP-Coated Ag Nanoparticles Using Mode-Mismatched Dual-Beam Thermal Lens Technique. Adv. Condens. Matter Phys. 2018, 2018, 3052793. [Google Scholar] [CrossRef]
- Shahriari, E.; Yunus, W.M.M.; Zamiri, R. The effect of nanoparticle size on thermal diffusivity of gold nano-fluid measured using thermal lens technique. J. Eur. Opt. Soc. Rapid Publ. 2013, 8, 13026. [Google Scholar] [CrossRef]
- Shahriari, E.; Varnamkhasti, M.G.; Zamiri, R. Characterization of thermal diffusivity and optical properties of Ag nanoparticles. Opt. Int. J. Light Electron Opt. 2015, 126, 2104–2107. [Google Scholar] [CrossRef]
- Hari, M.; Joseph, S.A.; Mathew, S.; Nithyaja, B.; Nampoori, V.P.N.; Radhakrishnan, P. Thermal diffusivity of nanofluids composed of rod-shaped silver nanoparticles. Int. J. Therm. Sci. 2013, 64, 188–194. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, J.; Pérez, J.L.; Cruz-Orea, A.; Fuentes, R.; Bautista-Hernández, A.; Pal, U. Thermal Diffusivity of Nanofluids Containing Au/Pd Bimetallic Nanoparticles of Different Compositions. J. Nanosci. Nanotechnol. 2006, 6, 685–690. [Google Scholar] [CrossRef] [PubMed]
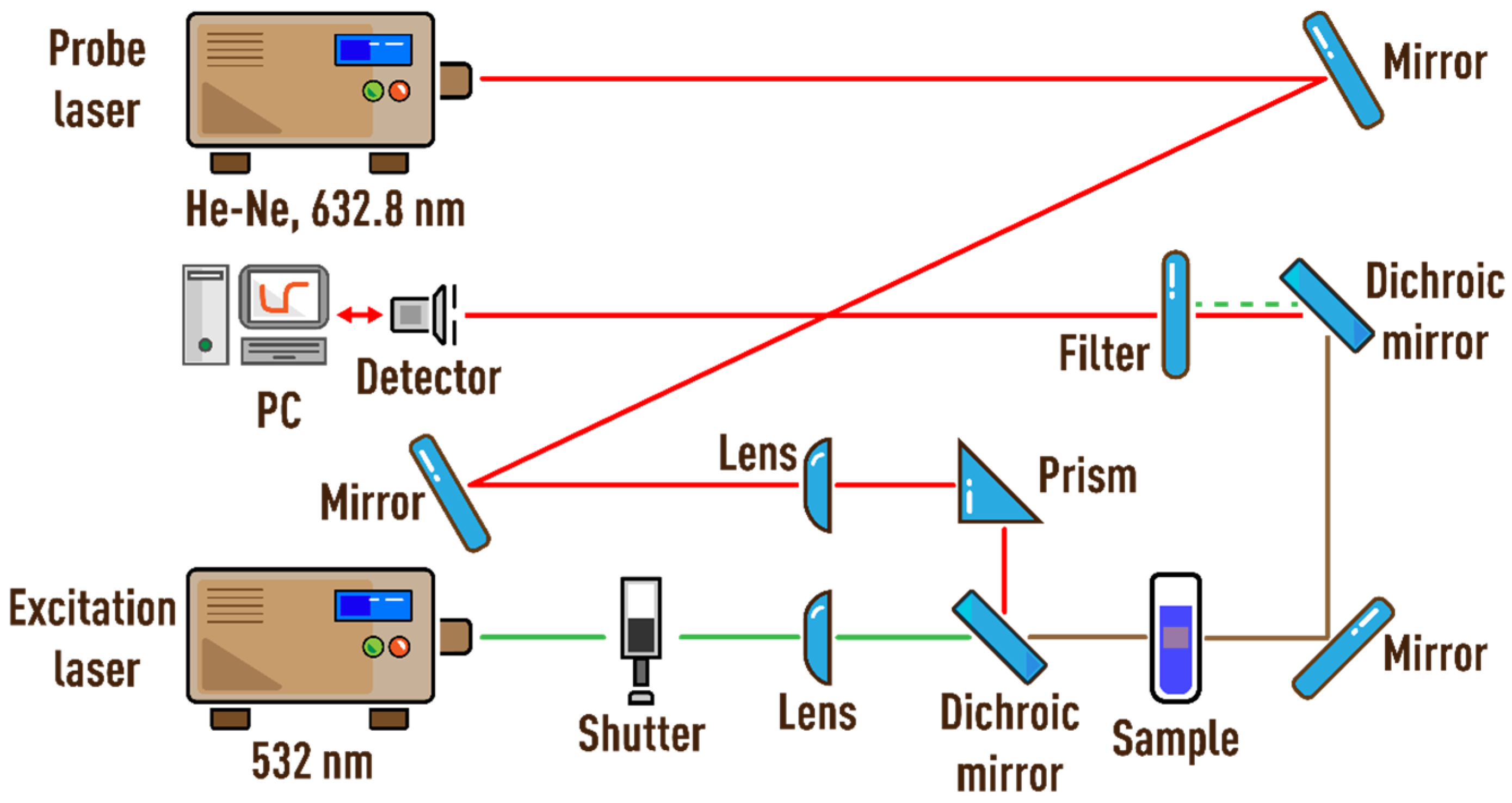



| Ludox | Average Particle Diameter dav, nm | Concentration, % w/w | Density, kg/m3 | Specific Surface, m2/g |
|---|---|---|---|---|
| SM-30 | 7 | 30 | 1.220 | 350 |
| HS-40 | 12 | 40 | 1.310 | 220 |
| TM-50 | 22 | 50 | 1.400 | 140 |
| Parameter | Value |
|---|---|
| Excitation laser | |
| Wavelength, λe (nm) | 532 |
| Focusing lens focal length, fe (mm) | 200 |
| Confocal distance, Zce (mm) | 10.9 |
| Laser power at cell, P (mW) | 120 |
| Spot size at the waist, ωe0 (µm) | 42 |
| Probe laser | |
| Wavelength, λp (nm) | 632.8 |
| Focusing lens focal length, fp (mm) | 300 |
| Confocal distance, Zcp (mm) | 2.7 |
| Laser power at cell (mW) | 4.5 |
| Spot size at the waist, ωp0 (µm) | 23 |
| Spot size at cell, ωp (µm) | 90 |
| Other constants | |
| Cell length (mm) | 10 |
| Sample-to-detector distance, Z2 (cm) | 230 |
| Mode mismatch factor, m | 4.59 |
| Geometric parameters, V | 4.89 |
| Modulator frequency (Hz) | 0.25 |
| Number of transient curves to average | 300 |
| Number of experiment repetitions | 5 |
| Solvent | Characteristic Time, ms | Thermal Diffusivity, mm2/s | ||||
|---|---|---|---|---|---|---|
| Calculation, Equation (2) | Experiment, Equation (7) | Δ, % | Theory [32] | Experiment | Δ, % | |
| Water | 3.10 | 3.04 ± 0.04 | 2 | 0.142 | 0.145 ± 0.002 | 2 |
| Ethanol | 4.95 | 4.95 ± 0.03 | <1 | 0.089 | 0.089 ± 0.001 | <1 |
| Chloroform | 5.44 | 5.44 ± 0.10 | <1 | 0.081 | 0.081 ± 0.002 | <1 |
| Toluene | 4.79 | 4.85 ± 0.23 | 1 | 0.092 | 0.091 ± 0.005 | 1 |
| Ludox | c, mg/mL | Not Correct, Equation (7) | Correct, Equation (10) | Δ(D), % | ||
|---|---|---|---|---|---|---|
| tc, ms | D, mm2/s | tc′, ms | D′, mm2/s | |||
| SM (dav = 7 nm) | 1.60 | 2.23 | 0.198 | 3.32 | 0.133 ± 0.002 | 49 |
| 3.98 | 1.48 | 0.298 | 3.24 | 0.136 ± 0.001 | 120 | |
| 8.79 | 0.38 | 1.161 | 3.20 | 0.138 ± 0.002 | 740 | |
| 14.39 | 0.20 | 2.205 | 2.88 | 0.153 ± 0.002 | 1340 | |
| 22.40 | 0.12 | 3.675 | 2.53 | 0.174 ± 0.004 | 2010 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khabibullin, V.R.; Usoltseva, L.O.; Mikheev, I.V.; Proskurnin, M.A. Thermal Diffusivity of Aqueous Dispersions of Silicon Oxide Nanoparticles by Dual-Beam Thermal Lens Spectrometry. Nanomaterials 2023, 13, 1006. https://doi.org/10.3390/nano13061006
Khabibullin VR, Usoltseva LO, Mikheev IV, Proskurnin MA. Thermal Diffusivity of Aqueous Dispersions of Silicon Oxide Nanoparticles by Dual-Beam Thermal Lens Spectrometry. Nanomaterials. 2023; 13(6):1006. https://doi.org/10.3390/nano13061006
Chicago/Turabian StyleKhabibullin, Vladislav R., Liliya O. Usoltseva, Ivan V. Mikheev, and Mikhail A. Proskurnin. 2023. "Thermal Diffusivity of Aqueous Dispersions of Silicon Oxide Nanoparticles by Dual-Beam Thermal Lens Spectrometry" Nanomaterials 13, no. 6: 1006. https://doi.org/10.3390/nano13061006
APA StyleKhabibullin, V. R., Usoltseva, L. O., Mikheev, I. V., & Proskurnin, M. A. (2023). Thermal Diffusivity of Aqueous Dispersions of Silicon Oxide Nanoparticles by Dual-Beam Thermal Lens Spectrometry. Nanomaterials, 13(6), 1006. https://doi.org/10.3390/nano13061006





