Accuracy of Measurements of Thermophysical Parameters by Dual-Beam Thermal-Lens Spectrometry
Abstract
1. Introduction
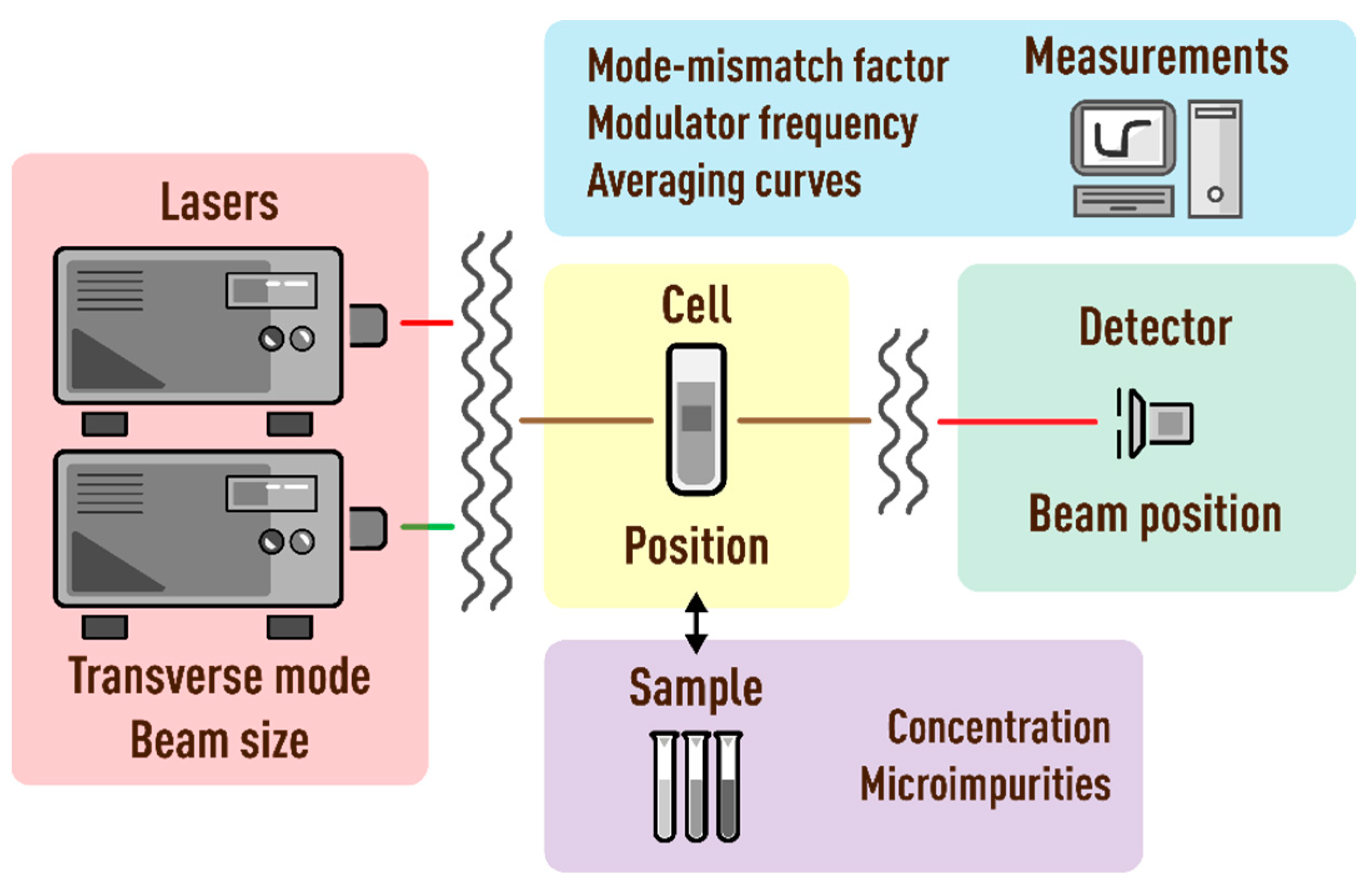
2. Materials and Method
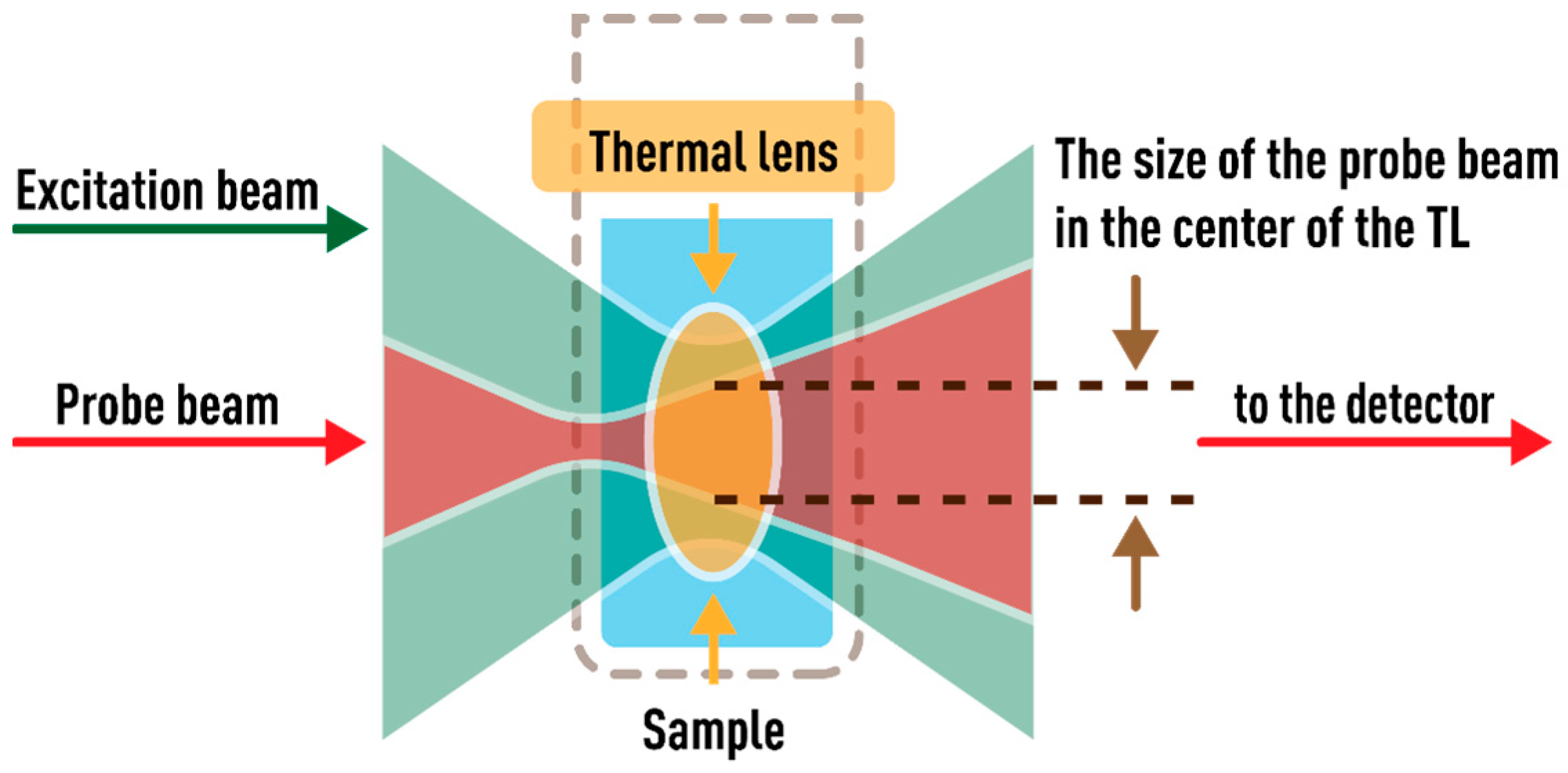
2.1. Thermal Lensing
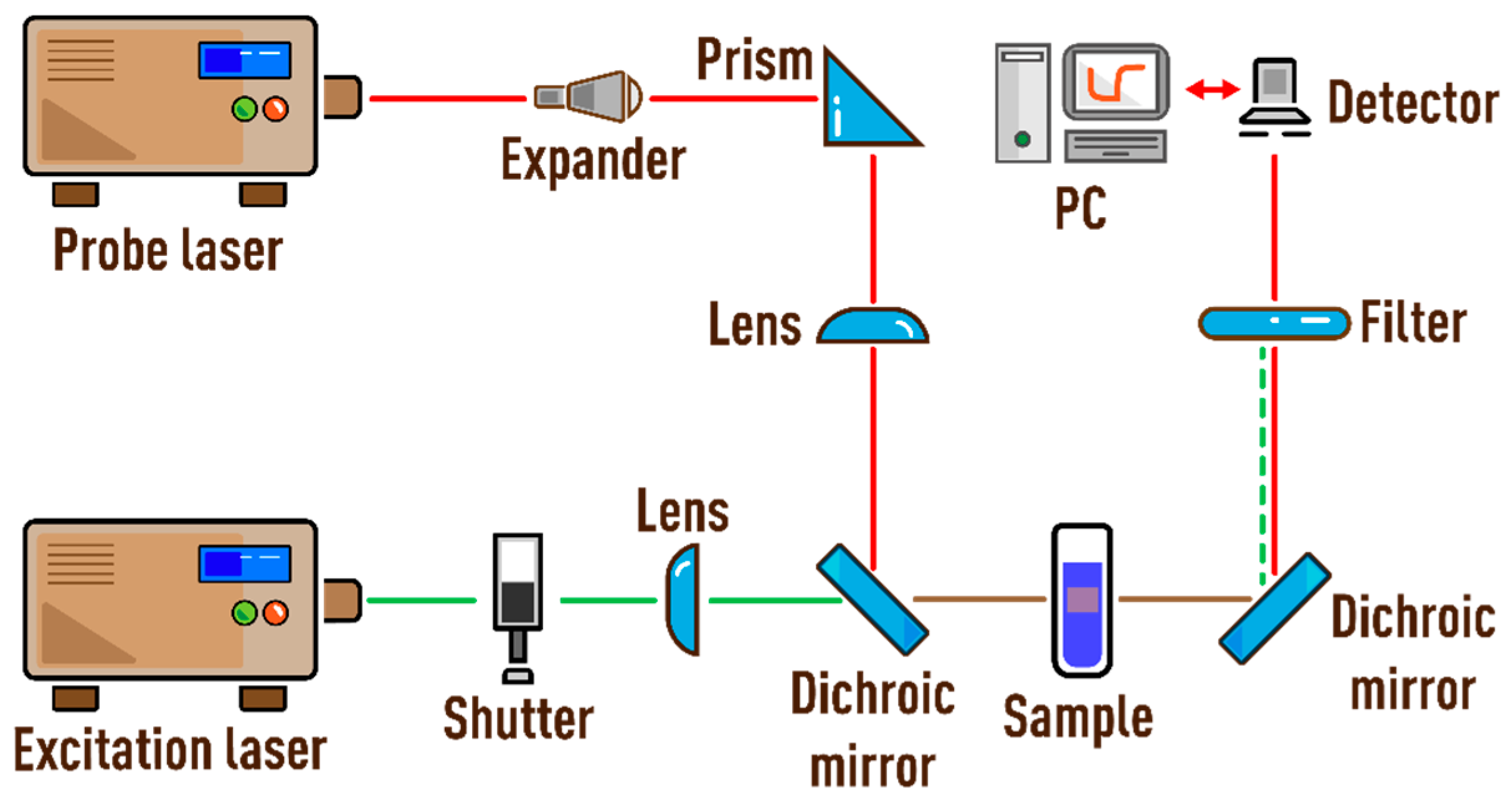
2.2. Spectrometers
2.3. Measurement Procedures
2.3.1. Auxiliary Measurements
2.4. Reagents
3. Results and Discussion
3.1. Excitation Radiation Source
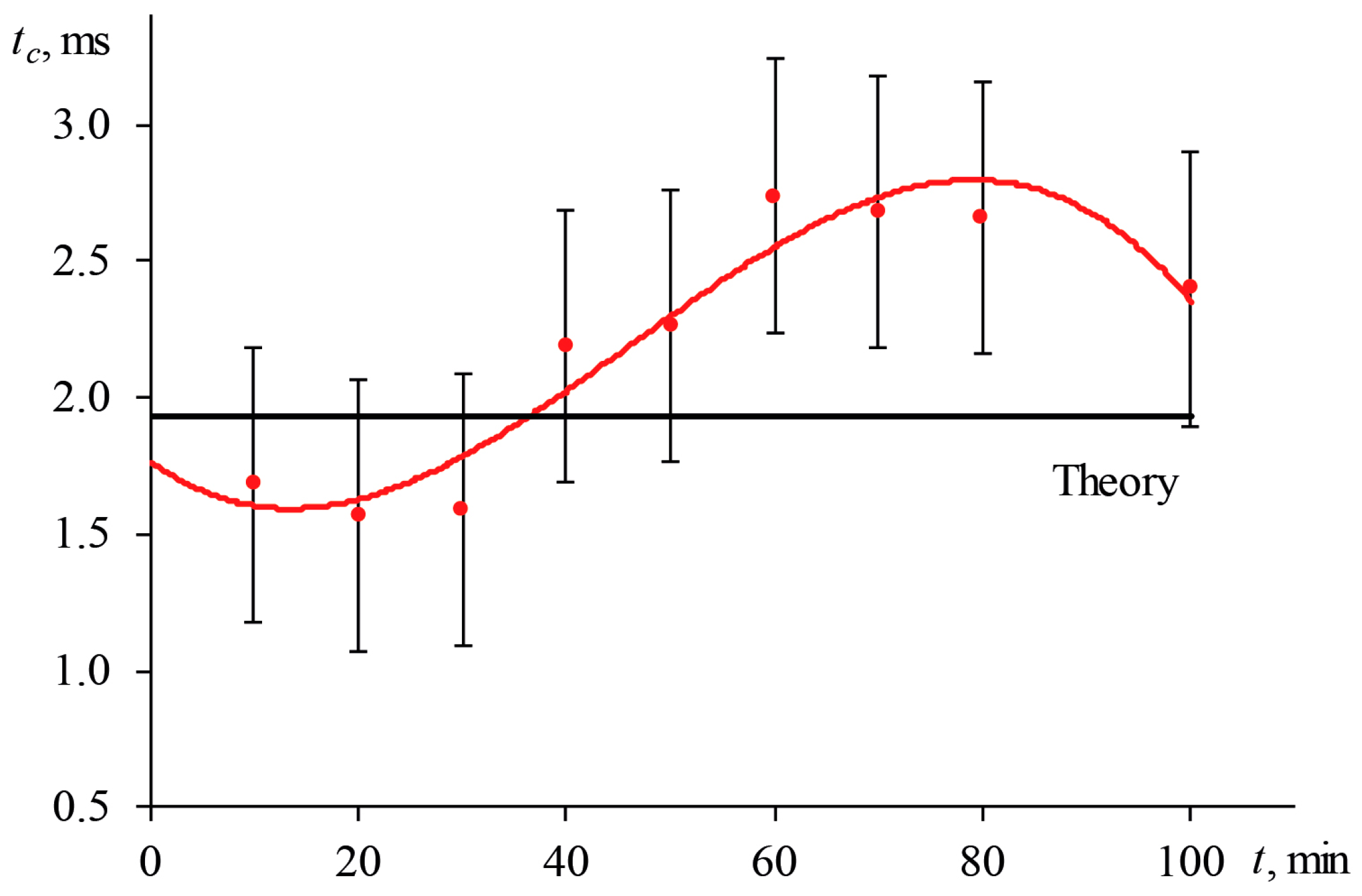
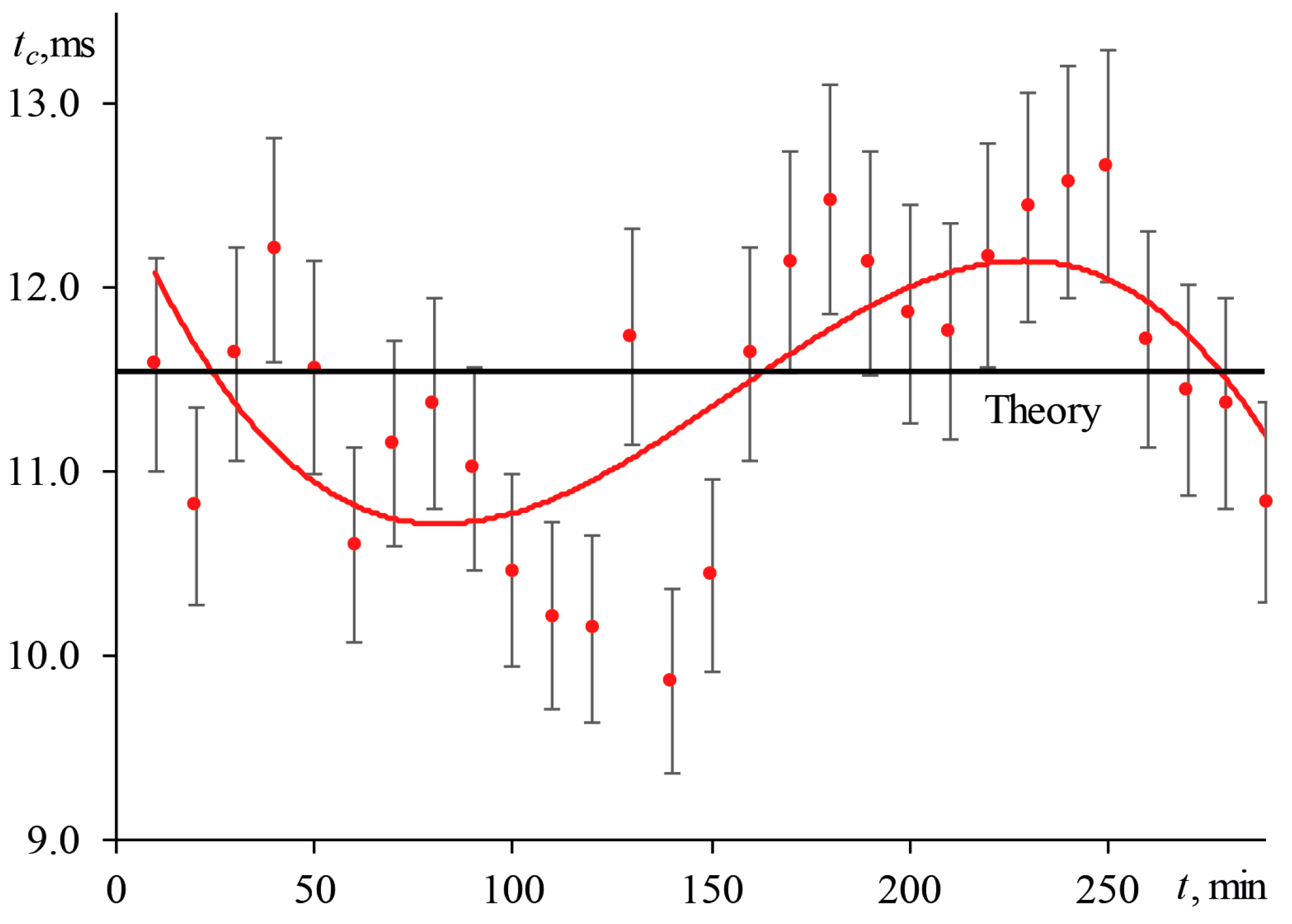
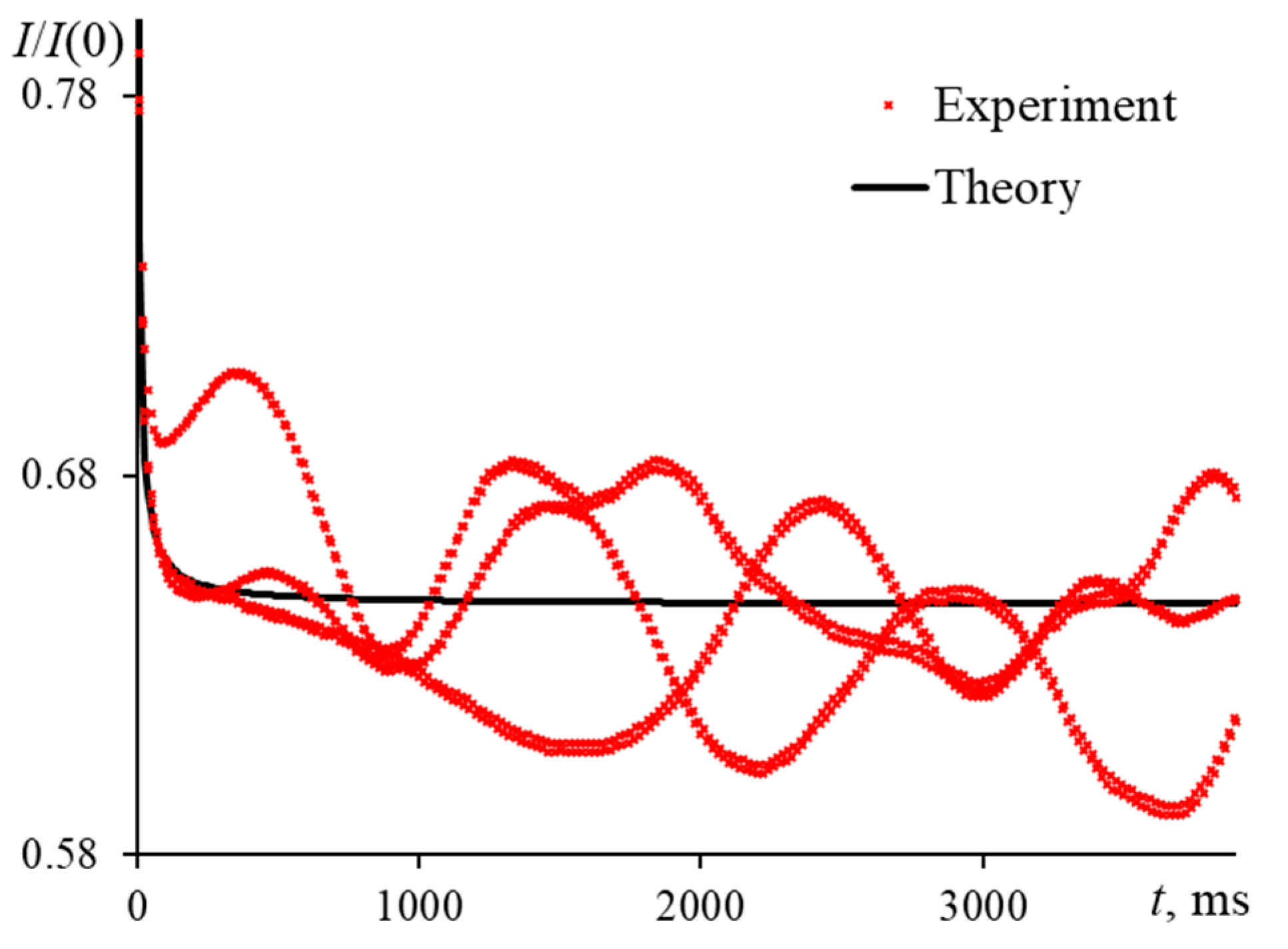
3.1.1. Periodic Changes of the Transverse Spatial Mode of Excitation Radiation
3.1.2. Error in Beam Radius Measurement
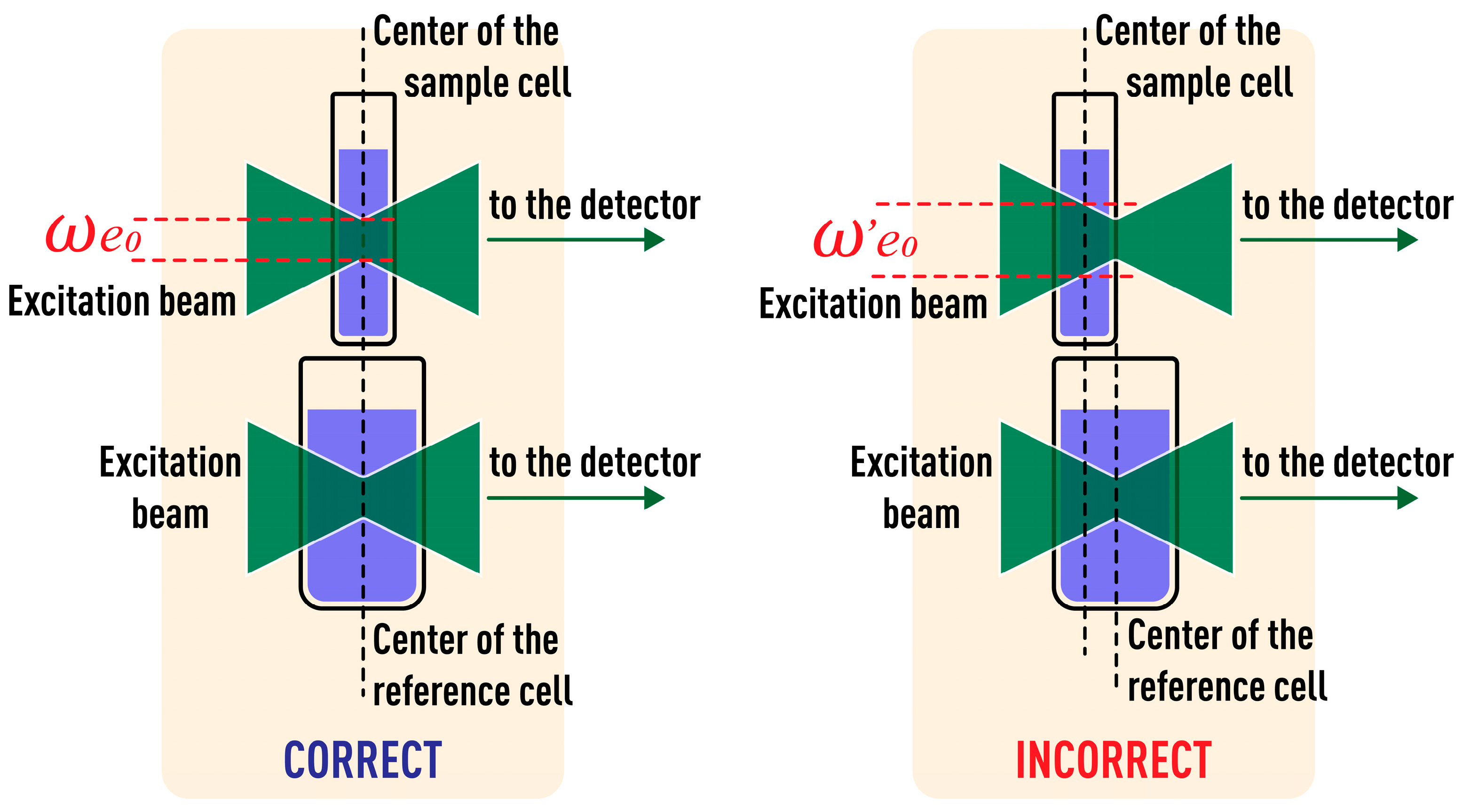
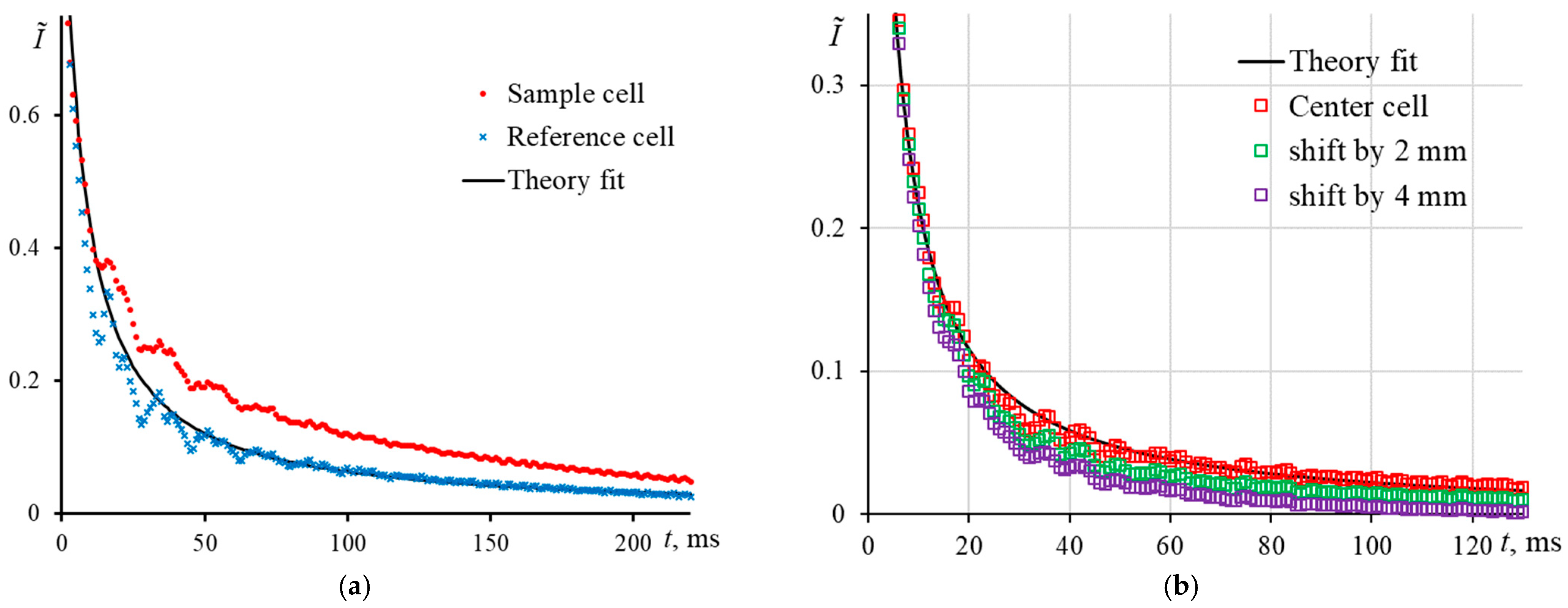
3.2. Cell Positioning
3.3. Detector
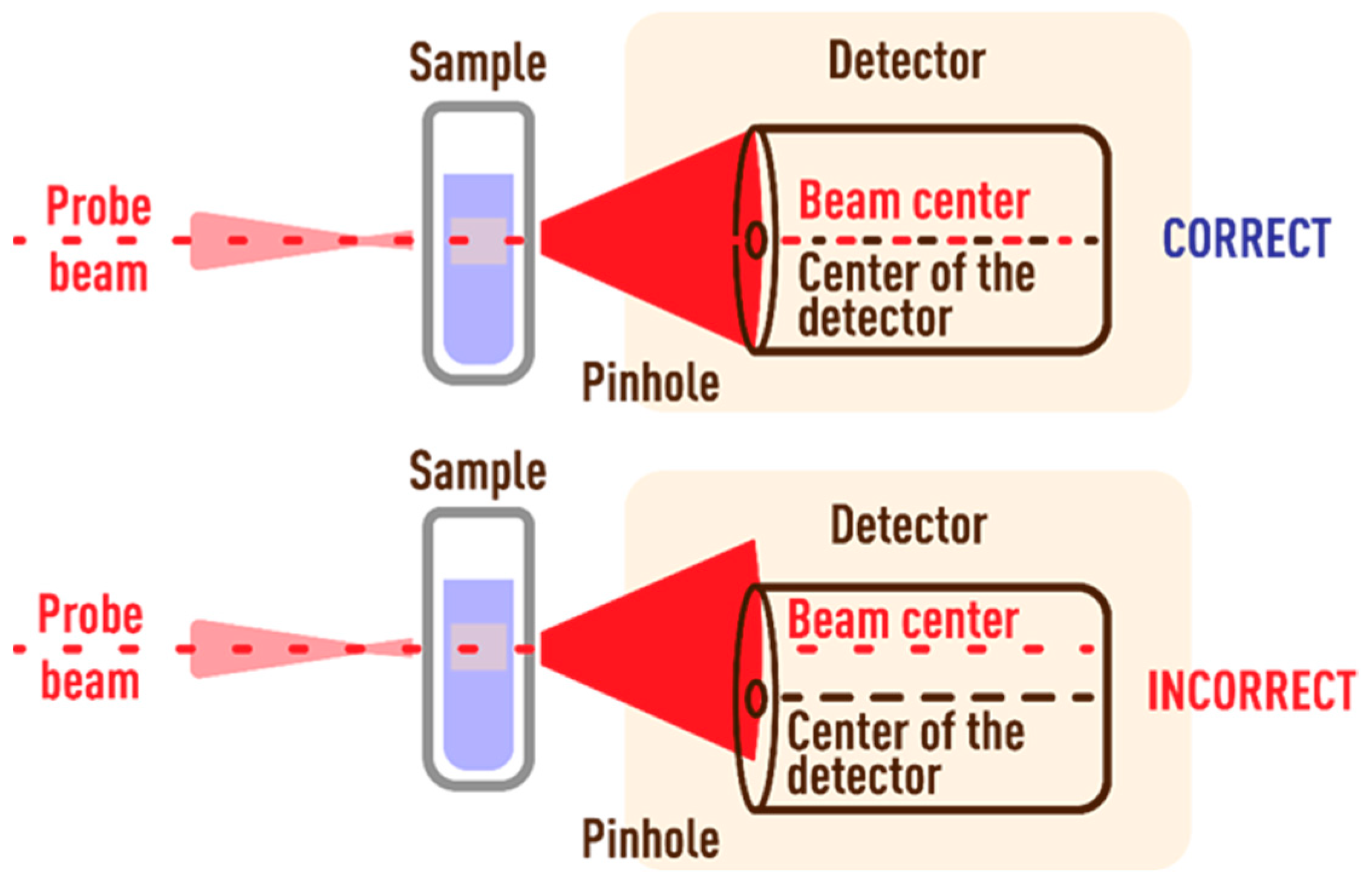
3.3.1. Probe-Beam Spot Offset Error
3.4. Sample
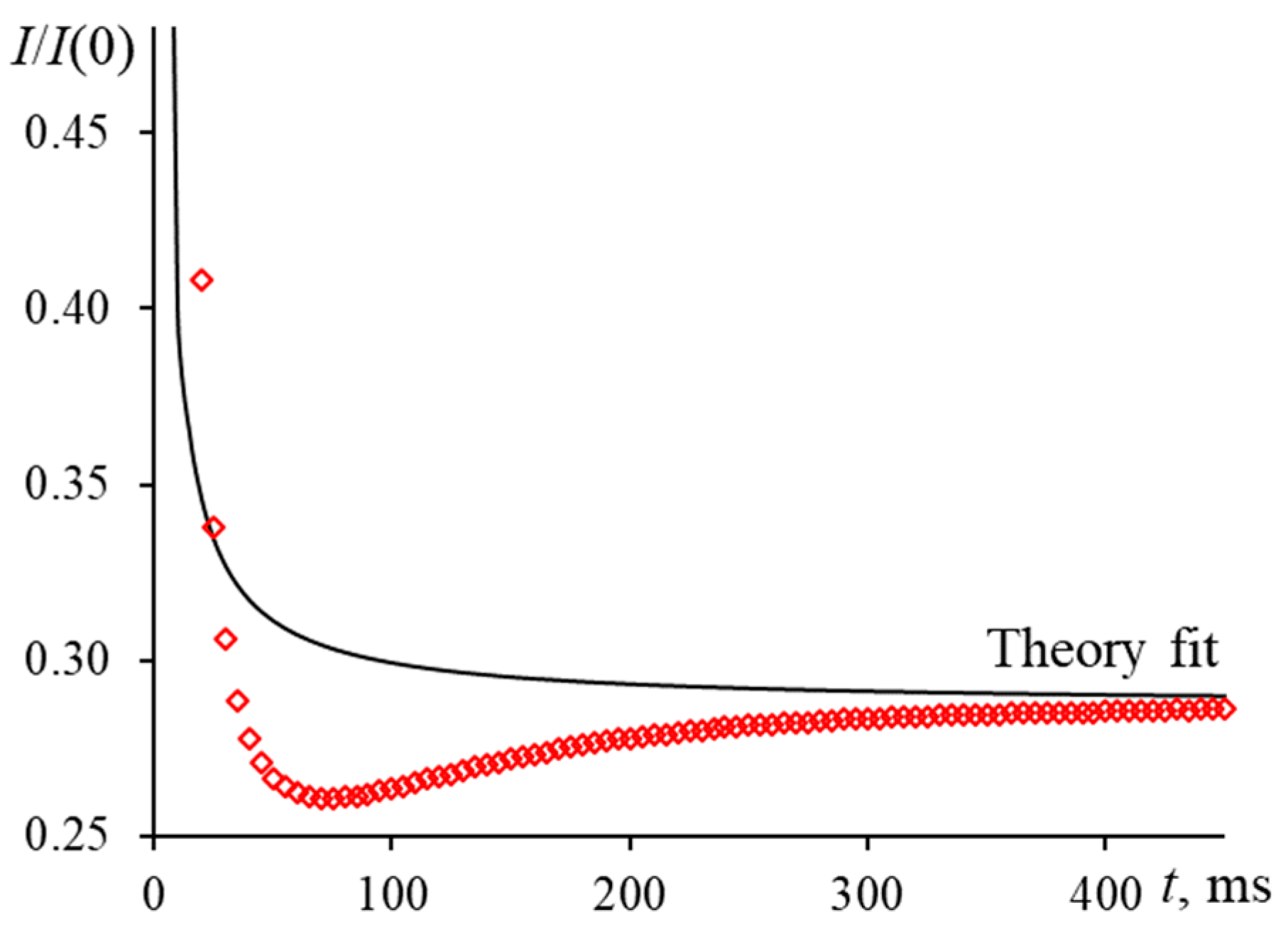
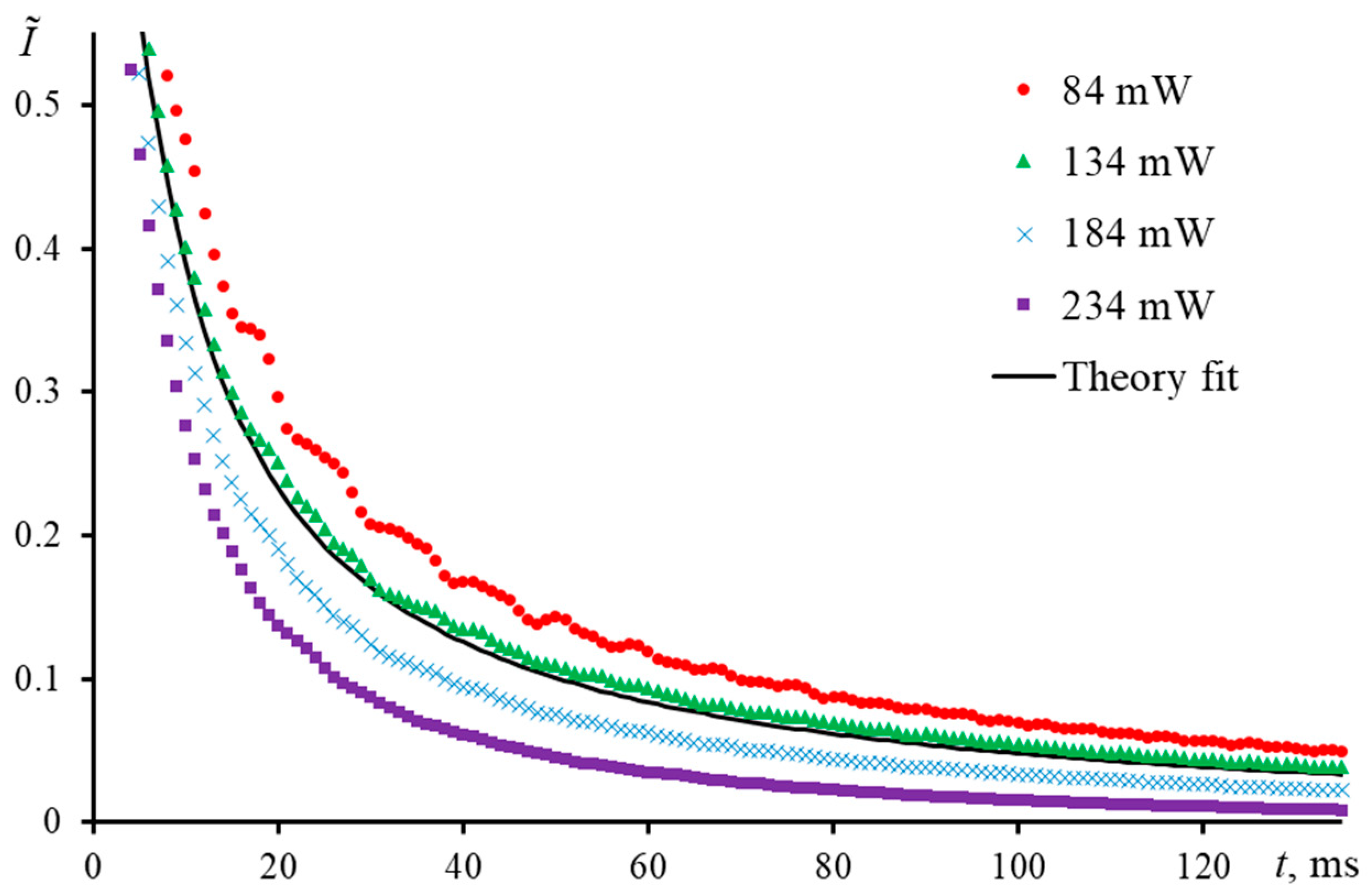
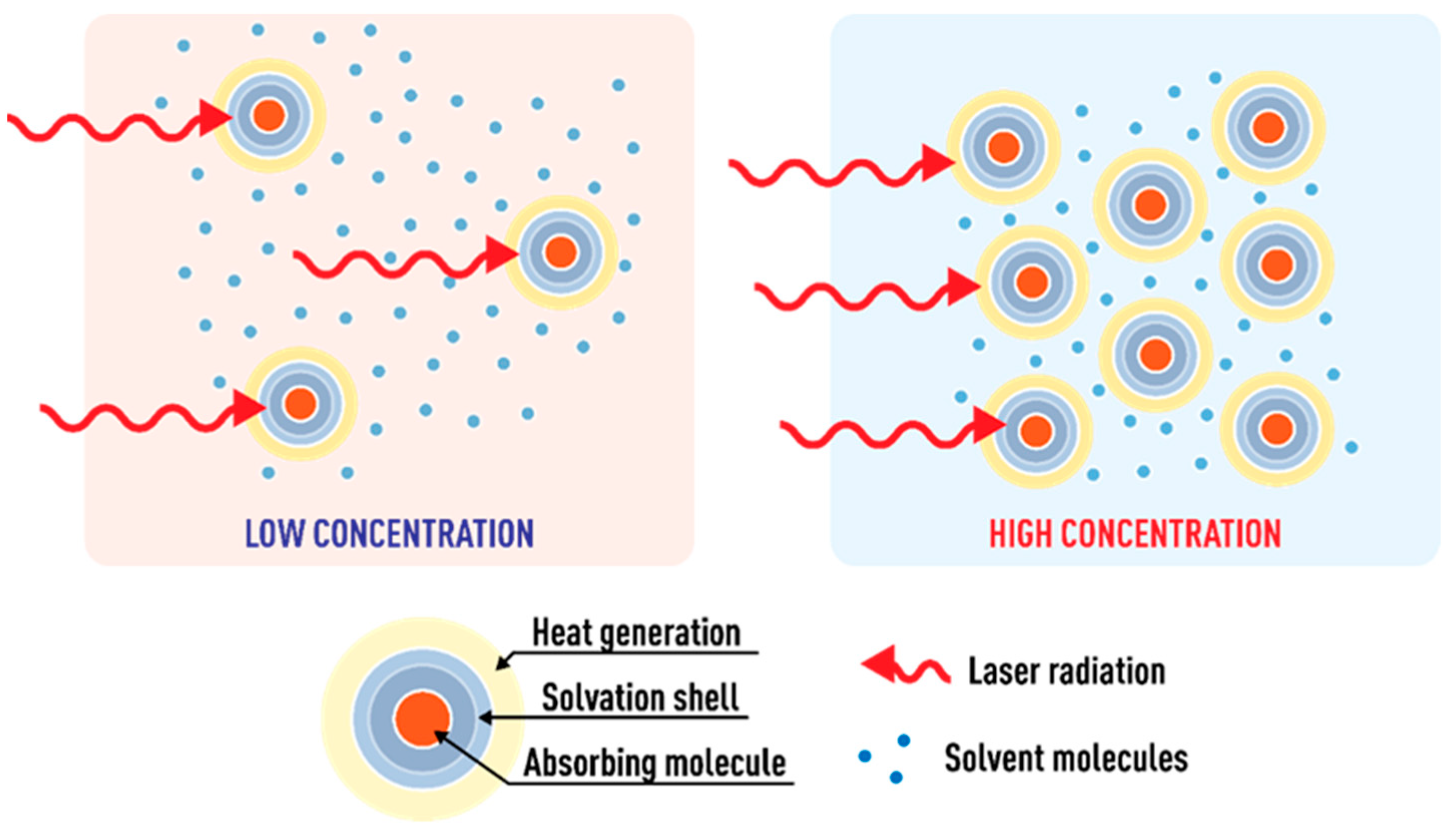
3.4.1. The Colorant Concentration (the Distance between Molecules)
3.4.2. Microimpurities and Dust
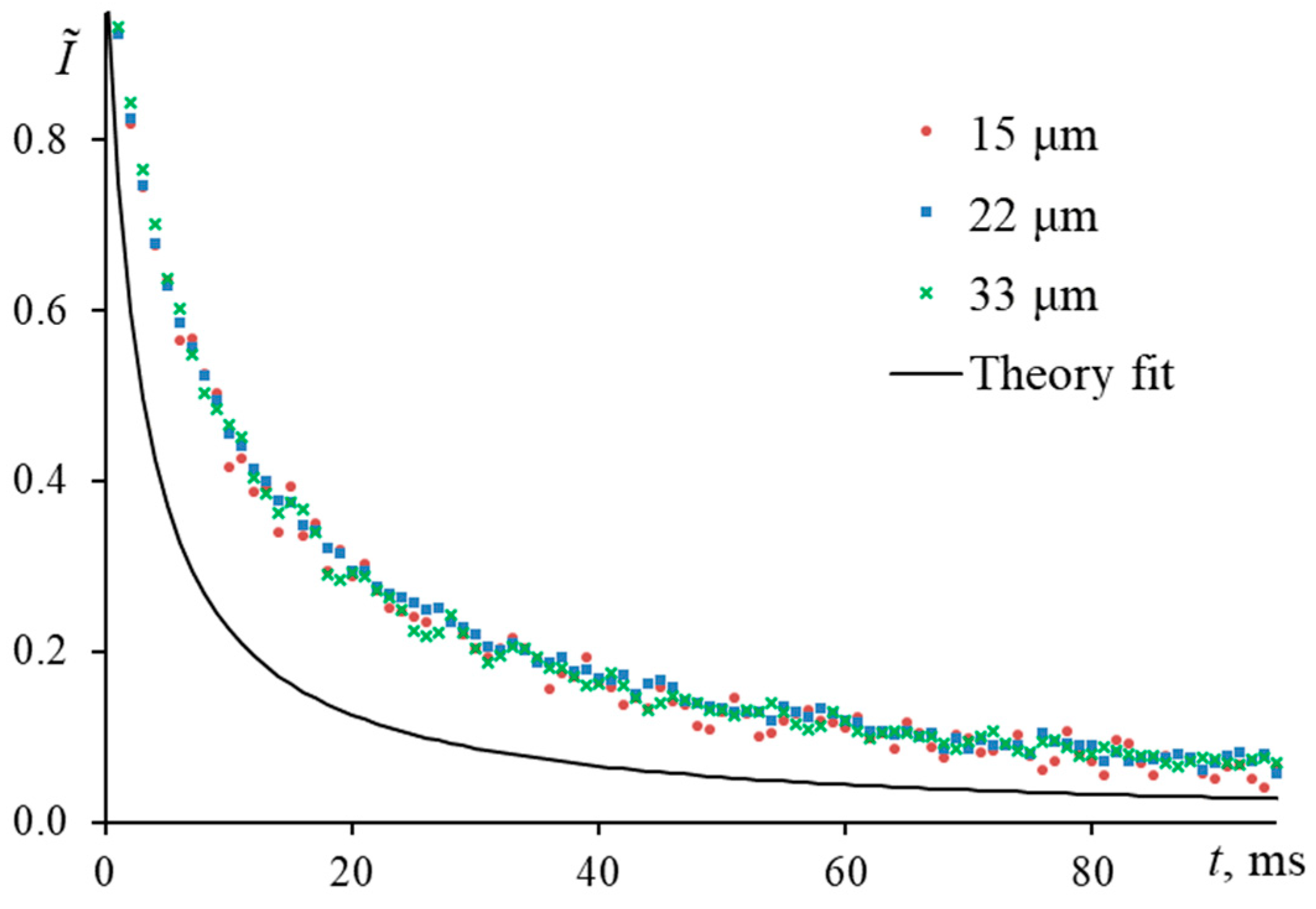
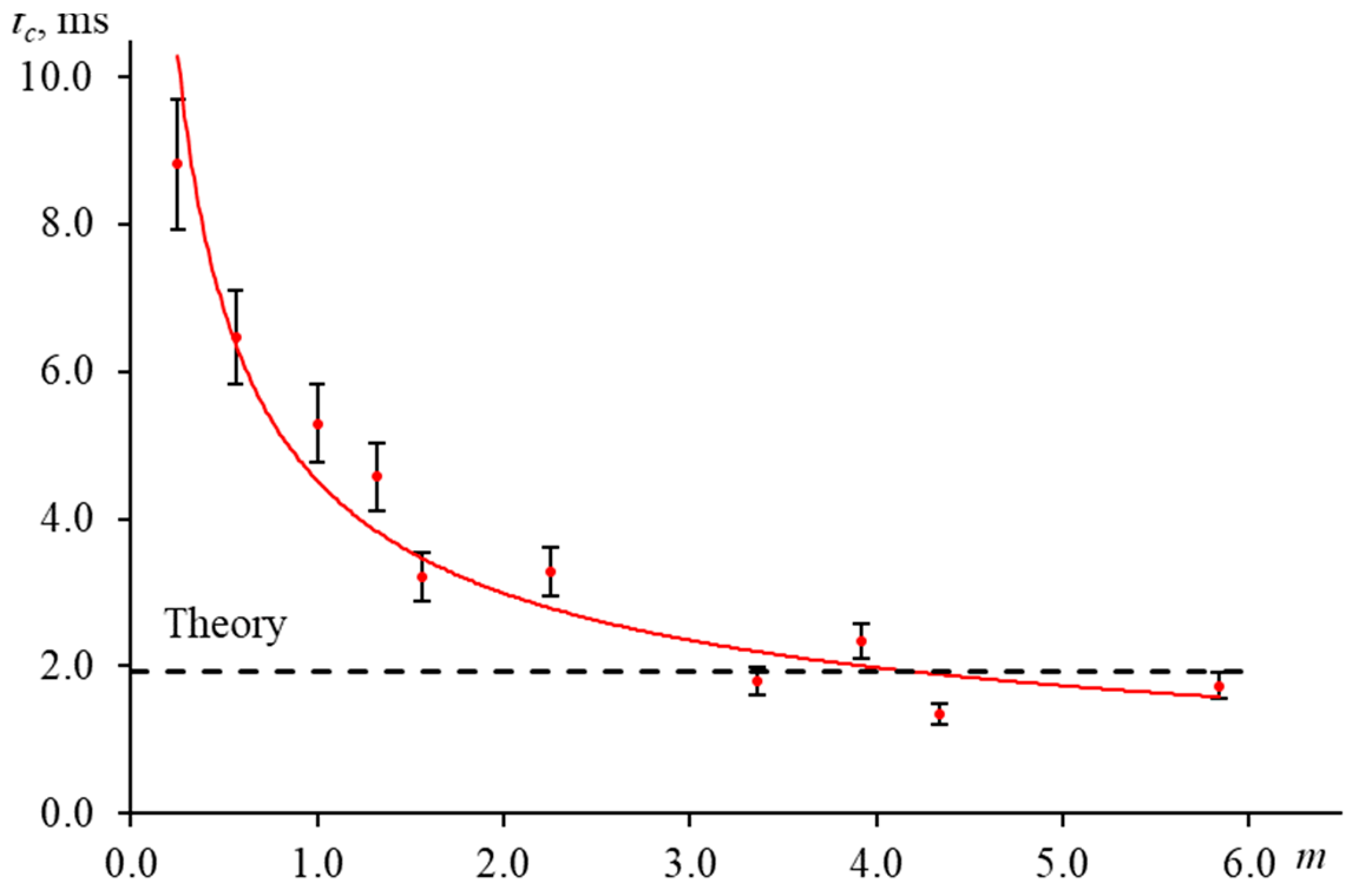
3.5. Time to Reach a Steady-State Thermal Lens
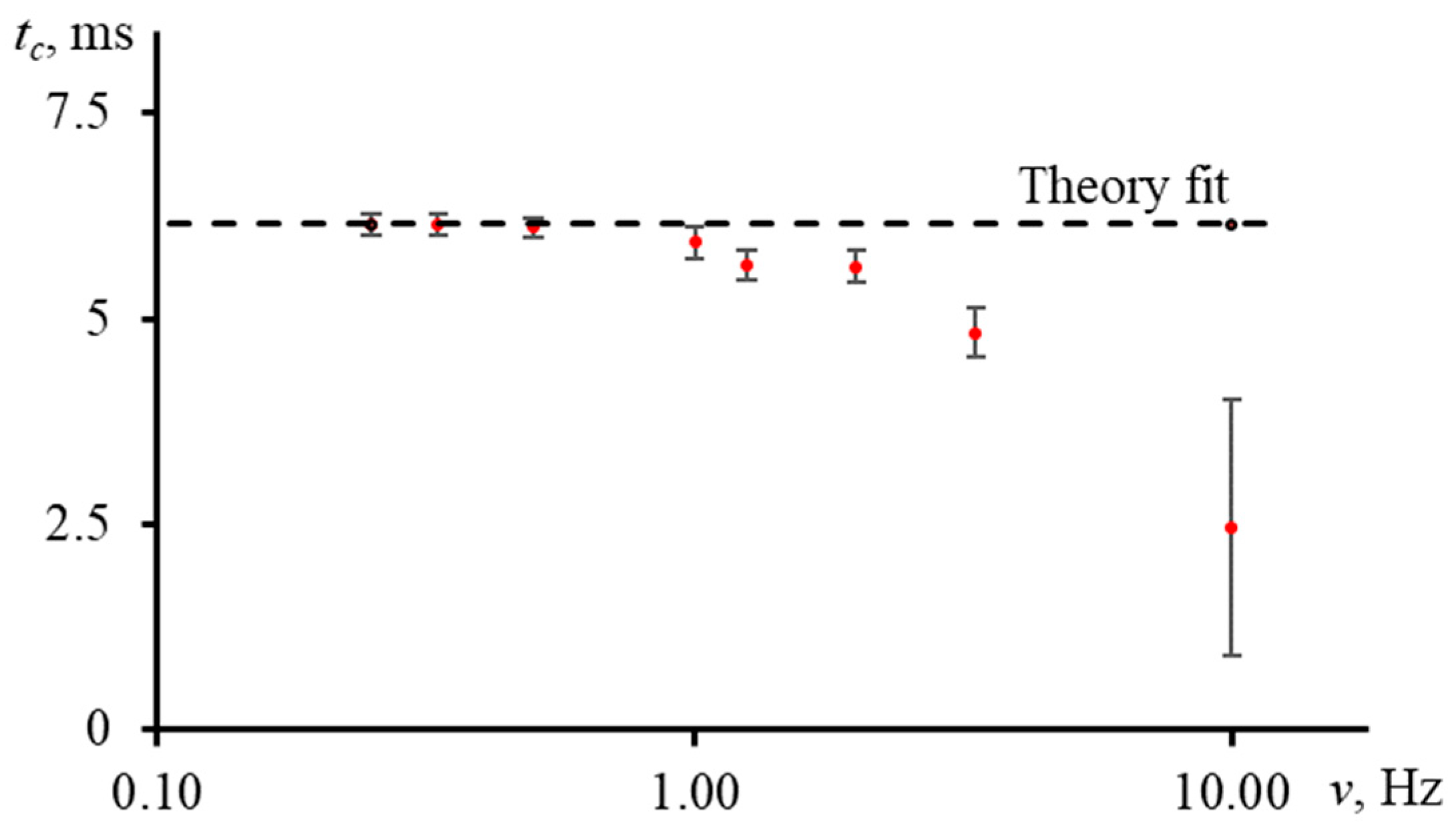
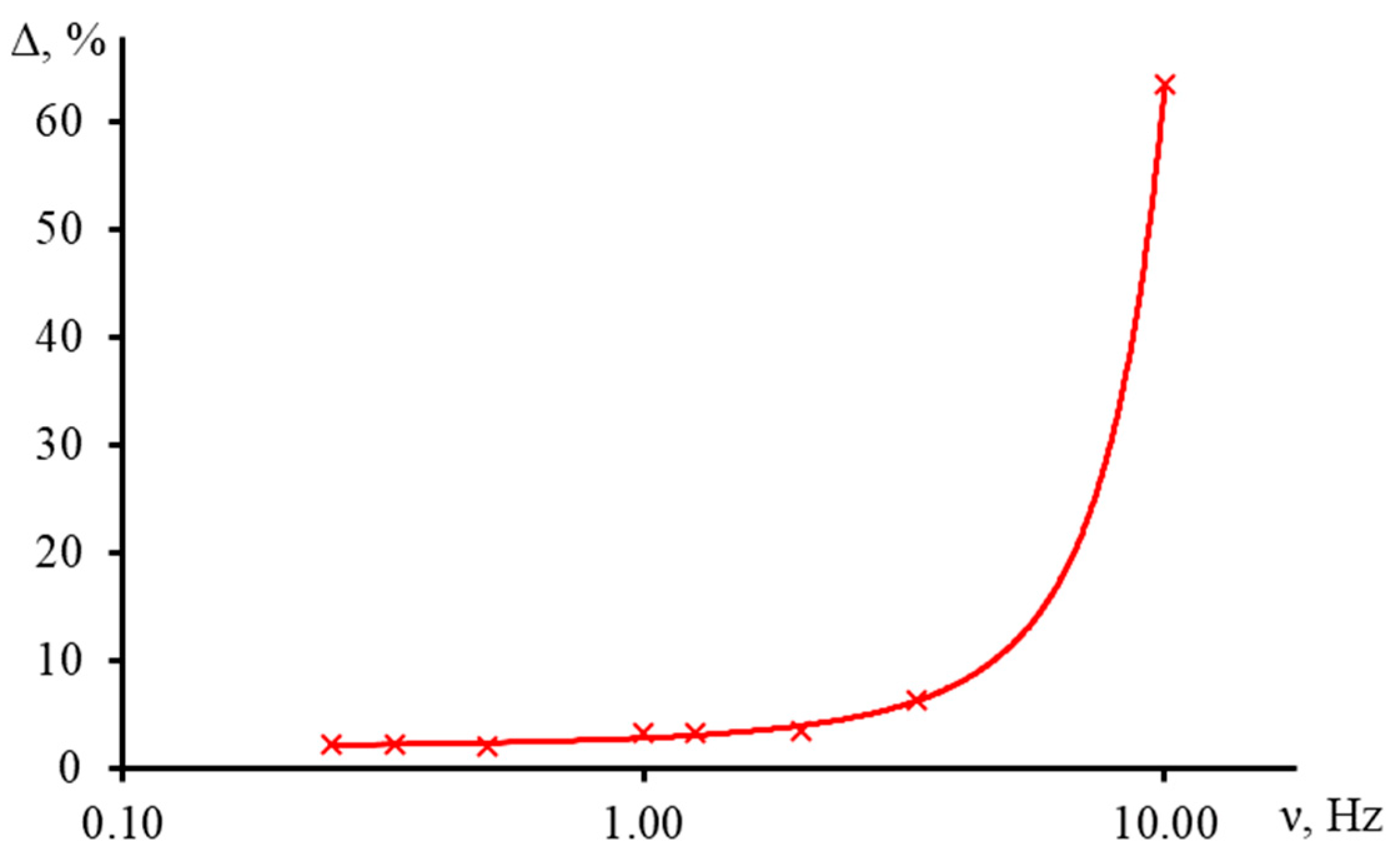
3.5.1. Shutter Frequency
3.5.2. Mode-Mismatch Factor
3.5.3. Measurement Time on the Accuracy of the Characteristic Time
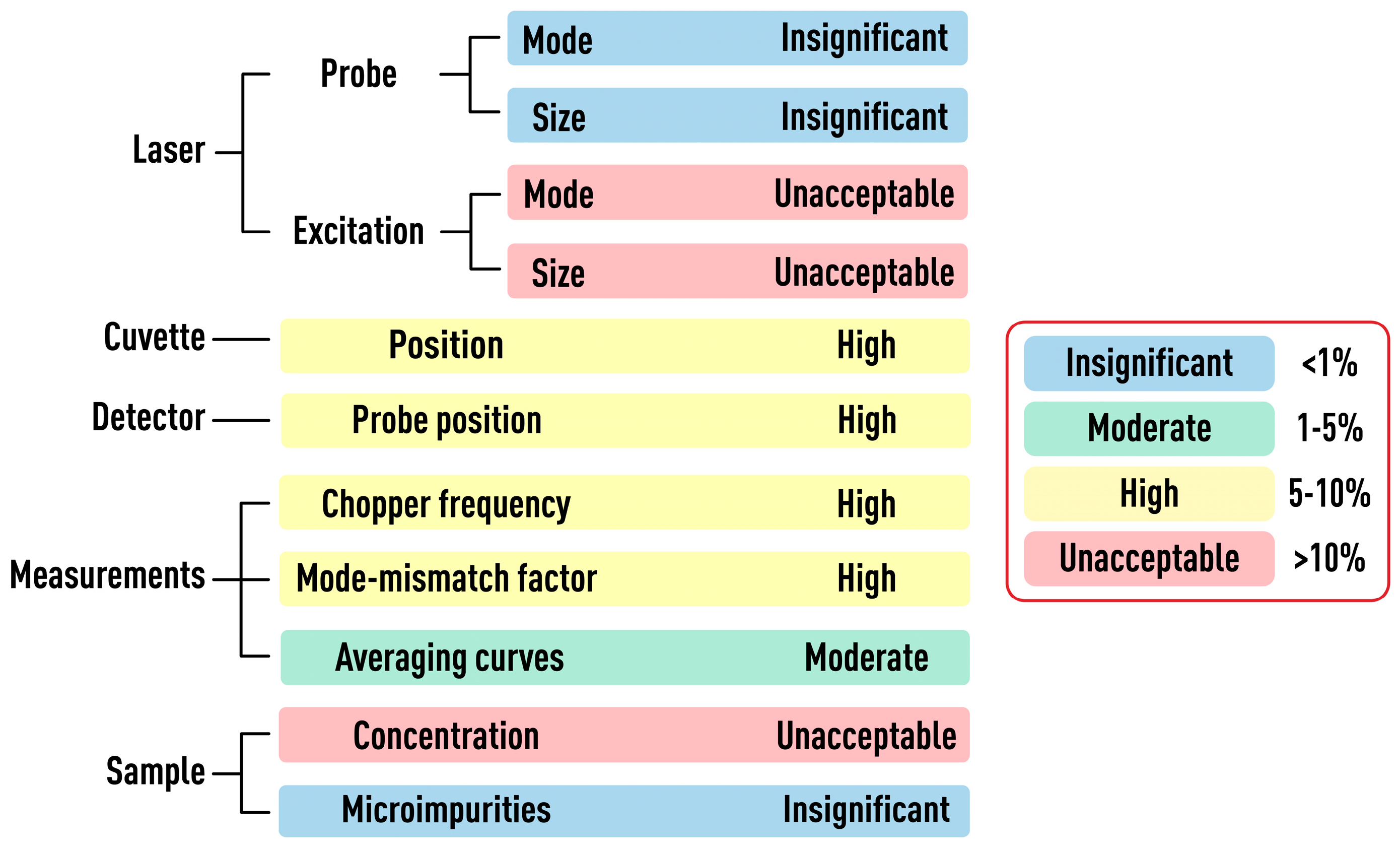
3.6. Summary
- Check of lasers for stability in divergence (at least once per month).
- Check of the radius of the beams in the cell (at least once per month).
- Check of coincidence of the centers of the excitation and probe beams (1.5–2 months).
- Check of the location of the excitation beam in the center of the cell with the sample (at least once every three months).
- Check of the location of the center of intensity of the probe beam at the center of the detector at least once every three months).
- Sample measurements (customized): checking the concentration range; working out the required frequency of the shutter; working off the measurement time and the number of transient curves for averaging.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.S.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, J.; Lee, D.; Baik, J.W.; Kim, C. Review on practical photoacoustic microscopy. Photoacoustics 2019, 15, 100141. [Google Scholar] [CrossRef] [PubMed]
- Bialkowski, S.E.; Astrath, N.G.C.; Proskurnin, M.A. Photothermal Spectroscopy Methods; Wiley: Hoboken, NJ, USA, 2019; p. 512. [Google Scholar]
- Franko, M.; Tran, C.D. Thermal Lens Spectroscopy. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Proskurnin, M.A.; Khabibullin, V.R.; Usoltseva, L.O.; Vyrko, E.A.; Mikheev, I.V.; Volkov, D.S. Photothermal and optoacoustic spectroscopy: State of the art and prospects. Phys. Uspekhi 2022, 65, 270. [Google Scholar] [CrossRef]
- Franko, M.; Goljat, L.; Liu, M.; Budasheva, H.; Zorz Furlan, M.; Korte, D. Recent Progress and Applications of Thermal Lens Spectrometry and Photothermal Beam Deflection Techniques in Environmental Sensing. Sensors 2023, 23, 472. [Google Scholar] [CrossRef]
- Deus, W.B.; Ventura, M.; Silva, J.R.; Andrade, L.H.C.; Catunda, T.; Lima, S.M. Monitoring of the ester production by near-near infrared thermal lens spectroscopy. Fuel 2019, 253, 1090–1096. [Google Scholar] [CrossRef]
- Constantino, R.; Lenzi, G.G.; Franco, M.G.; Lenzi, E.K.; Bento, A.C.; Astrath, N.G.C.; Malacarne, L.C.; Baesso, M.L. Thermal Lens Temperature Scanning technique for evaluation of oxidative stability and time of transesterification during biodiesel synthesis. Fuel 2017, 202, 78–84. [Google Scholar] [CrossRef]
- Franko, M.; van de Bovenkamp, P.; Bicanic, D. Determination of trans-beta-carotene and other carotenoids in blood plasma using high-performance liquid chromatography and thermal lens detection. J. Chromatogr. B Biomed. Sci. Appl. 1998, 718, 47–54. [Google Scholar] [CrossRef]
- Luterotti, S.; Franko, M.; Bicanic, D. Fast quality screening of vegetable oils by HPLC-thermal lens spectrometric detection. J. Am. Oil Chem. Soc. 2002, 79, 1027–1031. [Google Scholar] [CrossRef]
- Martelanc, M.; Ziberna, L.; Passamonti, S.; Franko, M. Application of high-performance liquid chromatography combined with ultra-sensitive thermal lens spectrometric detection for simultaneous biliverdin and bilirubin assessment at trace levels in human serum. Talanta 2016, 154, 92–98. [Google Scholar] [CrossRef]
- Sato, K.; Yamanaka, M.; Hagino, T.; Tokeshi, M.; Kimura, H.; Kitamori, T. Microchip-based enzyme-linked immunosorbent assay (microELISA) system with thermal lens detection. Lab Chip 2004, 4, 570–575. [Google Scholar] [CrossRef]
- Cassano, C.L.; Mawatari, K.; Kitamori, T.; Fan, Z.H. Thermal lens microscopy as a detector in microdevices. Electrophoresis 2014, 35, 2279–2291. [Google Scholar] [CrossRef] [PubMed]
- Proskurnin, M.A.; Bendrysheva, S.N.; Ragozina, N.; Heissler, S.; Faubel, W.; Pyell, U. Optimization of instrumental parameters of a near-field thermal-lens detector for capillary electrophoresis. Appl. Spectrosc. 2005, 59, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Nedosekin, D.A.; Bendrysheva, S.N.; Faubel, W.; Proskurnin, M.A.; Pyell, U. Indirect thermal lens detection for capillary electrophoresis. Talanta 2007, 71, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Shokoufi, N.; Shemirani, F. Laser induced-thermal lens spectrometry after cloud point extraction for the determination of trace amounts of rhodium. Talanta 2007, 73, 662–667. [Google Scholar] [CrossRef]
- Shokoufi, N.; Hamdamali, A. Laser induced-thermal lens spectrometry in combination with dispersive liquid-liquid microextraction for trace analysis. Anal. Chim. Acta 2010, 681, 56–62. [Google Scholar] [CrossRef]
- Andrade, A.A.; Coutinho, M.F.; de Castro, M.P.P.; Vargas, H.; Rohling, J.H.; Novatski, A.; Astrath, N.G.C.; Pereira, J.R.D.; Bento, A.C.; Baesso, M.L.; et al. Luminescence quantum efficiency investigation of low silica calcium aluminosilicate glasses doped with Eu2O3 by thermal lens spectrometry. J. Non-Cryst. Solids 2006, 352, 3624–3627. [Google Scholar] [CrossRef]
- Figueiredo, M.S.; Santos, F.A.; Yukimitu, K.; Moraes, J.C.S.; Silva, J.R.; Baesso, M.L.; Nunes, L.A.O.; Andrade, L.H.C.; Lima, S.M. Luminescence quantum efficiency at 1.5 μm of Er3+-doped tellurite glass determined by thermal lens spectroscopy. Opt. Mater. 2013, 35, 2400–2404. [Google Scholar] [CrossRef]
- Yu, F.; Kachanov, A.A.; Koulikov, S.; Wainright, A.; Zare, R.N. Ultraviolet thermal lensing detection of amino acids. J. Chromatogr. A 2009, 1216, 3423–3430. [Google Scholar] [CrossRef]
- Ventura, M.; Silva, J.R.; Catunda, T.; Andrade, L.H.C.; Lima, S.M. Identification of overtone and combination bands of organic solvents by thermal lens spectroscopy with tunable Ti: Sapphire laser excitation. J. Mol. Liq. 2021, 328, 115414. [Google Scholar] [CrossRef]
- Silva, J.R.; Andrade, L.H.C.; Lima, S.M.; Guyot, Y.; Giannini, N.; Sheik-Bahae, M. Investigation of allowed and forbidden electronic transitions in rare earth doped materials for laser cooling application by thermal lens spectroscopy. Opt. Mater. 2019, 95, 109195. [Google Scholar] [CrossRef]
- Ventura, M.; Silva, J.R.; Andrade, L.H.C.; Scorza Junior, R.P.; Lima, S.M. Near-near-infrared thermal lens spectroscopy to assess overtones and combination bands of sulfentrazone pesticide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 188, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.M.; Lowe, R.D.; Snook, R.D. Thermal lens investigation of the temperature dependence of the refractive index of aqueous electrolyte solutions. Anal. Chim. Acta 1997, 356, 277–288. [Google Scholar] [CrossRef]
- Colcombe, S.M.; Snook, R.D. Thermal lens investigation of the temperature dependence of the refractive index of organo–aqueous solutions. Anal. Chim. Acta 1999, 390, 155–161. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Chernysh, V.V.; Pakhomova, S.V.; Kononets, M.Y.; Sheshenev, A.A. Investigation of the reaction of copper(I) with 2,9-dimethyl-1,10-phenanthroline at trace level by thermal lensing. Talanta 2002, 57, 831–839. [Google Scholar] [CrossRef]
- Ventura, M.; Deus, W.B.; Silva, J.R.; Andrade, L.H.C.; Catunda, T.; Lima, S.M. Determination of the biodiesel content in diesel/biodiesel blends by using the near-near-infrared thermal lens spectroscopy. Fuel 2018, 212, 309–314. [Google Scholar] [CrossRef]
- Arnaud, N.; Georges, J. Cw-laser thermal lens spectrometry in binary mixtures of water and organic solvents: Composition dependence of the steady-state and time-resolved signals. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004, 60, 1817–1823. [Google Scholar] [CrossRef]
- Arnaud, N.; Georges, J. Investigation of the thermal lens effect in water-ethanol mixtures: Composition dependence of the refractive index gradient, the enhancement factor and the Soret effect. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2001, 57, 1295–1301. [Google Scholar] [CrossRef]
- Kononets, M.Y.; Proskurnin, M.A.; Bendrysheva, S.N.; Chernysh, V.V. Investigation of adsorption of nanogram quantities of iron(II) tris-(1,10-phenanthrolinate) on glasses and silica by thermal lens spectrometry. Talanta 2001, 53, 1221–1227. [Google Scholar] [CrossRef]
- Mikheev, I.V.; Usoltseva, L.O.; Ivshukov, D.A.; Volkov, D.S.; Korobov, M.V.; Proskurnin, M.A. Approach to the Assessment of Size-Dependent Thermal Properties of Disperse Solutions: Time-Resolved Photothermal Lensing of Aqueous Pristine Fullerenes C60and C70. J. Phys. Chem. C 2016, 120, 28270–28287. [Google Scholar] [CrossRef]
- Almeida, A.S.; Rivera, G.; Sousa, C.A.; Santos, F.E.P.; Souza, D.N. Thermal lens spectroscopy dosimetry at high doses using a commercial transparent glass. Radiat. Meas. 2019, 124, 85–90. [Google Scholar] [CrossRef]
- Shahriari, E.; Varnamkhasti, M.G.; Zamiri, R. Characterization of thermal diffusivity and optical properties of Ag nanoparticles. Opt. Int. J. Light Electron Opt. 2015, 126, 2104–2107. [Google Scholar] [CrossRef]
- Sampaio, J.A.; Gama, S.; Baesso, M.L.; Catunda, T. Fluorescence quantum efficiency of Er3+ in low silica calcium aluminate glasses determined by mode-mismatched thermal lens spectrometry. J. Non-Cryst. Solids 2005, 351, 1594–1602. [Google Scholar] [CrossRef]
- Brennetot, R.; Georges, J. Pulsed-laser mode-mismatched dual-beam thermal lens spectrometry: Comparison of the time-dependent and maximum signals with theoretical predictions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54, 111–122. [Google Scholar] [CrossRef]
- Lopes, C.S.; Lenart, V.M.; Turchiello, R.F.; Gómez, S.L. Determination of the Thermal Diffusivity of Plasmonic Nanofluids Containing PVP-Coated Ag Nanoparticles Using Mode-Mismatched Dual-Beam Thermal Lens Technique. Adv. Condens. Matter Phys. 2018, 2018, 3052793. [Google Scholar] [CrossRef]
- Gutierrez Fuentes, R.; Pescador Rojas, J.A.; Jiménez-Pérez, J.L.; Sanchez Ramirez, J.F.; Cruz-Orea, A.; Mendoza-Alvarez, J.G. Study of thermal diffusivity of nanofluids with bimetallic nanoparticles with Au(core)/Ag(shell) structure. Appl. Surf. Sci. 2008, 255, 781–783. [Google Scholar] [CrossRef]
- Saavedra, R.; Soto, C.; Gómez, R.; Muñoz, A. Determination of lead(II) by thermal lens spectroscopy (TLS) using 2-(2’-thiazolylazo)-p-cresol (TAC) as chromophore reagent. Microchem. J. 2013, 110, 308–313. [Google Scholar] [CrossRef]
- Lima, S.M.; Steimacher, A.; Medina, A.N.; Baesso, M.L.; Petrovich, M.N.; Rutt, H.N.; Hewak, D.W. Thermo-optical properties measurements in chalcogenide glasses using thermal relaxation and thermal lens methods. J. Non-Cryst. Solids 2004, 348, 108–112. [Google Scholar] [CrossRef]
- Abbas Ghaleb, K.; Georges, J. Investigation of the optimum optical design for pulsed-laser crossed-beam thermal lens spectrometry in infinite and finite samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004, 60, 863–872. [Google Scholar] [CrossRef]
- Qiu, L.; Ouyang, Y.; Li, F. Experimental Techniques Overview. In Micro and Nano Thermal Transport; Qiu, L., Feng, Y., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 19–45. [Google Scholar]
- Proskurnin, M.A.; Volkov, D.S.; Gor’kova, T.A.; Bendrysheva, S.N.; Smirnova, A.P.; Nedosekin, D.A. Advances in thermal lens spectrometry. J. Anal. Chem. 2015, 70, 249–276. [Google Scholar] [CrossRef]
- Usoltseva, L.O.; Korobov, M.V.; Proskurnin, M.A. Photothermal spectroscopy: A promising tool for nanofluids. J. Appl. Phys. 2020, 128, 190901. [Google Scholar] [CrossRef]
- Mathew, S.; Francis, F.; Joseph, S.A.; Kala, M.S. Enhanced thermal diffusivity of water based ZnO nanoflower/rGO nanofluid using the dual-beam thermal lens technique. Nano-Struct. Nano-Objects 2021, 28, 100784. [Google Scholar] [CrossRef]
- Raj, V.; Swapna, M.S.; Kumar, K.S.; Sankararaman, S. Time series analysis of duty cycle induced randomness in thermal lens system. Optik 2020, 212, 164720. [Google Scholar] [CrossRef]
- Ramírez, J.F.S.; Pérez, J.L.J.; Valdez, R.C.; Orea, A.C.; Fuentes, R.G.; Herrera-Pérez, J.L. Thermal Diffusivity Measurements in Fluids Containing Metallic Nanoparticles using Transient Thermal Lens. Int. J. Thermophys. 2006, 27, 1181–1188. [Google Scholar] [CrossRef]
- Silva, W.C.; Rocha, A.M.; Castro, M.P.P.; Sthel, M.S.; Vargas, H.; David, G.F.; Perez, V.H. Unconventional characterization of biodiesel from several sources by thermal lens spectroscopy to determine thermal diffusivity: Phenomenological correlation among their physicochemical and rheological properties. Fuel 2014, 130, 105–111. [Google Scholar] [CrossRef]
- Savi, E.L.; Herculano, L.S.; Lukasievicz, G.V.B.; Regatieri, H.R.; Torquato, A.S.; Malacarne, L.C.; Astrath, N.G.C. Assessing thermal and optical properties of biodiesel by thermal lens spectrometry: Theoretical and experimental aspects. Fuel 2018, 217, 404–408. [Google Scholar] [CrossRef]
- Starobor, A.V.; Mironov, E.A.; Volkov, M.R.; Karimov, D.N.; Ivanov, I.A.; Lovchev, A.V.; Naumov, A.K.; Semashko, V.V.; Palashov, O.V. Thermal lens investigation in EuF2.11, PrF3, and Na0.38Ho0.62F2.24 crystals for magnetooptical applications. Opt. Mater. 2020, 99, 109542. [Google Scholar] [CrossRef]
- Francis, F.; Anila, E.I.; Joseph, S.A. Dependence of thermal diffusivity on nanoparticle shape deduced through thermal lens technique taking ZnO nanoparticles and nanorods as inclusions in homogeneous dye solution. Optik 2020, 219, 165210. [Google Scholar] [CrossRef]
- Thomas, L.; John, J.; Kumar, B.R.; George, N.A.; Kurian, A. Thermal Diffusivity of Gold Nanoparticle Reduced by Polyvinyl Alcohol Using Dual Beam Thermal Lens Technique. Mater. Today Proc. 2015, 2, 1017–1020. [Google Scholar] [CrossRef]
- Luna-Sánchez, J.L.; Jiménez-Pérez, J.L.; Carbajal-Valdez, R.; Lopez-Gamboa, G.; Pérez-González, M.; Correa-Pacheco, Z.N. Green synthesis of silver nanoparticles using Jalapeño Chili extract and thermal lens study of acrylic resin nanocomposites. Thermochim. Acta 2019, 678, 178314. [Google Scholar] [CrossRef]
- Nideep, T.K.; Ramya, M.; Nampoori, V.P.N.; Kailasnath, M. The size dependent thermal diffusivity of water soluble CdTe quantum dots using dual beam thermal lens spectroscopy. Phys. E Low-Dimens. Syst. Nanostructures 2020, 116, 113724. [Google Scholar] [CrossRef]
- Martins, V.M.; Brasse, G.; Doualan, J.L.; Braud, A.; Camy, P.; Messias, D.N.; Catunda, T.; Moncorgé, R. Thermal conductivity of Nd3+ and Yb3+ doped laser materials measured by using the thermal lens technique. Opt. Mater. 2014, 37, 211–213. [Google Scholar] [CrossRef]
- Martins, V.M.; Messias, D.N.; Doualan, J.L.; Braud, A.; Camy, P.; Dantas, N.O.; Catunda, T.; Pilla, V.; Andrade, A.A.; Moncorgé, R. Thermo-optical properties of Nd3+ doped phosphate glass determined by thermal lens and lifetime measurements. J. Lumin. 2015, 162, 104–107. [Google Scholar] [CrossRef]
- Sampaio, J.A.; Catunda, T.; Gama, S.; Baesso, M.L. Thermo-optical properties of OH-free erbium-doped low silica calcium aluminosilicate glasses measured by thermal lens technique. J. Non-Cryst. Solids 2001, 284, 210–216. [Google Scholar] [CrossRef]
- Anjos, V.; Andrade, A.A.; Bell, M.J.V. Thermal lens investigation in amorphous SiN. Appl. Surf. Sci. 2008, 255, 698–700. [Google Scholar] [CrossRef]
- Ramis-Ramos, G. Analytical characteristics, applications and perspectives in thermal lens spectrometry. Anal. Chim. Acta 1993, 283, 623–634. [Google Scholar] [CrossRef]
- Cedeno, E.; Cabrera, H.; Delgadillo-Lopez, A.E.; Delgado-Vasallo, O.; Mansanares, A.M.; Calderon, A.; Marin, E. High sensitivity thermal lens microscopy: Cr-VI trace detection in water. Talanta 2017, 170, 260–265. [Google Scholar] [CrossRef]
- Shokoufi, N.; Yoosefian, J. Selective determination of Sm (III) in lanthanide mixtures by thermal lens microscopy. J. Ind. Eng. Chem. 2016, 35, 153–157. [Google Scholar] [CrossRef]
- Franko, M.; Liu, M.; Boskin, A.; Delneri, A.; Proskurnin, M.A. Fast Screening Techniques for Neurotoxigenic Substances and Other Toxicants and Pollutants Based on Thermal Lensing and Microfluidic Chips. Anal. Sci. 2016, 32, 23–30. [Google Scholar] [CrossRef]
- Liu, M.; Malovrh, S.; Franko, M. Microfluidic flow-injection thermal-lens microscopy for high-throughput and sensitive analysis of sub-μL samples. Anal. Methods 2016, 8, 5053–5060. [Google Scholar] [CrossRef]
- Solimini, D. Accuracy and sensitivity of the thermal lens method for measuring absorption. Appl. Opt. 1966, 5, 1931–1939. [Google Scholar] [CrossRef]
- Liu, M.; Franko, M. Progress in thermal lens spectrometry and its applications in microscale analytical devices. Crit. Rev. Anal. Chem. 2014, 44, 328–353. [Google Scholar] [CrossRef] [PubMed]
- Mohebbifar, M.R. Study of the effect of temperature on thermophysical properties of ethyl myristate by dual-beam thermal lens technique. Optik 2021, 247, 168000. [Google Scholar] [CrossRef]
- Cruz, R.A.; Filadelpho, M.C.; Castro, M.P.; Andrade, A.A.; Souza, C.M.; Catunda, T. Very low optical absorptions and analyte concentrations in water measured by Optimized Thermal Lens Spectrometry. Talanta 2011, 85, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Novak, U.; Plazl, I.; Franko, M. Optimization of a Thermal Lens Microscope for Detection in a Microfluidic Chip. Int. J. Thermophys. 2013, 35, 2011–2022. [Google Scholar] [CrossRef]
- Hannachi, R. Photothermal lens spectrometry: Experimental optimization and direct quantification of permanganate in water. Sens. Actuat. B 2021, 333, 129542. [Google Scholar] [CrossRef]
- Nossir, N.; Dalil-Essakali, L.; Belafhal, A. Analytical study of flat-topped beam characterization using the thermal lens method in sample liquids. Optik 2018, 166, 323–337. [Google Scholar] [CrossRef]
- Shen, J.; Lowe, R.D.; Snook, R.D. A model for cw laser induced mode-mismatched dual-beam thermal lens spectrometry. Chem. Phys. 1992, 165, 385–396. [Google Scholar] [CrossRef]
- Ivshukov, D.A.; Mikheev, I.V.; Volkov, D.S.; Korotkov, A.S.; Proskurnin, M.A. Two-Laser Thermal Lens Spectrometry with Signal Back-Synchronization. J. Anal. Chem. 2018, 73, 407–426. [Google Scholar] [CrossRef]
- De Araujo, M.A.; Silva, R.; de Lima, E.; Pereira, D.P.; de Oliveira, P.C. Measurement of Gaussian laser beam radius using the knife-edge technique: Improvement on data analysis. Appl. Opt. 2009, 48, 393–396. [Google Scholar] [CrossRef]
- Skinner, D.R.; Whitcher, R.E. Measurement of the radius of a high-power laser beam near the focus of a lens. J. Phys. E Sci. Instrum. 1972, 5, 237–238. [Google Scholar] [CrossRef]
- Khosrofian, J.M.; Garetz, B.A. Measurement of a Gaussian laser beam diameter through the direct inversion of knife-edge data. Appl. Opt. 1983, 22, 3406. [Google Scholar] [CrossRef] [PubMed]
- Tishchenko, K.; Muratova, M.; Volkov, D.; Filichkina, V.; Nedosekin, D.; Zharov, V.; Proskurnin, M. Multi-wavelength thermal-lens spectrometry for high-accuracy measurements of absorptivities and quantum yields of photodegradation of a hemoprotein–lipid complex. Arab. J. Chem. 2017, 10, 781–791. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Slyadnev, M.N.; Tokeshi, M.; Kitamori, T. Optimisation of thermal lens microscopic measurements in a microchip. Anal. Chim. Acta 2003, 480, 79–95. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Kuznetsova, V.V. Optimisation of the optical scheme of a dual-beam thermal lens spectrometer using expert estimation. Anal. Chim. Acta 2000, 418, 101–111. [Google Scholar] [CrossRef]
- Dovichi, N.J. Laser-based microchemical analysis. Rev. Sci. Instrum. 1990, 61, 3653–3668. [Google Scholar] [CrossRef]
- Dovichi, N.J.; Harris, J.M. Thermal lens calorimetry for flowing samples. Anal. Chem. 1981, 53, 689–692. [Google Scholar] [CrossRef]
- Dovichi, N.J.; Harris, J.M. Time-resolved thermal lens calorimetry. Anal. Chem. 1981, 53, 106–109. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Chernysh, V.V.; Filichkina, V.A. Some Metrological Aspects of the Optimization of Thermal-Lens Procedures. J. Anal. Chem. 2004, 59, 818–827. [Google Scholar] [CrossRef]
- Lima, S.M.; Sampaio, J.A.; Catunda, T.; Bento, A.C.; Miranda, L.C.M.; Baesso, M.L. Mode-mismatched thermal lens spectrometry for thermo-optical properties measurement in optical glasses: A review. J. Non-Cryst. Solids 2000, 273, 215–227. [Google Scholar] [CrossRef]
- Ventura, M.; Simionatto, E.; Andrade, L.H.C.; Simionatto, E.L.; Riva, D.; Lima, S.M. The use of thermal lens spectroscopy to assess oil–biodiesel blends. Fuel 2013, 103, 506–511. [Google Scholar] [CrossRef]
- Savi, E.L.; Malacarne, L.C.; Baesso, M.L.; Pintro, P.T.M.; Croge, C.; Shen, J.; Astrath, N.G.C. Investigation into photostability of soybean oils by thermal lens spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 145, 125–129. [Google Scholar] [CrossRef]
- Lenart, V.M.; Astrath, N.G.C.; Turchiello, R.F.; Goya, G.F.; Gómez, S.L. Thermal diffusivity of ferrofluids as a function of particle size determined using the mode-mismatched dual-beam thermal lens technique. J. Appl. Phys. 2018, 123, 085107. [Google Scholar] [CrossRef]
- Kumar, P.; Khan, A.; Goswami, D. Importance of molecular heat convection in time resolved thermal lens study of highly absorbing samples. Chem. Phys. 2014, 441, 5–10. [Google Scholar] [CrossRef]
- Malacarne, L.C.; Astrath, N.G.; Medina, A.N.; Herculano, L.S.; Baesso, M.L.; Pedreira, P.R.; Shen, J.; Wen, Q.; Michaelian, K.H.; Fairbridge, C. Soret effect and photochemical reaction in liquids with laser-induced local heating. Opt. Express 2011, 19, 4047–4058. [Google Scholar] [CrossRef]
- Martín-Biosca, Y.; Medina-Hernández, M.J.; García-Alvarez-Coque, M.C.; Ramis-Ramos, G. Effect of the nature of the solvent on the limit of detection in thermal lens spectrometry. Anal. Chim. Acta 1994, 296, 285–294. [Google Scholar] [CrossRef]
- Cabrera, H.; Goljat, L.; Korte, D.; Marin, E.; Franko, M. A multi-thermal-lens approach to evaluation of multi-pass probe beam configuration in thermal lens spectrometry. Anal. Chim. Acta 2020, 1100, 182–190. [Google Scholar] [CrossRef]
- Prakash, A.; Pathrose, B.P.; Nampoori, V.P.N.; Radhakrishnan, P.; Mujeeb, A. Thermal diffusivity of neutral red dye using dual beam thermal lens technique: A comparison on the effects using nano pulsed laser ablated silver and gold nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2019, 107, 203–208. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Usoltseva, L.O.; Volkov, D.S.; Nedosekin, D.A.; Korobov, M.V.; Zharov, V.P. Photothermal and Heat-Transfer Properties of Aqueous Detonation Nanodiamonds by Photothermal Microscopy and Transient Spectroscopy. J. Phys. Chem. C 2021, 125, 7808–7823. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, N.; Srinivasan, P.; Kumar, S.; Balaguru Rayappan, J.B.; Kailasam, K. Heptazine based organic framework as a chemiresistive sensor for ammonia detection at room temperature. J. Mater. Chem. A 2018, 6, 18389–18395. [Google Scholar] [CrossRef]
- Georges, J. A single and simple mathematical expression of the signal for cw-laser thermal lens spectrometry. Talanta 1994, 41, 2015–2023. [Google Scholar] [CrossRef]
- Augustine, A.K.; Mathew, S.; Girijavallabhan, C.P.; Radhakrishnan, P.; Nampoori, V.P.N.; Kailasnath, M. Size dependent variation of thermal diffusivity of CdSe nanoparticles based nanofluid using laser induced mode-matched thermal lens technique. J. Opt. 2014, 44, 85–91. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P. Cake Filtration and Filter Media Filtration. In Liquid Filtration; Cheremisinoff, N.P., Ed.; Butterworth-Heinemann: Woburn, UK, 1998; pp. 59–87. [Google Scholar]
- Cheremisinoff, N.P. An Introduction to Liquid Filtration. In Liquid Filtration; Cheremisinoff, N.P., Ed.; Butterworth-Heinemann: Woburn, UK, 1998; pp. 1–18. [Google Scholar]
- Li, X.; Ding, X.; Bian, C.; Wu, S.; Chen, M.; Wang, W.; Wang, J.; Cheng, L. Hydrophobic drug adsorption loss to syringe filters from a perspective of drug delivery. J. Pharmacol. Toxicol. Methods 2019, 95, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Jithin, J.; Swapna, M.S.; Sankararaman, S. Effect of duty cycle on photothermal phenomenon—A thermal lens study. Optik 2019, 186, 187–193. [Google Scholar] [CrossRef]





| Property | Value |
|---|---|
| Wavelength, nm | 532.0 ± 1.0 |
| Radiation power, mW | (10.0–500.0) ± 0.3 |
| Polarization | >100:1 |
| Mode, TEM00 | >95% |
| Property | Value |
|---|---|
| Wavelength, nm | 632.8 |
| Divergence of the beam in the cell | <1% |
| Radiation power, mW | 5.00 ± 0.01 |
| Polarization | >500:1 |
| Mode, TEM00 | >95% |
| Parameter | Configuration | ||
|---|---|---|---|
| Narrow-Focused | Middle-Focused | Wide-Focused | |
| Focal length of the excitation laser lens (fe), mm | 200.0 | 300.0 | 400.0 |
| Radius of the excitation laser in the waist (), µm | 33.0 ± 1.0 | 42.0 ± 1.0 | 82.0 ± 1.0 |
| Rayleigh length for excitation laser (), mm | 6.6 ± 0.5 | 10.4 ± 0.5 | 39.7 ± 1.0 |
| Focal length of the probe laser lens (fp), mm | 200.0 | 300.0 | |
| Radius of the probe laser in the waist (), µm | 14.5 ± 0.5 | 23.5 ± 0.5 | |
| Rayleigh length for probe laser (), mm | 1.0 ± 0.5 | 2.7 ± 0.5 | |
| Radius of the probe laser in the cell (), µm | 60.0 ± 1.0 | 60.0 ± 1.0 | 100 ± 1.0 |
| Distance from laser to sample for probe laser, m | 1.0 | 4.2 | |
| Distance from laser to sample for excitation laser, m | 0.7 | 1.2 | |
| Sample-detector distance (), m | 0.60 | 2.3–3.1 | |
| Excitation laser power, mW | 20.0 ± 0.3 | 200.0 ± 0.3 | |
| Probe laser power, mW | 4.95 ± 0.01 | 7.10 ± 0.01 | |
| Shutter frequency, Hz | 1 | ||
| Cell length, mm | 10.00 and 5.00 1 | ||
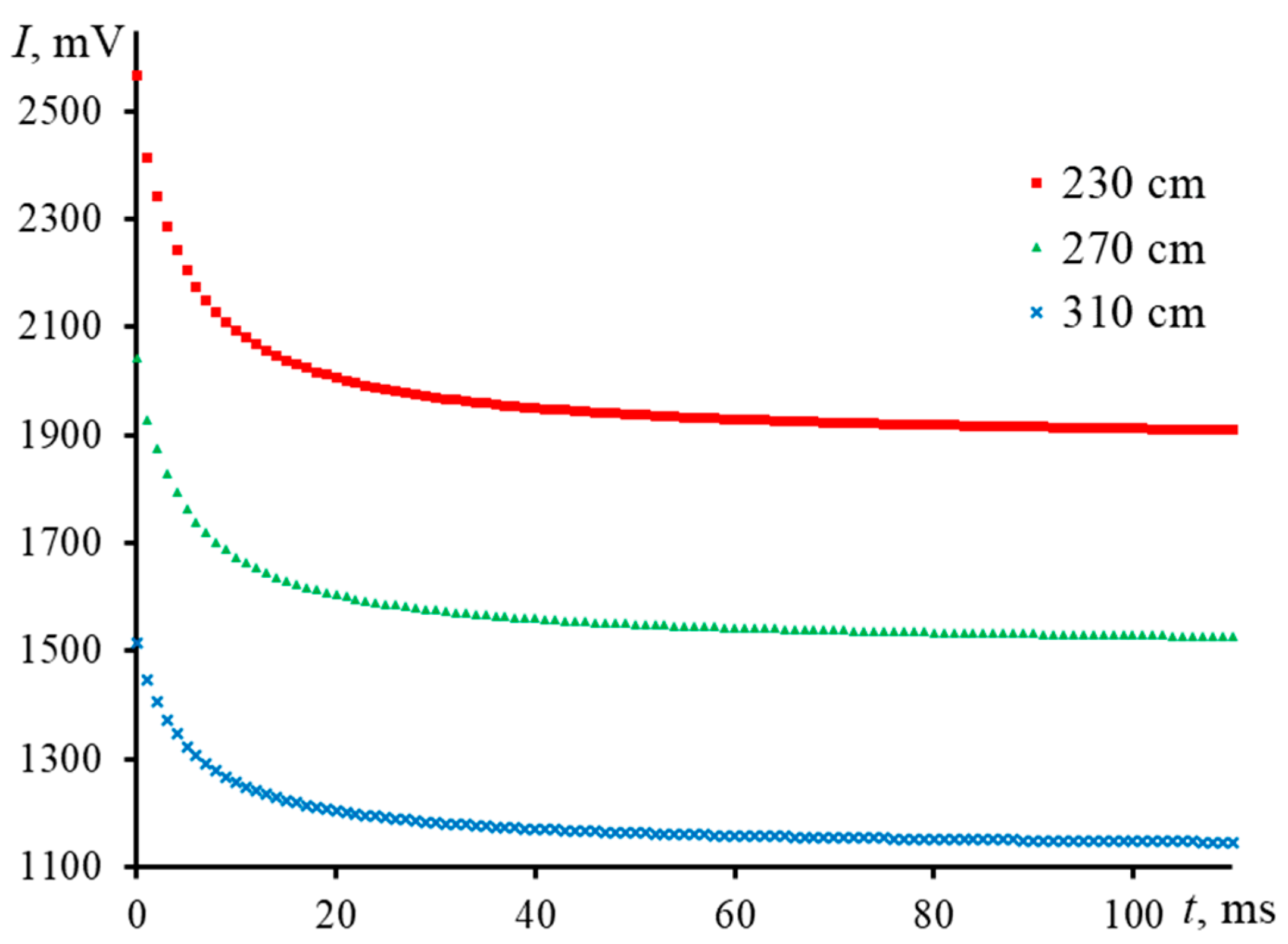
| Z2, cm | RSD, % | D, mm2/s | RSD, % | |
|---|---|---|---|---|
| 230 | 3.12 ± 0.05 | 1.6 | 0.141 ± 0.004 | 2.8 |
| 270 | 3.03 ± 0.07 | 2.3 | 0.145 ± 0.004 | 2.8 |
| 310 | 3.13 ± 0.07 | 2.2 | 0.141 ± 0.003 | 2.1 |
| Ferroin Concentration (μmol/L) | A | ||
|---|---|---|---|
| 0.01 | 0.00011 | 0.0002 | 0.0015 |
| 0.1 | 0.0011 | 0.002 | 0.015 |
| 1 | 0.011 | 0.02 | 0.15 |
| 5 | 0.055 | 0.08 | 0.74 |
| 10 | 0.073 | 0.11 | 0.98 |
| 25 | 0.190 | 0.28 | 2.55 |
| 50 | 0.395 | 0.59 | 5.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khabibullin, V.R.; Franko, M.; Proskurnin, M.A. Accuracy of Measurements of Thermophysical Parameters by Dual-Beam Thermal-Lens Spectrometry. Nanomaterials 2023, 13, 430. https://doi.org/10.3390/nano13030430
Khabibullin VR, Franko M, Proskurnin MA. Accuracy of Measurements of Thermophysical Parameters by Dual-Beam Thermal-Lens Spectrometry. Nanomaterials. 2023; 13(3):430. https://doi.org/10.3390/nano13030430
Chicago/Turabian StyleKhabibullin, Vladislav R., Mladen Franko, and Mikhail A. Proskurnin. 2023. "Accuracy of Measurements of Thermophysical Parameters by Dual-Beam Thermal-Lens Spectrometry" Nanomaterials 13, no. 3: 430. https://doi.org/10.3390/nano13030430
APA StyleKhabibullin, V. R., Franko, M., & Proskurnin, M. A. (2023). Accuracy of Measurements of Thermophysical Parameters by Dual-Beam Thermal-Lens Spectrometry. Nanomaterials, 13(3), 430. https://doi.org/10.3390/nano13030430







