Core and Shell Contributions to the Phonon Spectra of CdTe/CdS Quantum Dots
Abstract
1. Introduction
2. Materials and Methods
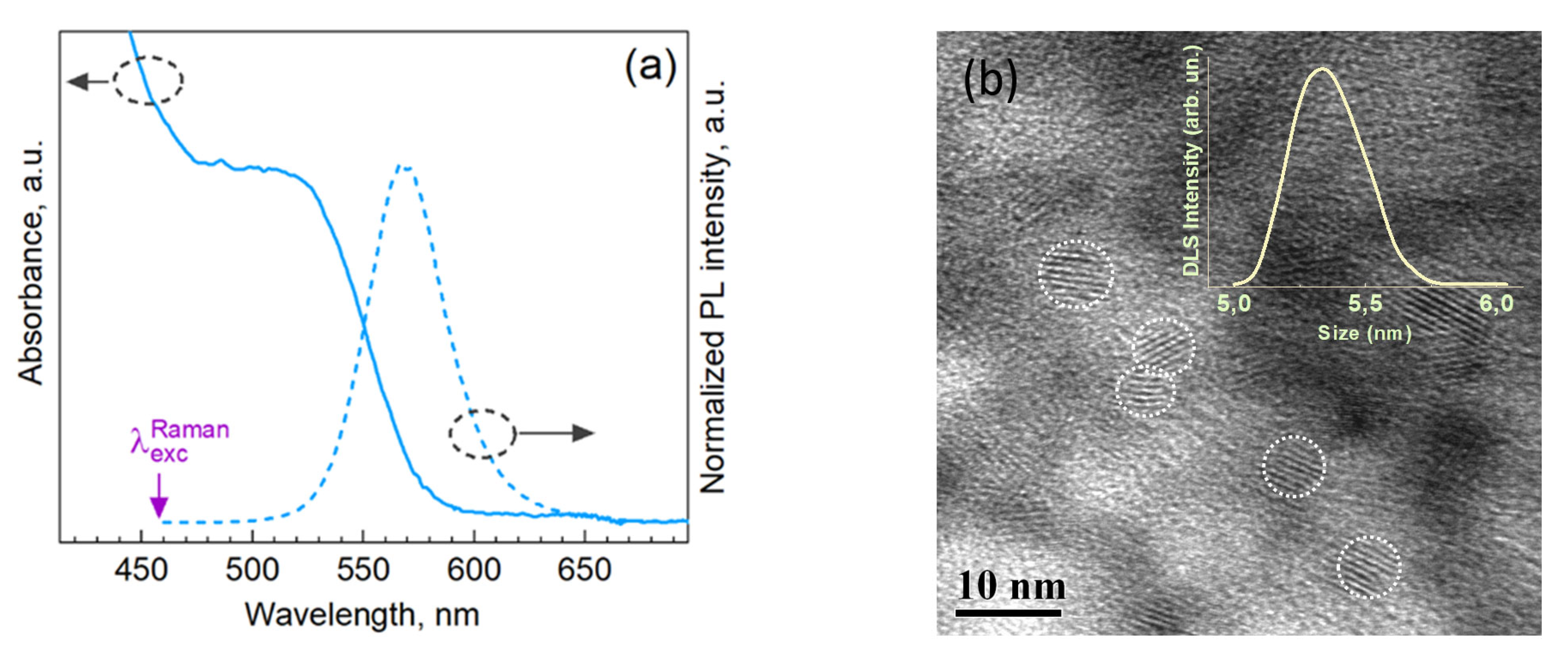
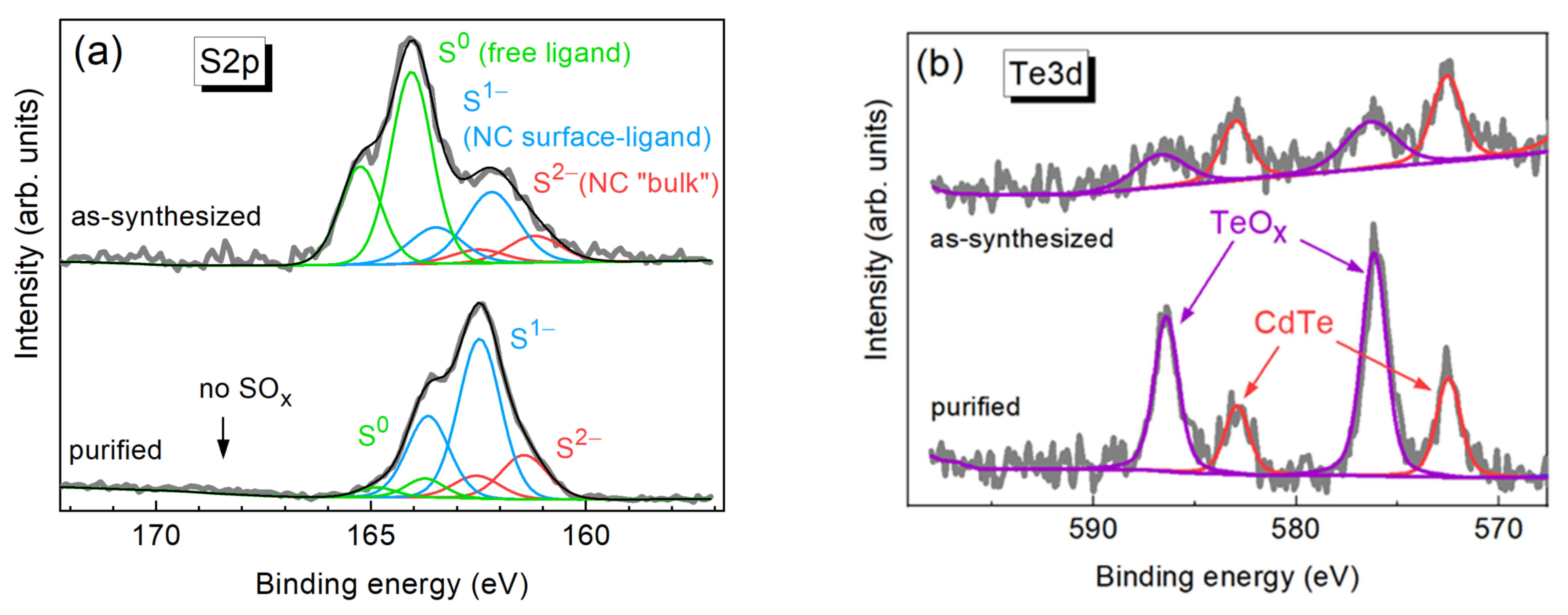
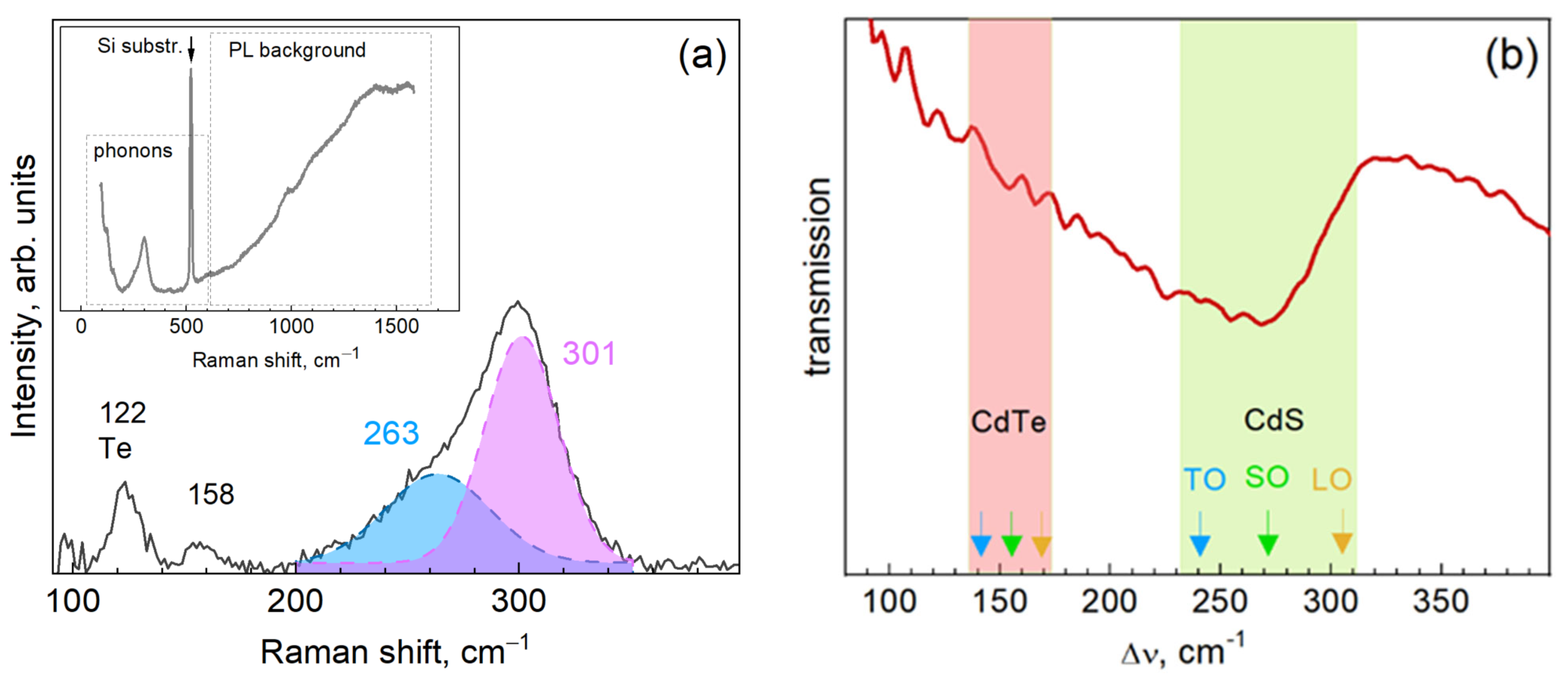
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malecha, K.; Lesiak, A.; Drzozga, K.; Cabaj, J.; Ba, M.; Podhorodecki, A. Optical Sensors Based on II-VI Quantum Dots. Nanomaterials 2019, 9, 192. [Google Scholar] [CrossRef]
- Ingrosso, C.; Panniello, A.M.; Comparelli, R.; Curri, M.L.; Striccoli, M. Colloidal inorganic nanocrystal based nanocomposites: Functional materials for micro and nanofabrication. Materials 2010, 3, 1316–1352. [Google Scholar] [CrossRef]
- Vyhnan, N.; Khalavka, Y. Size-dependent temperature sensitivity of photoluminescence peak position of CdTe quantum dots. Luminescence 2014, 29, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Enders, F.; Budweg, A.; Zeng, P.; Lauth, J.; Smith, T.A.; Brida, D.; Boldt, K. Switchable dissociation of excitons bound at strained CdTe/CdS interfaces. Nanoscale 2018, 10, 22362–22373. [Google Scholar] [CrossRef]
- Boldt, K.; Jander, S.; Hoppe, K.; Weller, H. Characterization of the organic ligand shell of semiconductor quantum dots by fluorescence quenching experiments. ACS Nano 2011, 5, 8115–8123. [Google Scholar] [CrossRef]
- Zeng, P.; Kirkwood, N.; Mulvaney, P.; Boldt, K.; Smith, T.A. Shell effects on hole-coupled electron transfer dynamics from CdSe/CdS quantum dots to methyl viologen. Nanoscale 2016, 8, 10380–10387. [Google Scholar] [CrossRef]
- Gorris, F.E.S.; Deffner, M.; Priyadarshi, S.; Klinke, C.; Weller, H.; Lange, H. Postdeposition Ligand Exchange Allows Tuning the Transport Properties of Large-Scale CuInSe 2 Quantum Dot Solids. Adv. Opt. Mater. 2020, 8, 1901058. [Google Scholar] [CrossRef]
- Tetyorkin, V.V.; Sukach, A.V.; Boiko, V.A.; Tkachuk, A.I. Characterization of grain boundaries in CdTe polycrystalline films. Semicond. Phys. Quantum Electron. Optoelectron. 2015, 18, 428–432. [Google Scholar] [CrossRef]
- Tschirner, N.; Lange, H.; Lambert, K.; Hens, Z.; Thomsen, C.; Colloidal, I.; Ncs, P. Raman spectroscopy of PbTe/CdTe nanocrystals. Phys. Status Solidi B 2011, 248, 2748–2750. [Google Scholar] [CrossRef]
- Azhniuk, Y.M.; Dzhagan, V.M.; Raevskaya, A.E.; Stroyuk, A.L.; Kuchmiy, S.Y.; Valakh, M.Y.; Zahn, D.R.T. Optical studies of CdSe/HgSe and CdSe/Ag 2 Se core/shell nanoparticles embedded in gelatin. J. Phys. Condens. Matter 2008, 20, 455203. [Google Scholar] [CrossRef]
- Dzhagan, V.M.; Azhniuk, Y.M.; Milekhin, A.G.; Zahn, D.R.T. Vibrational spectroscopy of compound semiconductor nanocrystals. J. Phys. D Appl. Phys. 2018, 51, 503001. [Google Scholar] [CrossRef]
- Boldt, K. Raman spectroscopy of colloidal semiconductor nanocrystals. Nano Futur. 2022, 6, 012003. [Google Scholar] [CrossRef]
- Tschirner, N.; Lange, H.; Schliwa, A.; Biermann, A.; Thomsen, C.; Lambert, K.; Gomes, R.; Hens, Z. Interfacial alloying in CdSe/CdS heteronanocrystals, a Raman spectroscopy analysis. Chem. Mater. 2012, 24, 311–318. [Google Scholar] [CrossRef]
- Dzhagan, V.M.; Valakh, M.Y.; Milekhin, A.G.; Yeryukov, N.A.; Zahn, D.R.T.; Cassette, E.; Pons, T.; Dubertret, B. Raman-and IR-active phonons in CdSe/CdS core/shell nanocrystals in the presence of interface alloying and strain. J. Phys. Chem. C 2013, 117, 18225–18233. [Google Scholar] [CrossRef]
- Cavanaugh, P.; Jen-La Plante, I.; Ippen, C.; Ma, R.; Kelley, D.F.; Kelley, A.M. Resonance Raman Study of Shell Morphology in InP/ZnSe/ZnS Core/Shell/Shell Nanocrystals. J. Phys. Chem. C 2021, 125, 10549–10557. [Google Scholar] [CrossRef]
- Silva, A.C.A.; Neto, E.S.F.; da Silva, S.W.; Morais, P.C.; Dantas, N.O. Modified Phonon Confinement Model and Its Application to CdSe/CdS Core–Shell Magic-Sized Quantum Dots Synthesized in Aqueous Solution by a New Route. J. Phys. Chem. C 2013, 117, 1904–1914. [Google Scholar] [CrossRef]
- Freire, P.T.C.; Silva, M.A.A.; Reynoso, V.C.S.; Vaz, A.R.; Lemos, V. Pressure Raman scattering of CdTe quantum dots. Phys. Rev. B 1997, 55, 6743. [Google Scholar] [CrossRef]
- Valakh, M.Y.; Litvinchuk, A.P. Resonance Raman Scattering in Solid Solutions of Semiconductors: The Effect of the polaron constants. Sov. Phys. Solid State 1985, 27, 1958–1961. [Google Scholar]
- Azhniuk, Y.M.; Hutych, Y.I.; Lopushansky, V.V.; Prymak, M.V.; Gomonnai, A.V.; Zahn, D.R.T. Raman Spectra of Quaternary CdS1-x-ySexTey Nanocrystals Embedded in Borosilicate Glass. Int. J. Spectrosc. 2012, 2012, 495896. [Google Scholar] [CrossRef]
- Azhniuk, Y.M.; Lopushansky, V.V.; Prymak, M.V.; Gomonnai, A.V.; Zahn, D.R.T. Glass-embedded quaternary CdSxSe1-x-yTey nanocrystals: Chemical composition derived from the Raman band intensities. J. Raman Spectrosc. 2017, 48, 485–493. [Google Scholar] [CrossRef]
- Raevskaya, A.E.; Stroyuk, O.L.; Panasiuk, Y.V.; Dzhagan, V.M.; Solonenko, D.I.; Schulze, S.; Zahn, D.R.T. A new route to very stable water-soluble ultra-small core/shell CdSe/CdS quantum dots. Nano-Struct. Nano-Objects 2018, 13, 146–154. [Google Scholar] [CrossRef]
- Lin, M.; Miscuglio, M.; Polovitsyn, A.; Leng, Y.; Mart, B.; Moreels, I.; Tan, P.; Krahne, R. Giant-Shell CdSe/CdS Nanocrystals: Exciton Coupling to Shell Phonons Investigated by Resonant Raman Spectroscopy. J. Phys. Chem. Lett. 2019, 10, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Lange, H.; Machón, M.; Artemyev, M.; Woggon, U.; Thomsen, C. Effect of ZnS shell on the Raman spectra from CdSe nanorods. Phys. Status Solidi—Rapid Res. Lett. 2007, 1, 274–276. [Google Scholar] [CrossRef]
- Schreder, B.; Schmidt, T.; Ptatschek, V.; Winkler, U.; Materny, A.; Umbach, E.; Lerch, M.; Muller, G.; Kiefer, W.; Spanhel, L. CdTe/CdS clusters with “core-shell” structure in colloids and films: The path of formation and thermal breakup. J. Phys. Chem. B 2000, 104, 1677–1685. [Google Scholar] [CrossRef]
- Dzhagan, V.; Valakh, M.; Mel’nik, N.; Rayevska, O.; Lokteva, I.; Kolny-Olesiak, J.; Zahn, D.R.T. Phonon Spectra of Small Colloidal II-VI Semiconductor Nanocrystals. Int. J. Spectrosc. 2012, 2012, 532385-1-6. [Google Scholar] [CrossRef]
- Kulakovich, O.S.; Korbutyak, D.V.; Kalytchuk, S.M.; Budzulyak, S.I.; Kapush, O.A.; Trishchuk, L.I.; Vaschenko, S.V.; Stankevich, V.V.; Ramanenka, A.A. Influence of conditions for synthesis of CdTe nanocrystals on their photoluminescence properties and plasmon effects. J. Appl. Spectrosc. 2012, 79, 774–780. [Google Scholar] [CrossRef]
- Dzhagan, V.; Kapush, O.; Isaieva, O.; Budzulyak, S.; Magda, O.; Kogutyuk, P.P.; Trishchuk, L.; Yefanov, V.; Valakh, M.; Yukhymchuk, V. Tuning the photoluminescence of CdTe quantum dots by controllable coupling to plasmonic Au nanoparticles. Phys. Chem. Solid State 2022, 4, 720–727. [Google Scholar] [CrossRef]
- Piryatinski, Y.P.; Malynovskyi, M.B.; Sevryukova, M.M.; Verbitsky, A.B.; Kapush, O.A.; Rozhin, A.G.; Lutsyk, P.M. Mixing of Excitons in Nanostructures Based on a Perylene Dye with CdTe Quantum Dots. Materials 2023, 16, 552. [Google Scholar] [CrossRef]
- Gaponik, N.; Talapin, D.V.; Rogach, A.L.; Hoppe, K.; Shevchenko, E.V.; Kornowski, A.; Eychmu, A.; Weller, H. Thiol-Capping of CdTe Nanocrystals: An Alternative to Organometallic Synthetic Routes. J. Phys. Chem. B 2002, 106, 7177–7185. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Khort, P.S.; Kutsevol, N.V.; Prokopets, V.M.; Kapush, O.; Dzhagan, V. Temperature Driven Plasmon-Exciton Coupling in Thermoresponsive Dextran-Graft-PNIPAM/Au Nanoparticle/CdTe Quantum Dots Hybrid Nanosystem. Plasmonics 2021, 16, 1137–1150. [Google Scholar] [CrossRef]
- Selyshchev, O.; Havryliuk, Y.; Valakh, M.Y.; Yukhymchuk, V.O.; Raievska, O.; Stroyuk, O.L.; Dzhagan, V.; Zahn, D.R.T. Raman and X-ray Photoemission Identification of Colloidal Metal Sulfides as Potential Secondary Phases in Nanocrystalline Cu2ZnSnS4 Photovoltaic Absorbers. ACS Appl. Nano Mater. 2020, 3, 5706–5717. [Google Scholar] [CrossRef]
- Dzhagan, V.; Kapush, O.; Mazur, N.; Havryliuk, Y.; Danylenko, M.I.; Budzulyak, S.; Yukhymchuk, V.; Valakh, M.; Litvinchuk, A.; Zahn, D.R.T. Colloidal Cu-Zn-Sn-Te Nanocrystals: Aqueous Synthesis and Raman Spectroscopy Study. Nanomaterials 2021, 11, 2923. [Google Scholar] [CrossRef] [PubMed]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database, NIST. Available online: https://srdata.nist.gov/xps/ (accessed on 14 January 2023).
- Korbutyak, D.V.; Kalytchuk, S.M.; Geru, I.I. Colloidal CdTe and CdSe Quantum Dots: Technology of Preparing and Optical Properties. J. Nanoelectron. Optoelectron. 2009, 4, 174–179. [Google Scholar] [CrossRef]
- Redígolo, M.L.; Arellano, W.A.; Barbosa, L.C.; Cruz, C.H.B.; Cesar, C.L.; Paula, A.M. de Temperature dependence of the absorption spectra in CdTe-doped glasses. Semicond. Sci. Technol. 1999, 14, 58–63. [Google Scholar] [CrossRef]
- Yavorskyi, R.S. Features of Optical Properties of High Stable CdTe Photovoltaic Absorber Layer. Phys. Chem. Solid State 2020, 21, 243–253. [Google Scholar] [CrossRef]
- Kamal, J.S.; Omari, A.; Van Hoecke, K.; Zhao, Q.; Vantomme, A.; Vanhaecke, F.; Capek, R.K.; Hens, Z. Size-Dependent Optical Properties of Zinc Blende Cadmium Telluride Quantum Dots. J. Phys. Chem. C 2012, 116, 5049–5054. [Google Scholar] [CrossRef]
- Bao, H.; Gong, Y.; Li, Z.; Gao, M. Enhancement Effect of Illumination on the Photoluminescence of Water-Soluble CdTe Nanocrystals: Toward Highly Fluorescent CdTe / CdS Core—Shell Structure. Chem. Mater. 2004, 16, 3853–3859. [Google Scholar] [CrossRef]
- Stroyuk, O.L.; Raevskaya, A.E.; Gaponik, N.; Selyshchev, O.; Dzhagan, V.M.; Schulze, S.; Zahn, D.R.T. Origin of the Broadband Photoluminescence of Pristine and Cu+/Ag+-Doped Ultra-small CdS and CdSe/CdS Quantum Dots. J. Phys. Chem. C 2018, 122, 10267–10277. [Google Scholar] [CrossRef]
- Czerlinski, G.H.; Fiess, A.S.D.H.A. A Mixed-valence Copper Complex with Thiol Compounds. J. Am. Chem. Soc. 1958, 1, 2920–2923. [Google Scholar]
- Briggs, D.; Seah, M.P. Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; John Wiley & Sons: Chicheste, UK, 1983. [Google Scholar]
- Drews, D.; Sahm, J.; Richter, W.; Zahn, D.R.T. Molecular-beam-epitaxy growth of CdTe on lnSb (110) monitored in situ by Raman spectroscopy. J. Appl. Phys. 1995, 78, 4060–4065. [Google Scholar] [CrossRef]
- Artamonov, V.V.; Valakh, M.Y.; Strel’chuk, V.V.; Baidullaeva, A.; Mozol’, P.E. Raman scattering by tellurium films on CdTe single crystals. J. Appl. Spectrosc. 1988, 48, 653–655. [Google Scholar] [CrossRef]
- De Paula, A.M.; Barbosa, L.C.; Cruz, C.H.B.; Alves, O.L.; Sanjurjo, J.A.; Cesar, C.L. Size effects on the phonon spectra of quantum dots in CdTe-doped glasses. Appl. Phys. Lett. 1996, 69, 357–359. [Google Scholar] [CrossRef]
- Mak, J.S.W.; Farah, A.A.; Chen, F.; Helmy, A.S. Photonic Crystal Fiber for Efficient Raman Scattering of CdTe Quantum Dots in Aqueous Solution. ACS Nano 2011, 5, 3823–3830. [Google Scholar] [CrossRef] [PubMed]
- Krahne, R.; Chilla, G.; Schüller, C.; Kudera, S.; Tari, D.; De Giorgi, M.; Heitmann, D.; Cingolani, R.; Manna, L. Shape Dependence of the Scattering Processes of Optical Phonons in Colloidal Nanocrystals Detected by Raman Spectroscopy. J. Nanoelectron. Optoelectron. 2006, 1, 104–107. [Google Scholar] [CrossRef]
- Barbosa, L.C.; Reynoso, V.C.S.; De Paula, A.M.; De Oliveira, C.R.M.; Alves, O.L.; Craievich, A.F.; Marotti, R.E.; Cruz, C.H.B.; Cesar, C.L. CdTe quantum dots by melt heat treatment in borosilicate glasses. J. Non. Cryst. Solids 1997, 219, 205–211. [Google Scholar] [CrossRef]
- Gilic, M.; Romcevic, N.; Romcevic, M.; Stojanovic, D.; Kostic, R.; Trajic, J.; Dobrowolski, W.D.; Karczewski, G.; Galazka, R. Optical properties of CdTe/ZnTe self-assembled quantum dots: Raman and photoluminescence spectroscopy. J. Alloys Compd. 2013, 579, 330–335. [Google Scholar] [CrossRef]
- Dzhagan, V.; Valakh, M.Y.; Kolny-Olesiak, J.; Lokteva, I.; Zahn, D.R.T. Resonant Raman study of phonons in high-quality colloidal CdTe nanoparticles. Appl. Phys. Lett. 2009, 94, 243101. [Google Scholar] [CrossRef]
- Azhniuk, Y.M.; Lopushansky, V.V.; Hutych, Y.I.; Prymak, M.V.; Gomonnai, A.V.; Zahn, D.R.T. Precipitates of selenium and tellurium in II-VI nanocrystal-doped glass probed by Raman scattering. Phys. Status Solidi B 2011, 248, 674–679. [Google Scholar] [CrossRef]
- Zahn, D.R.T.; Mackey, K.J.; Williams, R.H.; Munder, H.; Geurts, J.; Richter, W. Formation of interfacial layers in InSb- CdTe heterostructures studied by Raman scattering. Appl. Phys. Lett. 2020, 50, 742–744. [Google Scholar] [CrossRef]
- Fischer, A.; Feng, Z.; Bykov, E.; Contreras-Puente, G.; Compaan, A.; Mason, A. Optical phonons in laser-deposited CdSxTe1-x films. Appl. Phys. Lett. 2021, 70, 3239–3241. [Google Scholar] [CrossRef]
- Kostić, R.; Romčević, N. Raman spectroscopy of CdS nanoparticles. Phys. Status Solidi C 2004, 1, 2646–2649. [Google Scholar] [CrossRef]
- Silva, A.C.A.; da Silva, S.W.; Morais, P.C.; Dantas, N.O. Shell Thickness Modulation in Ultrasmall CdSe/CdSxSe1-x/CdS Core/Shell QuantumDots via 1-Thioglycerol. ACS Nano 2014, 8, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Kelley, D.F.; Kelley, A.M. Resonance Raman excitation profiles of CdS in pure CdS and CdSe/CdS core/shell quantum dots: CdS-localized excitons. J. Chem. Phys. 2017, 147, 224702. [Google Scholar] [CrossRef] [PubMed]
- Dzhagan, V.; Kempken, B.; Valakh, M.; Parisi, J.; Kolny-Olesiak, J.; Zahn, D.R.T. Probing the structure of CuInS2-ZnS core-shell and similar nanocrystals by Raman spectroscopy. Appl. Surf. Sci. 2017, 395, 24–28. [Google Scholar] [CrossRef]
- Pandya, R.; Chen, R.; Cheminal, A.; Dufour, M.; Richter, J.M.; Thomas, T.H.; Ahmed, S.; Sadhanala, A.; Booker, E.P.; Divitini, G.; et al. Exciton-Phonon Interactions Govern Charge-Transfer-State Dynamics in CdSe/CdTe Two-Dimensional Colloidal Heterostructures. J. Am. Chem. Soc. 2018, 140, 14097–14111. [Google Scholar] [CrossRef]
- Ca, N.X.; Hien, N.T.; Luyen, N.T.; Lien, V.T.K.; Thanh, L.D.; Do, P.V.; Bau, N.Q.; Pham, T.T. Photoluminescence properties of CdTe/CdTeSe/CdSecore/alloyed/shell type-II quantum dots. J. Alloys Compd. 2019, 787, 823–830. [Google Scholar] [CrossRef]
- Gong, K.; Kelley, D.F.; Kelley, A.M. Nonuniform Excitonic Charge Distribution Enhances Exciton-Phonon Coupling in ZnSe/CdSe Alloyed Quantum Dots. J. Phys. Chem. Lett. 2017, 8, 626–630. [Google Scholar] [CrossRef]
- Polit, J.; Sheregii, E.M.; Cebulski, J.; Robouch, B.V.; Marcelli, A.; Guidi, M.C.; Burattini, E.; Piccinini, M.; Mycielski, A.; Kisiel, A.; et al. Phonon and vibrational spectra of hydrogenated CdTe. J. Appl. Phys. 2008, 100, 013521. [Google Scholar] [CrossRef]
- Rolo, A.G.; Vasilevskiy, M.I.; Gaponik, N.P.; Rogach, A.L.; Gomes, M.J.M. Confined optical vibrations in CdTe quantum dots and clusters. Phys. Status Solidi Basic Res. 2002, 229, 433–437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzhagan, V.; Mazur, N.; Kapush, O.; Selyshchev, O.; Karnaukhov, A.; Yeshchenko, O.A.; Danylenko, M.I.; Yukhymchuk, V.; Zahn, D.R.T. Core and Shell Contributions to the Phonon Spectra of CdTe/CdS Quantum Dots. Nanomaterials 2023, 13, 921. https://doi.org/10.3390/nano13050921
Dzhagan V, Mazur N, Kapush O, Selyshchev O, Karnaukhov A, Yeshchenko OA, Danylenko MI, Yukhymchuk V, Zahn DRT. Core and Shell Contributions to the Phonon Spectra of CdTe/CdS Quantum Dots. Nanomaterials. 2023; 13(5):921. https://doi.org/10.3390/nano13050921
Chicago/Turabian StyleDzhagan, Volodymyr, Nazar Mazur, Olga Kapush, Oleksandr Selyshchev, Anatolii Karnaukhov, Oleg A. Yeshchenko, Mykola I. Danylenko, Volodymyr Yukhymchuk, and Dietrich R. T. Zahn. 2023. "Core and Shell Contributions to the Phonon Spectra of CdTe/CdS Quantum Dots" Nanomaterials 13, no. 5: 921. https://doi.org/10.3390/nano13050921
APA StyleDzhagan, V., Mazur, N., Kapush, O., Selyshchev, O., Karnaukhov, A., Yeshchenko, O. A., Danylenko, M. I., Yukhymchuk, V., & Zahn, D. R. T. (2023). Core and Shell Contributions to the Phonon Spectra of CdTe/CdS Quantum Dots. Nanomaterials, 13(5), 921. https://doi.org/10.3390/nano13050921




