Diamane-like Films Based on Twisted G/BN Bilayers: DFT Modelling of Atomic Structures and Electronic Properties
Abstract
1. Introduction
2. Computational Methodology
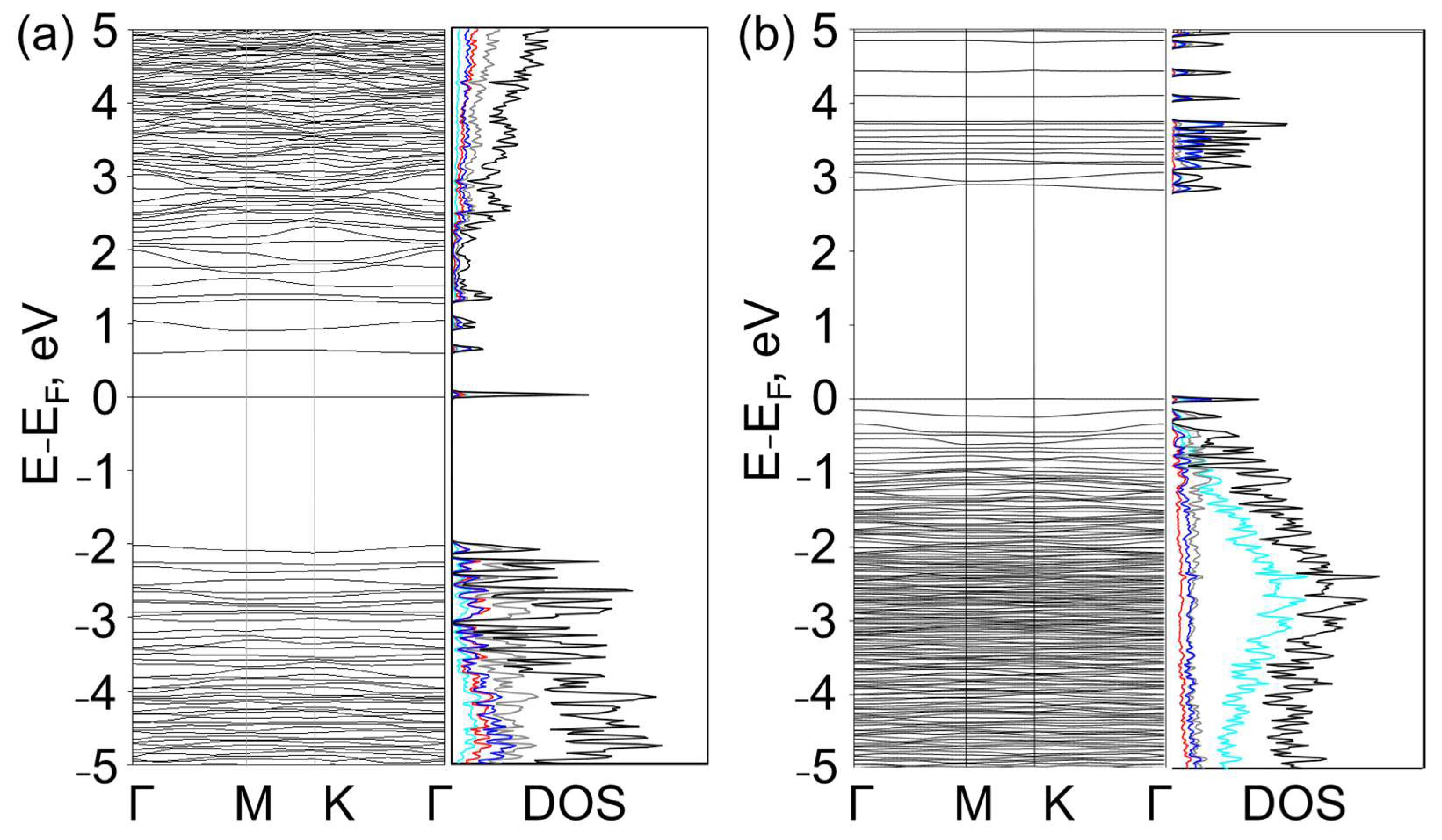
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chernozatonskii, L.A.; Sorokin, P.B.; Kvashnin, A.G.; Kvashnin, D.G. Diamond-like C2H Nanolayer, Diamane: Simulation of the Structure and Properties. JETP Lett. 2009, 90, 134–138. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Demin, V.A.; Kvashnin, D.G. Fully Hydrogenated and Fluorinated Bigraphenes–Diamanes: Theoretical and Experimental Studies. C 2021, 7, 17. [Google Scholar] [CrossRef]
- Kvashnin, A.G.; Sorokin, P.B. Lonsdaleite Films with Nanometer Thickness. J. Phys. Chem. Lett. 2014, 5, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Chernozatonskii, L.A.; Sorokin, P.B.; Kuzubov, A.A.; Sorokin, B.P.; Kvashnin, A.G.; Kvashnin, D.G.; Avramov, P.V.; Yakobson, B.I. Influence of Size Effect on the Electronic and Elastic Properties of Diamond Films with Nanometer Thickness. J. Phys. Chem. C 2011, 115, 132–136. [Google Scholar] [CrossRef]
- Raeisi, M.; Mortazavi, B.; Podryabinkin, E.V.; Shojaei, F.; Zhuang, X.; Shapeev, A.V. High Thermal Conductivity in Semiconducting Janus and Non-Janus Diamanes. Carbon 2020, 167, 51–61. [Google Scholar] [CrossRef]
- Piazza, F.; Monthioux, M.; Puech, P.; Gerber, I.C.; Gough, K. Progress on Diamane and Diamanoid Thin Film Pressureless Synthesis. C 2021, 7, 9. [Google Scholar] [CrossRef]
- Sorokin, P.B.; Yakobson, B.I. Two-Dimensional Diamond—Diamane: Current State and Further Prospects. Nano Lett. 2021, 21, 5475–5484. [Google Scholar] [CrossRef]
- Piazza, F.; Cruz, K.; Monthioux, M.; Puech, P.; Gerber, I. Raman Evidence for the Successful Synthesis of Diamane. Carbon 2020, 169, 129–133. [Google Scholar] [CrossRef]
- Bakharev, P.V.; Huang, M.; Saxena, M.; Lee, S.W.; Joo, S.H.; Park, S.O.; Dong, J.; Camacho-Mojica, D.C.; Jin, S.; Kwon, Y.; et al. Chemically Induced Transformation of Chemical Vapour Deposition Grown Bilayer Graphene into Fluorinated Single-Layer Diamond. Nat. Nanotechnol. 2020, 15, 59–66. [Google Scholar] [CrossRef]
- Ke, F.; Zhang, L.; Chen, Y.; Yin, K.; Wang, C.; Tzeng, Y.-K.; Lin, Y.; Dong, H.; Liu, Z.; Tse, J.S.; et al. Synthesis of Atomically Thin Hexagonal Diamond with Compression. Nano Lett. 2020, 20, 5916–5921. [Google Scholar] [CrossRef]
- Martins, L.G.P.; Matos, M.J.S.; Paschoal, A.R.; Freire, P.T.C.; Andrade, N.F.; Aguiar, A.L.; Kong, J.; Neves, B.R.A.; de Oliveira, A.B.; Mazzoni, M.S.C.; et al. Raman Evidence for Pressure-Induced Formation of Diamondene. Nat. Commun. 2017, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Barboza, A.P.M.; Guimaraes, M.H.D.; Massote, D.V.P.; Campos, L.C.; Neto, N.M.B.; Cancado, L.G.; Lacerda, R.G.; Chacham, H.; Mazzoni, M.S.C.; Neves, B.R.A. Room-Temperature Compression-Induced Diamondization of Few-Layer Graphene. Adv. Mater. 2011, 23, 3014–3017. [Google Scholar] [CrossRef]
- Cellini, F.; Lavini, F.; Cao, T.; de Heer, W.; Berger, C.; Bongiorno, A.; Riedo, E. Epitaxial Two-Layer Graphene under Pressure: Diamene Stiffer than Diamond. FlatChem 2018, 10, 8–13. [Google Scholar] [CrossRef]
- Emelin, E.V.; Cho, H.D.; Korepanov, V.I.; Varlamova, L.A.; Erohin, S.V.; Kim, D.Y.; Sorokin, P.B.; Panin, G.N. Formation of Diamane Nanostructures in Bilayer Graphene on Langasite under Irradiation with a Focused Electron Beam. Nanomaterials 2022, 12, 4408. [Google Scholar] [CrossRef] [PubMed]
- McCann, E.; Koshino, M. The Electronic Properties of Bilayer Graphene. Rep. Prog. Phys. 2013, 76, 056503. [Google Scholar] [CrossRef] [PubMed]
- Laref, A.; Alsagri, M.; Alay-e-Abbas, S.M.; Laref, S.; Huang, H.M.; Xiong, Y.C.; Yang, J.T.; Khandy, S.A.; Rai, D.P.; Varshney, D.; et al. Electronic Structure and Optical Characteristics of AA Stacked Bilayer Graphene: A First Principles Calculations. Optik 2020, 206, 163755. [Google Scholar] [CrossRef]
- Campanera, J.M.; Savini, G.; Suarez-Martinez, I.; Heggie, M.I. Density Functional Calculations on the Intricacies of Moiré Patterns on Graphite. Phys. Rev. B 2007, 75, 235449. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Demin, V.A.; Kvashnin, D.G. Ultrawide-Bandgap Moiré Diamanes Based on Bigraphenes with the Twist Angles Θ ∼ 30°. Appl. Phys. Lett. 2020, 117, 253104. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Katin, K.P.; Demin, V.A.; Maslov, M.M. Moiré Diamanes Based on the Hydrogenated or Fluorinated Twisted Bigraphene: The Features of Atomic and Electronic Structures, Raman and Infrared Spectra. Appl. Surf. Sci. 2021, 537, 148011. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Demin, V.A.; Kvashnin, A.G.; Kvashnin, D.G. Diamane Quasicrystals. Appl. Surf. Sci. 2022, 572, 151362. [Google Scholar] [CrossRef]
- Artyukh, A.A.; Chernozatonskii, L.A. Mechanical Characteristics of Diamond-Like Moiré Films. JETP Lett. 2022, 116, 737–744. [Google Scholar] [CrossRef]
- Chowdhury, S.; Demin, V.A.; Chernozatonskii, L.A.; Kvashnin, A.G. Ultra-Low Thermal Conductivity of Moiré Diamanes. Membranes 2022, 12, 925. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.; Massatt, D.; Fang, S.; Cazeaux, P.; Luskin, M.; Kaxiras, E. Twistronics: Manipulating the Electronic Properties of Two-Dimensional Layered Structures through Their Twist Angle. Phys. Rev. B 2017, 95, 075420. [Google Scholar] [CrossRef]
- Hennighausen, Z.; Kar, S. Twistronics: A Turning Point in 2D Quantum Materials. Electron. Struct. 2021, 3, 014004. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, R.; Li, M.; Li, L.; Geng, D.; Hu, W. Recent Advances in the Controlled Chemical Vapor Deposition Growth of Bilayer 2D Single Crystals. J. Mater. Chem. C 2022, 10, 13324–13350. [Google Scholar] [CrossRef]
- Shu, H.; Liu, X. Tuning Electronic and Optical Properties of Graphene/h-BN Heterobilayer via Surface Modification. Appl. Surf. Sci. 2022, 605, 154591. [Google Scholar] [CrossRef]
- Pinto, A.K.M.; Pontes, J.M.; Matos, M.J.S.; Mazzoni, M.S.C.; Azevedo, S. BCN Diamondol-like Compounds: Stability Trends and Electronic Properties. Comput. Mater. Sci. 2022, 215, 111737. [Google Scholar] [CrossRef]
- Barboza, A.P.M.; Souza, A.C.R.; Matos, M.J.S.; Brant, J.C.; Barbosa, T.C.; Chacham, H.; Mazzoni, M.S.C.; Neves, B.R.A. Graphene/h-BN Heterostructures under Pressure: From van Der Waals to Covalent. Carbon 2019, 155, 108–113. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular-Dynamics Simulation of the Liquid-Metal—Amorphous-Semiconductor Transition in Germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Neek-Amal, M.; Peeters, F.M. Graphene on Boron-Nitride: Moiré Pattern in the van Der Waals Energy. Appl. Phys. Lett. 2014, 104, 041909. [Google Scholar] [CrossRef]
- Liu, J.; Luo, C.; Lu, H.; Huang, Z.; Long, G.; Peng, X. Influence of Hexagonal Boron Nitride on Electronic Structure of Graphene. Molecules 2022, 27, 3740. [Google Scholar] [CrossRef]
- Kvashnin, A.G.; Chernozatonskii, L.A.; Yakobson, B.I.; Sorokin, P.B. Phase Diagram of Quasi-Two-Dimensional Carbon, from Graphene to Diamond. Nano Lett. 2014, 14, 676–681. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, X.C.; Guo, W. Fluorinating Hexagonal Boron Nitride into Diamond-Like Nanofilms with Tunable Band Gap and Ferromagnetism. J. Am. Chem. Soc. 2011, 133, 14831–14838. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Demin, V.A. Diamond-Like Films from Twisted Few-Layer Graphene. JETP Lett. 2022, 115, 161–166. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A.; Katin, K.P.; Kochaev, A.I.; Maslov, M.M. Moiré and Non-Twisted Sp-Hybridized Structures Based on Hexagonal Boron Nitride Bilayers: Ab Initio Insight into Infrared and Raman Spectra, Bands Structures and Mechanical Properties. Appl. Surf. Sci. 2022, 606, 154909. [Google Scholar] [CrossRef]
- Li, P.; Yi Wang, W.; Zou, C.; Gao, X.; Wang, J.; Fan, X.; Song, H.; Li, J. Lattice Distortion Optimized Hybridization and Superlubricity of MoS2/MoSe2 Heterointerfaces via Moiré Patterns. Appl. Surf. Sci. 2023, 613, 155760. [Google Scholar] [CrossRef]
- Kvashnin, D.G.; Kvashnina, O.P.; Avramov, P.V.; Sorokin, P.B.; Kvashnin, A.G. Novel Hybrid C/BN Two-Dimensional Heterostructures. Nanotechnology 2017, 28, 085205. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Arraga, L.A.; Lado, J.L.; Guinea, F.; San-Jose, P. Electrically Controllable Magnetism in Twisted Bilayer Graphene. Phys. Rev. Lett. 2017, 119, 107201. [Google Scholar] [CrossRef] [PubMed]
- Sboychakov, A.O.; Rozhkov, A.V.; Rakhmanov, A.L.; Nori, F. Externally Controlled Magnetism and Band Gap in Twisted Bilayer Graphene. Phys. Rev. Lett. 2018, 120, 266402. [Google Scholar] [CrossRef] [PubMed]
- Leenaerts, O.; Peelaers, H.; Hernández-Nieves, A.D.; Partoens, B.; Peeters, F.M. First-Principles Investigation of Graphene Fluoride and Graphane. Phys. Rev. B 2010, 82, 195436. [Google Scholar] [CrossRef]
- Muniz, A.R.; Maroudas, D. Superlattices of Fluorinated Interlayer-Bonded Domains in Twisted Bilayer Graphene. J. Phys. Chem. C 2013, 117, 7315–7325. [Google Scholar] [CrossRef]
- Andriotis, A.N.; Mpourmpakis, G.; Richter, E.; Menon, M. Surface Conductivity of Hydrogenated Diamond Films. Phys. Rev. Lett. 2008, 100, 106801. [Google Scholar] [CrossRef]
- Cao, Y.; Fatemi, V.; Fang, S.; Watanabe, K.; Taniguchi, T.; Kaxiras, E.; Jarillo-Herrero, P. Unconventional Superconductivity in Magic-Angle Graphene Superlattices. Nature 2018, 556, 43–50. [Google Scholar] [CrossRef]
- Yankowitz, M.; Chen, S.; Polshyn, H.; Zhang, Y.; Watanabe, K.; Taniguchi, T.; Graf, D.; Young, A.F.; Dean, C.R. Tuning Superconductivity in Twisted Bilayer Graphene. Science 2019, 363, 1059–1064. [Google Scholar] [CrossRef]
- Wang, X.; Ding, G.; Khandy, S.A.; Cheng, Z.; Zhang, G.; Wang, X.-L.; Chen, H. Unique Topological Nodal Line States and Associated Exceptional Thermoelectric Power Factor Platform in Nb3GeTe6 Monolayer and Bulk. Nanoscale 2020, 12, 16910–16916. [Google Scholar] [CrossRef]
- Tang, S.; Wang, H.; Zhang, Y.; Li, A.; Xie, H.; Liu, X.; Liu, L.; Li, T.; Huang, F.; Xie, X.; et al. Precisely Aligned Graphene Grown on Hexagonal Boron Nitride by Catalyst Free Chemical Vapor Deposition. Sci. Rep. 2013, 3, 2666. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, G.; Li, C.; Cheng, M.; Yang, W.; Wu, S.; Xie, G.; Zhang, J.; Zhao, J.; Lu, X.; et al. Thermally Induced Graphene Rotation on Hexagonal Boron Nitride. Phys. Rev. Lett. 2016, 116, 126101. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Pandey, R.; Wang, N.; Kumar, V.; Sunday, O.J.; Bystrzejewski, M.; Zhu, Y.; Mishra, Y.K. Progress in Diamanes and Diamanoids Nanosystems for Emerging Technologies. Adv. Sci. 2022, 9, 2105770. [Google Scholar] [CrossRef] [PubMed]
- Lavini, F.; Rejhon, M.; Riedo, E. Two-Dimensional Diamonds from Sp2-to-Sp3 Phase Transitions. Nat. Rev. Mater. 2022, 7, 814–832. [Google Scholar] [CrossRef]
- Leconte, N.; Park, Y.; An, J.; Jung, J. Commensuration Torques and Lubricity in Double Moire Systems. arXiv 2023, arXiv:2301.04105. [Google Scholar]

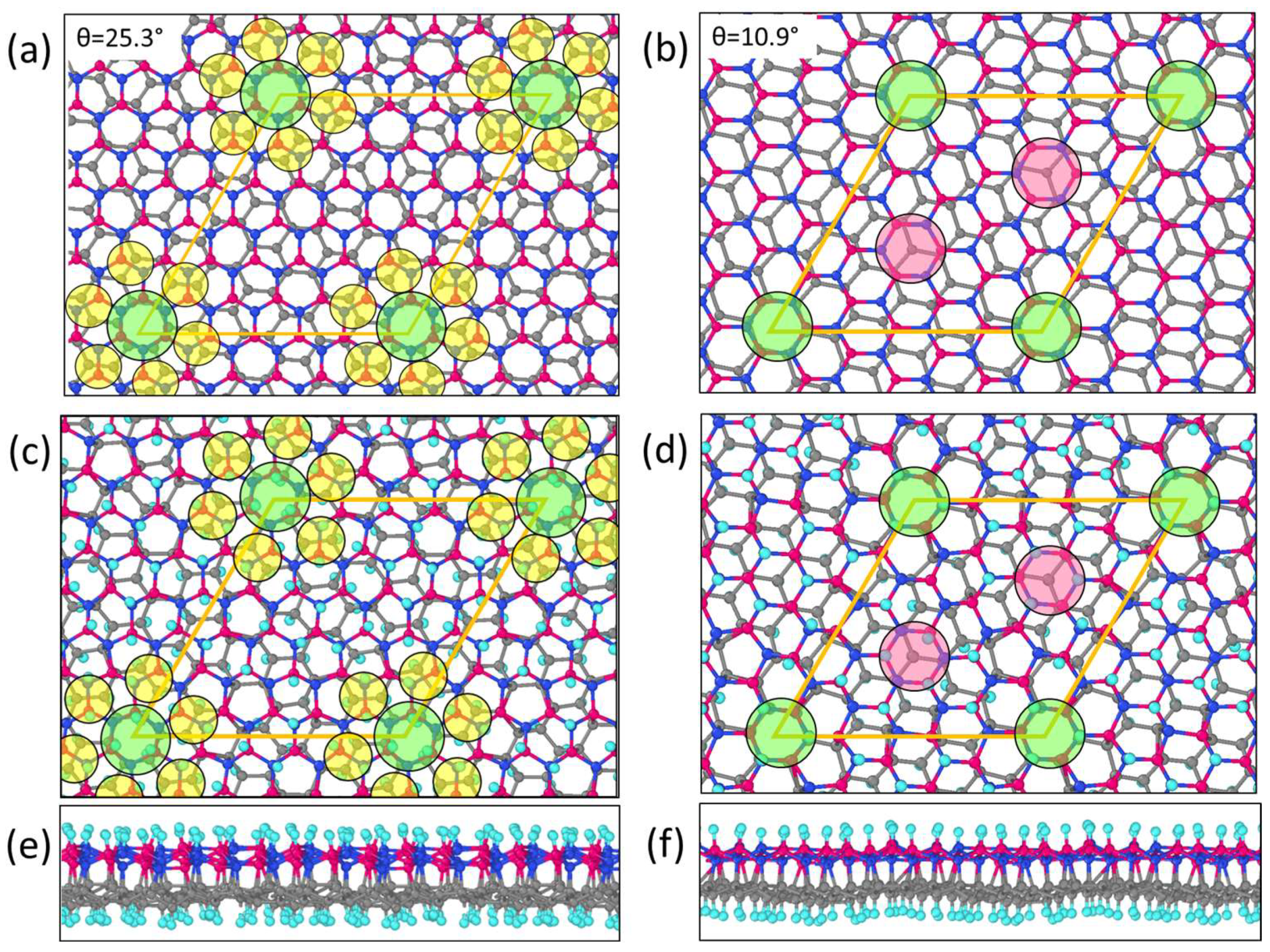

| Unit Cell | Twisted Angle θ, ° | Mismatch δ | Lattice Parameter a, Å | Ef, eV |
|---|---|---|---|---|
| C56B27N27 | 10.9 | −0.0017 | 13.02 | −0.0406 |
| C74B36N36 | 25.3 | 0.0028 | 15.03 | −0.0420 |
| Unit Cell | Twisted Angle θ, ° | Lattice Parameter a, Å | Ef, eV/atom | Eg, eV |
|---|---|---|---|---|
| Structures with C-B bonds in AA-stacked area | ||||
| C56B27N27H46 | 10.9 | 13.33 | 0.2025 | 2.8 |
| C74B36N36H74 | 25.3 | 15.33 | 0.2282 | 2.7 |
| C56B27N27F46 | 10.9 | 13.48 | −0.2624 | 2.9 |
| C74B36N36F74 | 25.3 | 15.67 | −0.2413 | 2.5 |
| Structures with C-N bonds in AA-stacked area | ||||
| C56B27N27H46 | 10.9 | 13.33 | 0.2047 | 2.5 |
| C74B36N36H74 | 25.3 | 15.31 | 0.2395 | 3.1 |
| C56B27N27F46 | 10.9 | 13.46 | −0.2597 | 3.0 |
| C74B36N36F74 | 25.3 | 15.31 | −0.2205 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demin, V.A.; Chernozatonskii, L.A. Diamane-like Films Based on Twisted G/BN Bilayers: DFT Modelling of Atomic Structures and Electronic Properties. Nanomaterials 2023, 13, 841. https://doi.org/10.3390/nano13050841
Demin VA, Chernozatonskii LA. Diamane-like Films Based on Twisted G/BN Bilayers: DFT Modelling of Atomic Structures and Electronic Properties. Nanomaterials. 2023; 13(5):841. https://doi.org/10.3390/nano13050841
Chicago/Turabian StyleDemin, Victor A., and Leonid A. Chernozatonskii. 2023. "Diamane-like Films Based on Twisted G/BN Bilayers: DFT Modelling of Atomic Structures and Electronic Properties" Nanomaterials 13, no. 5: 841. https://doi.org/10.3390/nano13050841
APA StyleDemin, V. A., & Chernozatonskii, L. A. (2023). Diamane-like Films Based on Twisted G/BN Bilayers: DFT Modelling of Atomic Structures and Electronic Properties. Nanomaterials, 13(5), 841. https://doi.org/10.3390/nano13050841






