Preparation of 3D Nd2O3-NiSe-Modified Nitrogen-Doped Carbon and Its Electrocatalytic Oxidation of Methanol and Urea
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental Section
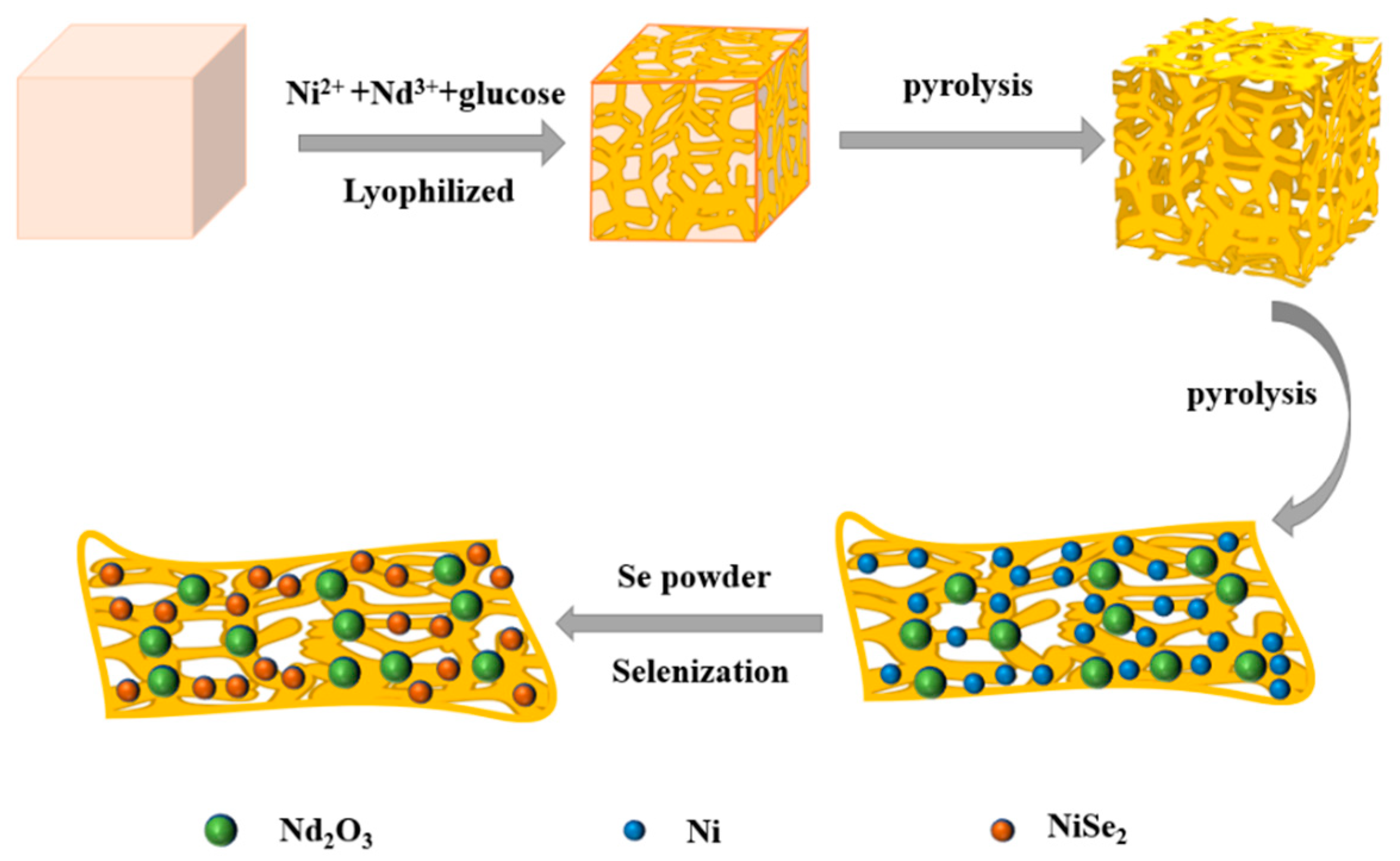
2.1. Preparation of Catalyst
2.2. Physical Property Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
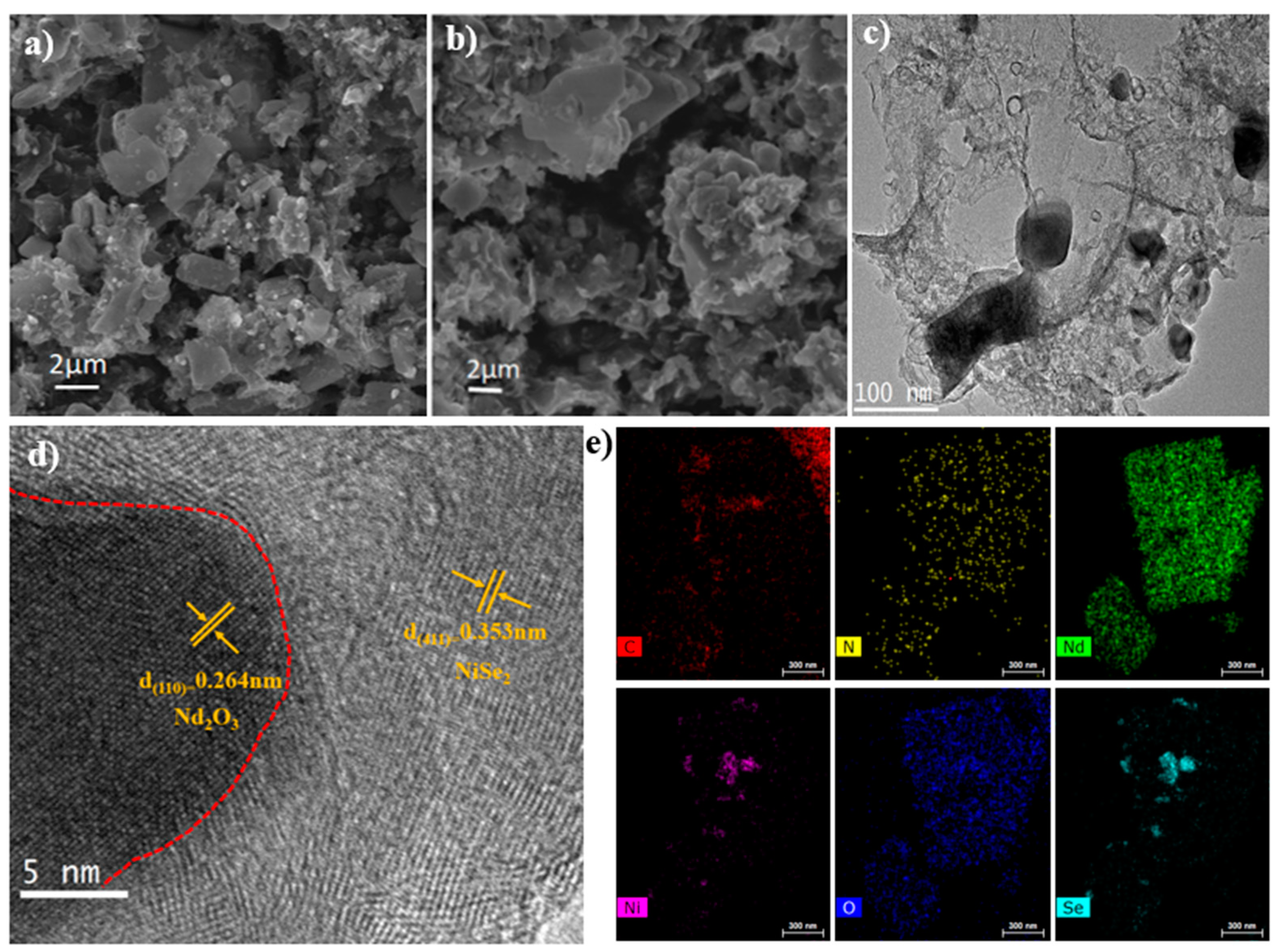
3.1. Physical Characterizations
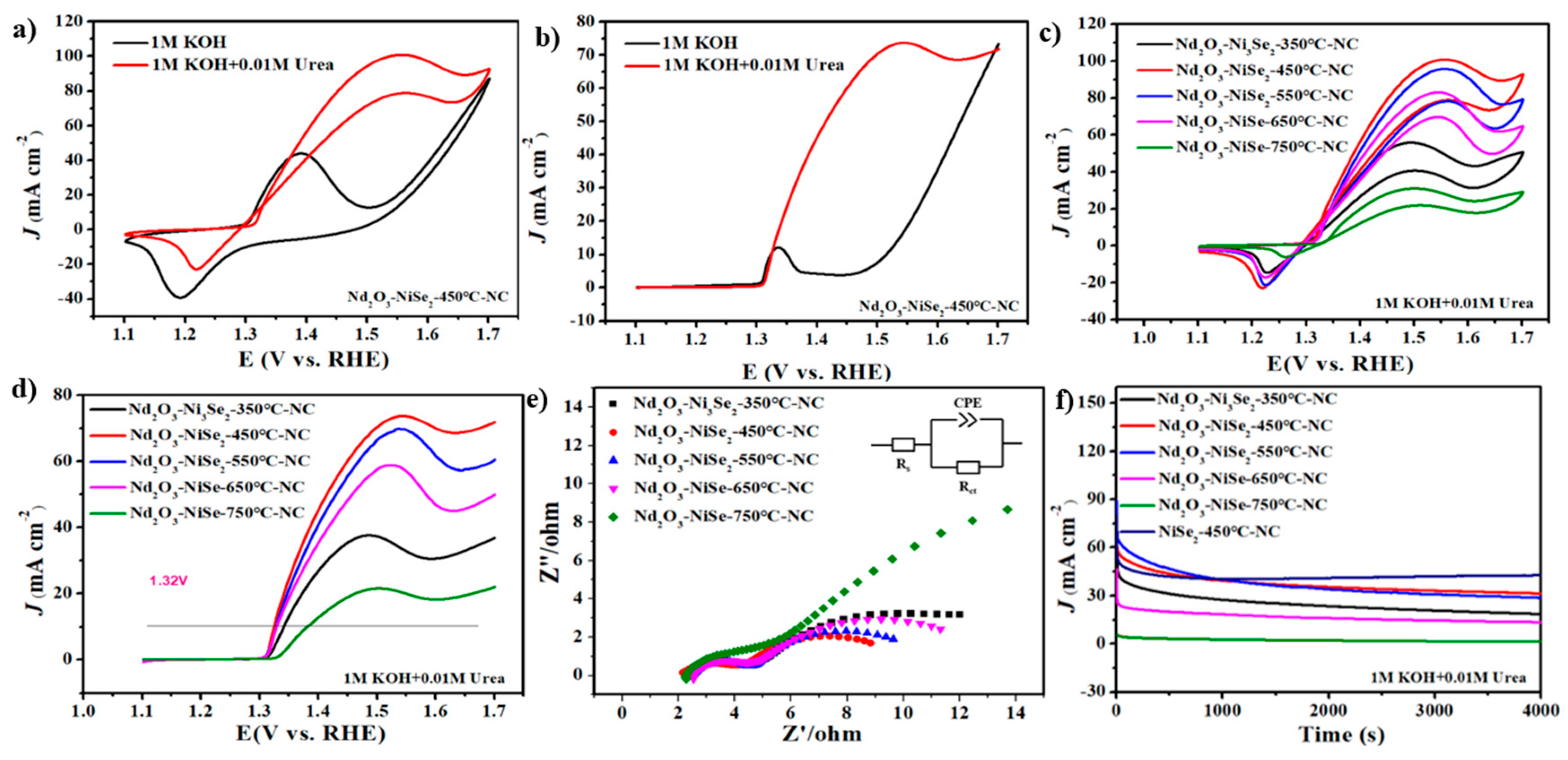
3.2. Electrochemical Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.J.; Theerthagiri, J.; Nithyadharseni, P.; Arunachalam, P.; Balaji, D.; Kumar, A.M.; Madhavan, J.; Mittal, V.; Choi, M.Y. Heteroatom-doped graphene-based materials for sustainable energy applications: A review. Renew. Sustain. Energy Rev. 2021, 143, 110849. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, S.J.; Theerthagiri, J.; Lee, Y.; Choi, M.Y. Architecting the AuPt alloys for hydrazine oxidation as an anolyte in fuel cell: Comparative analysis of hydrazine splitting and water splitting for energy-saving H2 generation. Appl. Catal. B Environ. 2022, 316, 121603. [Google Scholar] [CrossRef]
- Xiong, L.; Bi, J.; Wang, L.; Yang, S. Improving the electrocatalytic property of CoP for hydrogen evolution by constructing porous ternary CeO2-CoP-C hybrid nanostructure via ionic exchange of MOF. Int. J. Hydrogen Energy 2018, 43, 20372–20381. [Google Scholar] [CrossRef]
- Ao, D.; Shi, Y.; Li, S.; Chang, Y.; Xu, A.; Jia, J.; Jia, M. 3D Co-Ni-C Network from Milk as Competitive Bifunctional Catalysts for Methanol and Urea Electrochemical Oxidation. Catalysts 2021, 11, 844. [Google Scholar] [CrossRef]
- Cabán-Acevedo, M.; Stone, M.L.; Schmidt, J.R.; Thomas, J.G.; Ding, Q.; Chang, H.C.; Tsai, M.L.; He, J.H.; Jin, S. Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide. Nat. Mater. 2015, 14, 1245–1251. [Google Scholar] [CrossRef]
- Alias, M.; Kamarudin, S.; Zainoodin, A.; Masdar, M. Active direct methanol fuel cell: An overview. Int. J. Hydrogen Energy 2020, 45, 19620–19641. [Google Scholar] [CrossRef]
- Sayed, E.T.; Eisa, T.; Mohamed, H.O.; Abdelkareem, M.A.; Allagui, A.; Alawadhi, H.; Chae, K.-J. Direct urea fuel cells: Challenges and opportunities. J. Power Sources 2019, 417, 159–175. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative Strategies for Electrocatalytic Water Splitting. Accounts Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Liu, D.; Liu, T.; Zhang, L.; Qu, F.; Du, G.; Asiri, A.M.; Sun, X. High-performance urea electrolysis towards less energy-intensive electrochemical hydrogen production using a bifunctional catalyst electrode. J. Mater. Chem. A 2017, 5, 3208–3213. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, L.; Qu, F.; Liu, Z.; Du, G.; Asiri, A.M.; Sun, X. A porous Ni3N nanosheet array as a high-performance non-noble-metal catalyst for urea-assisted electrochemical hydrogen production. Inorg. Chem. Front. 2017, 4, 1120–1124. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Jung, W.; Zhou, W.; Shao, Z. Electrochemical Water Splitting: Bridging the Gaps between Fundamental Research and Industrial Applications. Energy Environ. Mater. 2022, e12441. [Google Scholar] [CrossRef]
- Jia, J.; Zhao, L.; Chang, Y.; Jia, M.; Wen, Z. Understanding the growth of NiSe nanoparticles on reduced graphene oxide as efficient electrocatalysts for methanol oxidation reaction. Ceram. Int. 2020, 46, 10023–10028. [Google Scholar] [CrossRef]
- Thiagarajan, V.; Karthikeyan, P.; Manoharan, R.; Sampath, S.; Hernández-Ramírez, A.; Sánchez-Castro, M.E.; Alonso-Lemus, I.L.; Rodríguez-Varela, F.J. Pt-Ru-NiTiO3 Nanoparticles Dispersed on Vulcan as High Performance Electrocatalysts for the Methanol Oxidation Reaction. Electrocatalysis 2018, 9, 582–592. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Liu, T.; Chen, L.; Li, Y.; Wang, H.; Chen, X.; Gong, M.; Liu, Z.; Yang, X. Deciphering and Suppressing Over-Oxidized Nitrogen in Nickel-Catalyzed Urea Electrolysis. Angew. Chem. Int. Ed. 2021, 60, 26656–26662. [Google Scholar] [CrossRef]
- Tang, C.; Zhao, Z.L.; Chen, J.; Li, B.; Chen, L.; Li, C.M. Se-Ni(OH)2-shelled vertically oriented NiSe nanowires as a superior electrocatalyst toward urea oxidation reaction of fuel cells. Electrochimica Acta 2017, 248, 243–249. [Google Scholar] [CrossRef]
- Forslund, R.P.; Alexander, C.T.; Abakumov, A.M.; Johnston, K.P.; Stevenson, K.J. Enhanced Electrocatalytic Activities by Substitutional Tuning of Nickel-Based Ruddlesden–Popper Catalysts for the Oxidation of Urea and Small Alcohols. ACS Catal. 2019, 9, 2664–2673. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Gnana Kumar, G.; Manthiram, A. 3D Hierarchical Core-Shell Nanostructured Arrays on Carbon Fibers as Catalysts for Direct Urea Fuel Cells. Adv. Energy Mater. 2018, 8, 1702207. [Google Scholar] [CrossRef]
- Wang, L.; Ren, L.; Wang, X.; Feng, X.; Zhou, J.; Wang, B. Multivariate MOF-Templated Pomegranate-Like Ni/C as Efficient Bifunctional Electrocatalyst for Hydrogen Evolution and Urea Oxidation. ACS Appl. Mater. Interfaces 2018, 10, 4750–4756. [Google Scholar] [CrossRef]
- Sun, H.; Liu, J.; Kim, H.; Song, S.; Fei, L.; Hu, Z.; Lin, H.J.; Chen, C.T.; Ciucci, F.; Jung, W. Ni-Doped CuO Nanoarrays Activate Urea Adsorption and Stabilizes Reaction Intermediates to Achieve High-Performance Urea Oxidation Catalysts. Adv. Sci. 2022, 9, e2204800. [Google Scholar] [CrossRef]
- Fang, M.; Gao, W.; Dong, G.; Xia, Z.; Yip, S.; Qin, Y.; Qu, Y.; Ho, J.C. Hierarchical NiMo-based 3D electrocatalysts for highly-efficient hydrogen evolution in alkaline conditions. Nano Energy 2016, 27, 247–254. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Xiong, Q.; Liu, G.; Zhao, C.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Vapour-phase hydrothermal synthesis of Ni2P nanocrystallines on carbon fiber cloth for high-efficiency H2 production and simultaneous urea decomposition. Electrochimica Acta 2017, 254, 44–49. [Google Scholar] [CrossRef]
- Zhao, Y.; Jalal, A.; Uzun, A. Interplay between Copper Nanoparticle Size and Oxygen Vacancy on Mg-Doped Ceria Controls Partial Hydrogenation Performance and Stability. ACS Catal. 2021, 11, 8116–8131. [Google Scholar] [CrossRef]
- Yoon, J.; Yoon, Y.S.; Kim, D.-J. Silver-Nanoparticle-Decorated NiOOH Nanorods for Electrocatalytic Urea Sensing. ACS Appl. Nano Mater. 2020, 3, 7651–7658. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Chen, Y.; Kim, H.; Xu, X.; Guan, D.; Hu, Z.; Zhang, L.; Shao, Z.; Jung, W. Boosting ethanol oxidation by NiOOH-CuO nano-heterostructure for energy-saving hydrogen production and biomass upgrading. Appl. Catal. B Environ. 2023, 325, 122388. [Google Scholar] [CrossRef]
- Cao, L.-M.; Cao, Q.-C.; Zhang, J.; Zhu, X.-Y.; Sun, R.-Z.; Du, Z.-Y.; He, C.-T. Electrochemically Controlled Synthesis of Ultrathin Nickel Hydroxide Nanosheets for Electrocatalytic Oxygen Evolution. Inorg. Chem. 2021, 60, 3365–3374. [Google Scholar] [CrossRef]
- Tran, M.H.; Park, B.J.; Kim, B.H.; Yoon, H.H. Mesoporous silica template-derived nickel-cobalt bimetallic catalyst for urea oxidation and its application in a direct urea/H2O2 fuel cell. Int. J. Hydrogen Energy 2020, 45, 1784–1792. [Google Scholar] [CrossRef]
- Tan, Y.; Wei, S.; Liu, X.; Pan, B.; Liu, S.; Wu, J.; Fu, M.; Jia, Y.; He, Y. Neodymium oxide (Nd2O3) coupled tubular g-C3N4, an efficient dual-function catalyst for photocatalytic hydrogen production and NO removal. Sci. Total. Environ. 2021, 773, 145583. [Google Scholar] [CrossRef]
- Majhi, K.C.; Yadav, M. Neodymium oxide doped neodymium phosphate as efficient electrocatalyst towards hydrogen evolution reaction in acidic medium. J. Environ. Chem. Eng. 2022, 10, 107416. [Google Scholar] [CrossRef]
- Li, P.; Fang, L.; Hou, N.; Li, J.; Yao, X.; Gan, T.; Fan, L.; Zhao, Y.; Li, Y. Improved Performance of Ni-Mo Based Anode for Direct Methanol Solid Oxide Fuel Cells with the Addition of Rare Earth Oxides. J. Electrochem. Soc. 2017, 164, F1142–F1148. [Google Scholar] [CrossRef]
- Ilanchezhiyan, P.; Kumar, G.M.; Siva, C.; Cho, H.; Lee, D.; Reddy, N.L.; Ramu, A.; Kang, T.; Kim, D. Neodymium (Nd) based oxide perovskite nanostructures for photocatalytic and photoelectrochemical water splitting functions. Environ. Res. 2021, 197, 111128. [Google Scholar]
- Chen, S.; Mi, J.-L.; Zhang, P.; Feng, Y.-H.; Yong, Y.-C.; Shi, W.-D. Control Synthesis of Nickel Selenides and Their Multiwalled Carbon Nanotubes Composites as Electrocatalysts for Enhanced Water Oxidation. J. Phys. Chem. C 2018, 122, 26096–26104. [Google Scholar] [CrossRef]
- Zhao, W.; Li, M.; Wu, H.; Feng, C.; Zhang, G. Rod-like nonstoichiometric Ni0.85Se as efficient electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 12653–12660. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, H.; Ren, J.; Jin, X.; Li, X.; Song, R. Synergistic engineering of morphology and electronic structure in constructing metal-organic framework-derived Ru doped cobalt-nickel oxide heterostructure towards efficient alkaline hydrogen evolution reaction. Chem. Eng. J. 2021, 426, 131300. [Google Scholar] [CrossRef]
- Chen, L.; Sagar, R.U.R.; Aslam, S.; Deng, Y.; Hussain, S.; Ali, W.; Liu, C.; Liang, T.; Hou, X. Neodymium-decorated graphene as an efficient electrocatalyst for hydrogen production. Nanoscale 2021, 13, 15471–15480. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Zhang, G.; Sun, D.; Zhou, J.; Song, H. Two-Dimensional NiSe2/N-Rich Carbon Nanocomposites Derived from Ni-Hexamine Frameworks for Superb Na-Ion Storage. ACS Appl. Mater. Interfaces 2018, 10, 34193–34201. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Y.; Wen, M.; Wu, Q.; Zhu, Q.; Fu, Y. Integrating CoNiSe2 Nanorod-arrays onto N-doped Sea-sponge-C spheres for highly efficient electrocatalysis of hydrogen evolution reaction. Chem. Eng. J. 2022, 446, 137335. [Google Scholar] [CrossRef]
- Luo, Q.; Zhao, Y.; Sun, L.; Wang, C.; Xin, H.; Song, J.; Li, D.; Ma, F. Interface oxygen vacancy enhanced alkaline hydrogen evolution activity of cobalt-iron phosphide/CeO2 hollow nanorods. Chem. Eng. J. 2022, 437, 135376. [Google Scholar] [CrossRef]
- Du, J.; You, S.; Li, X.; Tang, B.; Jiang, B.; Yu, Y.; Cai, Z.; Ren, N.; Zou, J. In Situ Crystallization of Active NiOOH/CoOOH Heterostructures with Hydroxide Ion Adsorption Sites on Velutipes-like CoSe/NiSe Nanorods as Catalysts for Oxygen Evolution and Cocatalysts for Methanol Oxidation. ACS Appl. Mater. Interfaces 2020, 12, 686–697. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Liu, H.; Feng, L. Efficient synergism of NiSe2 nanoparticle/NiO nanosheet for energy-relevant water and urea electrocatalysis. Appl. Catal. B Environ. 2020, 276, 119165. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Chang, Y.; Xu, A.; Jia, J.; Jia, M. Preparation of 3D Nd2O3-NiSe-Modified Nitrogen-Doped Carbon and Its Electrocatalytic Oxidation of Methanol and Urea. Nanomaterials 2023, 13, 814. https://doi.org/10.3390/nano13050814
Zhang S, Chang Y, Xu A, Jia J, Jia M. Preparation of 3D Nd2O3-NiSe-Modified Nitrogen-Doped Carbon and Its Electrocatalytic Oxidation of Methanol and Urea. Nanomaterials. 2023; 13(5):814. https://doi.org/10.3390/nano13050814
Chicago/Turabian StyleZhang, Simin, Ying Chang, Aiju Xu, Jingchun Jia, and Meilin Jia. 2023. "Preparation of 3D Nd2O3-NiSe-Modified Nitrogen-Doped Carbon and Its Electrocatalytic Oxidation of Methanol and Urea" Nanomaterials 13, no. 5: 814. https://doi.org/10.3390/nano13050814
APA StyleZhang, S., Chang, Y., Xu, A., Jia, J., & Jia, M. (2023). Preparation of 3D Nd2O3-NiSe-Modified Nitrogen-Doped Carbon and Its Electrocatalytic Oxidation of Methanol and Urea. Nanomaterials, 13(5), 814. https://doi.org/10.3390/nano13050814




