Effect of Iodide-Based Organic Salts and Ionic Liquid Additives in Dye-Sensitized Solar Cell Performance
Abstract
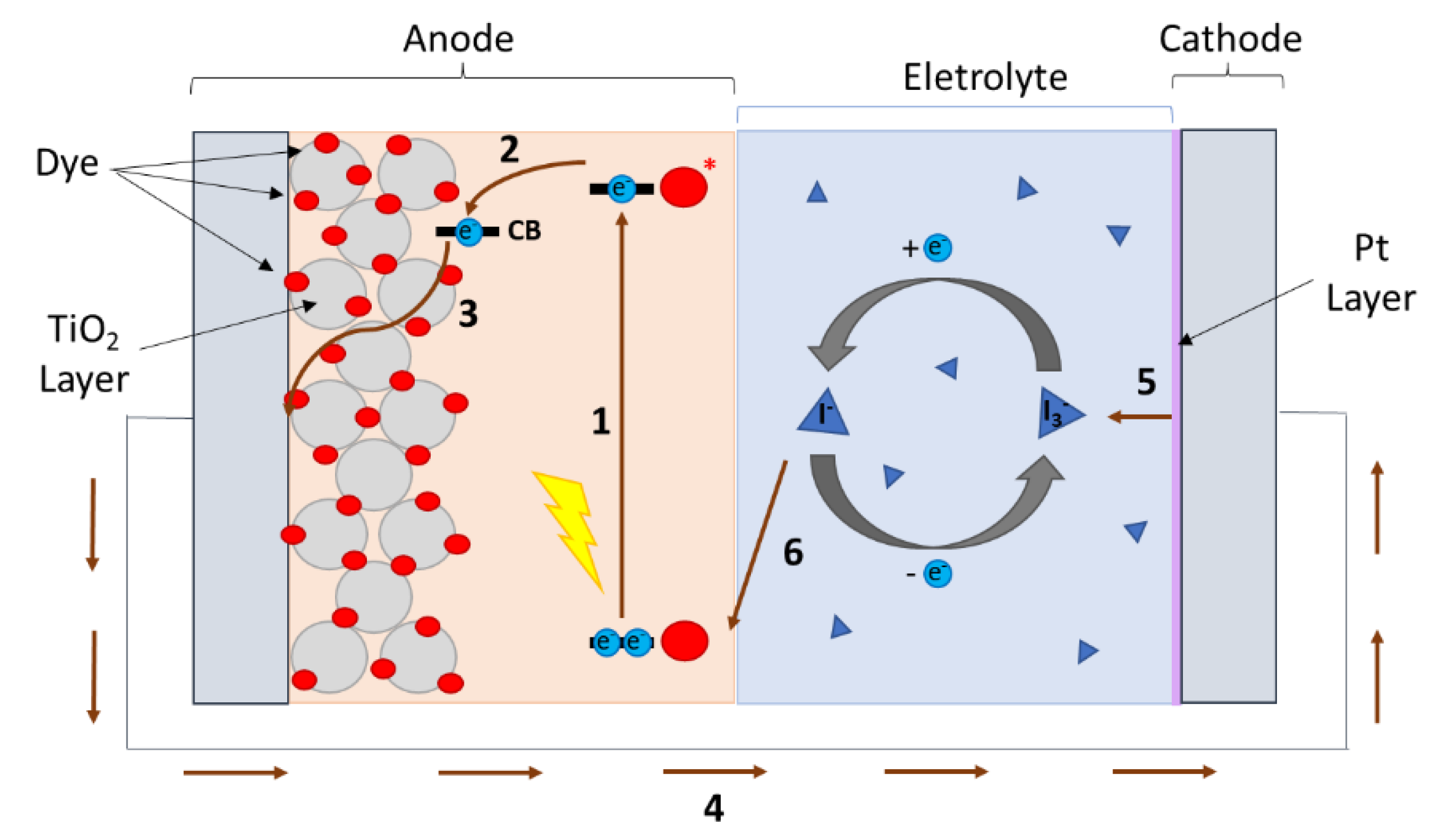
:1. Introduction
2. Materials and Methods
2.1. General Information and Instruments
2.2. DSSC Fabrication and Photovoltaic Characterization
2.3. Electrochemical Impedance Spectroscopy
2.4. Descriptor Analysis
3. Results and Discussion
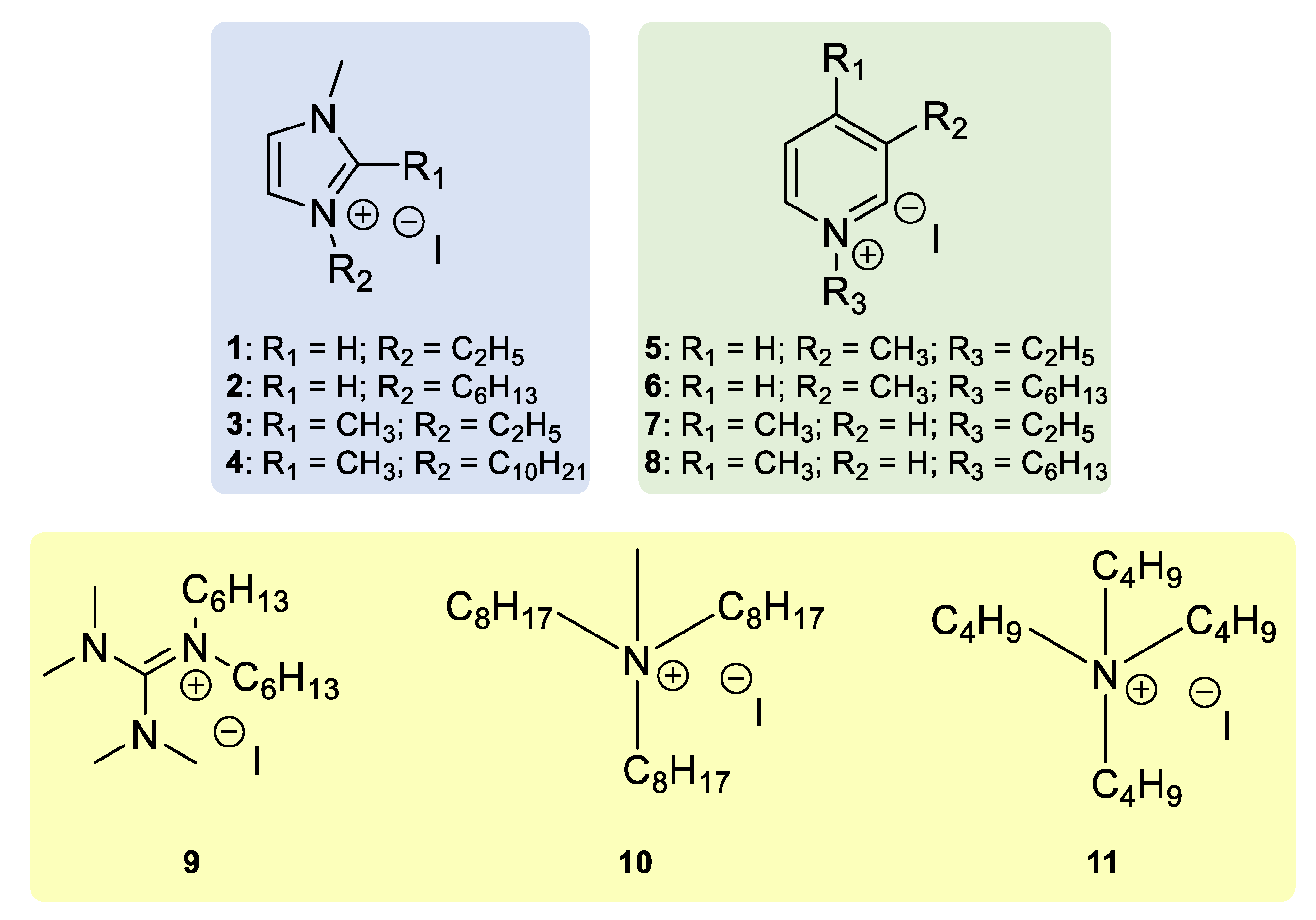
3.1. Preparation of Iodide Salts
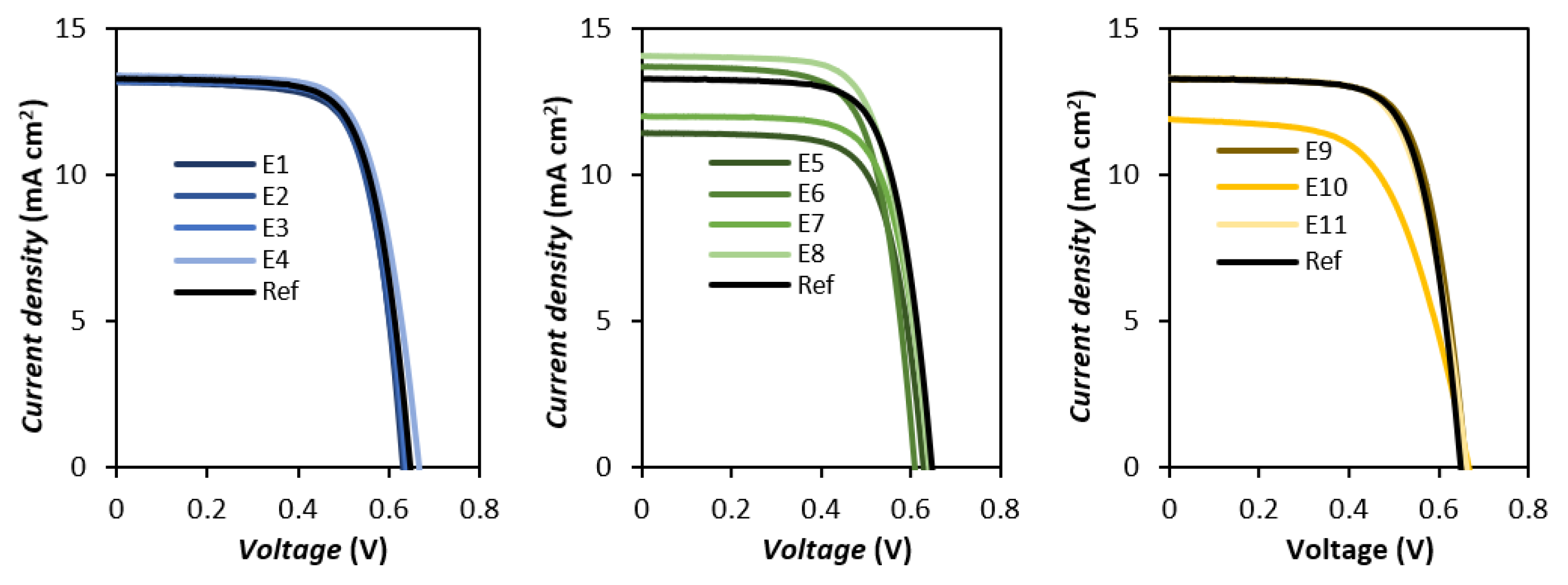
3.2. Photovoltaic Performance
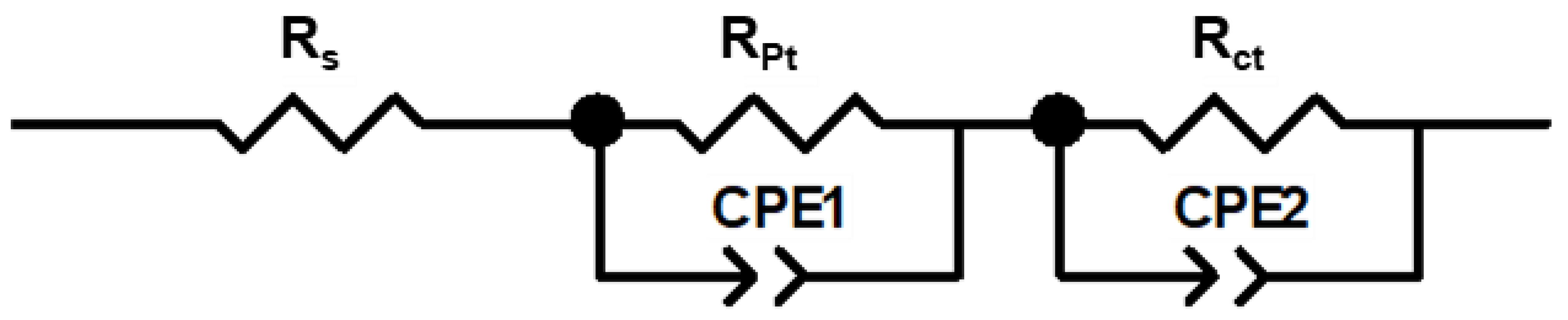
3.3. Electrochemical Impedance Measurements
3.4. Descriptor Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devadiga, D.; Selvakumar, M.; Shetty, P.; Santosh, M.S. Dye-Sensitized Solar Cell for Indoor Applications: A Mini-Review. J. Electron. Mater. 2021, 50, 3187–3206. [Google Scholar] [CrossRef]
- Aslam, A.; Mehmood, U.; Arshad, M.H.; Ishfaq, A.; Zaheer, J.; Khan, A.U.; Sufyan, M. Dye-sensitized solar cells (DSSCs) as a potential photovoltaic technology for the self-powered internet of things (IoTs) applications. Sol. Energy 2020, 207, 874–892. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, S.H. Energy Generation Performance of Window-Type Dye-Sensitized Solar Cells by Color and Transmittance. Sustainability 2020, 12, 8961. [Google Scholar] [CrossRef]
- Bella, F.; Galliano, S.; Gerbaldi, C.; Viscardi, G. Cobalt-Based Electrolytes for Dye-Sensitized Solar Cells: Recent Advances towards Stable Devices. Energies 2016, 9, 384. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, W.X.; Zhang, L.; Jiang, R.; Mijangos, E.; Saygili, Y.; Hammarstrom, L.; Hagfeldt, A.; Boschloo, G. A small electron donor in cobalt complex electrolyte significantly improves efficiency in dye-sensitized solar cells. Nat. Commun. 2016, 7, 13934. [Google Scholar] [CrossRef]
- Saygili, Y.; Soderberg, M.; Pellet, N.; Giordano, F.; Cao, Y.M.; Munoz-Garcia, A.B.; Zakeeruddin, S.M.; Vlachopoulos, N.; Pavone, M.; Boschloo, G.; et al. Copper Bipyridyl Redox Mediators for Dye-Sensitized Solar Cells with High Photovoltage. J. Am. Chem. Soc. 2016, 138, 15087–15096. [Google Scholar] [CrossRef]
- Zhang, D.; Stojanovic, M.; Ren, Y.M.; Cao, Y.M.; Eickemeyer, F.T.; Socie, E.; Vlachopoulos, N.; Moser, J.E.; Zakeeruddin, S.M.; Hagfeldt, A.; et al. A molecular photosensitizer achieves a V-oc of 1.24V enabling highly efficient and stable dye-sensitized solar cells with copper(II/I)-based electrolyte. Nat. Commun. 2021, 12, 1777. [Google Scholar] [CrossRef]
- Xiang, W.C.; Huang, W.C.; Bach, U.; Spiccia, L. Stable high efficiency dye-sensitized solar cells based on a cobalt polymer gel electrolyte. Chem. Commun. 2013, 49, 8997–8999. [Google Scholar] [CrossRef]
- Hwang, D.K.; Nam, J.E.; Jo, H.J.; Sung, S.J. Quasi-solid state electrolyte for semi-transparent bifacial dye-sensitized solar cell with over 10% power conversion efficiency. J. Power Sources 2017, 361, 87–95. [Google Scholar] [CrossRef]
- Buraidah, M.H.; Shah, S.; Teo, L.P.; Chowdhury, F.I.; Careem, M.A.; Albinsson, I.; Mellander, B.E.; Arof, A.K. High efficient dye sensitized solar cells using phthaloylchitosan based gel polymer electrolytes. Electrochim. Acta 2017, 245, 846–853. [Google Scholar] [CrossRef]
- Li, X.; Xu, B.; Liu, P.; Hu, Y.; Kloo, L.; Hua, J.L.; Sun, L.C.; Tian, H. Molecular engineering of D-A-pi-A sensitizers for highly efficient solid-state dye-sensitized solar cells. J. Mater. Chem. A 2017, 5, 3157–3166. [Google Scholar] [CrossRef]
- Cao, Y.M.; Saygili, Y.; Ummadisingu, A.; Teuscher, J.; Luo, J.S.; Pellet, N.; Giordano, F.; Zakeeruddin, S.M.; Moser, J.E.; Freitag, M.; et al. 11% efficiency solid-state dye-sensitized solar cells with copper(II/I) hole transport materials. Nat. Commun. 2017, 8, 15390. [Google Scholar] [CrossRef] [PubMed]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Harikisun, R.; Desilvestro, H. Long-term stability of dye solar cells. Sol. Energy 2011, 85, 1179–1188. [Google Scholar] [CrossRef]
- Venkatesan, S.; Lin, W.H.; Teng, H.S.; Lee, Y.L. High-Efficiency Bifacial Dye-Sensitized Solar Cells for Application under Indoor Light Conditions. ACS Appl. Mater. Interfaces 2019, 11, 42780–42789. [Google Scholar] [CrossRef]
- Mohanty, S.P.; Bhargava, P. Impact of Electrolytes Based on Different Solvents on the Long Term Stability of Dye Sensitized Solar Cells. Electrochim. Acta 2015, 168, 111–115. [Google Scholar] [CrossRef]
- Lee, H.S.; Bae, S.H.; Jo, Y.; Kim, K.J.; Jun, Y.; Han, C.H. A high temperature stable electrolyte system for dye-sensitized solar cells. Electrochim. Acta 2010, 55, 7159–7165. [Google Scholar] [CrossRef]
- Subramania, A.; Vijayakumar, E.; Sivasankar, N.; Priya, A.R.S.; Kim, K.J. Effect of different compositions of ethylene carbonate and propylene carbonate containing iodide/triiodide redox electrolyte on the photovoltaic performance of DSSC. Ionics 2013, 19, 1649–1653. [Google Scholar] [CrossRef]
- Tedla, A.; Tai, Y.A. Influence of binary solvent system on the stability and efficiency of liquid dye sensitized solar cells. J. Photochem. Photobiol. A-Chem. 2018, 358, 70–75. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Antoniadou, M.; Ibrahim, I.; Karagianni, C.S.; Falaras, P. Universal electrolyte for DSSC operation under both simulated solar and indoor fluorescent lighting. Mater. Chem. Phys. 2022, 277, 125543. [Google Scholar] [CrossRef]
- Abu Talip, R.A.; Yahya, W.Z.N.; Bustam, M.A. Ionic Liquids Roles and Perspectives in Electrolyte for Dye-Sensitized Solar Cells. Sustainability 2020, 12, 7598. [Google Scholar] [CrossRef]
- Bai, Y.; Cao, Y.M.; Zhang, J.; Wang, M.; Li, R.Z.; Wang, P.; Zakeeruddin, S.M.; Gratzel, M. High-performance dye-sensitized solar cells based on solvent-free electrolytes produced from eutectic melts. Nat. Mater. 2008, 7, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wenger, B.; Humphry-Baker, R.; Moser, J.E.; Teuscher, J.; Kantlehner, W.; Mezger, J.; Stoyanov, E.V.; Zakeeruddin, S.M.; Gratzel, M. Charge separation and efficient light energy conversion in sensitized mesoscopic solar cells based on binary ionic liquids. J. Am. Chem. Soc. 2005, 127, 6850–6856. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.Y.; Ma, P.; Cheng, H.B.; Tan, G.Y.; Wu, J.X.; Zheng, J.X.; Zhou, X.W.; Fang, S.B.; Dai, Y.H.; Lin, Y. Synthesis of Low-Viscosity Ionic Liquids for Application in Dye-Sensitized Solar Cells. Chem.-Asian J. 2019, 14, 4201–4206. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Athanassov, Y.; Armand, M.; Bonhote, P.; Pettersson, H.; Azam, A.; Gratzel, M. The performance and stability of ambient temperature molten salts for solar cell applications. J. Electrochem. Soc. 1996, 143, 3099–3108. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Shi, D.; Zhang, J.; Wang, M.; Jing, X.; Humphry-Baker, R.; Wang, P.; Zakeeruddin, S.M.; Grätzel, M. Enhance the Optical Absorptivity of Nanocrystalline TiO2 Film with High Molar Extinction Coefficient Ruthenium Sensitizers for High Performance Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2008, 130, 10720–10728. [Google Scholar] [CrossRef]
- Sauvage, F.; Chhor, S.; Marchioro, A.; Moser, J.E.; Graetzel, M. Butyronitrile-Based Electrolyte for Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2011, 133, 13103–13109. [Google Scholar] [CrossRef]
- Chen, X.J.; Xu, D.; Qiu, L.H.; Li, S.C.; Zhang, W.; Yan, F. Imidazolium functionalized TEMPO/iodide hybrid redox couple for highly efficient dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 8759–8765. [Google Scholar] [CrossRef]
- Yu, Z.; Gorlov, M.; Nissfolk, J.; Boschloo, G.; Kloo, L. Investigation of Iodine Concentration Effects in Electrolytes for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 10612–10620. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; De Angelis, F.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Ito, S.; Bessho, T.; Gratzel, M. Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847. [Google Scholar] [CrossRef]
- Yella, A.; Mai, C.L.; Zakeeruddin, S.M.; Chang, S.N.; Hsieh, C.H.; Yeh, C.Y.; Gratzel, M. Molecular Engineering of Push-Pull Porphyrin Dyes for Highly Efficient Dye-Sensitized Solar Cells: The Role of Benzene Spacers. Angew. Chem.-Int. Edit. 2014, 53, 2973–2977. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.S.; Tang, Y.Y.; Wu, W.J.; Wang, Y.Q.; Liu, J.C.; Li, X.; Tian, H.; Zhu, W.H. Porphyrin Cosensitization for a Photovoltaic Efficiency of 11.5%: A Record for Non-Ruthenium Solar Cells Based on Iodine Electrolyte. J. Am. Chem. Soc. 2015, 137, 14055–14058. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.M.; Zhou, H.R.; Eom, Y.K.; Kim, C.H.; Kim, H.K. 14.2% Efficiency Dye-Sensitized Solar Cells by Co-sensitizing Novel Thieno 3,2-b indole-Based Organic Dyes with a Promising Porphyrin Sensitizer. Adv. Energy Mater. 2020, 10, 12. [Google Scholar] [CrossRef]
- Bradley, D.; Williams, G.; Lawton, M. Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants. J. Org. Chem. 2010, 75, 8351–8354. [Google Scholar] [CrossRef]
- Pinto, A.L.; Oliveira, J.; Araujo, P.; Calogero, G.; de Freitas, V.; Pina, F.; Parola, A.J.; Lima, J.C. Study of the multi-equilibria of red wine colorants pyranoanthocyanins and evaluation of their potential in dye-sensitized solar cells. Sol. Energy 2019, 191, 100–108. [Google Scholar] [CrossRef]
- JChem 21.4.5 2021, ChemAxon. Available online: https://www.chemaxon.com/ (accessed on 13 December 2020).
- Moriwaki, H.; Tian, Y.S.; Kawashita, N.; Takagi, T. Mordred: A molecular descriptor calculator. J. Cheminform. 2018, 10, 1–14. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Malta, G.; Peixoto, D.; Ferreira, L.M.; Baptista, P.V.; Fernandes, A.R.; Branco, P.S. Synthesis of new hetero-arylidene-9(10H)-anthrone derivatives and their biological evaluation. Bioorg. Chem. 2020, 99, 103849. [Google Scholar] [CrossRef]
- Huang, J.; Riisager, A.; Wasserscheid, P.; Fehrmann, R. Reversible physical absorption of SO2 by ionic liquids. Chem. Commun. 2006, 38, 4027–4029. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Riano, S.; Binnemans, K. Separation of precious metals by split-anion extraction using water-saturated ionic liquids. Green Chem. 2020, 22, 8375–8388. [Google Scholar] [CrossRef]
- Jeon, S.; Jo, Y.; Kim, K.-J.; Jun, Y.; Han, C.-H. High Performance Dye-Sensitized Solar Cells with Alkylpyridinium Iodide Salts in Electrolytes. ACS Appl. Mater. Interfaces 2011, 3, 512–516. [Google Scholar] [CrossRef]
- Liu, Y.; Hagfeldt, A.; Xiao, X.R.; Lindquist, S.E. Investigation of influence of redox species on the interfacial energetics of a dye-sensitized nanoporous TiO2 solar cell. Sol. Energy Mater. Sol. Cells 1998, 55, 267–281. [Google Scholar] [CrossRef]
- Boschloo, G.; Haggman, L.; Hagfeldt, A. Quantification of the effect of 4-tert-butylpyridine addition to I-/I-3(-) redox electrolytes in dye-sensitized nanostructured TiO2 solar cells. J. Phys. Chem. B 2006, 110, 13144–13150. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lindblad, R.; Gabrielsson, E.; Boschloo, G.; Rensmo, H.; Sun, L.C.; Hagfeldt, A.; Edvinsson, T.; Johansson, E.M.J. Experimental and Theoretical Investigation of the Function of 4-tert-Butyl Pyridine for Interface Energy Level Adjustment in Efficient Solid-State Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 11572–11579. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.A.P.; Nguyen, N.P.; Nguyen, L.T.; Nguyen, P.H.; Le, T.K.; Huynh, T.V.; Lund, T.; Tsai, D.H.; Wei, T.C.; Nguyen, P.T. Direct experimental evidence for the adsorption of 4-tert-butylpyridine and 2,2′-bipyridine on TiO2 surface and their influence on dye-sensitized solar cells’ performance. Appl. Surf. Sci. 2020, 509, 144878. [Google Scholar] [CrossRef]
- Stergiopoulos, T.; Rozi, E.; Karagianni, C.S.; Falaras, P. Influence of electrolyte co-additives on the performance of dye-sensitized solar cells. Nanoscale Res. Lett. 2011, 6, 307. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Lu, J.; Yin, J.; Lu, G.; Cui, Y.M.; Wang, S.S.; Deng, S.Y.; Shan, D.; Tao, H.L.; Sun, Y.M. Influence of 4-tert-butylpyridine/guanidinium thiocyanate co-additives on band edge shift and recombination of dye-sensitized solar cells: Experimental and theoretical aspects. Electrochim. Acta 2015, 185, 69–75. [Google Scholar] [CrossRef]
- Zhang, C.N.; Huang, Y.; Huo, Z.P.; Chen, S.H.; Dai, S.Y. Photoelectrochemical Effects of Guanidinium Thiocyanate on Dye-Sensitized Solar Cell Performance and Stability. J. Phys. Chem. C 2009, 113, 21779–21783. [Google Scholar] [CrossRef]
- Kopidakis, N.; Neale, N.R.; Frank, A.J. Effect of an adsorbent on recombination and band-edge movement in dye-sensitized TiO2 solar cells: Evidence for surface passivation. J. Phys. Chem. B 2006, 110, 12485–12489. [Google Scholar] [CrossRef]
- Pan, M.M.; Huang, N.; Zhao, X.Z.; Fu, J.; Zhong, X.L. Enhanced Efficiency of Dye-Sensitized Solar Cell by High Surface Area Anatase-TiO2-Modified P25 Paste. J. Nanomater. 2013, 2013, 3. [Google Scholar] [CrossRef]
- Dissanayake, M.; Umair, K.; Senadeera, G.K.R.; Kumari, J. Effect of electrolyte conductivity, co-additives and mixed cation iodide salts on efficiency enhancement in dye sensitized solar cells with acetonitrile-free electrolyte. J. Photochem. Photobiol. A-Chem. 2021, 415, 113308. [Google Scholar] [CrossRef]
- Longo, C.; Nogueira, A.F.; De Paoli, M.A.; Cachet, H. Solid-state and flexible dye-sensitized TiO2 solar cells: A study by electrochemical impedance spectroscopy. J. Phys. Chem. B 2002, 106, 5925–5930. [Google Scholar] [CrossRef]
- Tian, H.J.; Hu, L.H.; Zhang, C.N.; Chen, S.H.; Sheng, J.A.; Mo, L.; Liu, W.Q.; Dai, S.Y. Enhanced photovoltaic performance of dye-sensitized solar cells using a highly crystallized mesoporous TiO2 electrode modified by boron doping. J. Mater. Chem. 2011, 21, 863–868. [Google Scholar] [CrossRef]
- Subramanian, A.; Wang, H.W. Effect of hydroxyl group attachment on TiO2 films for dye-sensitized solar cells. Appl. Surf. Sci. 2012, 258, 7833–7838. [Google Scholar] [CrossRef]
- Mingsukang, M.A.; Buraidah, M.H.; Careem, M.A. Development of gel polymer electrolytes for application in quantum dot-sensitized solar cells. Ionics 2017, 23, 347–355. [Google Scholar] [CrossRef]
- Yun, S.N.; Hagfeldt, A.; Ma, T.L. Pt-Free Counter Electrode for Dye-Sensitized Solar Cells with High Efficiency. Adv. Mater. 2014, 26, 6210–6237. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, J.Y.; Lee, D.K.; Kim, B.; Kim, H.; Ko, M.J. Importance of 4-tert-Butylpyridine in Electrolyte for Dye-Sensitized Solar Cells Employing SnO2 Electrode. J. Phys. Chem. C 2012, 116, 22759–22766. [Google Scholar] [CrossRef]
- Zhang, S.F.; Yang, X.D.; Zhang, K.; Chen, H.; Yanagida, M.; Han, L.Y. Effects of 4-tert-butylpyridine on the quasi-Fermi levels of TiO2 films in the presence of different cations in dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2011, 13, 19310–19313. [Google Scholar] [CrossRef]
- Nakade, S.; Kanzaki, T.; Kubo, W.; Kitamura, T.; Wada, Y.; Yanagida, S. Role of electrolytes on charge recombination in dye-sensitized TiO2 solar cell (1): The case of solar cells using the I-/I3(-) redox couple. J. Phys. Chem. B 2005, 109, 3480–3487. [Google Scholar] [CrossRef]
- Stanton, D.T.; Jurs, P.C. Development and use of charged partial surface-area structural descriptors in computer-assisted quantitative structure property relationship studies. Anal. Chem. 1990, 62, 2323–2329. [Google Scholar] [CrossRef]
- Stanton, D.T.; Egolf, L.M.; Jurs, P.C.; Hicks, M.G. Computer-assidted prediction of normal boiling points of pyrans and pyrroles. J. Chem. Inf. Comput. Sci. 1992, 32, 306–316. [Google Scholar] [CrossRef]
- Atkins, P.W.; Atkins, P.C.F.L.C.P.; Press, O.U.; Atkins, P.C.U.O.F.P.W. Quanta: A Handbook of Concepts; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Sangster, J.M. Octanol-Water Partition Coefficients: Fundamentals and Physical Chemistry; Wiley: Hoboken, NJ, USA, 1997. [Google Scholar]
- Wang, H.X.; Bell, J.; Desilvestro, J.; Bertoz, M.; Evans, G. Effect of inorganic iodides on performance of dye-sensitized solar cells. J. Phys. Chem. C 2007, 111, 15125–15131. [Google Scholar] [CrossRef]
- Son, K.M.; Kang, M.G.; Vittal, R.; Lee, J.; Kim, K.J. Effects of substituents of imidazolium cations on the performance of dye-sensitized TiO2 solar cells. J. Appl. Electrochem. 2008, 38, 1647–1652. [Google Scholar] [CrossRef]
- Denizalti, S.; Ali, A.K.; Ela, C.; Ekmekci, M.; Erten-Ela, S. Dye-sensitized solar cells using ionic liquids as redox mediator. Chem. Phys. Lett. 2018, 691, 373–378. [Google Scholar] [CrossRef]

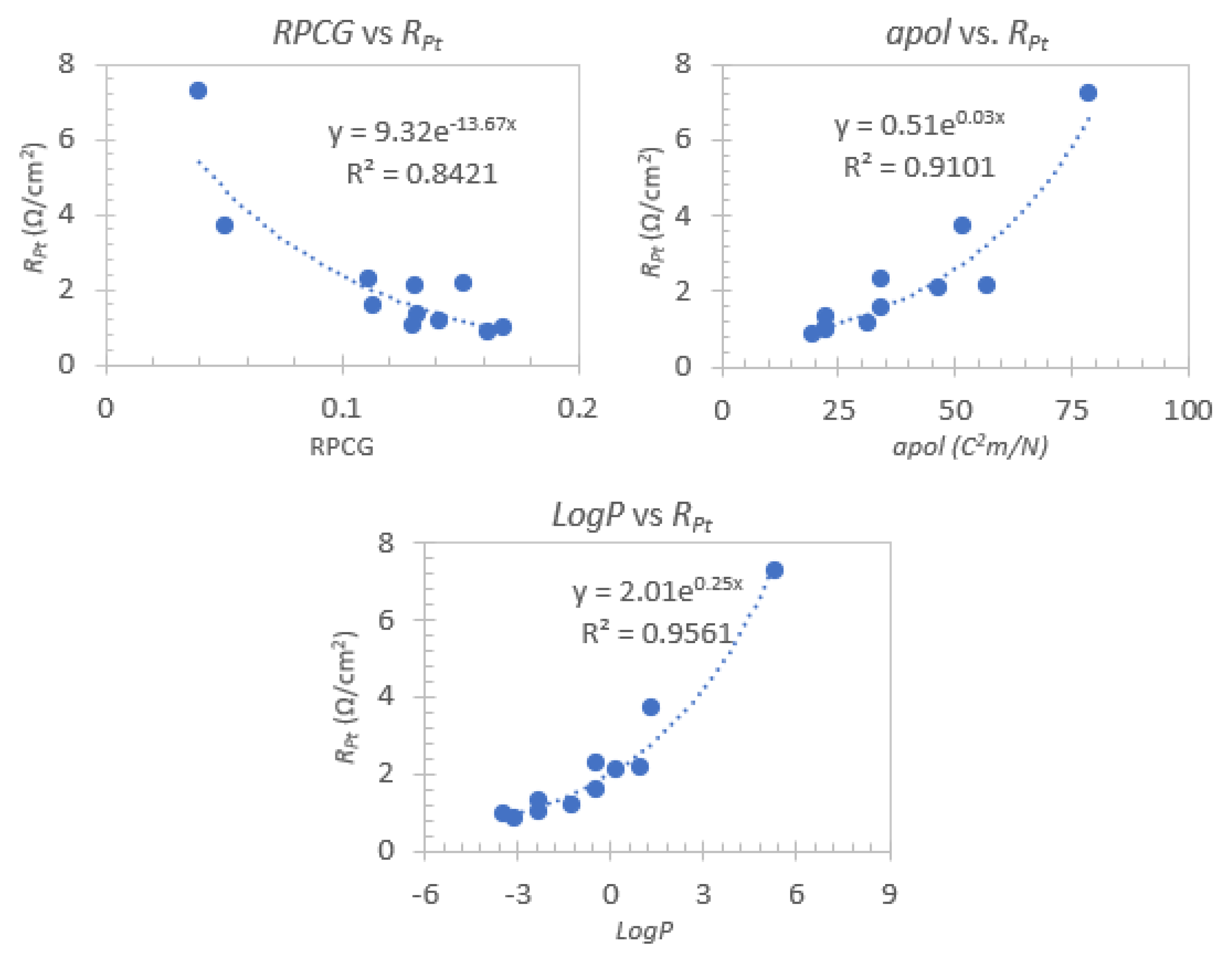
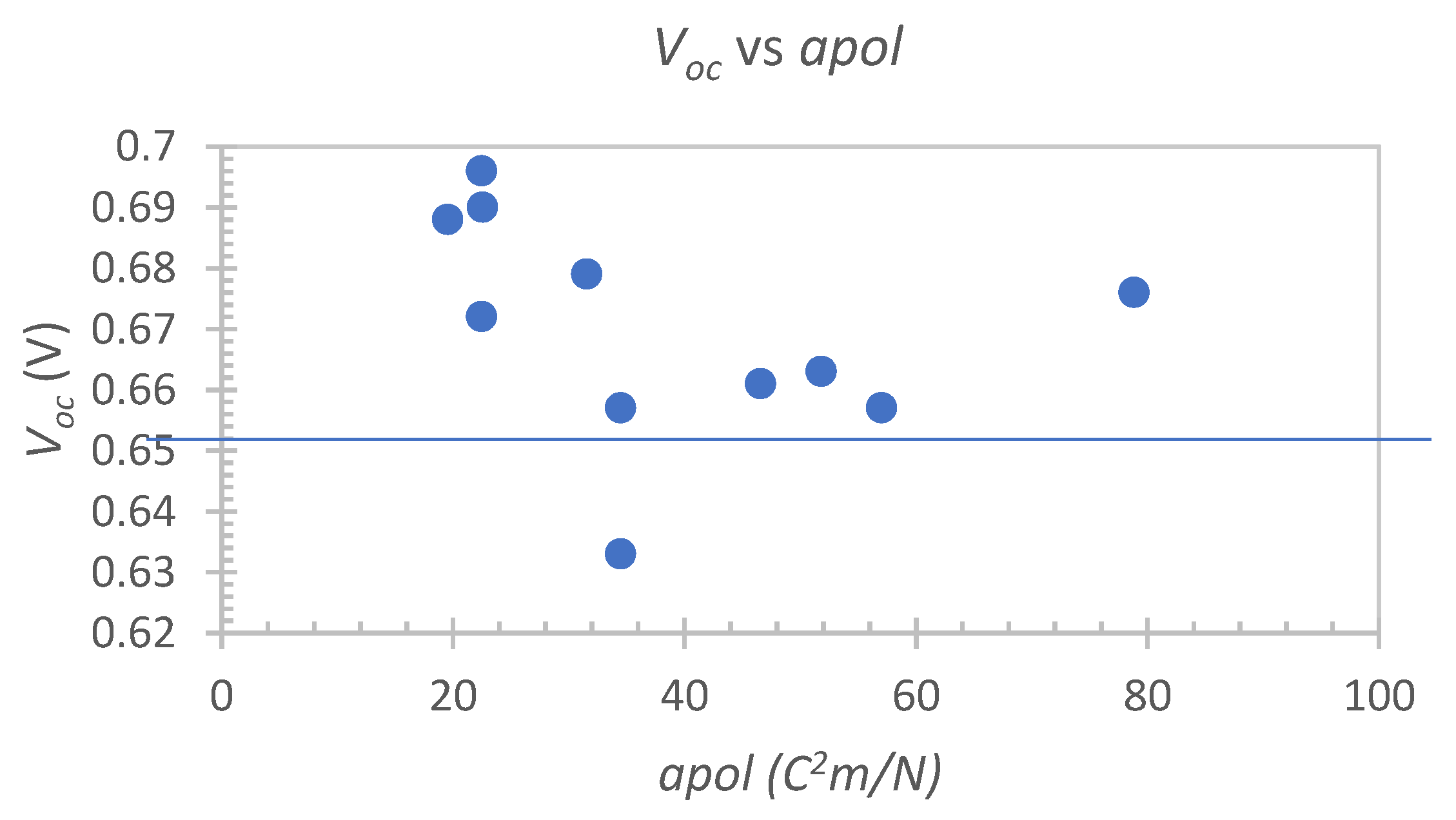
| Electrolyte Mixture | Dye | Voc (V) | Jsc (mA/cm2) | FF | η (%) | Ref. |
|---|---|---|---|---|---|---|
| Solvent | ||||||
| I2/NBB/GuNCS in [DMIM]I/[C3MIM]I/[EMIM][TCB] | Z907Na | 0.741 | 14.26 | 0.77 | 8.20 | [22] |
| I2/NBB in [C3MIM]I/EMImTCM | Z907Na | 0.752 | 12.81 | 0.76 | 7.40 | [23] |
| I2/NMBI/LiI/GuNCS in HeMImI | N3 | 0.725 | 13.52 | 0.70 | 6.82 | [24] |
| Additive | ||||||
| [DMIM]I/I2/GuNCS/4-TBP/LiI in ACN/VN (85/15) | C101 | 0.778 | 17.94 | 0.79 | 11.0 | [26] |
| [DMIM]I/I2/NBB/GuNCS/NaI in BN | C106 | 0.733 | 17.90 | 0.76 | 10.0 | [27] |
| [C3MIM]I/[MIm-TEMPO][TFSI]/NOBF4/LiTFSI/NBB in MPN | D205 | 0.729 | 18.4 | 0.61 | 8.2 | [28] |
| Name | Cation | Voc (V) | Jsc (mA/cm2) | FF | η (%) |
|---|---|---|---|---|---|
| E1 | [C2MIM] | 0.465 ± 0.008 | 14.35 ± 0.24 | 0.64 ± 0.013 | 4.27 ± 0.04 |
| E2 | [C6MIM] | 0.454 ± 0.022 | 14.00 ± 0.89 | 0.61 ± 0.010 | 3.85 ± 0.05 |
| E3 | [C2DMIM] | 0.483 ± 0.008 | 14.78 ± 0.13 | 0.63 ± 0.011 | 4.48 ± 0.03 |
| E4 | [C10DMIM] | 0.464 ± 0.005 | 14.57 ± 0.07 | 0.62 ± 0.007 | 4.20 ± 0.02 |
| E5 | [C2-3PIC] | 0.461 ± 0.007 | 14.52 ± 0.09 | 0.64 ± 0.010 | 4.25 ± 0.03 |
| E6 | [C6-3PIC] | 0.493 ± 0.011 | 14.13 ± 0.34 | 0.64 ± 0.009 | 4.45 ± 0.18 |
| E7 | [C2-4PIC] | 0.470 ± 0.014 | 13.92 ± 0.27 | 0.65 ± 0.006 | 4.27 ± 0.19 |
| E8 | [C6-4PIC] | 0.475 ± 0.006 | 13.89 ± 0.64 | 0.63 ± 0.020 | 4.16 ± 0.28 |
| E9 | [C6-TMG] | 0.472 ± 0.007 | 13.81 ± 0.08 | 0.63 ± 0.006 | 4.13 ± 0.09 |
| E10 | [ALIQUAT] | 0.492 ± 0.013 | 13.75 ± 0.21 | 0.56 ± 0.022 | 3.78 ± 0.11 |
| E11 | [TBA] | 0.493 ± 0.008 | 15.08 ± 0.03 | 0.57 ± 0.004 | 4.21 ± 0.10 |
| Ref | --- | 0.470 ± 0.005 | 14.55 ± 0.55 | 0.61 ± 0.021 | 4.15 ± 0.12 |
| Name | Cation | Voc (mV) | Jsc (mA/cm2) | FF | η (%) |
|---|---|---|---|---|---|
| E1 | [C2MIM] | 0.688 ± 0.013 | 13.06 ± 0.15 | 0.71 ± 0.005 | 6.37 ± 0.06 |
| E2 | [C6MIM] | 0.679 ± 0.010 | 13.41 ± 0.10 | 0.70 ± 0.007 | 6.39 ± 0.05 |
| E3 | [C2DMIM] | 0.690 ± 0.008 | 13.05 ± 0.13 | 0.72 ± 0.005 | 6.48 ± 0.09 |
| E4 | [C10DMIM] | 0.661 ± 0.008 | 13.26 ± 0.20 | 0.69 ± 0.009 | 6.08 ± 0.14 |
| E5 | [C2-3PIC] | 0.672 ± 0.004 | 11.19 ± 0.25 | 0.70 ± 0.006 | 5.28 ± 0.14 |
| E6 | [C6-3PIC] | 0.657 ± 0.009 | 13.36 ± 0.31 | 0.69 ± 0.012 | 6.07 ± 0.14 |
| E7 | [C2-4PIC] | 0.696 ± 0.007 | 12.02 ± 0.04 | 0.69 ± 0.018 | 5.75 ± 0.10 |
| E8 | [C6-4PIC] | 0.633 ± 0.007 | 13.94 ± 0.11 | 0.69 ± 0.010 | 6.13 ± 0.11 |
| E9 | [C6-TMG] | 0.657 ± 0.009 | 13.40 ± 0.07 | 0.69 ± 0.018 | 6.09 ± 0.10 |
| E10 | [ALIQUAT] | 0.676 ± 0.013 | 11.71 ± 0.26 | 0.55 ± 0.033 | 4.33 ± 0.27 |
| E11 | [TBA] | 0.663 ± 0.013 | 13.08 ± 0.22 | 0.68 ± 0.016 | 5.90 ± 0.09 |
| Ref | --- | 0.655 ± 0.008 | 13.07 ± 0.18 | 0.70 ± 0.005 | 6.00 ± 0.05 |
| Name | Cation | Rs (Ω/cm2) | RPt (Ω/cm2) | Rct (Ω/cm2) | τe (ms) |
|---|---|---|---|---|---|
| E1/E1 * | [C2MIM] | 1.363/1.140 | 0.855/1.486 | 8.581/12.046 | 4.57/7.88 |
| E2/E2 * | [C6MIM] | 1.380/1.224 | 1.152/1.740 | 12.154/16.158 | 6.03/9.54 |
| E3/E3 * | [C2DMIM] | 1.312/1.459 | 0.975/1.137 | 11.323/15.531 | 7.14/9.70 |
| E4/E4 * | [C10DMIM] | 1.014/1.594 | 2.080/2.495 | 16.523/17.273 | 10.27/10.87 |
| E5/E5 * | [C2-3PIC] | 1.636/1.308 | 1.318/2.483 | 6.860/16.654 | 3.05/8.36 |
| E6/E6 * | [C6-3PIC] | 1.479/1.135 | 1.554/2.258 | 10.684/9.829 | 5.24/5.90 |
| E7/E7 * | [C2-4PIC] | 0.965/1.675 | 1.019/2.768 | 7.462/9.624 | 3.72/5.55 |
| E8/E8 * | [C6-4PIC] | 1.611/1.511 | 2.297/3.269 | 10.016/13.508 | 5.30/7.99 |
| E9/E9 * | [C6-TMG] | 1.085/1.398 | 2.146/2.436 | 8.291/17.885 | 5.46/10.86 |
| E10/E10 * | [ALIQUAT] | 1.443/1.457 | 7.244/12.123 | 8.942/15.415 | 7.77/11.70 |
| E11/E11 * | [TBA] | 1.030/1.373 | 3.702/3.489 | 24.912/17.775 | 21.79/11.24 |
| Ref/Ref * | --- | 1.483/1.434 | 1.076/1.501 | 7.338/7.56 | 6.09/5.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarrato, J.; Pinto, A.L.; Cruz, H.; Jordão, N.; Malta, G.; Branco, P.S.; Lima, J.C.; Branco, L.C. Effect of Iodide-Based Organic Salts and Ionic Liquid Additives in Dye-Sensitized Solar Cell Performance. Nanomaterials 2022, 12, 2988. https://doi.org/10.3390/nano12172988
Sarrato J, Pinto AL, Cruz H, Jordão N, Malta G, Branco PS, Lima JC, Branco LC. Effect of Iodide-Based Organic Salts and Ionic Liquid Additives in Dye-Sensitized Solar Cell Performance. Nanomaterials. 2022; 12(17):2988. https://doi.org/10.3390/nano12172988
Chicago/Turabian StyleSarrato, João, Ana Lucia Pinto, Hugo Cruz, Noémi Jordão, Gabriela Malta, Paula S. Branco, J. Carlos Lima, and Luis C. Branco. 2022. "Effect of Iodide-Based Organic Salts and Ionic Liquid Additives in Dye-Sensitized Solar Cell Performance" Nanomaterials 12, no. 17: 2988. https://doi.org/10.3390/nano12172988
APA StyleSarrato, J., Pinto, A. L., Cruz, H., Jordão, N., Malta, G., Branco, P. S., Lima, J. C., & Branco, L. C. (2022). Effect of Iodide-Based Organic Salts and Ionic Liquid Additives in Dye-Sensitized Solar Cell Performance. Nanomaterials, 12(17), 2988. https://doi.org/10.3390/nano12172988









