Titanium Dioxide Nanoparticles Modulate Systemic Immune Response and Increase Levels of Reduced Glutathione in Mice after Seven-Week Inhalation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
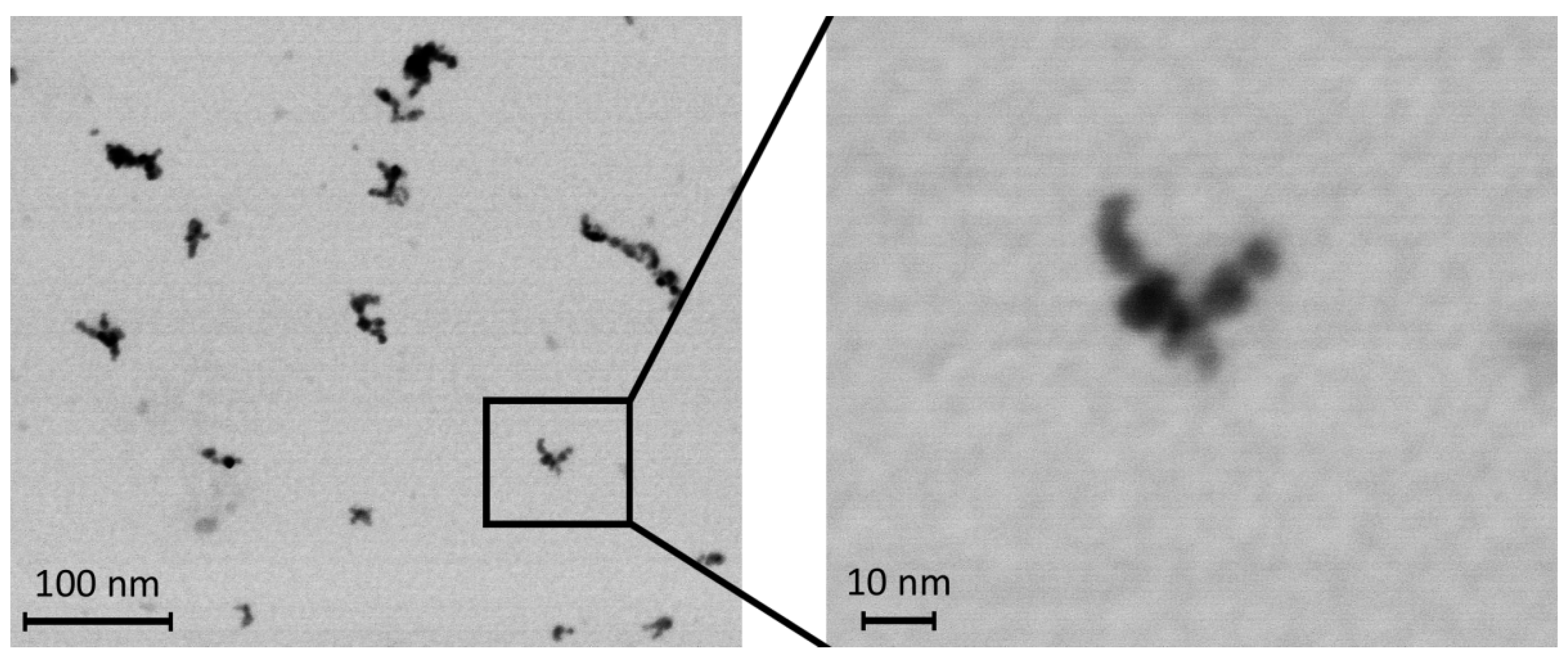
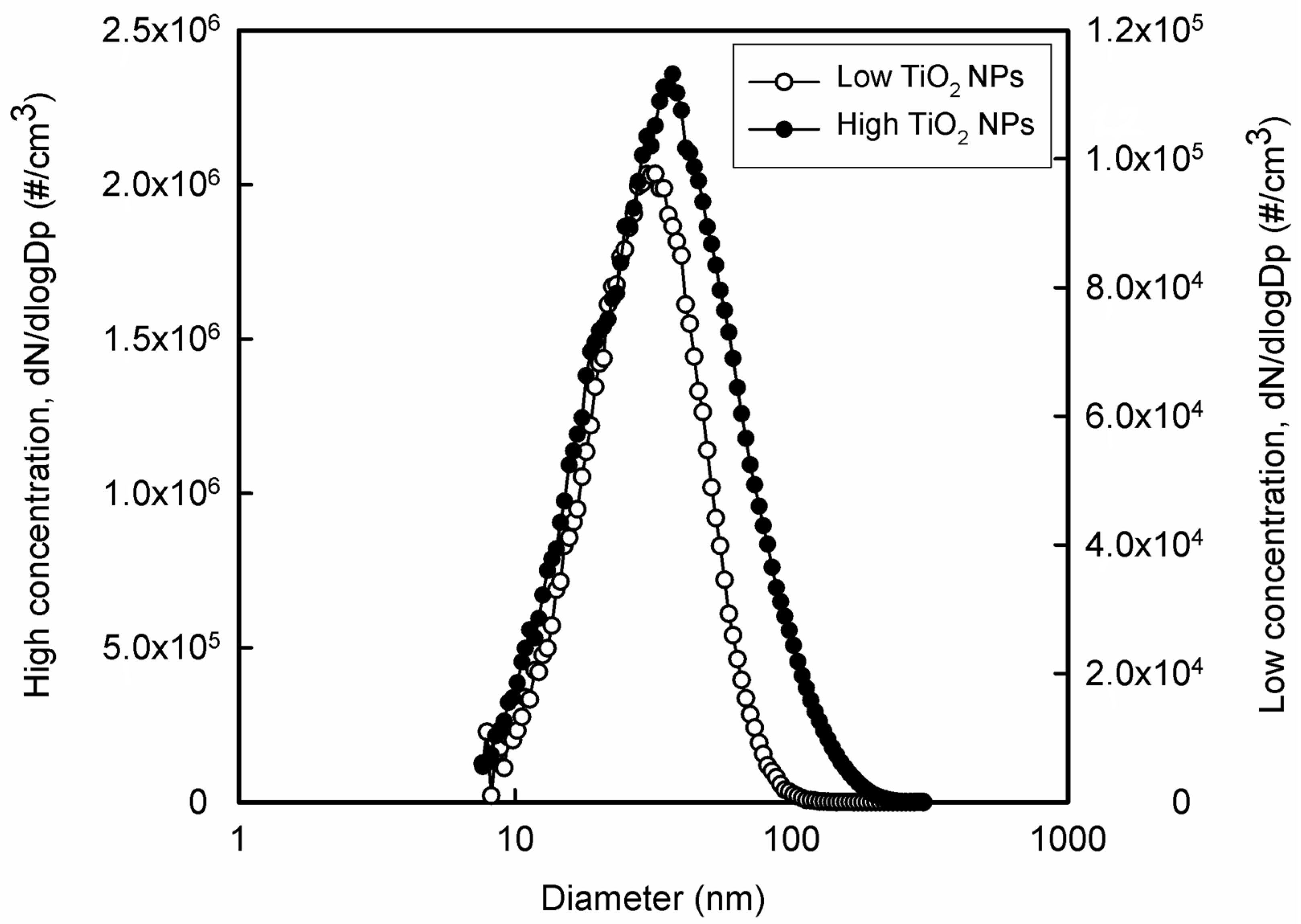
2.2. Preparation of NPs
2.3. Exposure to TiO2 NPs
2.4. Immunoassays
2.4.1. Phagocytic Activity of Granulocytes and Monocytes and Respiratory Burst of Phagocytes
2.4.2. Phenotypic Analysis of Spleen, Thymus, and Bone Marrow
2.4.3. In Vitro Production of Cytokines and Chemokines
2.5. Antioxidant Status, Reduced Glutathione, and Oxidized Glutathione
2.6. Statistical Analysis
3. Results
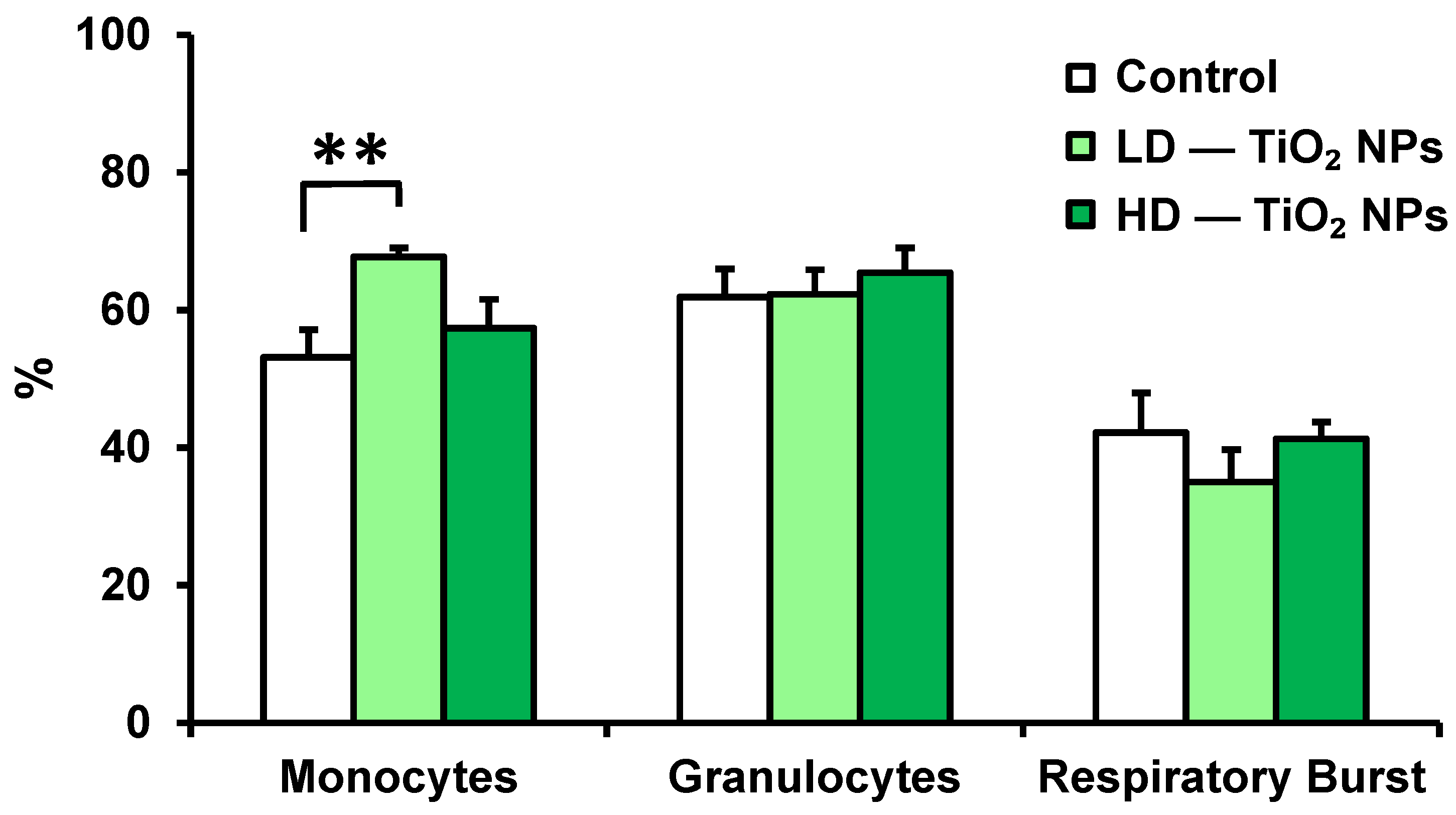
3.1. Phagocytic Activity of Blood Monocytes, Granulocytes, and Respiratory Burst
3.2. Phenotypic Analysis of Spleen, Thymus, and Bone Marrow
3.3. In Vitro Production of Cytokines
3.4. Antioxidant Status of Blood—Reduced Glutathione and Oxidized Glutathione
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | cluster of differentiation |
| Con A | concanavalin A |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GRO-α/KC/CXCL1 | Chemokine growth-regulated protein alpha |
| GSH | reduced form of glutathione |
| GSSG | oxidized form of glutathione |
| HD | high dose |
| IFN-γ | interferon-γ |
| IL | interleukin |
| IP-10/CXCL10 | Interferon gamma-inducible protein 10 |
| LD | low dose |
| MCP | Monocyte chemotactic protein |
| MIP | Macrophage inflammatory protein |
| NK-cells | natural killer cells |
| NP | nanoparticle |
| RANTES/CCL5 | Regulated on activation normal T-cell expressed and secreted |
| ROS | reactive oxygen species |
| Th | T-helper lymphocyte |
| TiO2 | titaium dioxide |
| TNF-α | tumor necrosis factor-α |
References
- Waghmode, M.S.; Gunjal, A.B.; Mulla, J.A.; Patil, N.N.; Nawani, N.N. Studies on the titanium dioxide nanoparticles: Biosynthesis, applications and remediation. SN Appl. Sci. 2019, 1, 310. [Google Scholar] [CrossRef]
- Nabi, G.; Aain, Q.U.; Khalid, N.R.; Tahir, M.B.; Rafique, M.; Rizwan, M.; Hussain, S.; Iqbal, T.; Majid, A. A Review on Novel Eco-Friendly Green Approach to Synthesis TiO2 Nanoparticles Using Different Extracts. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1552–1564. [Google Scholar] [CrossRef]
- Laux, P.; Tentschert, J.; Riebeling, C.; Braeuning, A.; Creutzenberg, O.; Epp, A.; Fessard, V.; Haas, K.H.; Haase, A.; Hund-Rinke, K.; et al. Nanomaterials: Certain aspects of application, risk assessment and risk communication. Arch. Toxicol. 2018, 92, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Dunphy-Guzman, K.A.; Taylor, M.R.; Banfield, J.F. Environmental risks of nanotechnology: National Nanotechnology Initiative Funding, 2000–2004. Environ. Sci. Technol. 2006, 40, 1401–1407. [Google Scholar] [CrossRef]
- Shakeel, M.; Jabeen, F.; Shabbir, S.; Asghar, M.S.; Khan, M.S.; Chaudhry, A.S. Toxicity of Nano-Titanium Dioxide (TiO2-NP) Through Various Routes of Exposure: A Review. Biol. Trace Elem. Res. 2016, 172, 1–36. [Google Scholar] [CrossRef]
- Yin, J.; Kang, C.; Li, Y.; Li, Q.; Zhang, X.; Li, W. Aerosol inhalation exposure study of respiratory toxicity induced by 20 nm anatase titanium dioxide nanoparticles. Toxicol. Res. 2014, 3, 367–374. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Chang, X.; Zhang, Y.; Ma, S.; Sui, J.; Yin, L.; Pu, Y.; Liang, G. Systemic immune effects of titanium dioxide nanoparticles after repeated intratracheal instillation in rat. Int. J. Mol. Sci. 2014, 15, 6961–6973. [Google Scholar] [CrossRef] [PubMed]
- NIOSH. Current Intelligence Bulletin 63: Occupational Exposure to Titanium Dioxide. Current Intelligence Bulletin 63. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. 2011, DHHS (NIOSH) Publication No. 2011-160. Available online: https://www.cdc.gov/niosh/docs/2011-160/ (accessed on 17 January 2023).
- Lee, J.H.; Kwon, M.; Ji, J.H.; Kang, C.S.; Ahn, K.H.; Han, J.H.; Yu, I.J. Exposure assessment of workplaces manufacturing nanosized TiO2 and silver. Inhal. Toxicol. 2011, 23, 226–236. [Google Scholar] [CrossRef]
- Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Cogliano, V.; WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of carbon black, titanium dioxide, and talc. Lancet Oncol. 2006, 7, 295–296. [Google Scholar] [CrossRef]
- EUR-Lex. Commission Delegated Regulation (EU) 2020/217 of 4 October 2019 Amending, for the Purposes of Its Adaptation to Technical and Scientific Progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on Classification, Labelling and Packaging of Substances and Mixtures and Correcting that Regulation. OJ L44, 18.2.2020, p.1. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:2020:044:TOC (accessed on 17 January 2023).
- Titanium Dioxide Manufacturers Association (TDMA). Available online: https://tdma.info/what-you-should-know-about-eu-titanium-dioxide-regulations/ (accessed on 17 January 2023).
- Fiordaliso, F.; Bigini, P.; Salmona, M.; Diomede, D. Toxicological impact of titanium dioxide nanoparticles and food-grade titanium dioxide (E171) on human and environmental health. Environ. Sci. Nano 2022, 9, 1199–1211. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Holzwarth, U.; Schleh, C.; Hirn, S.; Wenk, A.; Schäffler, M.; Haberl, N.; Semmler-Behnke, M.; Gibson, N. Quantitative biokinetics over a 28 day period of freshly generated, pristine, 20 nm titanium dioxide nanoparticle aerosols in healthy adult rats after a single two-hour inhalation exposure. Part. Fibre Toxicol. 2019, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Geraets, L.; Oomen, A.G.; Krystek, P.; Jacobsen, N.R.; Wallin, H.; Laurentie, M.; Verharen, H.W.; Brandon, E.F.; de Jong, W.H. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Part. Fibre Toxicol. 2014, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Winkler, H.C.; Notter, T.; Meyer, U.; Naegeli, H. Critical review of the safety assessment of titanium dioxide additives in food. J. Nanobiotechnol. 2018, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.N.; Shah, Z.; Hussain, M.; Khan, M. Hazardous Effects of Titanium Dioxide Nanoparticles in Ecosystem. Bioinorg. Chem. Appl. 2017, 2017, 4101735. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Liu, J.; Feng, X.; Wei, L.; Shao, L. A review on potential neurotoxicity of titanium dioxide nanoparticles. Nanoscale Res. Lett. 2015, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Fuster, E.; Candela, H.; Estévez, J.; Vilanova, E.; Sogorb, M.A. Titanium Dioxide, but Not Zinc Oxide, Nanoparticles Cause Severe Transcriptomic Alterations in T98G Human Glioblastoma Cells. Int. J. Mol. Sci. 2021, 22, 2084. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Shabbir, S.; Kulyar, M.F.; Bhutta, Z.A.; Boruah, P.; Asif, M. Toxicological Consequences of Titanium Dioxide Nanoparticles (TiO2 NPs) and Their Jeopardy to Human Population. Bionanoscience 2021, 11, 621–632. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef]
- Habib, G.M.; Shi, Z.Z.; Lieberman, M.W. Glutathione protects cells against arsenite-induced toxicity. Free Radic. Biol. Med. 2007, 42, 191–201. [Google Scholar] [CrossRef]
- Dar, G.I.; Saeed, M.; Wu, A. Toxicity of TiO2 Nanoparticles. In TiO2 Nanoparticles Applications in Nanobiotechnology and Nanomedicine; Wu, A., Ren, W., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 67–103. [Google Scholar] [CrossRef]
- Hu, H.; Fan, X.; Yin, Y.; Guo, Q.; Yang, D.; Wei, X.; Zhang, B.; Liu, J.; Wu, Q.; Oh, Y.; et al. Mechanisms of titanium dioxide nanoparticle-induced oxidative stress and modulation of plasma glucose in mice. Environ. Toxicol. 2019, 34, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Lappas, C.M. The immunomodulatory effects of titanium dioxide and silver nanoparticles. Food Chem. Toxicol. 2015, 85, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.M.; Chiasson, S.; Girard, D. Activation of human neutrophils by titanium dioxide (TiO2) nanoparticles. Toxicol. In Vitro 2010, 24, 1002–1008. [Google Scholar] [CrossRef]
- Sang, X.; Zheng, L.; Sun, Q.; Li, N.; Cui, Y.; Hu, R.; Gao, G.; Cheng, Z.; Cheng, J.; Gui, S.; et al. The chronic spleen injury of mice following long-term exposure to titanium dioxide nanoparticles. J. Biomed. Mater. Res. A 2012, 100, 894–902. [Google Scholar] [CrossRef]
- Moon, E.Y.; Yi, G.H.; Kang, J.S.; Lim, J.S.; Kim, H.M.; Pyo, S. An increase in mouse tumor growth by an in vivo immunomodulating effect of titanium dioxide nanoparticles. J. Immunotoxicol. 2011, 8, 56–67. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, J.; Ma, L.; Li, N.; Liu, H.; Wang, J.; Zheng, L.; Liu, C.; Wang, X.; Zhao, X.; et al. Toxicological characteristics of nanoparticulate anatase titanium dioxide in mice. Biomaterials 2010, 31, 894–899. [Google Scholar] [CrossRef]
- Grassian, V.H.; O’Shaughnessy, P.T.; Adamcakova-Dodd, A.; Pettibone, J.M.; Thorne, P.S. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ. Health Perspect. 2007, 115, 397–402. [Google Scholar] [CrossRef]
- Nemmar, A.; Melghit, K.; Ali, B.H. The acute proinflammatory and prothrombotic effects of pulmonary exposure to rutile TiO2 nanorods in rats. Exp. Biol. Med. 2008, 233, 610–619. [Google Scholar] [CrossRef]
- Gustafsson, Å.; Lindstedt, E.; Elfsmark, L.S.; Bucht, A. Lung exposure of titanium dioxide nanoparticles induces innate immune activation and long-lasting lymphocyte response in the Dark Agouti rat. J. Immunotoxicol. 2011, 8, 111–121. [Google Scholar] [CrossRef]
- Park, E.J.; Yoon, J.; Choi, K.; Yi, J.; Park, K. Induction of chronic inflammation in mice treated with titanium dioxide nanoparticles by intratracheal instillation. Toxicology 2009, 260, 37–46. [Google Scholar] [CrossRef]
- Akhtar, M.K.; Vemury, S.; Pratsinis, S.E. Competition between TiCl4 hydrolysis and oxidation and its effect on product TiO2 powder. AIChE J. 1994, 40, 1183–1192. [Google Scholar] [CrossRef]
- Xia, B.; Li, W.; Zhang, B.; Xie, Y. Low temperature vapor-phase preparation of TiO2 nanopowders. J. Mater. Sci. 1999, 34, 3505–3511. [Google Scholar] [CrossRef]
- Akhtar, M.K.; Xiong, Y.; Pratsinis, S.E. Vapor synthesis of titania powder by titanium tetrachloride oxidation. AIChE J. 1991, 37, 1561–1570. [Google Scholar] [CrossRef]
- Xiong, Y.; Akhtar, M.K.; Pratsinis, S.E. Formation of agglomerate particles by coagulation and sintering—Part II. The evolution of the morphology of aerosol-made titania, silica and silica-doped titania powders. J. Aerosol Sci. 1993, 24, 301–313. [Google Scholar] [CrossRef]
- Nakaso, K.; Okuyama, K.; Shimada, M.; Pratsinis, S.E. Effect of Reaction Temperature on CVD-Made TiO2 Primary Particle Diameter. Chem. Eng. Sci. 2003, 58, 3327–3335. [Google Scholar] [CrossRef]
- Schleich, D.M.; Walter, B. Formation of titania nanoparticles by vapor phase reactions of titanium tetraisopropoxide in oxygen/ozone containing atmospheres. Nanostruct. Mater. 1997, 8, 579–586. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yu, J.H.; Lee, J.S. The Characteristics of Nanosized TiO2 Powders Synthesized by Chemical Vapor Condensation. NanoStruct. Mater. 1999, 12, 471–474. [Google Scholar] [CrossRef]
- Choi, J.G.; Park, K.J. Effect of Reaction Atmosphere on Particle Morphology of TiO2 Produced by Thermal Decomposition of Titanium Tetraisopropoxide. J. Nanopart. Res. 2006, 8, 269–278. [Google Scholar] [CrossRef]
- Komiyama, H.; Kanai, T.; Inoue, H. Preparation of Porous, Amorphous, and Ultrafine TiO2 Particles by Chemical Vapor Deposition. Chem. Lett. 1984, 13, 1283–1286. [Google Scholar] [CrossRef]
- Koivisto, A.J.; Mäkinen, M.; Rossi, E.M.; Lindberg, H.K.; Miettinen, M.; Falck, G.C.-M.; Norppa, H.; Alenius, H.; Korpi, A.; Riikonen, J.; et al. Aerosol Characterization and Lung Deposition of Synthesized TiO2 Nanoparticles for Murine Inhalation Studies. J. Nanopart. Res. 2011, 13, 2949–2961. [Google Scholar] [CrossRef]
- Moravec, P.; Schwarz, J.; Vodicka, P.; Kostejn, M. Study of TiO2 nanoparticle generation for follow-up inhalation experiments with laboratory animals. Aerosol Sci. Technol. 2016, 50, 1068–1076. [Google Scholar] [CrossRef]
- Schmid, O.; Stoeger, T. Surface area is the biologically most effective dose metric for acute nanoparticle toxicity in the lung. J. Aerosol Sci. 2016, 99, 133–143. [Google Scholar] [CrossRef]
- Vecera, Z.; Mikuska, P.; Moravec, P.; Smolik, J. Unique exposure system for the whole body inhalation experiments with small animals. In Proceedings of the 3rd International Conference NANOCON 2011, Brno, Czech Republic, 21–23 September 2011; pp. 652–654. [Google Scholar]
- Dumková, J.; Smutná, T.; Vrlíková, L.; Le Coustumer, P.; Večeřa, Z.; Dočekal, B.; Mikuška, P.; Čapka, L.; Fictum, P.; Hampl, A.; et al. Sub-chronic inhalation of lead oxide nanoparticles revealed their broad distribution and tissue-specific subcellular localization in target organs. Part. Fibre Toxicol. 2017, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Tulinska, J.; Masanova, V.; Liskova, A.; Mikusova, M.L.; Rollerova, E.; Krivosikova, Z.; Stefikova, K.; Uhnakova, I.; Ursinyova, M.; Babickova, J.; et al. Six-week inhalation of CdO nanoparticles in mice: The effects on immune response, oxidative stress, antioxidative defense, fibrotic response, and bones. Food Chem. Toxicol. 2020, 136, 110954. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Yamano, S.; Takeda, T.; Goto, Y.; Hirai, S.; Furukawa, Y.; Kikuchi, Y.; Kasai, T.; Misumi, K.; Suzuki, M.; Takanobu, K.; et al. Lack of pulmonary fibrogenicity and carcinogenicity of titanium dioxide nanoparticles in 26-week inhalation study in rasH2 mouse model. bioRxiv 2021, 2021.12.23.473959. [Google Scholar] [CrossRef]
- Farrera, C.; Fadeel, B. It takes two to tango: Understanding the interactions between engineered nanomaterials and the immune system. Eur. J. Pharm. Biopharm. 2015, 95 Pt A, 3–12. [Google Scholar] [CrossRef]
- You, D.J.; Lee, H.Y.; Bonner, J.C. Macrophages: First Innate Immune Responders to Nanomaterials. In Molecular and Integrative Toxicology; Bonner, J., Brown, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 15–34. [Google Scholar] [CrossRef]
- Sund, J.; Palomäki, J.; Ahonen, N.; Savolainen, K.; Alenius, H.; Puustinen, A. Phagocytosis of nano-sized titanium dioxide triggers changes in protein acetylation. J. Proteom. 2014, 108, 469–483. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Pu, Y.; Yin, L.; Li, Y.; Zhang, X.; Liang, G.; Li, X.; Zhang, J. Small-sized titanium dioxide nanoparticles mediate immune toxicity in rat pulmonary alveolar macrophages in vivo. J. Nanosci. Nanotechnol. 2010, 10, 5161–5169. [Google Scholar] [CrossRef] [PubMed]
- Ghanbary, F.; Seydi, E.; Naserzadeh, P.; Salimi, A. Toxicity of nanotitanium dioxide (TiO2-NP) on human monocytes and their mitochondria. Environ. Sci. Pollut. Res. 2018, 25, 6739–6750. [Google Scholar] [CrossRef] [PubMed]
- Ispanixtlahuatl-Meráz, O.; Delgado-Buenrostro, N.L.; Déciga-Alcaraz, A.; Ramos-Godinez, M.D.P.; Oliva-Rico, D.; López-Villegas, E.O.; Vázquez-Zapién, G.J.; Mata-Miranda, M.M.; Ilhuicatzi-Alvarado, D.; Moreno-Fierros, L.; et al. Differential response of immobile (pneumocytes) and mobile (monocytes) barriers against 2 types of metal oxide nanoparticles. Chem. Biol. Interact. 2021, 347, 109596. [Google Scholar] [CrossRef] [PubMed]
- Luster, M.I. A historical perspective of immunotoxicology. J. Immunotoxicol. 2014, 11, 197–202. [Google Scholar] [CrossRef]
- Boraschi, D.; Costantino, L.; Italiani, P. Interaction of nanoparticles with immunocompetent cells: Nanosafety considerations. Nanomedicine 2012, 7, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Yang, H.-L.; Lin, B.-C.; Zhang, W.; Tian, L.; Zhang, H.-S.; Xi, Z.-G. Toxic effect comparison of three typical sterilization nanoparticles on oxidative stress and immune inflammation response in rats. Toxicol. Res. 2015, 4, 486–493. [Google Scholar] [CrossRef]
- Alijagic, A.; Gaglio, D.; Napodano, E.; Russo, R.; Costa, C.; Benada, O.; Kofroňová, O.; Pinsino, A. Titanium dioxide nanoparticles temporarily influence the sea urchin immunological state suppressing inflammatory-relate gene transcription and boosting antioxidant metabolic activity. J. Hazard. Mater. 2020, 384, 121389. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Sun, M.; Yang, Y.; Wang, F.; Ma, X.; Li, J.; Wang, Y.; Ding, Q.; Ying, H.; Song, H.; et al. Titanium dioxide nanoparticles prime a specific activation state of macrophages. Nanotoxicology 2017, 11, 737–750. [Google Scholar] [CrossRef]
- Mishra, V.; Baranwal, V.; Mishra, R.K.; Sharma, S.; Paul, B.; Pandey, A.C. Titanium dioxide nanoparticles augment allergic airway inflammation and Socs3 expression via NF-κB pathway in murine model of asthma. Biomaterials 2016, 92, 90–102. [Google Scholar] [CrossRef]
- Latha, T.S.; Reddy, M.C.; Durbaka, P.V.R.; Muthukonda, S.V.; Lomada, D. Immunomodulatory properties of titanium dioxide nanostructural materials. Indian J. Pharmacol. 2017, 49, 458–464. [Google Scholar] [CrossRef]
- Hashiguchi, S.; Yoshida, H.; Akashi, T.; Komemoto, K.; Ueda, T.; Ikarashi, Y.; Miyauchi, A.; Konno, K.; Yamanaka, S.; Hirose, A.; et al. Titanium dioxide nanoparticles exacerbate pneumonia in respiratory syncytial virus (RSV)-infected mice. Environ. Toxicol. Pharmacol. 2015, 39, 879–886. [Google Scholar] [CrossRef]
- Wang, J.X.; Fan, Y.B.; Gao, Y.; Hu, Q.H.; Wang, T.C. TiO2 nanoparticles translocation and potential toxicological effect in rats after intraarticular injection. Biomaterials 2009, 30, 4590–4600. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, A.; Samyuktha, L.; Atya, K.; Neha, H.; Rakesh, K.S.; Shantveer, G.U.; Kaiser, J. In Vivo Interactions of Nanosized Titania Anatase and Rutile Particles Following Oral Administration. Nano Prog. 2020, 2, 11–20. [Google Scholar]
- Sauer, U.G.; Vogel, S.; Aumann, A.; Hess, A.; Kolle, S.N.; Ma-Hock, L.; Wohlleben, W.; Dammann, M.; Strauss, V.; Treumann, S.; et al. Applicability of rat precision-cut lung slices in evaluating nanomaterial cytotoxicity, apoptosis, oxidative stress, and inflammation. Toxicol. Appl. Pharmacol. 2014, 276, 1–20. [Google Scholar] [CrossRef]
- Murugadoss, S.; Mülhopt, S.; Diabaté, S.; Ghosh, M.; Paur, H.R.; Stapf, D.; Weiss, C.; Hoet, P.H. Agglomeration State of Titanium-Dioxide (TiO2) Nanomaterials Influences the Dose Deposition and Cytotoxic Responses in Human Bronchial Epithelial Cells at the Air-Liquid Interface. Nanomaterials 2021, 11, 3226. [Google Scholar] [CrossRef]
- Relier, C.; Dubreuil, M.; Lozano Garcìa, O.; Cordelli, E.; Mejia, J.; Eleuteri, P.; Robidel, F.; Loret, T.; Pacchierotti, F.; Lucas, S.; et al. Study of TiO2 P25 Nanoparticles Genotoxicity on Lung, Blood, and Liver Cells in Lung Overload and Non-Overload Conditions After Repeated Respiratory Exposure in Rats. Toxicol. Sci. 2017, 156, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C.; Liu, Y.; Jiao, F.; Li, W.; Lao, F.; Li, Y.; Li, B.; Ge, C.; Zhou, G.; et al. Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicol. Lett. 2008, 183, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Jiao, F.; Lao, F.; Li, W.; Gu, Y.; Li, Y.; Ge, C.; Zhou, G.; Li, B.; et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO2 nanoparticles. Toxicology 2008, 254, 82–90. [Google Scholar] [CrossRef]
- Carmo, T.L.L.; Siqueira, P.R.; Azevedo, V.C.; Tavares, D.; Pesenti, E.C.; Cestari, M.M.; Martinez, C.B.R.; Fernandes, M.N. Overview of the toxic effects of titanium dioxide nanoparticles in blood, liver, muscles, and brain of a Neotropical detritivorous fish. Environ. Toxicol. 2019, 34, 457–468. [Google Scholar] [CrossRef]
- Modrzynska, J.; Mortensen, A.; Berthing, T.; Ravn-Haren, G.; Szarek, J.; Saber, A.T.; Vogel, U. Effect on Mouse Liver Morphology of CeO2, TiO2 and Carbon Black Nanoparticles Translocated from Lungs or Deposited Intravenously. Appl. Nano 2021, 2, 222–241. [Google Scholar] [CrossRef]
- Nemmar, A.; Melghit, K.; Al-Salam, S.; Zia, S.; Dhanasekaran, S.; Attoub, S.; Al-Amri, I.; Ali, B.H. Acute respiratory and systemic toxicity of pulmonary exposure to rutile Fe-doped TiO2 nanorods. Toxicology 2011, 279, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Eydner, M.; Schaudien, D.; Creutzenberg, O.; Ernst, H.; Hansen, T.; Baumgärtner, W.; Rittinghausen, S. Impacts after inhalation of nano- and fine-sized titanium dioxide particles: Morphological changes, translocation within the rat lung, and evaluation of particle deposition using the relative deposition index. Inhal. Toxicol. 2012, 24, 557–569. [Google Scholar] [CrossRef] [PubMed]
| Organ | Parameter | Control (Mean ± SEM) | LD—TiO2 NPs (Mean ± SEM) | HD—TiO2 NPs (Mean ± SEM) |
|---|---|---|---|---|
| Spleen | CD3+ | 42.7 ± 2.0 | 36.5 ± 2.7 * | 34.5 ± 3.4 * |
| CD3+CD4+ | 26.4 ± 1.6 | 21.8 ± 2.2 * | 22.5 ± 2.4 | |
| CD3+CD8+ | 31.1 ± 2.3 | 26.6 ± 2.8 | 27.4 ± 3.0 | |
| CD3−CD19+ | 34.7 ± 2.8 | 38.0 ± 2.7 | 36.6 ± 2.1 | |
| CD3−CD335+ | 1.5 ± 0.2 | 2.7 ± 1.2 | 1.4 ± 0.1 | |
| Thymus | CD3+ | 39.5 ± 3.4 | 43.6 ± 2.9 | 35.9 ± 2.8 |
| CD3+CD4+ | 33.5 ± 3.0 | 36.8 ± 2.7 | 31.0 ± 2.2 | |
| CD3+CD8+ | 31.0 ± 2.9 | 36.2 ± 3.3 | 26.1 ± 3.0 | |
| Bone marrow | CD3+ | 3.6 ± 0.4 | 3.4 ± 0.5 | 3.7 ± 0.6 |
| CD3−CD19+ | 2.6 ± 0.4 | 2.8 ± 0.4 | 2.4 ± 0.5 | |
| CD3−CD335+ | 1.7 ± 0.3 | 1.2 ± 0.2 | 1.4 ± 0.3 |
| Cytokine | Control (Mean ± SEM) | LD—TiO2 NPs (Mean ± SEM) | HD—TiO2 NPs (Mean ± SEM) |
|---|---|---|---|
| IL-2 | 287.0 ± 102.0 | 276.1 ± 88.8 | 180.8 ± 54.7 |
| IL-4 | 713.6 ± 189.1 | 965.2 ± 236.4 | 315.4 ± 121.5 * |
| IL-6 | 94.4 ± 33.4 | 52.9 ± 8.1 | 43.0 ± 12.0 |
| IL-10 | 77.2 ± 28.0 | 54.1 ± 17.5 | 46.4 ± 21.5 |
| IL-13 | 166.6 ± 74.2 | 97.8 ± 41.8 | 73.2 ± 40.8 |
| IL-17A | 63.8 ± 23.8 | 44.1 ± 26.9 | 30.7 ± 20.9 |
| IL-18 | 1808.4 ± 301.8 | 2161.7 ± 264.4 | 1139.1 ± 226.6 * |
| IFN-γ | 262.2 ± 159.0 | 134.1 ± 41.9 | 104.4 ± 54.7 |
| TNF-α | 56.0 ± 15.6 | 43.6 ± 6.1 | 31.8 ± 6.0 |
| GM-CSF | 25.0 ± 11.4 | 12.2 ± 3.1 | 9.8 ± 3.5 |
| Eotaxin/CCL11 | 40.5 ± 11.2 | 34.7 ± 4.5 | 24.5 ± 6.0 |
| MIP-1α/CCL3 | 127.2 ± 40.4 | 98.7 ± 12.0 | 77.0 ± 22.8 |
| MIP-1β/CCL4 | 272.4 ± 103.9 | 200.9 ± 27.7 | 141.1 ± 34.9 |
| MIP-2/CCL8 | 8.8 ± 2.4 | 5.4 ± 0.6 | 6.2 ± 0.9 |
| RANTES/CCL5 | 68.8 ± 12.7 | 52.3 ± 6.8 | 43.4 ± 6.4 |
| IP-10/CXCL10 | 122.1 ± 41.1 | 129.2 ± 44.6 | 88.1 ± 30.8 |
| MCP-1/CCL2 | 216.5 ± 34.6 | 433.3 ± 110.6 | 243.6 ± 60.6 |
| MCP-3/CCL7 | 71.4 ± 19.5 | 119.0 ± 40.0 | 82.6 ± 31.2 |
| Parameter | Control (Mean ± SEM) | LD—TiO2 NPs (Mean ± SEM) | HD—TiO2 NPs (Mean ± SEM) |
|---|---|---|---|
| GSH | 785.5 ± 110.4 | 1037.6 ± 165.9 | 1368.6 ± 108.5 ** |
| GSSG | 60.3 ± 3.5 | 61.5 ± 7.0 | 61.4 ± 5.1 |
| GSH/GSSG | 5.3 ± 0.7 | 7.6 ± 1.1 * | 10.5 ± 1.1 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehotska Mikusova, M.; Busova, M.; Tulinska, J.; Masanova, V.; Liskova, A.; Uhnakova, I.; Dusinska, M.; Krivosikova, Z.; Rollerova, E.; Alacova, R.; et al. Titanium Dioxide Nanoparticles Modulate Systemic Immune Response and Increase Levels of Reduced Glutathione in Mice after Seven-Week Inhalation. Nanomaterials 2023, 13, 767. https://doi.org/10.3390/nano13040767
Lehotska Mikusova M, Busova M, Tulinska J, Masanova V, Liskova A, Uhnakova I, Dusinska M, Krivosikova Z, Rollerova E, Alacova R, et al. Titanium Dioxide Nanoparticles Modulate Systemic Immune Response and Increase Levels of Reduced Glutathione in Mice after Seven-Week Inhalation. Nanomaterials. 2023; 13(4):767. https://doi.org/10.3390/nano13040767
Chicago/Turabian StyleLehotska Mikusova, Miroslava, Milena Busova, Jana Tulinska, Vlasta Masanova, Aurelia Liskova, Iveta Uhnakova, Maria Dusinska, Zora Krivosikova, Eva Rollerova, Radka Alacova, and et al. 2023. "Titanium Dioxide Nanoparticles Modulate Systemic Immune Response and Increase Levels of Reduced Glutathione in Mice after Seven-Week Inhalation" Nanomaterials 13, no. 4: 767. https://doi.org/10.3390/nano13040767
APA StyleLehotska Mikusova, M., Busova, M., Tulinska, J., Masanova, V., Liskova, A., Uhnakova, I., Dusinska, M., Krivosikova, Z., Rollerova, E., Alacova, R., Wsolova, L., Horvathova, M., Szabova, M., Lukan, N., Vecera, Z., Coufalik, P., Krumal, K., Alexa, L., Thon, V., ... Mikuska, P. (2023). Titanium Dioxide Nanoparticles Modulate Systemic Immune Response and Increase Levels of Reduced Glutathione in Mice after Seven-Week Inhalation. Nanomaterials, 13(4), 767. https://doi.org/10.3390/nano13040767










