Single-Step Synthesis of Graphitic Carbon Nitride Nanomaterials by Directly Calcining the Mixture of Urea and Thiourea: Application for Rhodamine B (RhB) Dye Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of g-C3N4 Samples
2.2.2. Characterization Techniques
2.2.3. Photocatalytic Degradation Experiments
3. Results and Discussion
3.1. XRD Analysis
3.2. Optical Properties Studies
3.2.1. PL Analysis
3.2.2. UV-Vis DRS Analysis
3.2.3. FTIR Analysis
3.3. TGA Analysis
3.4. FE-SEM and HR-TEM Analysis
3.5. Photocatalytic Activity Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, F.; Sun, S.; Xu, H.; Li, M.; Hao, X.; Shao, G.; Wang, H.; Chen, D.; Lu, H.; Zhang, R. Investigation on g-C3N4/rGO/TiO2 nanocomposite with enhanced photocatalytic degradation performance. J. Phys. Chem. Solids 2021, 156, 110181. [Google Scholar] [CrossRef]
- Pham, T.H.; Myung, Y.; Van Le, Q.; Kim, T. Visible-light photocatalysis of Ag-doped graphitic carbon nitride for photodegradation of micropollutants in wastewater. Chemosphere 2022, 301, 134626. [Google Scholar] [CrossRef]
- Thines, R.K.; Mubarak, N.M.; Nizamuddin, S.; Sahu, J.N.; Abdullah, E.C.; Ganesan, P. Application potential of carbon nanomaterials in water and wastewater treatment: A review. J. Taiwan Inst. Chem. Eng. 2017, 72, 116–133. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, P.; Ma, R.; Luo, C.; Wen, T.; Zhao, G.; Cheng, W.; Wang, X. Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: A critical review. Catal. Today 2018, 335, 65–77. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef]
- Sewnet, A.; Abebe, M.; Asaithambi, P.; Alemayehu, E. Visible-Light-Driven g-C3N4/TiO2 Based Heterojunction Nanocomposites for Photocatalytic Degradation of Organic Dyes in Wastewater: A Review. Air Soil Water Res. 2022, 15, 11786221221117266. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. Synthesis and characterization of solar photoactive TiO2 nanoparticles with enhanced structural and optical properties. Adv. Powder Technol. 2017, 29, 211–219. [Google Scholar] [CrossRef]
- Saravanan, R.; Garcia, F.; Stephen, A. Basic principles, mechanism, and challenges of photocatalysis. In Nanocomposites for Visible Light-Induced Photocatalysis; Springer: Cham, Switzerland, 2017; pp. 19–40. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Shandilya, P.; Jeong, D.Y.; Lim, J.H.; Singh, P. Review on fabrication of graphitic carbon nitride based efficient nanocomposites for photodegradation of aqueous phase organic pollutants. J. Ind. Eng. Chem. 2018, 67, 28–51. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal-doped/codoped TiO2, and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrog. Energy 2019, 45, 7764–7778. [Google Scholar] [CrossRef]
- Hwang, K.J.; Lee, J.W.; Shim, W.G.; Jang, H.D.; Lee, S.I.; Yoo, S.J. Adsorption and photocatalysis of nanocrystalline TiO2 particles prepared by sol-gel method for methylene blue degradation. Adv. Powder Technol. 2012, 23, 414–418. [Google Scholar] [CrossRef]
- Ameta, R.; Solanki, M.S.; Benjamin, S.; Ameta, S.C. Photocatalysis. In Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology; Academic Press: Cambridge, MA, USA, 2018; pp. 135–175. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.-W.; Mohite, B.M. Role of Nanotechnology in Photocatalysis Application. Recent Pat. Nanotechnol. 2023, 17, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.W.; Fujishima, A.; Terashima, C. Enhanced Photocatalytic Degradation Activity Using the V2O5/RGO Composite. Nanomaterials 2023, 13, 338. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A review. Appl. Catal. B 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Iqbal, S.; Ahmad, N.; Javed, M.; Qamar, M.A.; Bahadur, A.; Ali, S.; Ahmad, Z.; Irfan, R.M.; Liu, G.; Akbar, M.B.; et al. Designing highly potential photocatalytic comprising silver deposited ZnO NPs with sulfurized graphitic carbon nitride (Ag/ZnO/S-g-C3N4) ternary composite. J. Environ. Chem. Eng. 2020, 9, 104919. [Google Scholar] [CrossRef]
- Yadav, A.A.; Kang, S.W.; Hunge, Y.M. Photocatalytic degradation of Rhodamine B using graphitic carbon nitride photocatalyst. J. Mater. Sci. Mater. Electron. 2021, 32, 15577–15585. [Google Scholar] [CrossRef]
- Ke, P.; Zeng, D.; Cui, J.; Li, X.; Chen, Y. Improvement in Structure and Visible Light Catalytic Performance of g-C3N4 Fabricated at a Higher Temperature. Catalysts 2022, 12, 247. [Google Scholar] [CrossRef]
- Fang, H.B.; Luo, Y.; Zheng, Y.Z.; Ma, W.; Tao, X. Facile Large-Scale Synthesis of Urea-Derived Porous Graphitic Carbon Nitride with Extraordinary Visible-Light Spectrum Photodegradation. Ind. Eng. Chem. Res. 2016, 55, 4506–4514. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Controllable Synthesis of Mesoporous Sulfur-Doped Carbon Nitride Materials for Enhanced Visible Light Photocatalytic Degradation. Langmuir 2017, 33, 7062–7078. [Google Scholar] [CrossRef]
- Papailias, I.; Giannakopoulou, T.; Todorova, N.; Demotikali, D.; Vaimakis, T.; Trapalis, C. Effect of processing temperature on structure and photocatalytic properties of g-C3N4. Appl. Surf. Sci. 2015, 358, 278–286. [Google Scholar] [CrossRef]
- Kadam, A.N.; Moniruzzaman, M.; Lee, S.W. Dual functional S-doped g-C3N4 pinhole porous nanosheets for selective fluorescence sensing of Ag+ and visible-light photocatalysis of dyes. Molecules 2019, 24, 450. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, L.; Praus, P.; Lima, M.J.; Sampaio, M.J.; Matýsek, D.; Ritz, M.; Dvorský, R.; Faria, J.L.; Silva, C.G. Graphitic carbon nitride nanosheets as highly efficient photocatalysts for phenol degradation under high-power visible LED irradiation. Mater. Res. Bull. 2018, 100, 322–332. [Google Scholar] [CrossRef]
- Yang, C.C.; Doong, R.A.; Chen, K.F.; Chen, G.S.; Tsai, Y.P. The photocatalytic degradation of methylene blue by green semiconductor films that is induced by irradiation by a light-emitting diode and visible light. J. Air Waste Manag. Assoc. 2018, 68, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gong, H.; Li, G.; Zeng, H.; Zhong, L.; Liu, K.; Cao, H.; Yan, H. Synthesis of graphitic carbon nitride by heating mixture of urea and thiourea for enhanced photocatalytic H2 production from water under visible light. Int. J. Hydrog. Energy 2016, 42, 143–151. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Xiao, X.; Guo, L.; Li, Y. Enhanced visible light photocatalytic hydrogen evolution of sulfur-doped polymeric g-C3N4 photocatalysts. Mater. Res. Bull. 2013, 48, 3919–3925. [Google Scholar] [CrossRef]
- Wang, L.; Hou, Y.; Xiao, S.; Bi, F.; Zhao, L.; Li, Y.; Zhang, X.; Gai, G.; Dong, X. One-step, high-yield synthesis of g-C3N4 nanosheets for enhanced visible light photocatalytic activity. RSC Adv. 2019, 9, 39304–39314. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Liu, Z.; Wang, C.; Liu, G.; Li, Q.; Feng, X. Enhanced photocatalytic activity of g-C3N4 2D nanosheets through thermal exfoliation using dicyandiamide as precursor. Ceram Int. 2018, 44, 20613–20619. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, E.; Shi, J.; Lin, X.; Sheng, L.; Zhang, M.; Wang, L.; Chen, J. A direct one-step synthesis of ultrathin g-C3N4 nanosheets from thiourea for boosting solar photocatalytic H2 evolution. Int. J. Hydrog. Energy 2019, 44, 7194–7204. [Google Scholar] [CrossRef]
- Paul, D.R.; Sharma, R.; Nehra, S.P.; Sharma, A. Effect of calcination temperature, pH and catalyst loading on photodegradation efficiency of urea derived graphitic carbon nitride towards methylene blue dye solution. RSC Adv. 2019, 9, 15381–15391. [Google Scholar] [CrossRef]
- Qin, H.; Lv, W.; Bai, J.; Zhou, Y.; Wen, Y.; He, Q.; Tang, J.; Wang, L.; Zhou, Q. Sulfur-doped porous graphitic carbon nitride heterojunction hybrids for enhanced photocatalytic H2 evolution. J. Mater. Sci. 2019, 54, 4811–4820. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Y.; Yan, L.; Su, Z. DFT Study on Sulfur-Doped g-C3N4 Nanosheets as a Photocatalyst for CO2 Reduction Reaction. J. Phys. Chem. C 2018, 122, 7712–7719. [Google Scholar] [CrossRef]
- Li, D.-F.; Huang, W.-Q.; Zou, L.-R.; Pan, A.; Huang, G.-F. Mesoporous g-C3N4 Nanosheets: Synthesis, Superior Adsorption Capacity, and Photocatalytic Activity. J. Nanosci. Nanotechnol. 2018, 18, 5502–5510. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Linh, P.H.; Do Chung, P.; Van Khien, N.; Thu, V.T.; Bach, T.N.; Hang, L.T.; Hung, N.M.; Lam, V.D. A simple approach for controlling the morphology of g-C3N4 nanosheets with enhanced photocatalytic properties. Diam. Relat. Mater. 2021, 111, 108214. [Google Scholar] [CrossRef]
- Lu, Z.; Zeng, L.; Song, W.; Qin, Z.; Zeng, D.; Xie, C. In situ synthesis of C-TiO2/g-C3N4 heterojunction nanocomposite as highly visible light active photocatalyst originated from effective interfacial charge transfer. Appl. Catal. B 2017, 202, 489–499. [Google Scholar] [CrossRef]
- Liu, R.; Bie, Y.; Qiao, Y.; Liu, T.; Song, Y. Design of g-C3N4/TiO2 nanotubes heterojunction for enhanced organic pollutants degradation in wastewater. Mater. Lett. 2019, 251, 126–130. [Google Scholar] [CrossRef]
- Hao, R.; Wang, G.; Jiang, C.; Tang, H.; Xu, Q. In situ hydrothermal synthesis of g-C3N4/TiO2 heterojunction photocatalysts with high specific surface area for Rhodamine B degradation. Appl. Surf. Sci. 2017, 411, 400–410. [Google Scholar] [CrossRef]
- Lan, Y.; Li, Z.; Li, D.; Yan, G.; Yang, Z.; Guo, S. Graphitic carbon nitride synthesized at different temperatures for enhanced visible-light photodegradation of 2-naphthol. Appl. Surf. Sci. 2019, 467–468, 411–422. [Google Scholar] [CrossRef]
- Su, Q.; Sun, J.; Wang, J.; Yang, Z.; Cheng, W.; Zhang, S. Urea-derived graphitic carbon nitride as an efficient heterogeneous catalyst for CO2 conversion into cyclic carbonates. Catal. Sci. Technol. 2013, 4, 1556–1562. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.; Yu, J. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B 2015, 176–177, 44–52. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wu, Z.; Wang, L. g-C3N4 based composite photocatalysts for photocatalytic CO2 reduction. Catal. Today 2018, 300, 160–172. [Google Scholar] [CrossRef]
- Zhou, Y.; Lv, W.; Zhu, B.; Tong, F.; Pan, J.; Bai, J.; Zhou, Q.; Qin, H. Template-Free One-Step Synthesis of g-C3N4 Nanosheets with Simultaneous Porous Network and S-Doping for Remarkable Visible-Light-Driven Hydrogen Evolution. ACS Sustain. Chem. Eng. 2019, 7, 5801–5807. [Google Scholar] [CrossRef]
- An, T.D.; Phuc N van Tri, N.N.; Phu, H.T.; Hung, N.P.; Vo, V. Sulfur-Doped g-C3N4 with Enhanced Visible-Light Photocatalytic Activity. Appl. Mech. Mater. 2019, 889, 43–50. [Google Scholar] [CrossRef]
- Guo, X.; Duan, J.; Wang, W.; Zhang, Z. Modified graphitic carbon nitride as the photocatalyst for wastewater treatment under visible light irradiation. Fuel 2020, 280, 118544. [Google Scholar] [CrossRef]
- Ong, W.; Tan, L.; Ng, Y.H.; Yong, S.; Chai, S. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.R.; Sharma, R.; Panchal, P.; Malik, R.; Sharma, A.; Tomer, V.K.; Nehra, S.P. Silver Doped Graphitic Carbon Nitride for the Enhanced Photocatalytic Activity Towards Organic Dyes. J. Nanosci. Nanotechnol. 2019, 19, 5241–5248. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Dong, F.; Zhao, Z. The multiple effects of precursors on the properties of polymeric carbon nitride. Int. J. Photoenergy 2013, 2013, 685038. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis A to Z-What we know and what we do not know in a scientific sense. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 157–178. [Google Scholar] [CrossRef]
- Padhiari, S.; Tripathy, M.; Hota, G. Nitrogen-Doped Reduced Graphene Oxide Covalently Coupled with Graphitic Carbon Nitride/Sulfur-Doped Graphitic Carbon Nitride Heterojunction Nanocatalysts for Photoreduction and Degradation of 4-Nitrophenol. ACS Appl. Nano Mater. 2021, 4, 7145–7161. [Google Scholar] [CrossRef]
- Liao, G.; Yao, W. Facile synthesis of porous isotype heterojunction g-C3N4 for enhanced photocatalytic degradation of RhB under visible light. Diam. Relat. Mater. 2022, 128, 109–227. [Google Scholar] [CrossRef]
- Lei, X.F.; Xue, X.X.; Yang, H. Preparation and characterization of Ag-doped TiO2 nanomaterials and their photocatalytic reduction of Cr(VI) under visible light. Appl. Surf. Sci. 2014, 321, 396–403. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; He, D.; Situ, Y.; Huang, H. Construction of heterostructured g-C3N4/Ag/TiO2 microspheres with enhanced photocatalysis performance under visible-light irradiation. ACS Appl. Mater. Interfaces 2014, 6, 14405–14414. [Google Scholar] [CrossRef]
- Mo, Z.; She, X.; Li, Y.; Liu, L.; Huang, L.; Chen, Z.; Zhang, Q.; Xu, H.; Li, H. Synthesis of g-C3N4 at different temperatures for superior visible/UV photocatalytic performance and photoelectrochemical sensing of MB solution. RSC Adv. 2015, 5, 101552–101562. [Google Scholar] [CrossRef]
- Narkbuakaew, T.; Sattayaporn, S.; Saito, N.; Sujaridworakun, P. Investigation of the Ag species and synergy of Ag-TiO2 and g-C3N4 for the enhancement of photocatalytic activity under UV-Visible light irradiation. Appl. Surf. Sci. 2022, 573, 151617. [Google Scholar] [CrossRef]
- Narkbuakaew, T.; Sujaridworakun, P. Synthesis of Tri-S-Triazine Based g-C3N4 Photocatalyst for Cationic Rhodamine B Degradation under Visible Light. Top. Catal. 2020, 63, 1086–1096. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Radošević, T.; Podlogar, M. Enhanced Photocatalytic Activity of Hybrid rGO@TiO2/CN Nanocomposite for Organic Pollutant Degradation under Solar Light Irradiation. Catalysts 2021, 11, 1023. [Google Scholar] [CrossRef]
- Madima, N.; Kefeni, K.K.; Mishra, S.B.; Mishra, A.K. TiO2-modified g-C3N4 nanocomposite for photocatalytic degradation of organic dyes in aqueous solution. Heliyon 2022, 8, e10683. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Peng, S.; Lu, G.; Li, S. Eosin Y-sensitized graphitic carbon nitride fabricated by heating urea for visible light photocatalytic hydrogen evolution: The effect of the pyrolysis temperature of urea. Phys. Chem. Chem. Phys. 2013, 15, 7657–7665. [Google Scholar] [CrossRef]
- Rattan, P.D.; Nehra, S.P. Graphitic carbon nitride: A sustainable photocatalyst for organic pollutant degradation and antibacterial applications. Environ. Sci. Pollut. Res. 2021, 28, 3888–3896. [Google Scholar] [CrossRef]
- Kadam, A.N.; Kim, H.; Lee, S.W. Low-temperature in situ fabrication of porous S-doped g-C3N4 nanosheets using gaseous-bubble template for enhanced visible-light photocatalysis. Ceram. Int. 2020, 46, 28481–28489. [Google Scholar] [CrossRef]
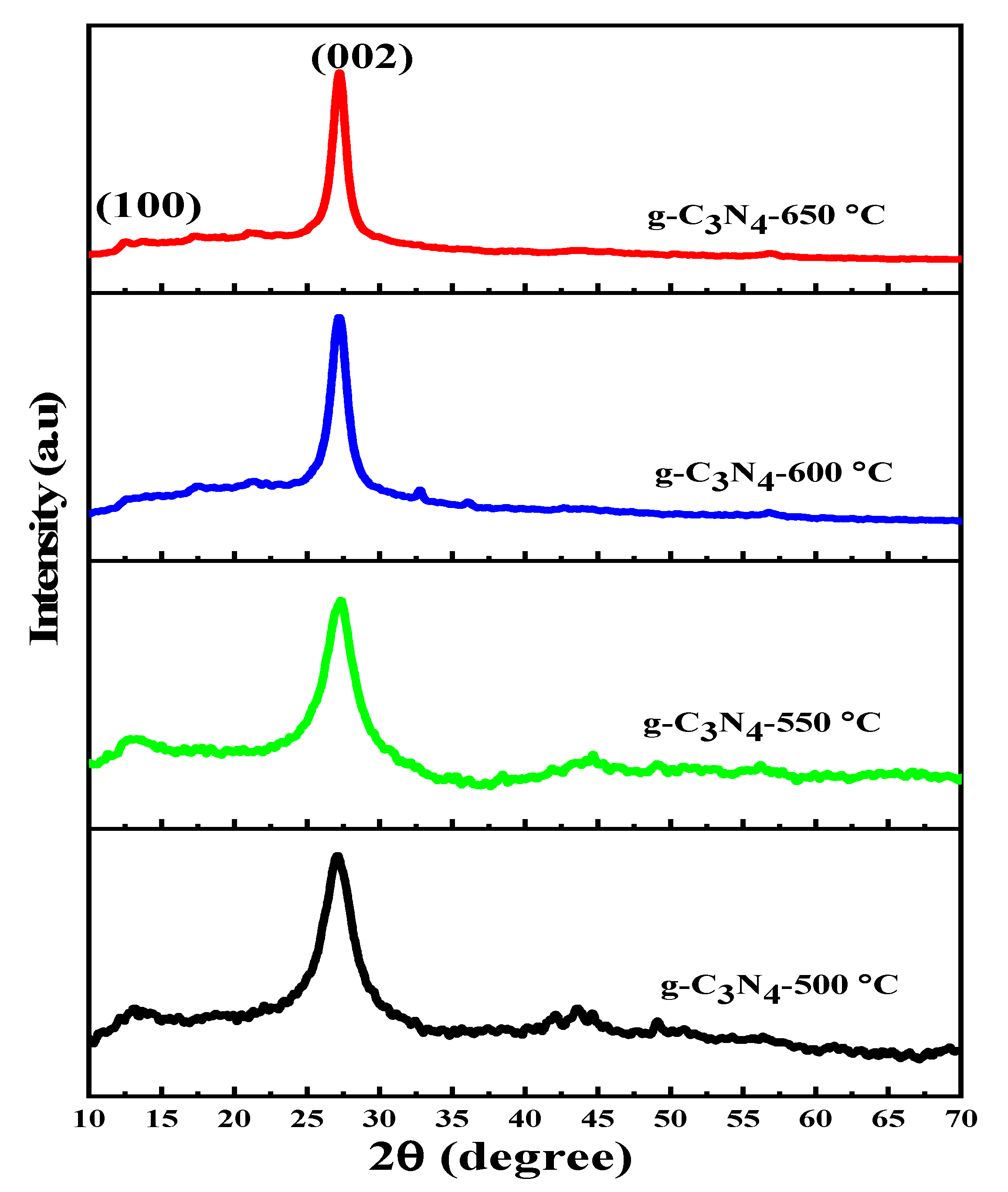





| Samples | 2θ (°) | β (FWHM) (Rad) | Crystalline Size (nm) | dhkl-Spacing (nm) | Eg (eV) |
|---|---|---|---|---|---|
| g-C3N4-500 °C | 27.08 | 0.064 | 2.23 | 0.329 | 2.78 |
| g-C3N4-550 °C | 27.30 | 0.056 | 2.55 | 0.320 | 2.75 |
| g-C3N4-600 °C | 27.19 | 0.033 | 4.32 | 0.328 | 2.82 |
| g-C3N4-650 °C | 27.25 | 0.026 | 5.49 | 0.327 | 2.89 |
| S. No. | Photocatalyst | Degradation Efficiency | Adsorption Efficiency | Reaction Rate Constant (k) (min−1) |
|---|---|---|---|---|
| 1 | g-C3N4-500 °C | 18.34% | 1.85% | 0.00113 |
| 2 | g-C3N4-550 °C | 22.20% | 0.66% | 0.00139 |
| 3 | g-C3N4-600 °C | 94.83% | 3.5% | 0.01646 |
| 4 | g-C3N4-650 °C | 94.80% | 1.55% | 0.01643 |
| Photocatalyst | Catalyst Dosage | Light Source | Irradiation Time | Pollutant Load | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|---|
| U-g-C3N4 | 10 mg | 300 W xenon lamp | 25 min | RhB (0.25 μmol/L) | 100% | [20] |
| CN-700 °C | 100 mg | 1000 W xenon lamp | 30 min | RhB (100 mg/L) | 99.11% | [19] |
| p–SCN–2 | 50 mg | 1000 W Xe lamp | 90 min | RhB (10 ppm) | 97.5% | [63] |
| 75:25SCN | 0.12 g | 100 W tungsten lamp | 420 min | RhB (30 mg/L) | 84.25% | [46] |
| g-C3N4-600 °C | 50 mg | 50 W LED lamp | 180 min | RhB (10 mg/L) | 94.83% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sewnet, A.; Alemayehu, E.; Abebe, M.; Mani, D.; Thomas, S.; Kalarikkal, N.; Lennartz, B. Single-Step Synthesis of Graphitic Carbon Nitride Nanomaterials by Directly Calcining the Mixture of Urea and Thiourea: Application for Rhodamine B (RhB) Dye Degradation. Nanomaterials 2023, 13, 762. https://doi.org/10.3390/nano13040762
Sewnet A, Alemayehu E, Abebe M, Mani D, Thomas S, Kalarikkal N, Lennartz B. Single-Step Synthesis of Graphitic Carbon Nitride Nanomaterials by Directly Calcining the Mixture of Urea and Thiourea: Application for Rhodamine B (RhB) Dye Degradation. Nanomaterials. 2023; 13(4):762. https://doi.org/10.3390/nano13040762
Chicago/Turabian StyleSewnet, Agidew, Esayas Alemayehu, Mulualem Abebe, Dhakshnamoorthy Mani, Sabu Thomas, Nandakumar Kalarikkal, and Bernd Lennartz. 2023. "Single-Step Synthesis of Graphitic Carbon Nitride Nanomaterials by Directly Calcining the Mixture of Urea and Thiourea: Application for Rhodamine B (RhB) Dye Degradation" Nanomaterials 13, no. 4: 762. https://doi.org/10.3390/nano13040762
APA StyleSewnet, A., Alemayehu, E., Abebe, M., Mani, D., Thomas, S., Kalarikkal, N., & Lennartz, B. (2023). Single-Step Synthesis of Graphitic Carbon Nitride Nanomaterials by Directly Calcining the Mixture of Urea and Thiourea: Application for Rhodamine B (RhB) Dye Degradation. Nanomaterials, 13(4), 762. https://doi.org/10.3390/nano13040762







