A “Special” Solvent to Prepare Alloyed Pd2Ni1 Nanoclusters on a MWCNT Catalyst for Enhanced Electrocatalytic Oxidation of Formic Acid
Abstract
:1. Introduction
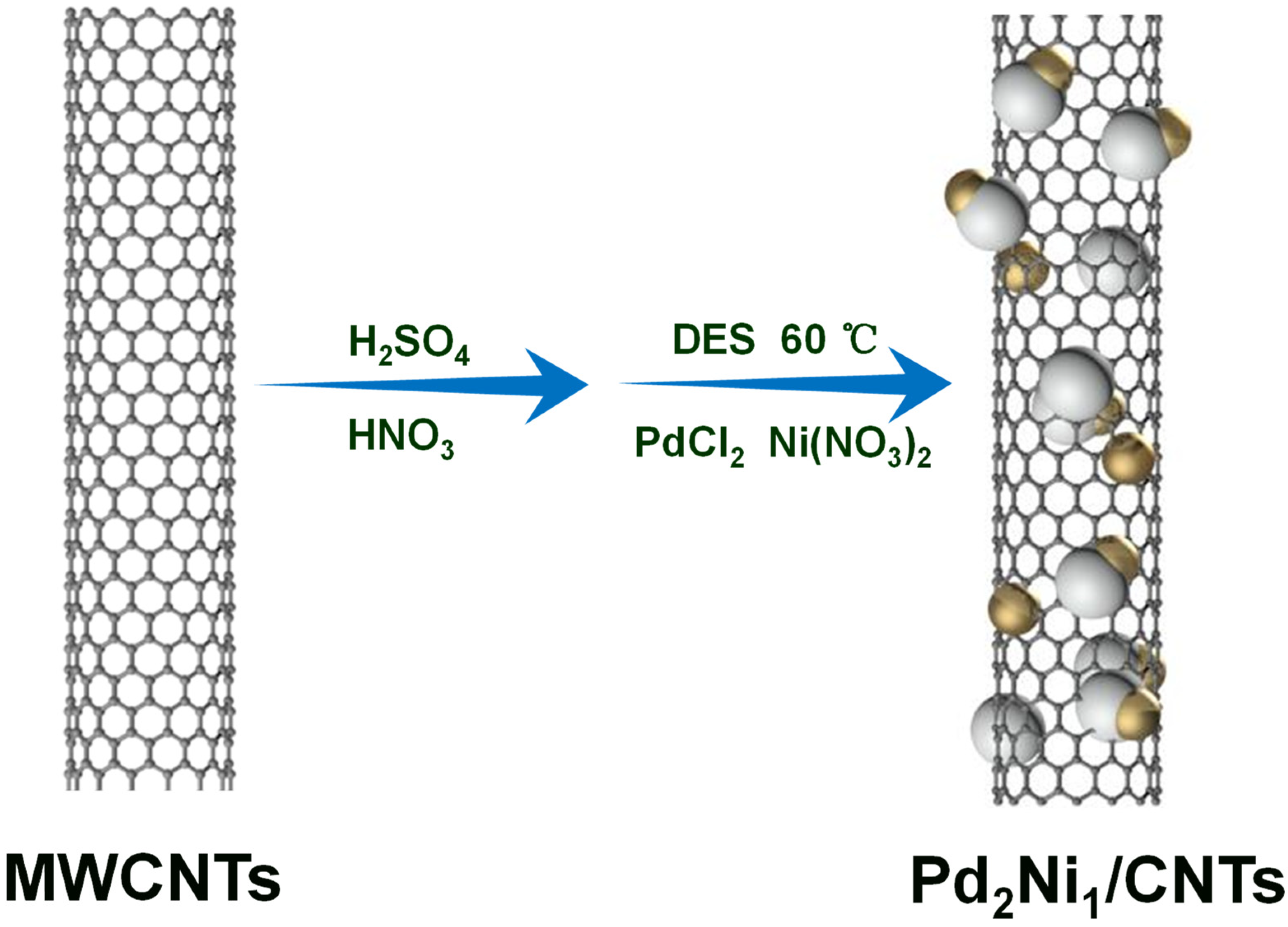
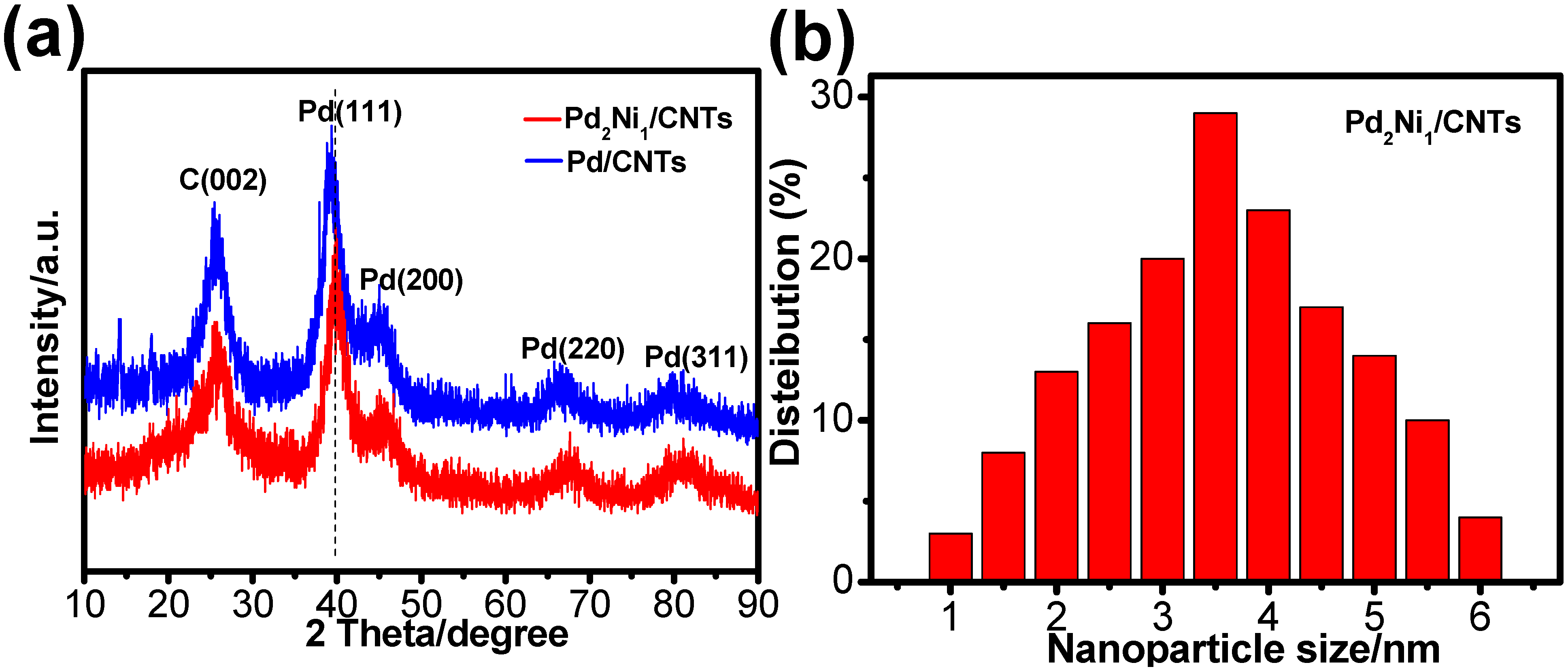
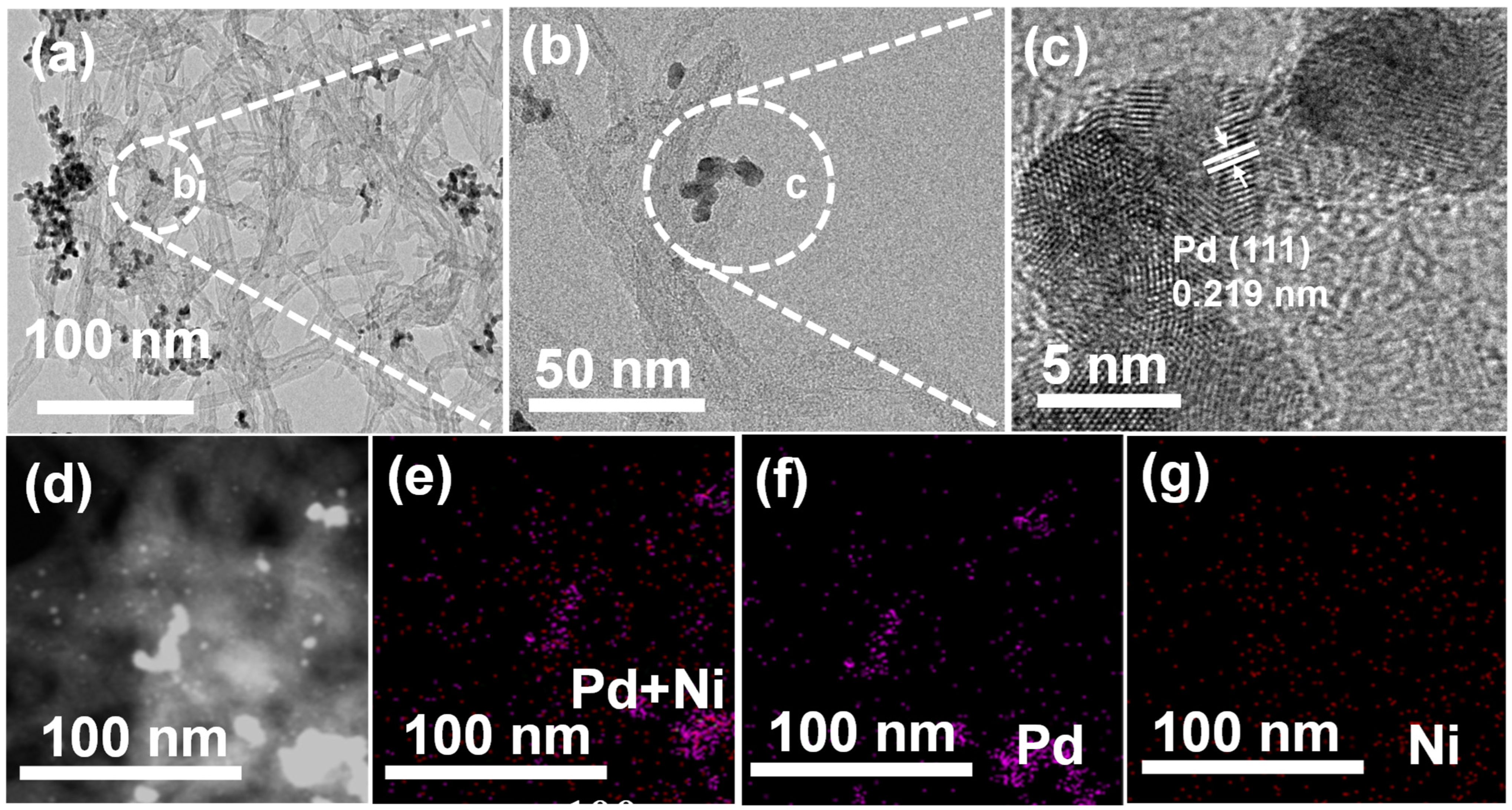
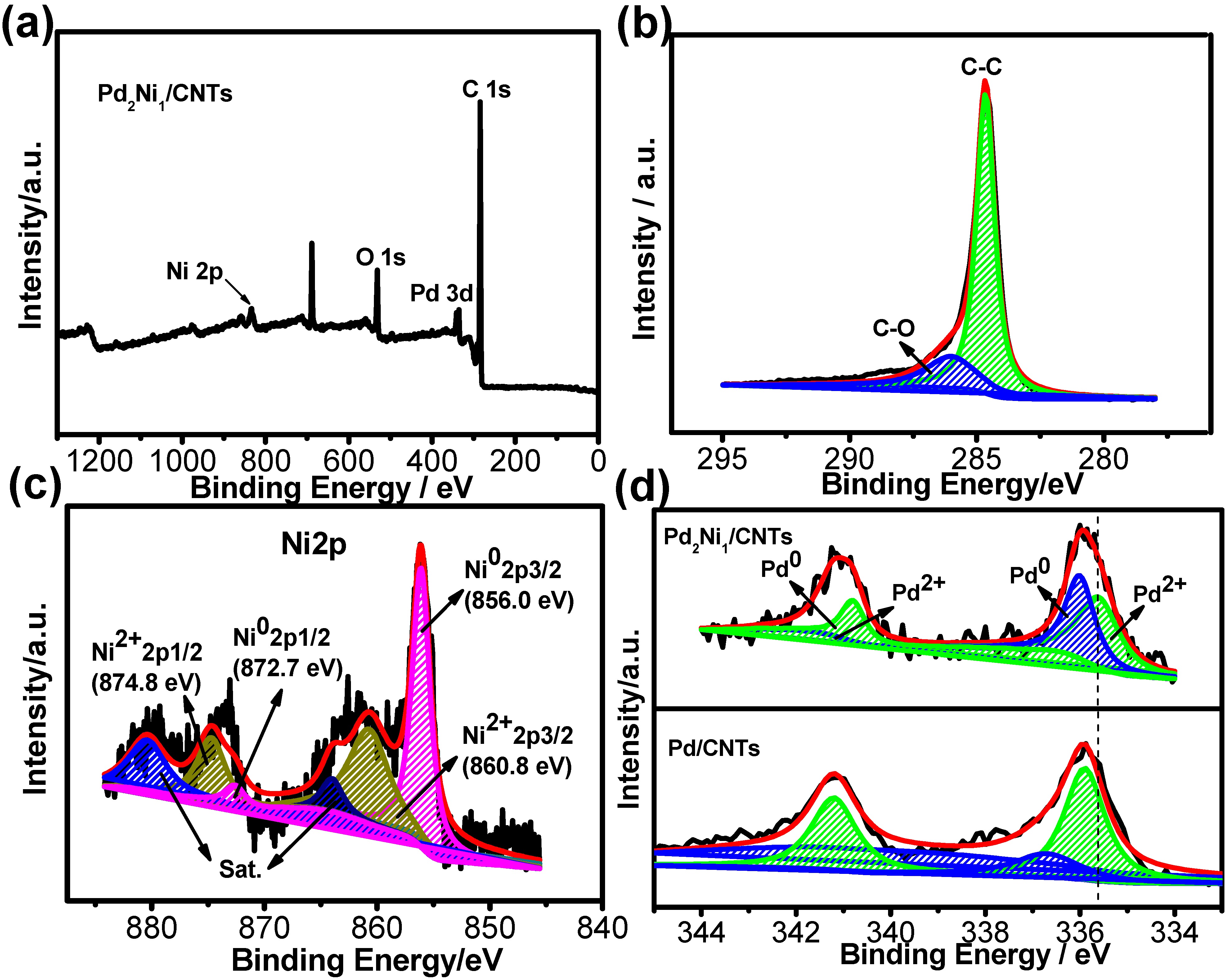
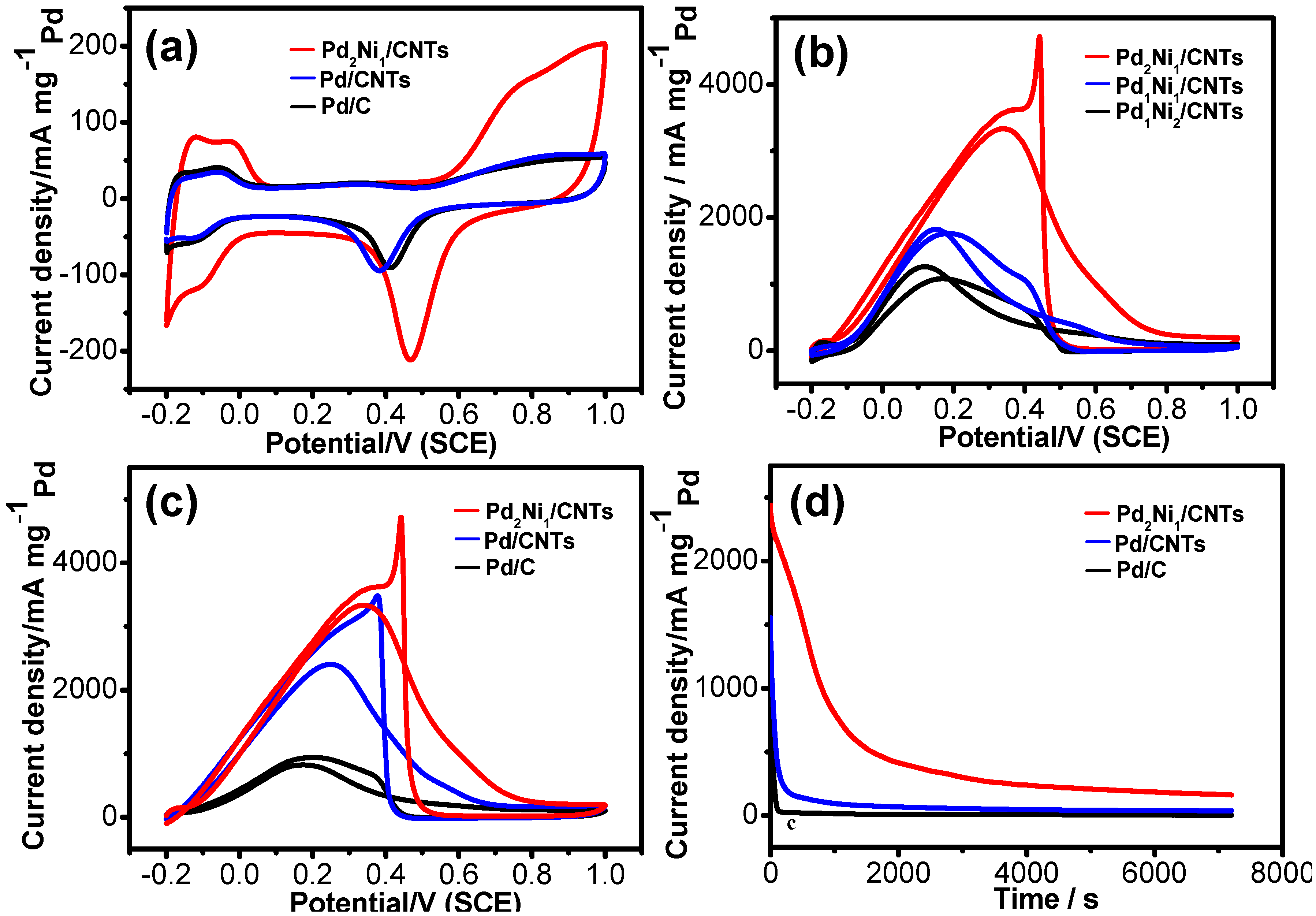
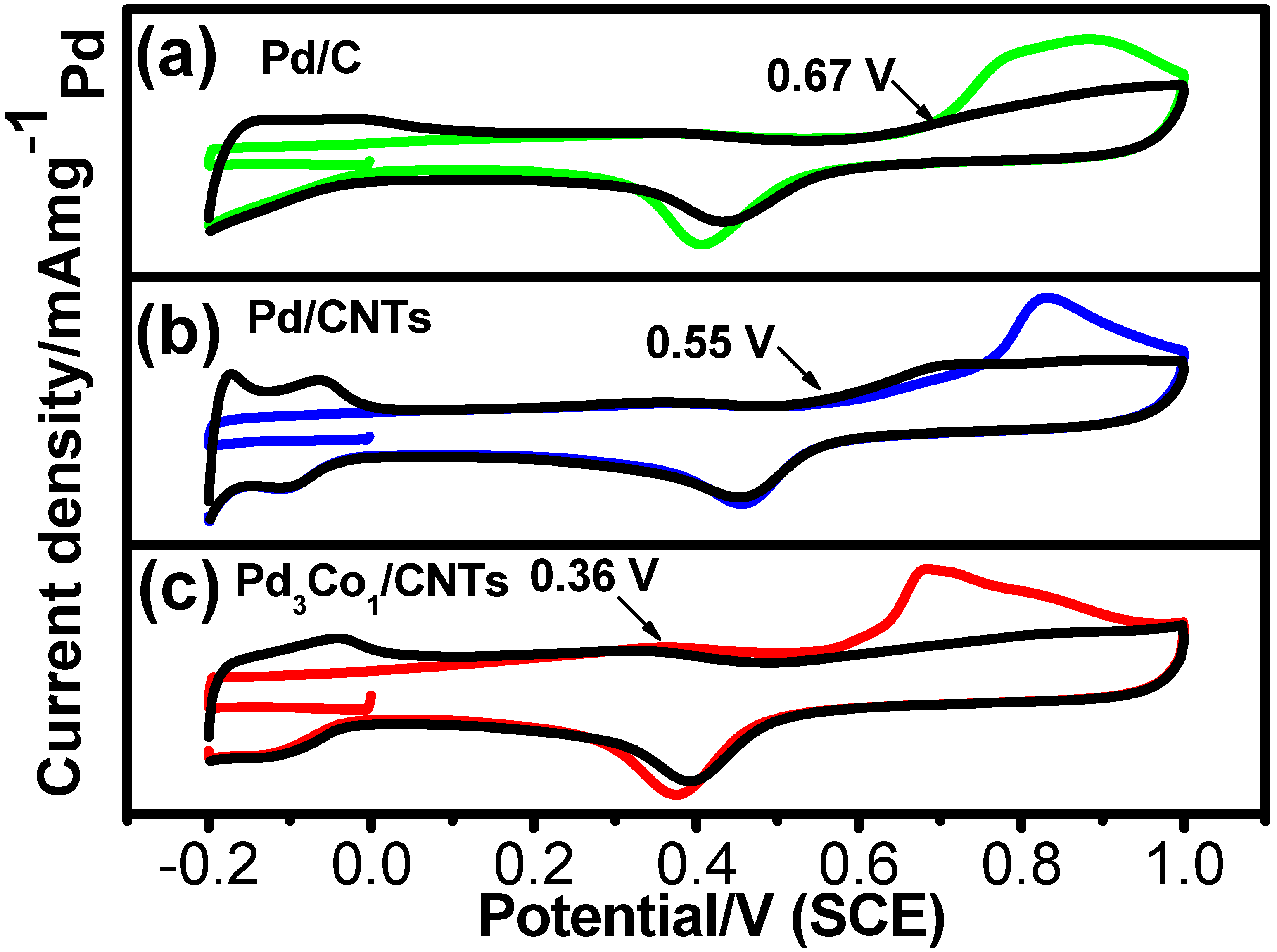
2. Results and Discussion
3. Experimental
3.1. Materials
3.2. Preparation of Catalysts
3.3. Physical Characterization
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.; Geng, S.; Li, L.; Zhang, Y.; Ren, G.; Huang, B.; Hu, Z.; Lee, J.F.; Lai, Y.H.; Chu, Y.H.; et al. A top-down strategy for amorphization of hydroxyl compounds for electrocatalytic oxygen evolution. Nat. Commun. 2022, 13, 1187. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Huang, B.; Jin, F.; Yang, Y.; Luo, M.; Sun, M.; Liu, Q.; Gao, F.; Guo, S. Atomic PdAu Interlayer Sandwiched into Pd/Pt Core/Shell Nanowires Achieves Superstable Oxygen Reduction Catalysis. ACS Nano 2020, 14, 11570–11578. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Dong, J.; Huang, Z.Q.; Xin, P.; Chen, W.; Wang, Y.; Li, Z.; Jin, Z.; Xing, W.; Zhuang, Z.; et al. Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation. Nat. Nanotechnol. 2020, 15, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gan, M.; Ma, L.; Zhao, W.; Zhang, Y.; Wang, L.; Hua, X. Facile synthesis of N-doped carbon nanotubes grafted on N-doped carbon nanosheets co-encapsulating cobalt and molybdenum carbide nanoparticles for efficient methanol oxidation. Mater. Today Chem. 2022, 23, 100665. [Google Scholar] [CrossRef]
- Lu, Q.; Li, J.; Eid, K.; Gu, X.; Wan, Z.; Li, W.; Al-Hajri, R.S.; Abdullah, A.M. Facile one-step aqueous-phase synthesis of porous PtBi nanosponges for efficient electrochemical methanol oxidation with a high CO tolerance. J. Electroanal. Chem. 2022, 916, 116361. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, L.; Wei, X.; Dong, S.; Ouyang, Y. Pd3Co1 Alloy Nanocluster on the MWCNT Catalyst for Efficient Formic Acid Electro-Oxidation. Nanomaterials 2022, 12, 4182. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, C.; Shen, T.; Guo, X.; Yang, C.; Wang, D.; Zhu, Y. Hollow Carbon Nanorod Confined Single Atom Rh for Direct Formic Acid Electrooxidation. Adv. Sci. 2022, 9, e2205299. [Google Scholar] [CrossRef]
- Al-Qodami, B.A.; Alalawy, H.H.; Al-Akraa, I.M.; Sayed, S.Y.; Allam, N.K.; Mohammad, A.M. Surface engineering of nanotubular ferric oxyhydroxide “goethite” on platinum anodes for durable formic acid fuel cells. Int. J. Hydrogen Energy 2022, 47, 264–275. [Google Scholar] [CrossRef]
- Pramanick, B.; Kumar, T.; Chowdhury, S.; Halder, A.; Siril, P.F. Graphene-Supported Palladium Nanostructures as Highly Active Catalysts for Formic Acid Oxidation Reaction. ACS Appl. Energy Mater. 2022, 5, 13480–13491. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Feng, H.; Zeng, H.; Tan, C.; Yao, J.; Zhang, J.; Jiang, L.; Sun, Y. PdAg Nanoparticles with Different Sizes: Facile One-Step Synthesis and High Electrocatalytic Activity for Formic Acid Oxidation. Chem. Asian J. 2021, 16, 34–38. [Google Scholar] [CrossRef]
- Chen, D.; Pei, S.; He, Z.; Shao, H.; Wang, J.; Wang, K.; Wang, Y.; Jin, Y. High Active PdSn Binary Alloyed Catalysts Supported on B and N Codoped Graphene for Formic Acid Electro-Oxidation. Catalysts 2020, 10, 751. [Google Scholar] [CrossRef]
- Ulas, B.; Caglar, A.; Kivrak, H. Determination of optimum Pd:Ni ratio for PdxNi100−x/CNTs formic acid electrooxidation catalysts synthesized via sodium borohydride reduction method. Int. J. Energy Res. 2019, 43, 3436–3445. [Google Scholar] [CrossRef]
- Gharib, A.; Arab, A. Electrodeposited Pd, Pd Cd, and Pd Bi nanostructures: Preparation, characterization, corrosion behavior, and their electrocatalytic activities for formic acid oxidation. J. Electroanal. Chem. 2020, 866, 114166. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Zhang, H.; Yin, S.; Xu, Y.; Li, X.; Wang, L.; Wang, H. B-Doped PdRu nanopillar assemblies for enhanced formic acid oxidation electrocatalysis. Nanoscale 2020, 12, 19159–19164. [Google Scholar] [CrossRef]
- Liu, X.; Bu, Y.; Cheng, T.; Gao, W.; Jiang, Q. Flower-like carbon supported Pd–Ni bimetal nanoparticles catalyst for formic acid electrooxidation. Electrochim. Acta 2019, 324, 134816. [Google Scholar] [CrossRef]
- Li, M.; Liu, R.; Han, G.; Tian, Y.; Chang, Y.; Xiao, Y. Facile Synthesis of Pd-Ni Nanoparticles on Reduced Graphene Oxide under Microwave Irradiation for Formic Acid Oxidation. Chin. J. Chem. 2017, 35, 1405–1410. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, Q.; Wu, X.; Fu, Y.; Deng, K.; Wang, X. Intimately coupled hybrid of graphitic carbon nitride nanoflakelets with reduced graphene oxide for supporting Pd nanoparticles: A stable nanocatalyst with high catalytic activity towards formic acid and methanol electrooxidation. Electrochim. Acta 2016, 200, 131–141. [Google Scholar] [CrossRef]
- Qian, H.; Wu, J.; Guo, Y.; Fang, W. PdAgPt Corner-Satellite Nanocrystals in Well-Controlled Morphologies and the Structure-Related Electrocatalytic Properties. Nanomaterials 2021, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Saadat, N.; Dhakal, H.N.; Tjong, J.; Jaffer, S.; Yang, W.; Sain, M. Recent advances and future perspectives of carbon materials for fuel cell. Renew. Sustain. Energy Rev. 2021, 138, 110535. [Google Scholar] [CrossRef]
- Li, Y.-H.; Deng, H.-C.; Zhou, Z.-H.; Yang, P.-P.; Fei, J.-J.; Xie, Y.-X. Pd12Ag1 nanoalloy on dendritic CNFs catalyst for boosting formic acid oxidation. Appl. Surf. Sci. 2023, 608, 155131. [Google Scholar] [CrossRef]
- Wagle, D.V.; Zhao, H.; Baker, G.A. Deep Eutectic Solvents: Sustainable Media for Nanoscale and Functional Materials. Acc. Chem. Res. 2014, 47, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhou, Z.; Zheng, T.; Gu, C.; Gong, X.; Zhang, Y.; Xie, Y.; Yang, N.; Fei, J. A novel strategy to synthesize Pt/CNTs nanocatalyst with highly improved activity for methanol electrooxidation. J. Electroanal. Chem. 2021, 897, 115557. [Google Scholar] [CrossRef]
- Yang, P.; Wei, L.; Xiao, X.; Zhou, Z.; Li, J.; Zhang, Y.; Xie, Y.; Yang, N.; Fei, J. Electrocatalytic oxidation of formic acid on Pd/CNTs nanocatalysts synthesized in special “non-aqueous” system. J. Electroanal. Chem. 2022, 906, 115980. [Google Scholar] [CrossRef]
- Yang, P.; Devasenathipathy, R.; Xu, W.; Wang, Z.; Chen, D.-H.; Zhang, X.; Fan, Y.; Chen, W. Pt1(CeO2)0.5 Nanoparticles Supported on Multiwalled Carbon Nanotubes for Methanol Electro-oxidation. ACS Appl. Nano Mater. 2021, 4, 10584–10591. [Google Scholar] [CrossRef]
- Yang, P.; Li, Y.; Chen, S.; Li, J.; Zhao, P.; Zhang, L.; Xie, Y.; Fei, J. One-step synthesis in deep eutectic solvents of Pt3Sn1-SnO2 alloy nanopore on carbon nanotubes for boosting electro-catalytic methanol oxidation. J. Electroanal. Chem. 2021, 887, 115164. [Google Scholar] [CrossRef]
- Wang, R.-X.; Fan, Y.-J.; Liang, Z.-R.; Zhang, J.-M.; Zhou, Z.-Y.; Sun, S.-G. PdSn nanocatalysts supported on carbon nanotubes synthesized in deep eutectic solvents with high activity for formic acid electrooxidation. RSC Adv. 2016, 6, 60400–60406. [Google Scholar] [CrossRef]
- Hung, T.-C.; Liu, Y.-R.; Chou, P.-C.; Lin, C.-W.; Hsieh, Y.-T. Electrochemical Sensing of Hydrazine Using Hollow Pd/Ag dendrites prepared by Galvanic replacement from Choline Chloride-based deep eutectic Solvents. J. Electroanal. Chem. 2022, 922, 116791. [Google Scholar] [CrossRef]
- Hammons, J.A.; Muselle, T.; Ustarroz, J.; Tzedaki, M.; Raes, M.; Hubin, A.; Terryn, H. Stability, Assembly, and Particle/Solvent Interactions of Pd Nanoparticles Electrodeposited from a Deep Eutectic Solvent. J. Phys. Chem. C 2013, 117, 14381–14389. [Google Scholar] [CrossRef]
- Bao, Y.; Zha, M.; Sun, P.; Hu, G.; Feng, L. PdNi/N-doped graphene aerogel with over wide potential activity for formic acid electrooxidation. J. Energy Chem. 2021, 59, 748–754. [Google Scholar] [CrossRef]
- Tian, J.; Jiang, H.; Deng, X.; Zhao, X.; Tian, J.; Shi, G.; Li, G.; Zhang, J.; Zhang, W. Response modulation of PdNi nano-film hydrogen sensors by thickness control. Appl. Surf. Sci. 2021, 562, 150064. [Google Scholar] [CrossRef]
- Kashif, M.; Quader, A.; Khan, M.A.; Ramay, S.M.; Atiq, S. Effectively coupled BiFeO3-MnFe2O4-Cr2O3 tri-phase multiferroic composites for efficient energy storage and fast switching. J. Alloy. Compd. 2022, 929, 167274. [Google Scholar] [CrossRef]
- Zhang, J.-M.; He, J.-J.; Wang, X.-Q.; Fan, Y.-J.; Zhang, X.-J.; Zhong, J.-P.; Chen, W.; Sun, S.-G. One-step synthesis in deep eutectic solvents of PtV alloy nanonetworks on carbon nanotubes with enhanced methanol electrooxidation performance. Int. J. Hydrogen Energy 2019, 44, 28709–28719. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Mao, Q.; Yu, H.; Deng, K.; Xu, Y.; Li, X.; Wang, Z.; Wang, L. Tensile strained PdNi bimetallene for energy-efficient hydrogen production integrated with formate oxidation. Chem. Eng. J. 2022, 450, 137995. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Liu, M.; Liu, H.; Chen, Q.; Yin, Y.; Jin, M. Construction of Pd-M (M = Ni, Ag, Cu) alloy surfaces for catalytic applications. Nano Res. 2017, 11, 780–790. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Gong, Y.; Wu, D.; Wu, G.; Xu, B.; Bi, L.; Yuan, W.; Cui, Z. Twisted palladium-copper nanochains toward efficient electrocatalytic oxidation of formic acid. J. Colloid Interface Sci. 2019, 537, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Berjis, A.; Mirza, B.; Anaraki-Ardakani, H. One-Pot Synthesis of Highly Functionalized Indole in Choline Chloride/Oxalic Acid as a Deep Eutectic Solvent. Polycycl. Aromat. Compd. 2022, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Wang, C.; Tang, J.; Chen, X.; Li, Y.; Shi, J.; Zhao, P.; Xie, Y.; Fei, J. A novel kaempferol electrochemical sensor based on glass carbon electrode modified by poly (3, 4-ethylenedioxythiophene) decorated with green synthesized MIL-100(Fe)-multi- walled carbon nanotubes composites. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 649, 129484. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Gong, Y.; Wu, D.; Li, Z.; Li, Q.; Zheng, L.; Chen, W. Palladium-cobalt nanodots anchored on graphene: In-situ synthesis, and application as an anode catalyst for direct formic acid fuel cells. Appl. Surf. Sci. 2019, 469, 305–311. [Google Scholar] [CrossRef]
- Zhang, Z.; Gong, Y.; Wu, D.; Li, Z.; Li, Q.; Zheng, L.; Chen, W.; Yuan, W.; Zhang, L. Facile fabrication of stable PdCu clusters uniformly decorated on graphene as an efficient electrocatalyst for formic acid oxidation. Int. J. Hydrogen Energy 2019, 44, 2731–2740. [Google Scholar] [CrossRef]
- Zheng, J.; Zeng, H.; Tan, C.; Zhang, T.; Zhao, B.; Guo, W.; Wang, H.; Sun, Y.; Jiang, L. Coral-like PdCu Alloy Nanoparticles Act as Stable Electrocatalysts for Highly Efficient Formic Acid Oxidation. ACS Sustain. Chem. Eng. 2019, 7, 15354–15360. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Zhao, Z.; Yuan, W.; Li, C. Facile one-pot surfactant-free synthesis of uniform Pd6Co nanocrystals on 3D graphene as an efficient electrocatalyst toward formic acid oxidation. Nanoscale 2016, 8, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhai, J.; Jiang, K.; Wang, J.; Cai, W. Pd-Cu/C electrocatalysts synthesized by one-pot polyol reduction toward formic acid oxidation: Structural characterization and electrocatalytic performance. Int. J. Hydrogen Energy 2015, 40, 1726–1734. [Google Scholar] [CrossRef]
- Sun, D.-D.; Si, L.; Fu, G.; Liu, C.; Sun, D.; Chen, Y.; Tang, Y.; Lu, T. Nanobranched porous palladium-tin intermetallics: One-step synthesis and their superior electrocatalysis towards formic acid oxidation. J. Power Sources 2015, 280, 141–146. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Y.; Liu, P.; Cui, Y.; Ge, X.; Jing, Q. Pd-Cu alloy with hierarchical network structure as enhanced electrocatalysts for formic acid oxidation. Int. J. Hydrogen Energy 2016, 41, 6773–6780. [Google Scholar] [CrossRef]
- Feng, A.-N.; Bai, J.; Shao, W.; Hong, W.; Tian, Z.; Xiao, Z. Surfactant-free Pd-Fe nanoparticles supported on reduced graphene oxide as nanocatalyst for formic acid oxidation. Int. J. Hydrogen Energy 2017, 42, 15196–15202. [Google Scholar]
- Zhu, F.-C.; Ma, G.; Bai, Z.; Hang, R.; Tang, B.; Zhang, Z.; Wang, X. High activity of carbon nanotubes supported binary and ternary Pd-based catalysts for methanol, ethanol and formic acid electro-oxidation. J. Power Sour. 2013, 242, 610–620. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Zhang, L.; Wei, X.; Dong, S.; Cao, W.; Ma, D.; Ouyang, Y.; Xie, Y.; Fei, J. A “Special” Solvent to Prepare Alloyed Pd2Ni1 Nanoclusters on a MWCNT Catalyst for Enhanced Electrocatalytic Oxidation of Formic Acid. Nanomaterials 2023, 13, 755. https://doi.org/10.3390/nano13040755
Yang P, Zhang L, Wei X, Dong S, Cao W, Ma D, Ouyang Y, Xie Y, Fei J. A “Special” Solvent to Prepare Alloyed Pd2Ni1 Nanoclusters on a MWCNT Catalyst for Enhanced Electrocatalytic Oxidation of Formic Acid. Nanomaterials. 2023; 13(4):755. https://doi.org/10.3390/nano13040755
Chicago/Turabian StyleYang, Pingping, Li Zhang, Xuejiao Wei, Shiming Dong, Wenting Cao, Dong Ma, Yuejun Ouyang, Yixi Xie, and Junjie Fei. 2023. "A “Special” Solvent to Prepare Alloyed Pd2Ni1 Nanoclusters on a MWCNT Catalyst for Enhanced Electrocatalytic Oxidation of Formic Acid" Nanomaterials 13, no. 4: 755. https://doi.org/10.3390/nano13040755
APA StyleYang, P., Zhang, L., Wei, X., Dong, S., Cao, W., Ma, D., Ouyang, Y., Xie, Y., & Fei, J. (2023). A “Special” Solvent to Prepare Alloyed Pd2Ni1 Nanoclusters on a MWCNT Catalyst for Enhanced Electrocatalytic Oxidation of Formic Acid. Nanomaterials, 13(4), 755. https://doi.org/10.3390/nano13040755







