Research Progress and Application of Polyimide-Based Nanocomposites
Abstract
:1. Introduction
2. PI Basic Information
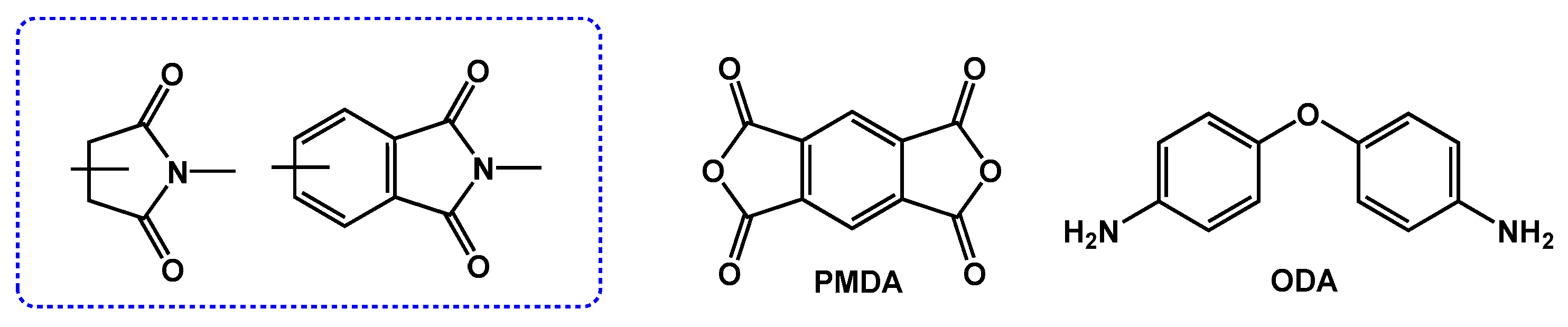
2.1. PI Molecular Structure
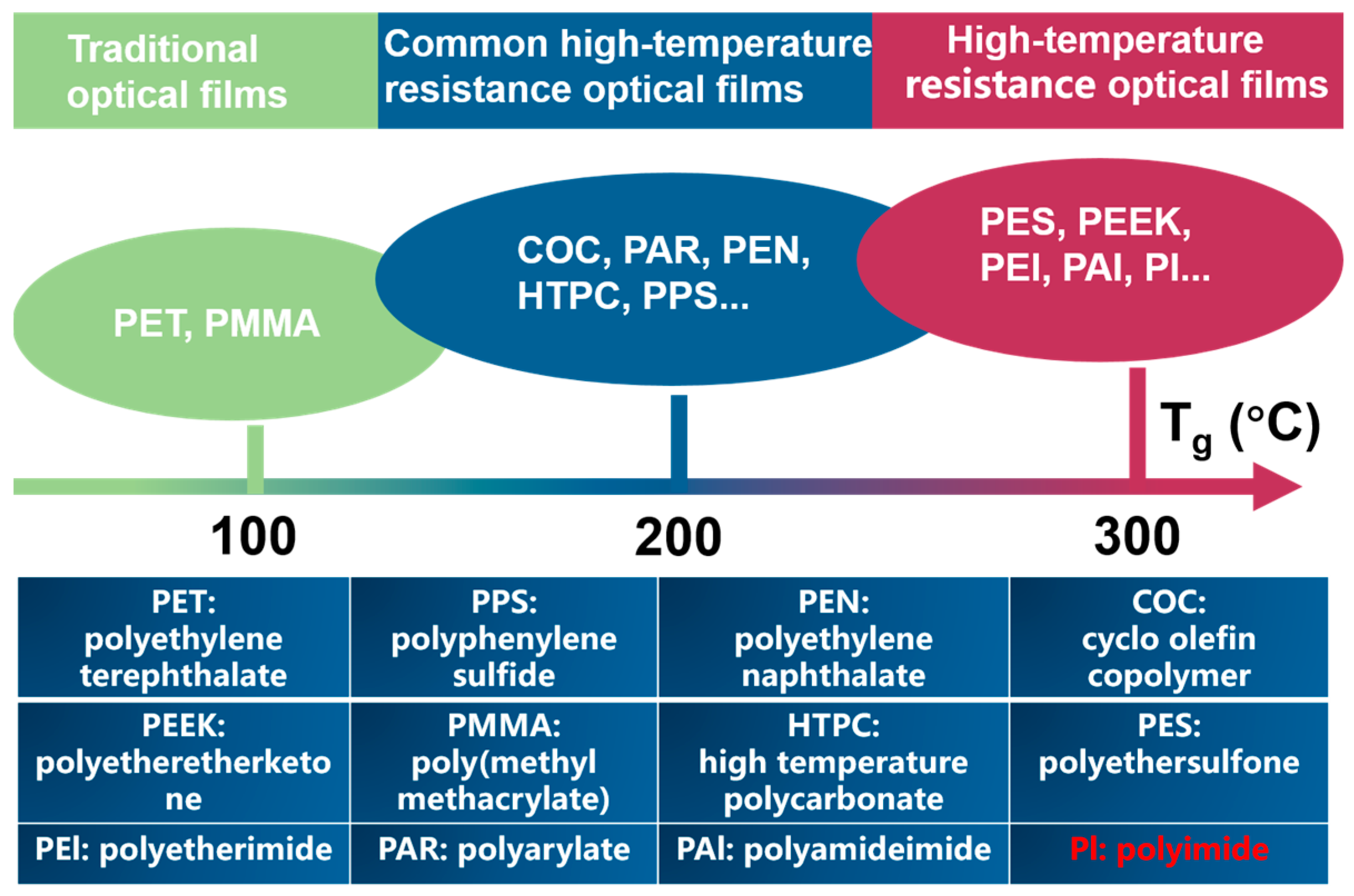
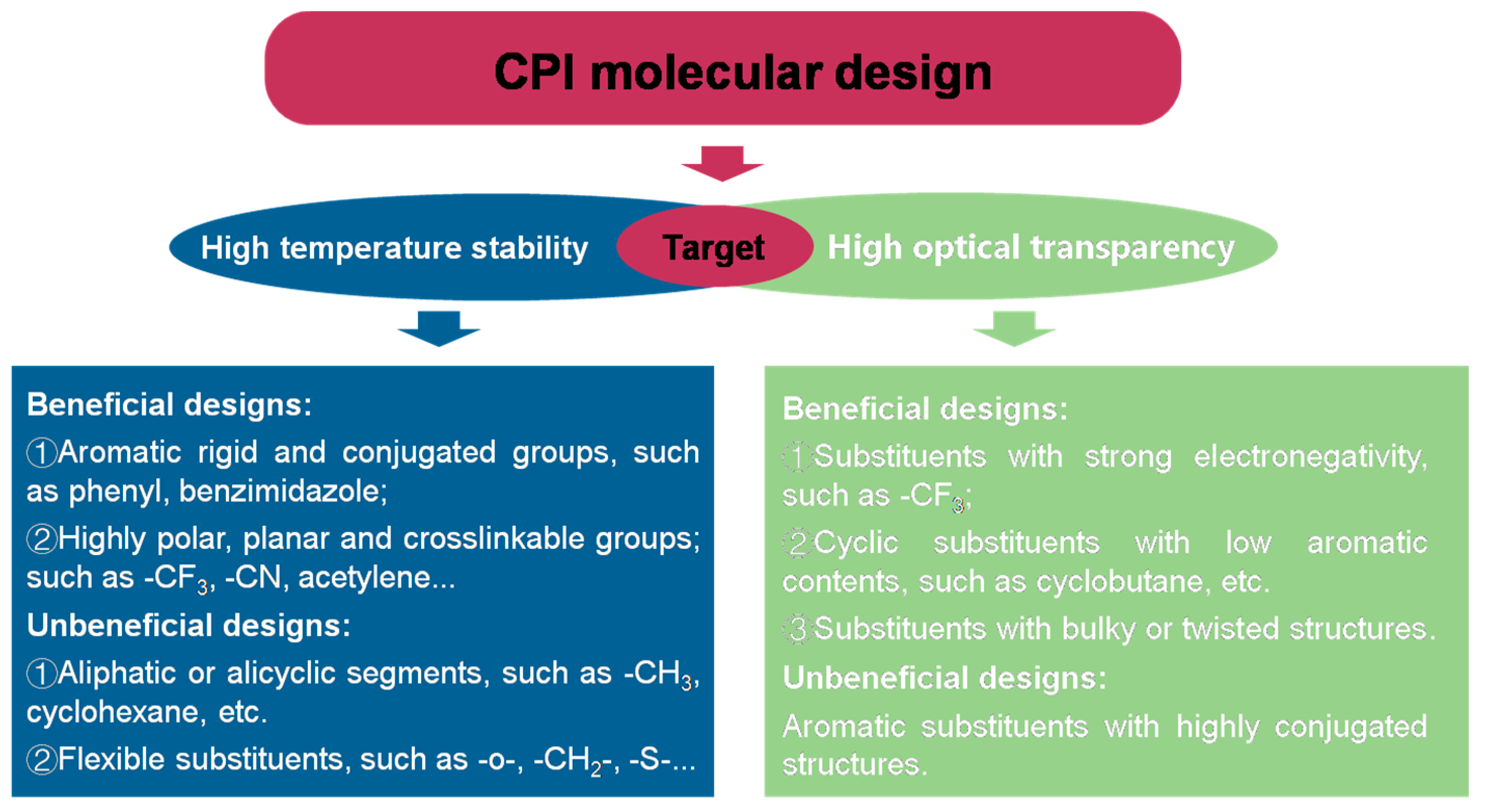
2.2. The Colorless PI
2.3. PI Preparation
2.3.1. Imidization Methods
- Direct thermal imidization
- Two-step method
- Chemical imidization
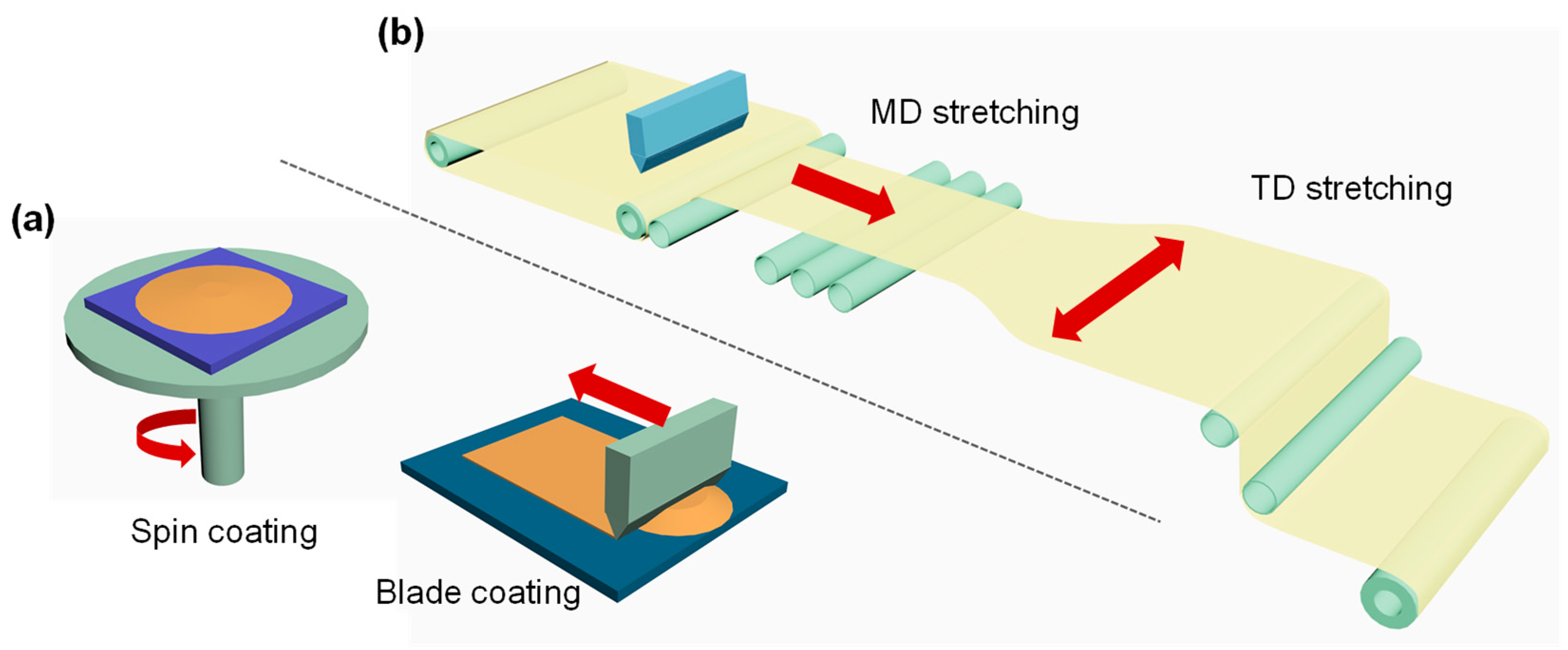
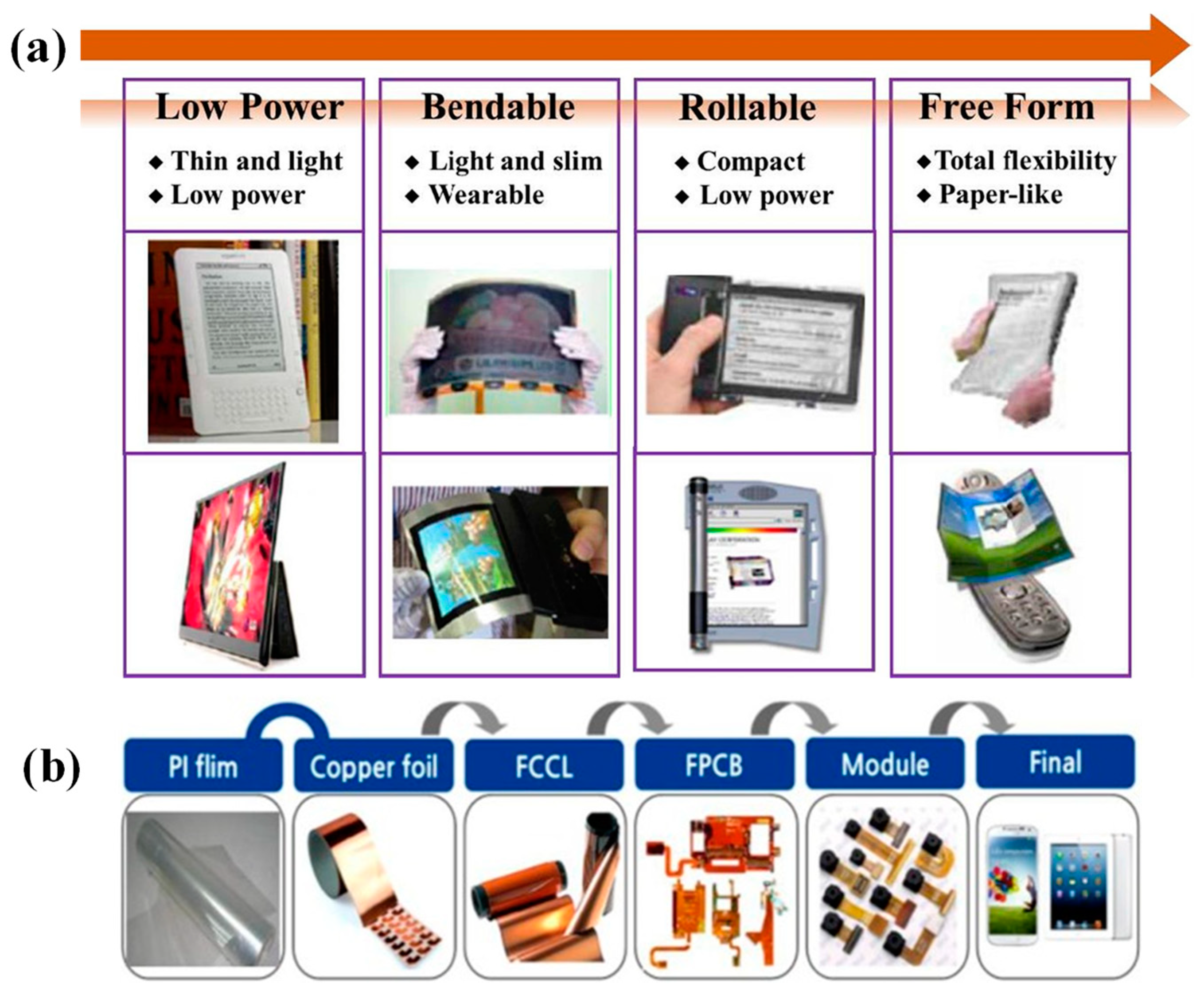
2.3.2. PI Film Preparation Techniques
- Slice-to-slice scale preparation of PI films in laboratory
- Industrial roll-to-roll scale preparation of PI films
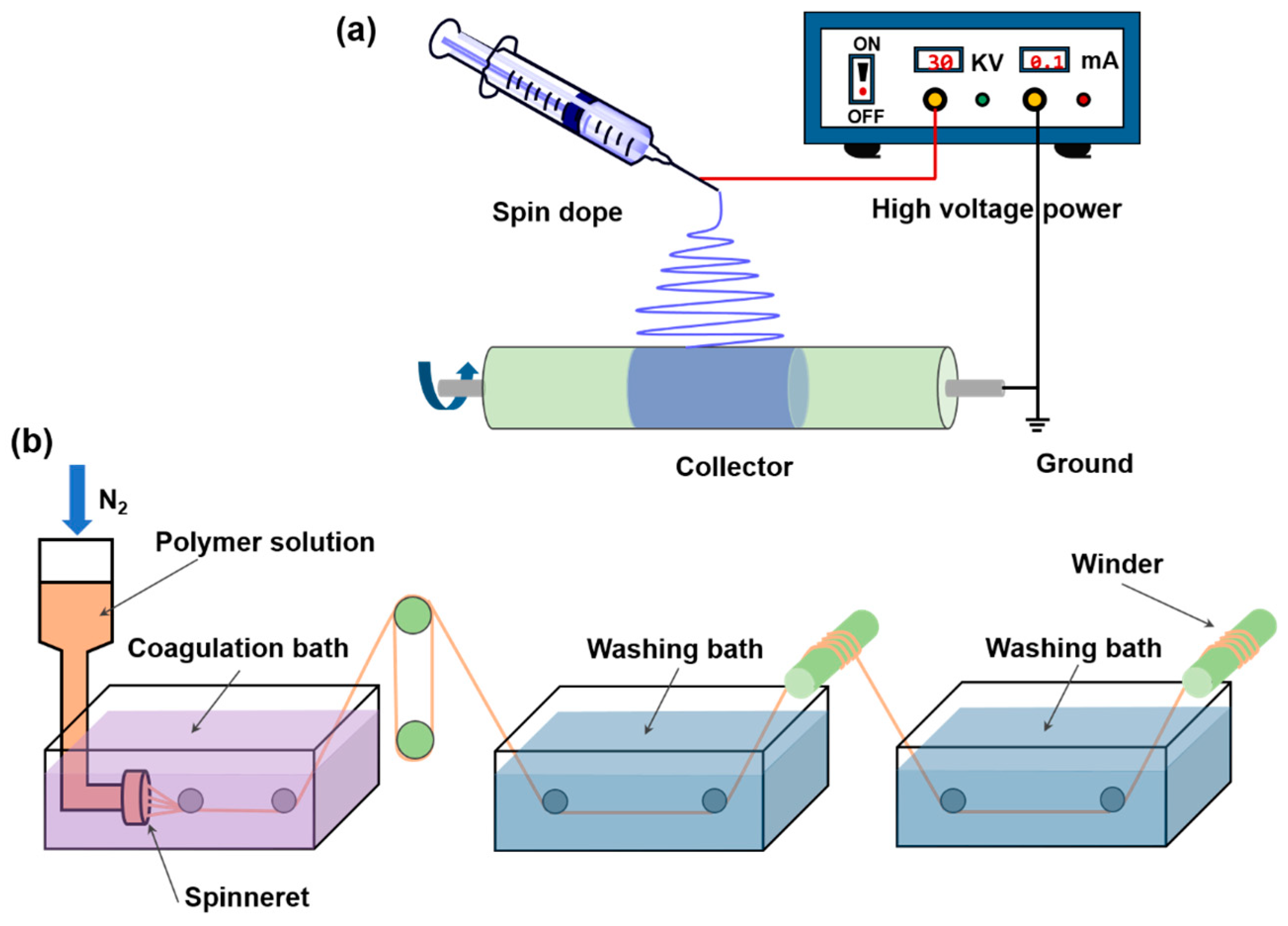
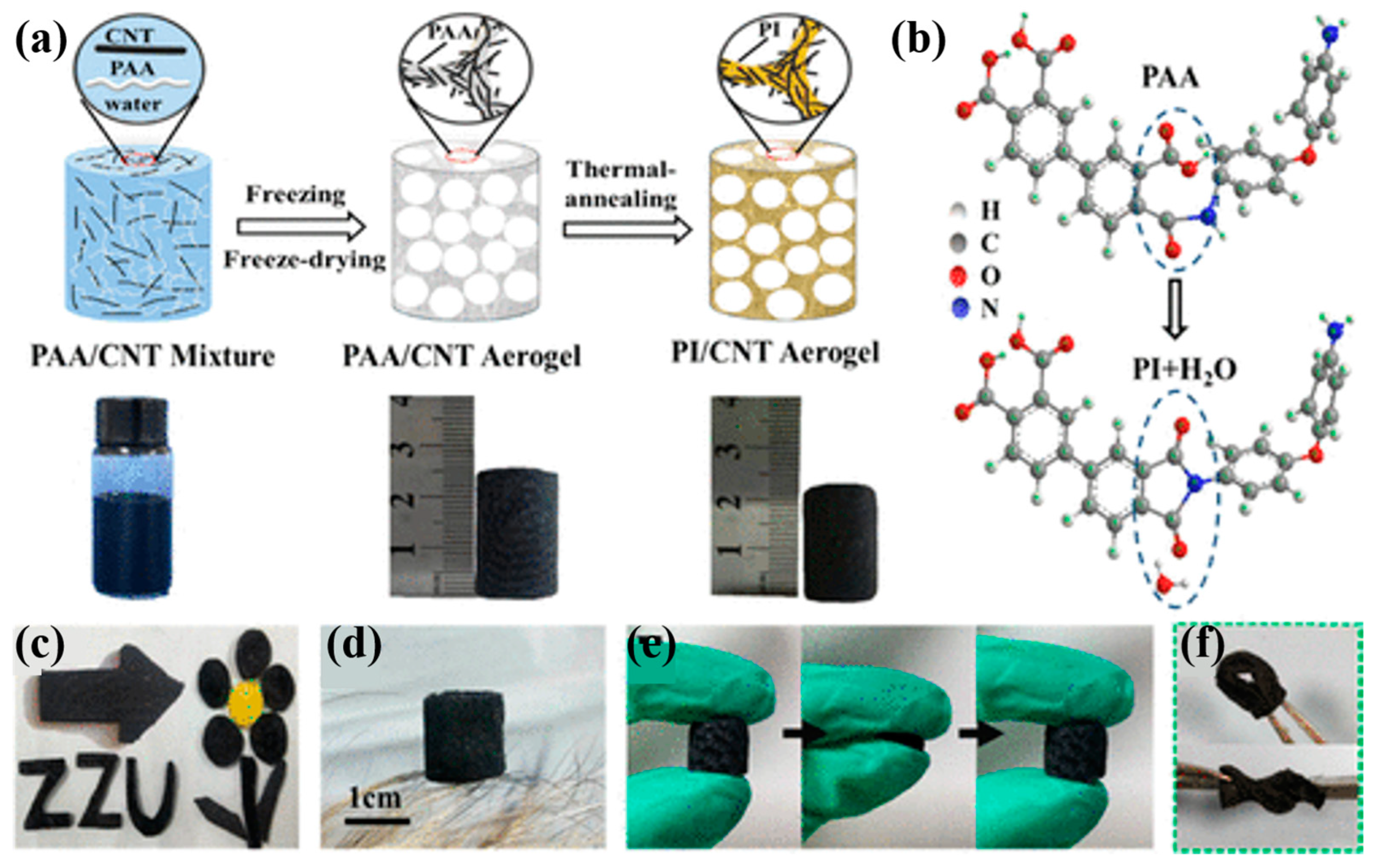
2.3.3. PI Nanofiber Preparation
3. PI-Based Composites and Their Preparation Method
3.1. In Situ Polymerization
3.2. Solution Blending
3.3. The Deposition Method
3.4. Electrospinning

3.5. Sol–Gel Method
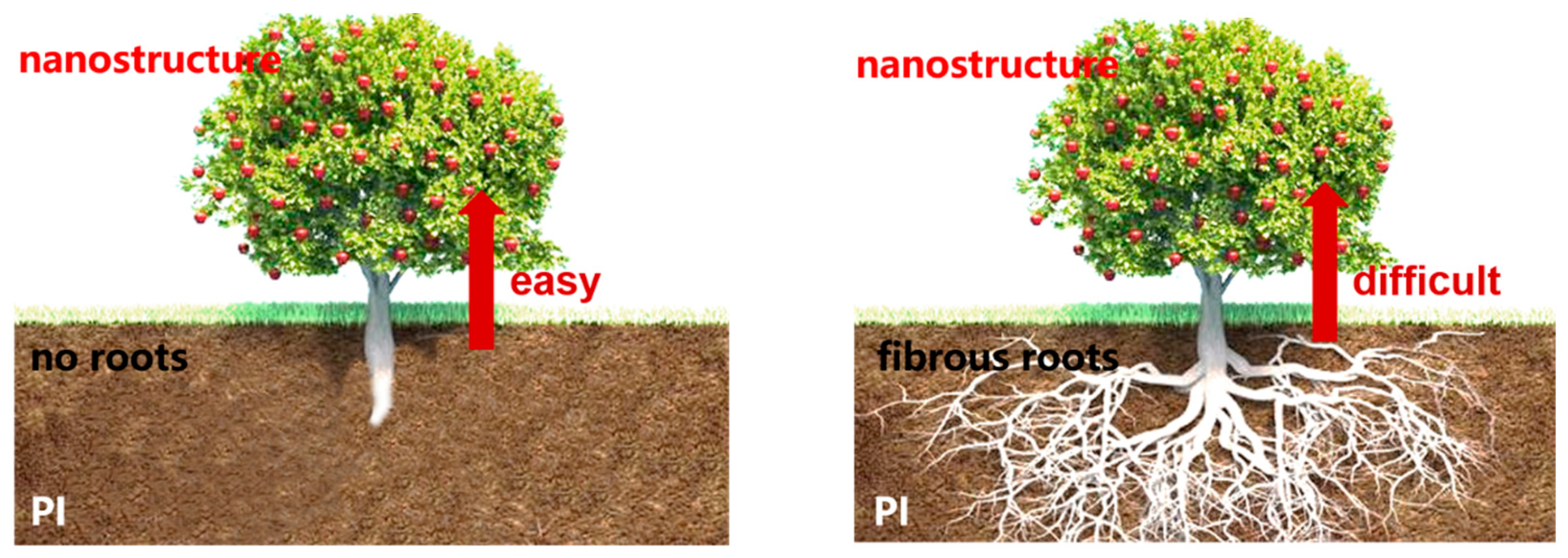
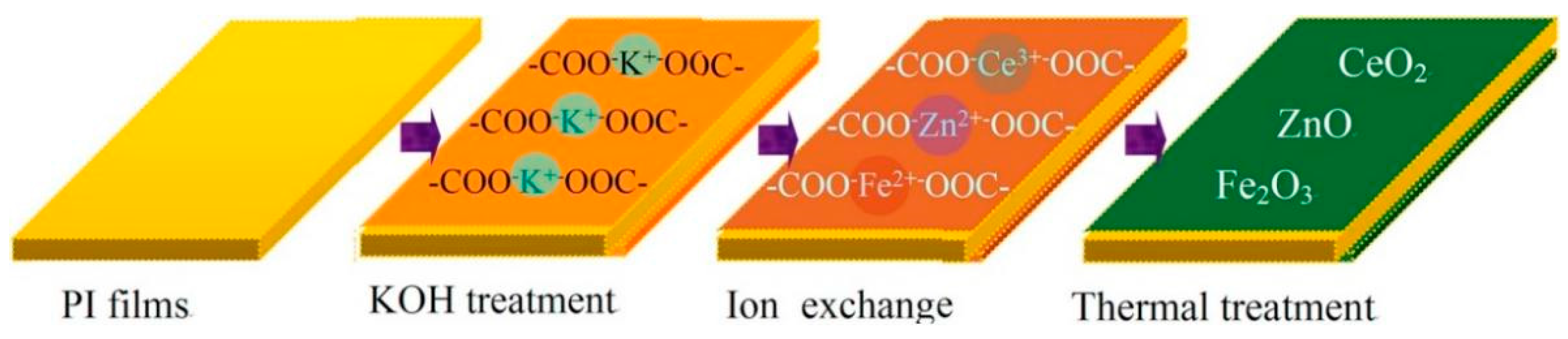
3.6. Surface Ion Exchange Method
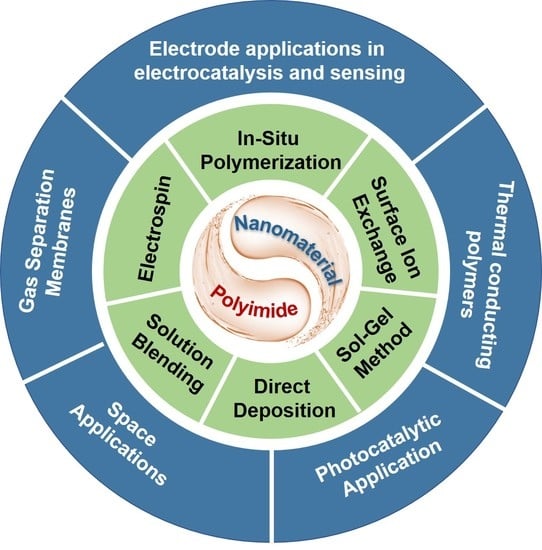
4. Application of PI-Based Composites
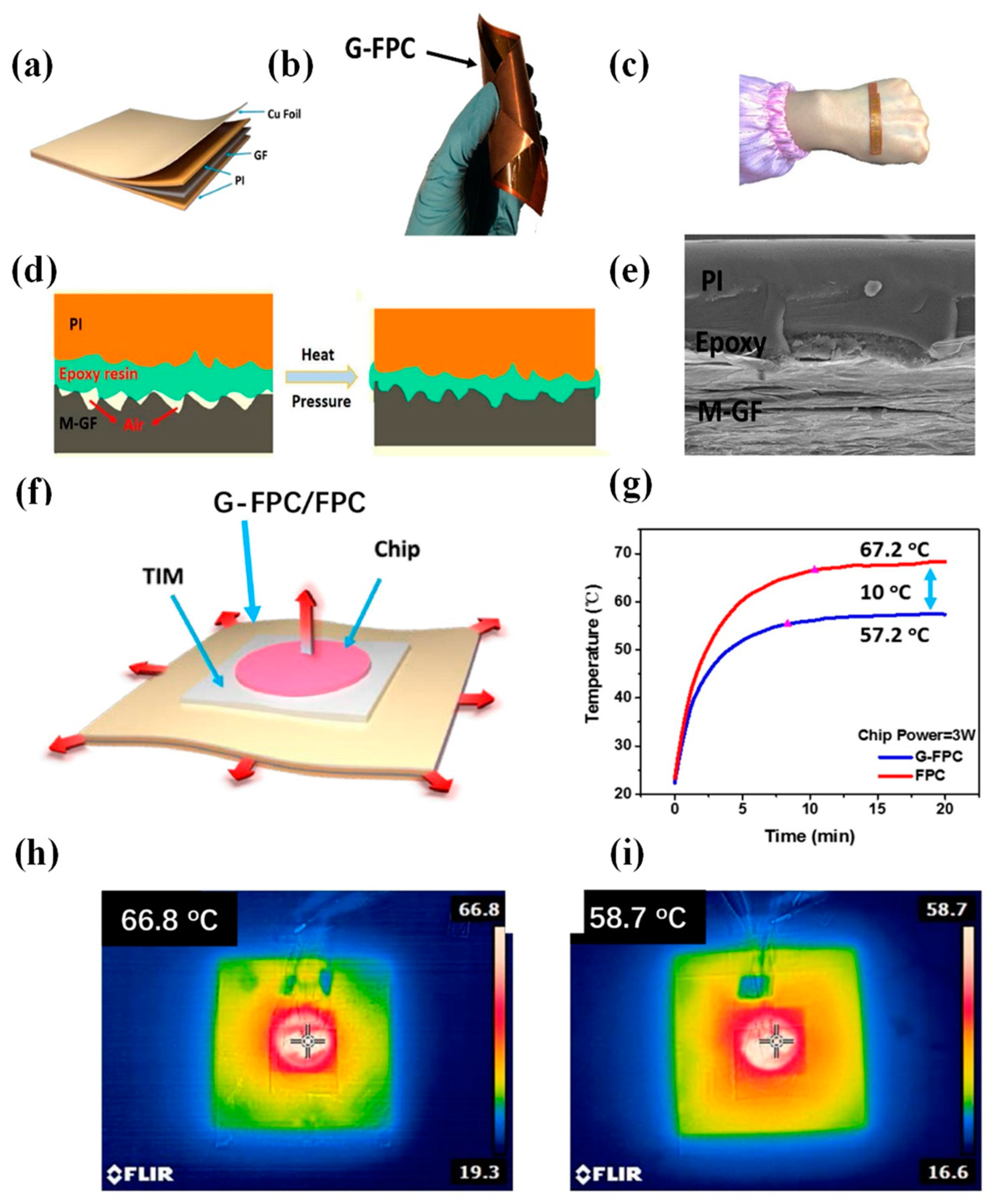
4.1. Thermal Conducting Polymers (LED Lighting and Microelectronics Packaging Technology)
4.2. Gas Separation Membranes
4.3. Space Applications
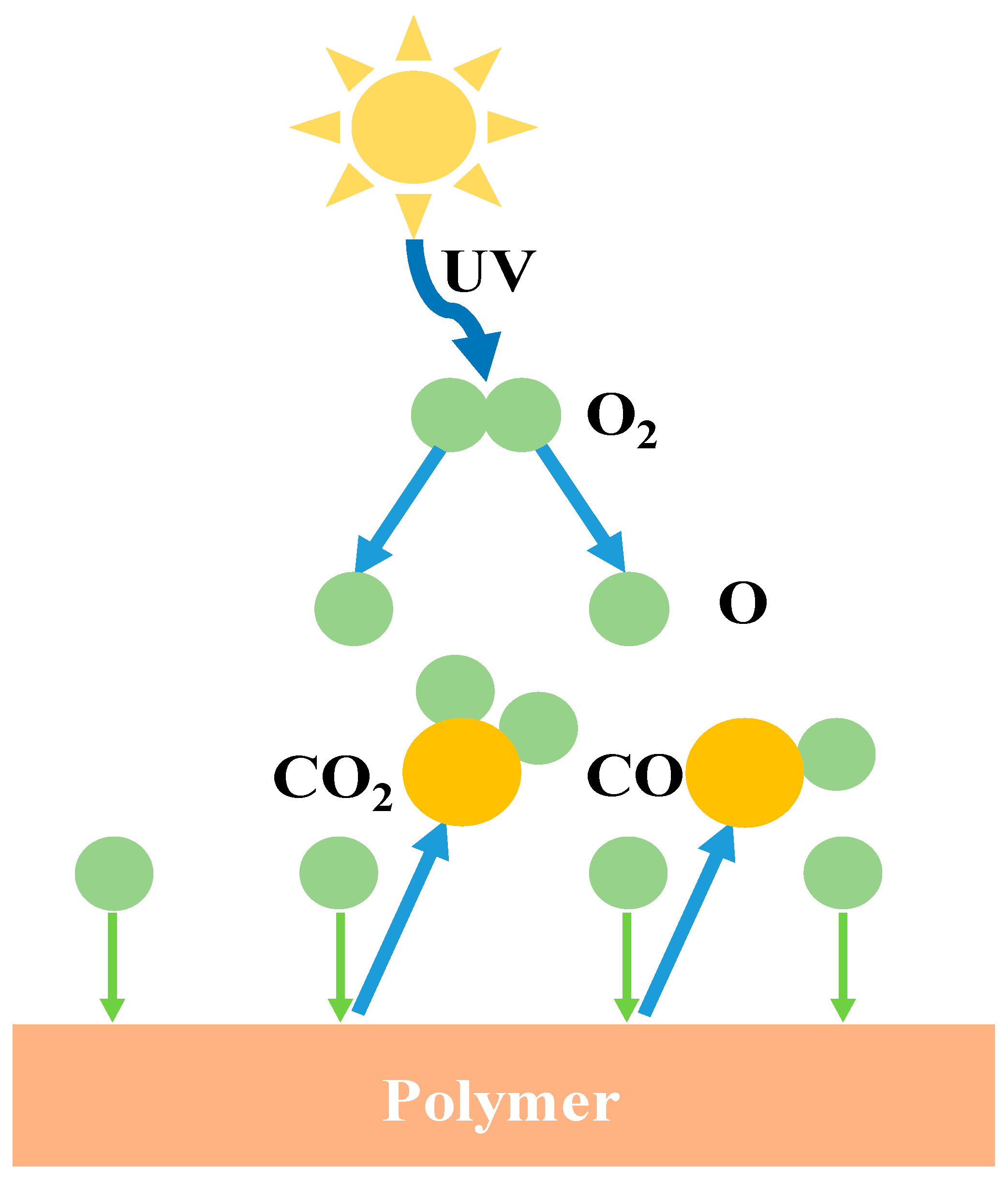
4.3.1. Atomic Oxygen-Resistant Film
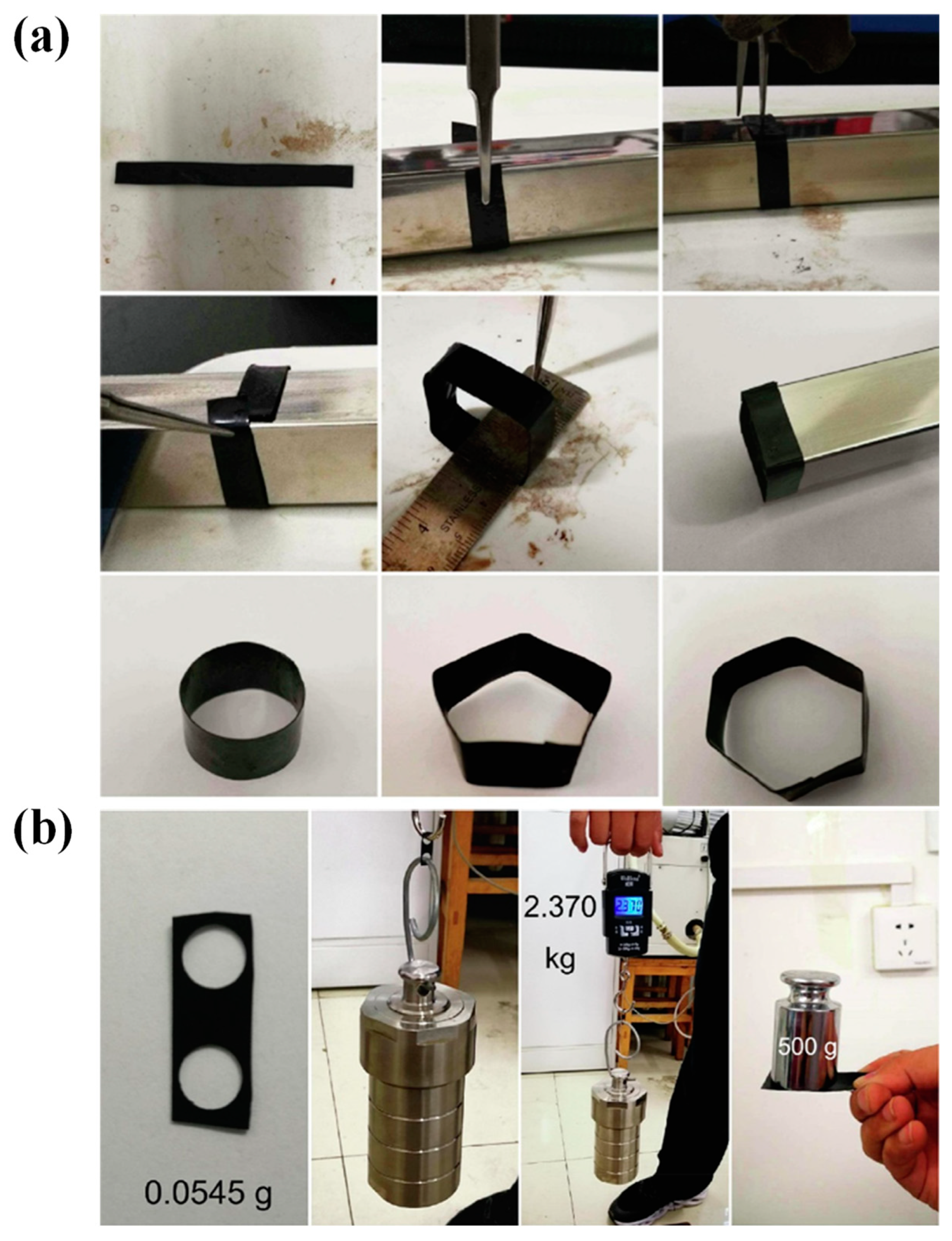
4.3.2. Shape Memory Materials
4.3.3. Corona Resistant Material
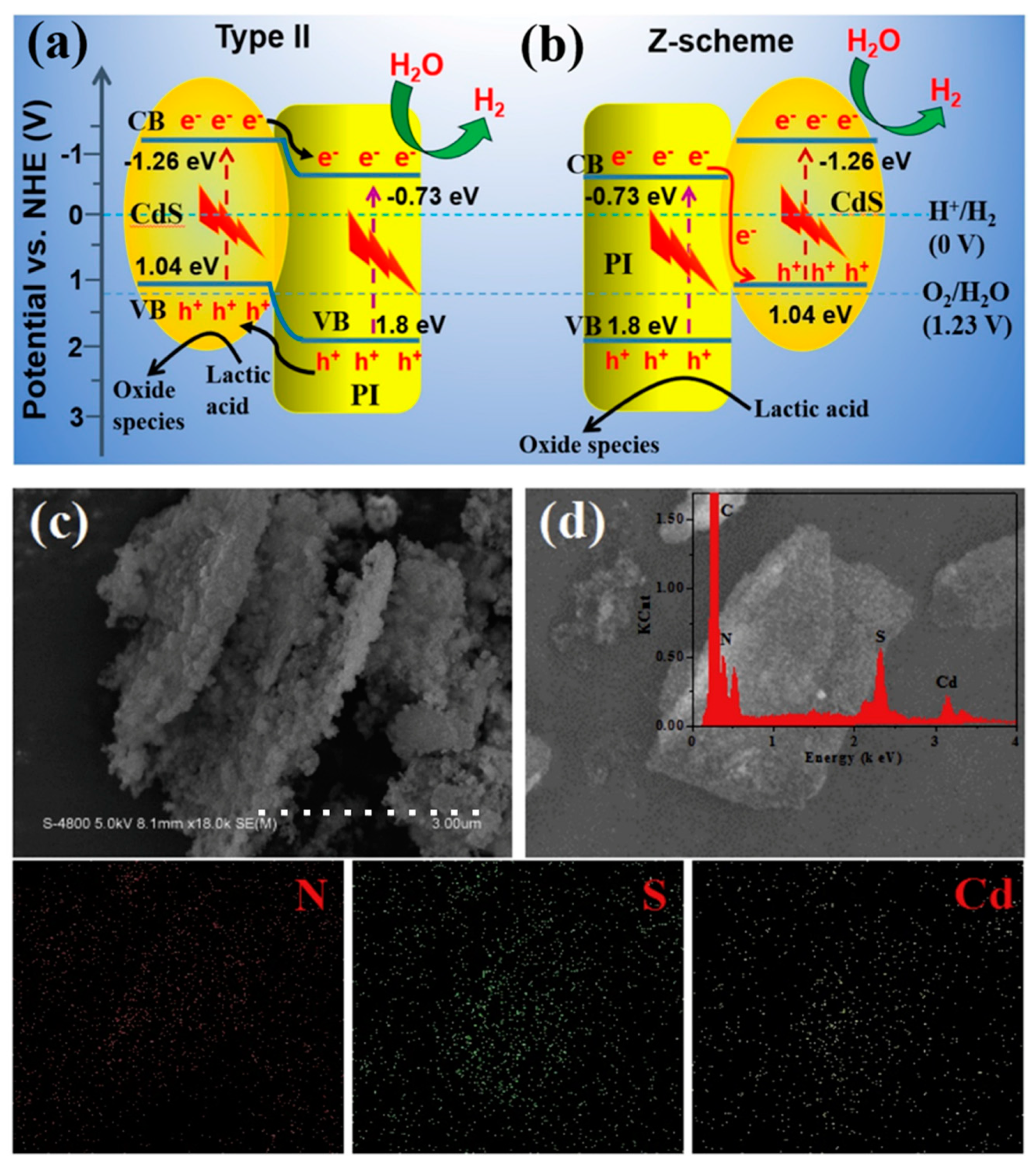
4.4. Photocatalytic Application
4.5. Electrode Applications in Electrocatalysis and Sensing
5. Conclusions and Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ohya, H.; Kudryavsev, V.V.; Semenova, S.I. Polyimide Membranes: Applications, Fabrications and Properties; CRC Press LLC: Milton, ON, Canada, 1997. [Google Scholar]
- Kash, L. (Ed.) Polyimides and Other High Temperature Polymers: Synthesis, Characterization and Applications; CRC Press: Mittal, ON, Canada, 2005; Volume 3. [Google Scholar]
- Yang, S.-Y. (Ed.) Advanced Polyimide Materials: Synthesis, Characterization, and Applications; Elsevier: Saint Louis, MI, USA, 2018. [Google Scholar]
- Akiyama, M.; Morofuji, Y.; Kamohara, T.; Nishikubo, K.; Ooishi, Y.; Tsubai, M.; Fukuda, O.; Ueno, N. Preparation of Oriented Aluminum Nitride Thin Films on Polyimide Films and Piezoelectric Response with High Thermal Stability and Flexibility. Adv. Funct. Mater. 2007, 17, 458–462. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Zhou, Z.-H.; Zhou, C.-G.; Sun, W.-J.; Gao, J.-F.; Dai, K.; Yan, D.-X.; Li, Z.-M. Lightweight and Robust Carbon Nanotube/Polyimide Foam for Efficient and Heat-Resistant Electromagnetic Interference Shielding and Microwave Absorption. ACS Appl. Mater. Interfaces 2020, 12, 8704–8712. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, A.; Wang, Z.; Li, K.; Yang, S. Closed-Cell Rigid Polyimide Foams for High-Temperature Applications: The Effect of Structure on Combined Properties. Polymers 2021, 13, 4434. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liu, H.; Wu, D.; Wang, X. Polyimide/Mxene Hybrid Aerogel-Based Phase-Change Composites for Solar-Driven Seawater Desalination. Chem. Eng. J. 2022, 440, 135862. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, T.-W.; Stryakhilev, D.; Lee, J.-S.; An, S.-G.; Pyo, Y.-S.; Lee, D.-B.; Mo, Y.G.; Jin, D.-U.; Chung, H.K. Flexible Full Color Organic Light-Emitting Diode Display on Polyimide Plastic Substrate Driven by Amorphous Indium Gallium Zinc Oxide Thin-Film Transistors. Appl. Phys. Lett. 2009, 95, 13503. [Google Scholar] [CrossRef]
- Wang, B.; He, B.; Wang, Z.; Qi, S.; Zhang, D.; Tian, G.; Wu, D. Enhanced Impact Properties of Hybrid Composites Reinforced by Carbon Fiber and Polyimide Fiber. Polymers 2021, 13, 2599. [Google Scholar] [CrossRef]
- He, B.; Wang, B.; Wang, Z.; Qi, S.; Tian, G.; Wu, D. Mechanical Properties of Hybrid Composites Reinforced by Carbon Fiber and High-Strength and High-Modulus Polyimide Fiber. Polymer 2020, 204, 122830. [Google Scholar] [CrossRef]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in Polyimide-Based Materials for Space Applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef]
- Sezer Hicyilmaz, A.; Celik Bedeloglu, A. Applications of Polyimide Coatings: A Review. SN Appl. Sci. 2021, 3, 363. [Google Scholar] [CrossRef]
- Harasimowicz, M.; Orluk, P.; Zakrzewska-Trznadel, G.; Chmielewski, A.G. Application of Polyimide Membranes for Biogas Purification and Enrichment. J. Hazard. Mater. 2007, 144, 698–702. [Google Scholar] [CrossRef]
- Guo, Y.; Qiu, H.; Ruan, K.; Zhang, Y.; Gu, J. Hierarchically Multifunctional Polyimide Composite Films with Strongly Enhanced Thermal Conductivity. Nanomicro Lett. 2022, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, T.; Higashihara, T.; Ueda, M. Polyimide Memory: A Pithy Guideline for Future Applications. Polym. Chem. 2012, 4, 16–30. [Google Scholar] [CrossRef]
- Ding, Y.; Hou, H.; Zhao, Y.; Zhu, Z.; Fong, H. Electrospun Polyimide Nanofibers and Their Applications. Prog. Polym. Sci. 2016, 61, 67–103. [Google Scholar] [CrossRef]
- Rahman, M.M.; Nayeem, M.A.; Nahid, S.; Bin Alvee, S.R.; Rashidul Hasan, R.; Rahman, M.A. On-Body Humidity Sensing Antenna with Polyimide for Ban Applications over 5g Networks. In Proceedings of the 2020 IEEE International IOT, Electronics and Mechatronics Conference (IEMTRONICS), Vancouver, BC, Canada, 9–12 September 2020. [Google Scholar]
- Peng, J.-J.; Qu, S.-W.; Xia, M.; Yang, S. Wide-Scanning Conformal Phased Array Antenna for Uav Radar Based on Polyimide Film. IEEE Antennas Wirel. Propag. Lett. 2020, 19, 1581–1585. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, D. Double Layered Radar Absorbing Structures of Silicon Carbide Fibers/Polyimide Composites. Synth. Met. 2018, 246, 213–219. [Google Scholar] [CrossRef]
- Pham, H.Q.; Kim, G.; Jung, H.M.; Song, S.W. Fluorinated Polyimide as a Novel High-Voltage Binder for High-Capacity Cathode of Lithium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1704690. [Google Scholar] [CrossRef]
- Jiang, X.; Bin, Y.; Matsuo, M. Electrical and Mechanical Properties of Polyimide–Carbon Nanotubes Composites Fabricated by in Situ Polymerization. Polymer 2005, 46, 7418–7424. [Google Scholar] [CrossRef]
- Wu, G.; Cheng, Y.; Wang, Z.; Wang, K.; Feng, A. In Situ Polymerization of Modified Graphene/Polyimide Composite with Improved Mechanical and Thermal Properties. J. Mater. Sci. 2017, 28, 576–581. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Yang, S.-Y.; Huang, Y.-L.; Tien, H.-W.; Chin, W.-K.; Ma, C.-C.M. Preparation and Properties of Graphene Oxide/Polyimide Composite Films with Low Dielectric Constant and Ultrahigh Strength Via in Situ Polymerization. J. Mater. Chem. 2011, 21, 13569–13575. [Google Scholar] [CrossRef]
- Ahmad, Z.; Mark, J.E. Polyimide−Ceramic Hybrid Composites by the Sol−Gel Route. Chem. Mater. 2001, 13, 3320–3330. [Google Scholar] [CrossRef]
- Yang, C.-P.; Su, Y.-Y.; Hsiao, S.-H. Synthesis and Properties of Low-Color Polyimide/Silica Hybrid Films. J. Appl. Polym. Sci. 2007, 104, 4046–4052. [Google Scholar] [CrossRef]
- Joly, C.; Goizet, S.; Schrotter, J.C.; Sanchez, J.; Escoubes, M. Sol-Gel Polyimide-Silica Composite Membrane: Gas Transport Properties. J. Membr. Sci. 1997, 130, 63–74. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, L.; Chen, G.; Fang, X. Aqueous Solution Blending Route for Preparing Flexible and Antistatic Polyimide/Carbon Nanotube Composite Films with Core-Shell Structured Polyimide/Graphene Microspheres. Polym. Compos. 2022, 43, 6062–6073. [Google Scholar] [CrossRef]
- Shayapat, J.; Chung, O.H.; Park, J.S. Electrospun Polyimide-Composite Separator for Lithium-Ion Batteries. Electrochim. Acta 2015, 170, 110–121. [Google Scholar] [CrossRef]
- Zhou, X.; Ding, C.; Cheng, C.; Liu, S.; Duan, G.; Xu, W.; Liu, K.; Hou, H. Mechanical and Thermal Properties of Electrospun Polyimide/Rgo Composite Nanofibers Via in-Situ Polymerization and in-Situ Thermal Conversion. Eur. Polym. J. 2020, 141, 110083. [Google Scholar] [CrossRef]
- Wu, D.C.; Shen, L.; Low, J.E.; Wong, S.Y.; Li, X.; Tjiu, W.C.; Liu, Y.; He, C.B. Multi-Walled Carbon Nanotube/Polyimide Composite Film Fabricated through Electrophoretic Deposition. Polymer 2010, 51, 2155–2160. [Google Scholar] [CrossRef]
- Wang, H.; Wei, M.; Zhong, Z.; Wang, Y. Atomic-Layer-Deposition-Enabled Thin-Film Composite Membranes of Polyimide Supported on Nanoporous Anodized Alumina. J. Membr. Sci. 2017, 535, 56–62. [Google Scholar] [CrossRef]
- Ikeda, S.; Yanagimoto, H.; Akamatsu, K.; Nawafune, H. Copper/Polyimide Heterojunctions: Controlling Interfacial Structures through an Additive-Based, All-Wet Chemical Process Using Ion-Doped Precursors. Adv. Funct. Mater. 2007, 17, 889–897. [Google Scholar] [CrossRef]
- Ding, Q.; Miao, Y.-E.; Liu, T. Morphology and Photocatalytic Property of Hierarchical Polyimide/ZnO Fibers Prepared Via a Direct Ion-Exchange Process. ACS Appl. Mater. Interfaces 2013, 5, 5617–5622. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Pan, Z.; Wu, X.; Chen, H.; Lv, X.; Li, P.; Liu, J.; Zhai, J. Enhancement of Thermal Stability and Energy Storage Capability of Flexible Ag Nanodot/Polyimide Nanocomposite Films Via in Situ Synthesis. J. Mater. Chem. C 2020, 8, 12607–12614. [Google Scholar] [CrossRef]
- Jeong, S.; Song, H.C.; Lee, W.W.; Choi, Y.; Ryu, B.-H. Preparation of Aqueous Ag Ink with Long-Term Dispersion Stability and Its Inkjet Printing for Fabricating Conductive Tracks on a Polyimide Film. J. Appl. Phys. 2010, 108, 102805. [Google Scholar] [CrossRef]
- Oh, S.H.; Legros, M.; Kiener, D.; Gruber, P.; Dehm, G. In Situ Tem Straining of Single Crystal Au Films on Polyimide: Change of Deformation Mechanisms at the Nanoscale. Acta Mater. 2007, 55, 5558–5571. [Google Scholar] [CrossRef]
- Yadav, D.; Borpatra Gohain, M.; Karki, S.; Ingole, P.G. A Novel Approach for the Development of Low-Cost Polymeric Thin-Film Nanocomposite Membranes for the Biomacromolecule Separation. ACS Omega 2022, 7, 47967–47985. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ryou, M.-H.; Son, B.; Lee, J.-N.; Lee, D.J.; Lee, Y.M.; Choi, J.W.; Park, J.-K. Co-Polyimide-Coated Polyethylene Separators for Enhanced Thermal Stability of Lithium Ion Batteries. Electrochim. Acta 2012, 85, 524–530. [Google Scholar] [CrossRef]
- Borpatra Gohain, M.; Karki, S.; Yadav, D.; Yadav, A.; Thakare, N.R.; Hazarika, S.; Lee, H.K.; Ingole, P.G. Development of Antifouling Thin-Film Composite/Nanocomposite Membranes for Removal of Phosphate and Malachite Green Dye. Membranes 2022, 12, 768. [Google Scholar] [CrossRef]
- Haight, R.; White, R.C.; Silverman, B.D.; Ho, P.S. Complex Formation and Growth at the Cr– and Cu–Polyimide Interface. J. Vac. Sci. Technol. A 1988, 6, 2188–2199. [Google Scholar] [CrossRef]
- Muthukrishnan, A.; Nabae, Y.; Hayakawa, T.; Okajima, T.; Ohsaka, T. Fe-Containing Polyimide-Based High-Performance ORR Catalysts in Acidic Medium: A Kinetic Approach to Study the Durability of Catalysts. Catal. Sci. Technol. 2014, 5, 475–483. [Google Scholar] [CrossRef]
- Nam, V.B.; Shin, J.; Choi, A.; Choi, H.; Ko, S.H.; Lee, D. High-Temperature, Thin, Flexible and Transparent Ni-Based Heaters Patterned by Laser-Induced Reductive Sintering on Colorless Polyimide. J. Mater. Chem. C 2021, 9, 5652–5661. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Z.; Ruan, K.; Gu, J. Multifunctional Ti3C2Tx-(Fe3O4/Polyimide) Composite Films with Janus Structure for Outstanding Electromagnetic Interference Shielding and Superior Visual Thermal Management. Nano Res. 2022, 15, 5601–5609. [Google Scholar] [CrossRef]
- Yoda, S.; Hasegawa, A.; Suda, H.; Uchimaru, Y.; Haraya, K.; Tsuji, T.; Otake, K. Preparation of a Platinum and Palladium/Polyimide Nanocomposite Film as a Precursor of Metal-Doped Carbon Molecular Sieve Membrane Via Supercritical Impregnation. Chem. Mater. 2004, 16, 2363–2368. [Google Scholar] [CrossRef]
- Nasreen, S.; Baczkowski, M.L.; Treich, G.M.; Tefferi, M.; Anastasia, C.; Ramprasad, R.; Cao, Y.; Sotzing, G.A. Sn-Polyester/Polyimide Hybrid Flexible Free-Standing Film as a Tunable Dielectric Material. Macromol. Rapid Commun. 2019, 40, 1800679. [Google Scholar] [CrossRef] [PubMed]
- Ishii, J.; Yokotsuka, H.; Saito, T.; Hasegawa, M. Strategy for Improvement of Non-Flammability in Functional Polyimides. J. Photopolym. Sci. Technol. 2011, 24, 287–291. [Google Scholar] [CrossRef]
- Sava, I.; Asandulesa, M.; Zocher, K.; Kruth, A.; Kolb, J.F.; Bodnar, W.; Witte, K.; Ishizaki, T.; Miron, C. Electrical and Mechanical Properties of Polyimide Films Treated by Plasma Formed in Water and Isopropanol. React. Funct. Polym. 2019, 134, 22–30. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, K.; Dou, S.; Zhao, J.; Yan, X.; Li, Y. Mechanical, Dielectric, and Thermal Attributes of Polyimides Stemmed out of 4, 4′-Diaminodiphenyl Ether. Crystals 2020, 10, 173. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced Polyimide Materials: Syntheses, Physical Properties and Applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar]
- Pakhuruddin, M.Z.; Ibrahim, K.; Aziz, A.A. Properties of Polyimide Substrate for Applications in Flexible Solar Cells. Optoelectron. Adv. Mater. Rapid Commun. 2013, 7, 377–380. [Google Scholar]
- Sekitani, T.; Zschieschang, U.; Klauk, H.; Someya, T. Flexible Organic Transistors and Circuits with Extreme Bending Stability. Nat. Mater. 2010, 9, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Thiel, R.C.; de Groot, H.J.M.; de Vries, J.W.C. Use of Kapton Film as a Cryogenic Construction Material. Cryogenics 1984, 24, 702–704. [Google Scholar] [CrossRef]
- Kim, J.H.; Lan, N.X.V.; Kulkarni, U.; Kim, C.; Cho, S.M.; Yoo, P.J.; Kim, D.; Schroeder, M.; Yi, G.R. Optically Transparent and Low-CTE Polyethersulfone-Based Nanocomposite Films for Flexible Display. Adv. Mater. Interfaces 2020, 7, 2001422. [Google Scholar] [CrossRef]
- Tan, Y.-y.; Zhang, Y.; Jiang, G.-l.; Zhi, X.-x.; Xiao, X.; Wu, L.; Jia, Y.-J.; Liu, J.-g.; Zhang, X.-m. Preparation and Properties of Inherently Black Polyimide Films with Extremely Low Coefficients of Thermal Expansion and Potential Applications for Black Flexible Copper Clad Laminates. Polymers 2020, 12, 576. [Google Scholar] [CrossRef]
- You, N.-H.; Chueh, C.-C.; Liu, C.-L.; Ueda, M.; Chen, W.-C. Synthesis and Memory Device Characteristics of New Sulfur Donor Containing Polyimides. Macromolecules 2009, 42, 4456–4463. [Google Scholar] [CrossRef]
- Ling, Q.-D.; Chang, F.-C.; Song, Y.; Zhu, C.-X.; Liaw, D.-J.; Chan, D.S.-H.; Kang, E.-T.; Neoh, K.-G. Synthesis and Dynamic Random Access Memory Behavior of a Functional Polyimide. J. Am. Chem. Soc. 2006, 128, 8732–8733. [Google Scholar] [CrossRef]
- Ke, F.; Song, N.; Liang, D.; Xu, H. A Method to Break Charge Transfer Complex of Polyimide: A Study on Solution Behavior. J. Appl. Polym. Sci. 2013, 127, 797–803. [Google Scholar] [CrossRef]
- Huang, J.-J.; Chen, Y.-P.; Lien, S.-Y.; Weng, K.-W.; Chao, C.-H. High Mechanical and Electrical Reliability of Bottom-Gate Microcrystalline Silicon Thin Film Transistors on Polyimide Substrate. Curr. Appl. Phys. 2011, 11, S266–S270. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Yuan, L.-L. Advanced Polyimide Films. In Advanced Polyimide Materials: Synthesis, Characterization, and Applications; Yang, S.-Y., Ed.; Chemical Industry Press: Beijing, China, 2018; pp. 1–66. [Google Scholar]
- Liu, J.-g.; Ni, H.-j.; Wang, Z.-h.; Yang, S.-y.; Zhou, W.-f. Colorless and Transparent High-Temperature-Resistant Polymer Optical Films–Current Status and Potential Applications in Optoelectronic Fabrications. In Optoelectronics—Materials and Devices; InTech: Rijeka, Croatia, 2015; pp. 57–81. [Google Scholar]
- Ni, H.-j.; Liu, J.-g.; Wang, Z.-h.; Yang, S.-y. A Review on Colorless and Optically Transparent Polyimide Films: Chemistry, Process and Engineering Applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Jin, Q.; Yamashita, T.; Horie, K.; Yokota, R.; Mita, I. Polyimides with Alicyclic Diamines. I. Syntheses and Thermal Properties. J. Polym. Sci. A Polym. Chem. 1993, 31, 2345–2351. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujii, M.; Ishii, J.; Yamaguchi, S.; Takezawa, E.; Kagayama, T.; Ishikawa, A. Colorless Polyimides Derived from 1s,2s,4r,5r-Cyclohexanetetracarboxylic Dianhydride, Self-Orientation Behavior During Solution Casting, and Their Optoelectronic Applications. Polymer 2014, 55, 4693–4708. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kasamatsu, K.; Koseki, K. Colorless Poly(Ester Imide)S Derived from Hydrogenated Trimellitic Anhydride. Eur. Polym. J. 2012, 48, 483–498. [Google Scholar] [CrossRef]
- Banerjee, S.; Madhra, M.K.; Salunke, A.K.; Jaiswal, D.K. Synthesis and Properties of Fluorinated Polyimides. 3. Derived from Novel 1,3-Bis[3′-Trifluoromethyl-4′(4″-Amino Benzoxy) Benzyl] Benzene and 4,4-Bis[3′-Trifluoromethyl-4′(4-Amino Benzoxy) Benzyl] Biphenyl. Polymer 2003, 44, 613–622. [Google Scholar] [CrossRef]
- Suzuki, H.; Abe, T.; Takaishi, K.; Narita, M.; Hamada, F. The Synthesis and X-Ray Structure of 1,2,3,4-Cyclobutane Tetracarboxylic Dianhydride and the Preparation of a New Type of Polyimide Showing Excellent Transparency and Heat Resistance. J. Polym. Sci. A 2000, 38, 108–116. [Google Scholar] [CrossRef]
- Kaneya, Y.; Arakawa, Y.; Suzuki, K. Polyimide Precursor Composition, Polyimide Film, and Transparent Flexible Film. Google Patent US20110059305A1, 5 August 2014. [Google Scholar]
- Uchida, A.; Hasegawa, M.; Takezawa, E.; Yamaguchi, S.; Ishikawa, A.; Kagayama, T. (1r*,2s*,4s*,5r*)-Cyclohexane-1,2:4, 5-Tetracarboxylic Dianhydride. Acta Crystallogr. Sect. Sect. E Struct. Rep. 2012, 68, o579. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Hirano, D.; Fujii, M.; Haga, M.; Takezawa, E.; Yamaguchi, S.; Ishikawa, A.; Kagayama, T. Solution-Processable Colorless Polyimides Derived from Hydrogenated Pyromellitic Dianhydride with Controlled Steric Structure. J. Polym. Sci. A 2013, 51, 575–592. [Google Scholar] [CrossRef]
- Li, T.-L.; Hsu, S.L.-C. Preparation and Properties of a High Temperature, Flexible and Colorless Ito Coated Polyimide Substrate. Eur. Polym. J. 2007, 43, 3368–3373. [Google Scholar] [CrossRef]
- Nishikawa, M.; Matsuki, Y.; Bessho, N.; Iimura, Y.; Kobayashi, S. Characteristics of Polyimide Liquid Crystal Alignment Films for Active Matrix-Lcd Use. J. Photopolym. Sci. Technol. 1995, 8, 233–240. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Song, Y.-Z.; Liu, J.-G.; Yang, S.-Y. Synthesis and Properties of Cost-Effective Light-Color and Highly Transparent Polyimide Films from Fluorine-Containing Tetralin Dianhydride and Aromatic Diamines. J. Photopolym. Sci. Technol. 2016, 29, 31–38. [Google Scholar] [CrossRef]
- Guo, Y.-z.; Song, H.-w.; Zhai, L.; Liu, J.-g.; Yang, S. Synthesis and Characterization of Novel Semi-Alicyclic Polyimides from Methyl-Substituted Tetralin Dianhydride and Aromatic Diamines. Polym. J. 2012, 44, 718–723. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Liu, X.; Wang, Y.; Gao, X.; Meng, X.; Tu, G. High Performance Soluble Polyimides from Ladder-Type Fluorinated Dianhydride with Polymorphism. Polymers 2018, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Acar, O.; Varis, S.; Isık, T.; Tirkes, S.; Demir, M.M. Synthesis and Characterization of Novel High Temperature Structural Adhesives Based on Nadic End Capped Mda-Btda-Oda Copolyimide. Mater. Res. Express 2018, 5, 105305. [Google Scholar] [CrossRef]
- Ni, H.; Xing, Y.; Dai, X.; Zhang, D.; Li, J.; Liu, J.; Yang, S.; Chen, X. Intrinsically Heat-Sealable Polyimide Films with Atomic Oxygen Resistance: Synthesis and Characterization. High Perform. Polym. 2020, 32, 902–913. [Google Scholar] [CrossRef]
- Chen, S.; Hu, P.; Greiner, A.; Cheng, C.; Cheng, H.; Chen, F.; Hou, H. Electrospun Nanofiber Belts Made from High Performance Copolyimide. Nanotechnology 2008, 19, 015604. [Google Scholar] [CrossRef]
- Yu, X.; Liang, W.; Cao, J.; Wu, D. Mixed Rigid and Flexible Component Design for High-Performance Polyimide Films. Polymers 2017, 9, 451. [Google Scholar] [CrossRef]
- Sheng, S.-R.; Zhang, W.; Lu, C.; Wan, J.; Liu, X.-L.; Song, C.-S. Synthesis and Characterization of New Cardo Poly(Ether Imide)S Derived from 9,9-Bis [4-(4-Aminophenoxy)Phenyl]Xanthene. J. Appl. Polym. Sci. 2012, 126, 297–303. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Song, C.; Wei, M.-H.; Huang, Z.-Z.; Sheng, S.-R. Organosoluble and Transparent Cardo Polyimides with High Tg Derived from 9,9-Bis(4-Aminophenyl)Xanthene. High Perform. Polym. 2019, 31, 909–918. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Ha, C.S. Recent Trends on Transparent Colorless Polyimides with Balanced Thermal and Optical Properties: Design and Synthesis. Macromol. Chem. Phys. 2019, 220, 1800313. [Google Scholar] [CrossRef]
- Hasegawa, M.; Takahashi, S.; Tsukuda, S.; Hirai, T.; Ishii, J.; Yamashina, Y.; Kawamura, Y. Symmetric and Asymmetric Spiro-Type Colorless Poly(Ester Imide)S with Low Coefficients of Thermal Expansion, High Glass Transition Temperatures, and Excellent Solution-Processability. Polymer 2019, 169, 167–184. [Google Scholar] [CrossRef]
- Yu, X.-H.; Liu, J.-N.; Wu, D.-Y. Colorless PI Structure Design and Evaluation for Achieving Low Cte Target. Mater. Today Commun. 2019, 21, 100562. [Google Scholar] [CrossRef]
- Hasegawa, M. Development of Solution-Processable, Optically Transparent Polyimides with Ultra-Low Linear Coefficients of Thermal Expansion. Polymers 2017, 9, 520. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Su, Y.-Y.; Hsiao, F.-Z. The Synthesis and Characterization of Fluorinated Polyimides Derived from 2′-Methyl-1,4-Bis-(4-Amino-2-Trifluoromethylphenoxy)Benzene and Various Aromatic Dianhydrides. J. Macromol. Sci. A 2020, 57, 579–588. [Google Scholar] [CrossRef]
- DeMeuse, M.T. (Ed.) Biaxial Stretching of Film: Principles and Applications; Woodhead Publishing Ltd.: Cambridge, MA, USA, 2011. [Google Scholar]
- Meador, M.A.B.; Malow, E.J.; Silva, R.; Wright, S.; Quade, D.; Vivod, S.L.; Guo, H.; Guo, J.; Cakmak, M. Mechanically Strong, Flexible Polyimide Aerogels Cross-Linked with Aromatic Triamine. ACS Appl. Mater. Interfaces 2012, 4, 536–544. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Zagho, M.M.; Elzatahry, A.A. Polymer-Based Electrospun Nanofibers for Biomedical Applications. Nanomaterials 2018, 8, 259. [Google Scholar] [CrossRef]
- Kadavil, H.; Zagho, M.; Elzatahry, A.; Altahtamouni, T. Sputtering of Electrospun Polymer-Based Nanofibers for Biomedical Applications: A Perspective. Nanomaterials 2019, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, W.; Tang, Y.; Xu, X.; Huang, Y.; Yu, D. Polymer-Based Nanofiber–Nanoparticle Hybrids and Their Medical Applications. Polymers 2022, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xiong, J.; Lei, S.; Wang, L.; Qin, X. Diameter Refinement of Electrospun Nanofibers: From Mechanism, Strategies to Applications. Adv. Fiber Mater. 2021, 4, 145–161. [Google Scholar] [CrossRef]
- Baheti, V.; Mishra, R.; Militky, J.; Behera, B.K. Influence of Noncellulosic Contents on Nano Scale Refinement of Waste Jute Fibers for Reinforcement in Polylactic Acid Films. Fibers Polym. 2014, 15, 1500–1506. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, J.; Pan, D.; Yang, G.; Su, F.; Ji, Y.; Liu, C.; Shen, C. Constructing Dual Thermal Conductive Networks in Electrospun Polyimide Membranes with Highly Thermally Conductivity but Electrical Insulation Properties. Adv. Compos. Hybrid Mater. 2021, 4, 1102–1112. [Google Scholar] [CrossRef]
- Rogalski, J.J.; Zhang, H.; Yao, J.; Bastiaansen, C.W.M.; Peijs, T. High-Modulus Rotary Jet Spun Co-Polyimide Nanofibers and Their Composites. Nanocomposites 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Kim, J.-K.; Lim, Y.-W.; Hwang, H.-B.; Kwon, H.-Y.; Bae, B.-S.; Jin, J. Squid Pen-Inspired Chitinous Functional Materials: Hierarchical Chitin Fibers by Centrifugal Jet-Spinning and Transparent Chitin Fiber-Reinforced Composite. APL Mater. 2018, 6, 016102. [Google Scholar] [CrossRef]
- Hatami, M. Production of Polyimide Ceria Nanocomposites by Development of Molecular Hook Technology in Nano-Sonochemistry. Ultrason. Sonochem. 2018, 44, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, G.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Preparation of Solution Blown Polyamic Acid Nanofibers and Their Imidization into Polyimide Nanofiber Mats. Nanomaterials 2017, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, H.; Yu, F.; Liu, Y.; Jia, L.; Zhao, W.; Li, P.; Wang, H.; Zhu, P.; Li, B. Blow Spinning of Polyimide/SiO2 Composite Fibrous Sponges with Excellent Adsorption Capacity and Recyclability. ACS Appl. Polym. Mater. 2022, 4, 8487–8495. [Google Scholar] [CrossRef]
- Zhang, M.; Niu, H.; Wu, D. Polyimide Fibers with High Strength and High Modulus: Preparation, Structures, Properties, and Applications. Macromol. Rapid. Commun. 2018, 39, 1800141. [Google Scholar] [CrossRef] [PubMed]
- Monsef, K.; Homayoonfal, M.; Davar, F. Engineering Arrangement of Nanoparticles within Nanocomposite Membranes Matrix: A Suggested Way to Enhance Water Flux. Polym.-Plast. Technol. Mater. 2020, 59, 733–752. [Google Scholar] [CrossRef]
- Niu, H.; Qi, S.; Han, E.; Tian, G.; Wang, X.; Wu, D. Fabrication of High-Performance Copolyimide Fibers from 3, 3′, 4, 4′-Biphenyltetracarboxylic Dianhydride, P-Phenylenediamine and 2-(4-Aminophenyl)-6-Amino-4 (3h)-Quinazolinone. Mater. Lett. 2012, 89, 63–65. [Google Scholar] [CrossRef]
- Xu, W.; Ding, Y.; Jiang, S.; Ye, W.; Liao, X.; Hou, H. High Permittivity Nanocomposites Fabricated from Electrospun Polyimide/BaTiO3 Hybrid Nanofibers. Polym. Compos. 2016, 37, 794–801. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre Diameter Fibres of Polymer, Produced by Electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Chen, Y.; Han, D.; Ouyang, W.; Chen, S.; Hou, H.; Zhao, Y.; Fong, H. Fabrication and Evaluation of Polyamide 6 Composites with Electrospun Polyimide Nanofibers as Skeletal Framework. Compos. B Eng. 2012, 43, 2382–2388. [Google Scholar] [CrossRef]
- Huang, C.; Chen, S.; Reneker, D.H.; Lai, C.; Hou, H. High-Strength Mats from Electrospun Poly(P-Phenylene Biphenyltetracarboximide) Nanofibers. Adv. Mater. 2006, 18, 668–671. [Google Scholar] [CrossRef]
- Miao, Y.-E.; Zhu, G.-N.; Hou, H.; Xia, Y.-Y.; Liu, T. Electrospun Polyimide Nanofiber-Based Nonwoven Separators for Lithium-Ion Batteries. J. Power Sources 2013, 226, 82–86. [Google Scholar] [CrossRef]
- Chen, S.; Han, D.; Hou, H. High Strength Electrospun Fibers. Polym. Adv. Technol. 2011, 22, 295–303. [Google Scholar] [CrossRef]
- Huang, C.; Wang, S.; Zhang, H.; Li, T.; Chen, S.; Lai, C.; Hou, H. High Strength Electrospun Polymer Nanofibers Made from Bpda–Pda Polyimide. Eur. Polym. J. 2006, 42, 1099–1104. [Google Scholar] [CrossRef]
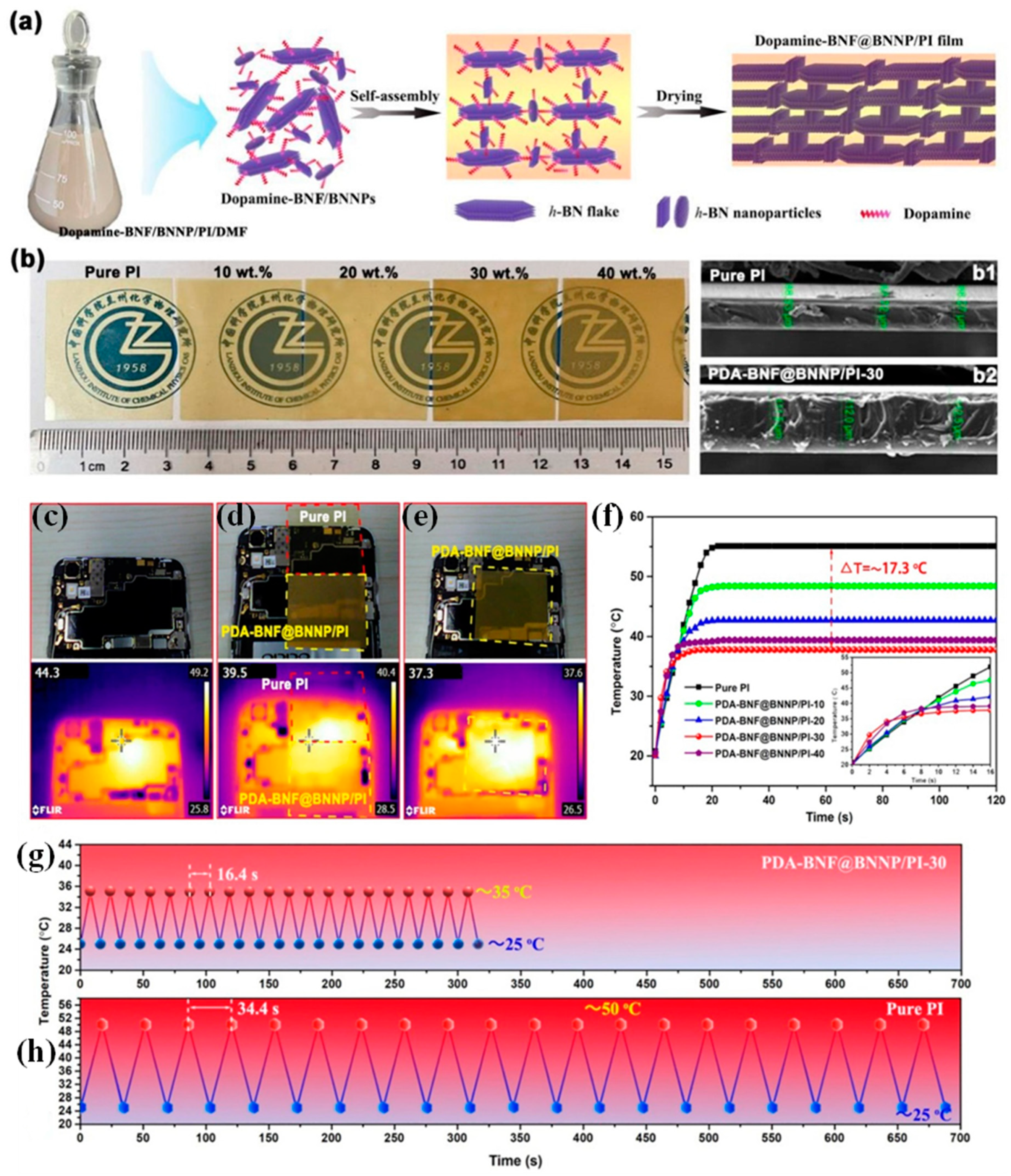
- Guo, Y.; Lyu, Z.; Yang, X.; Lu, Y.; Ruan, K.; Wu, Y.; Kong, J.; Gu, J. Enhanced Thermal Conductivities and Decreased Thermal Resistances of Functionalized Boron Nitride/Polyimide Composites. Compos. B Eng. 2019, 164, 732–739. [Google Scholar] [CrossRef]
- Liu, X.; Ji, T.; Li, N.; Liu, Y.; Yin, J.; Su, B.; Zhao, J.; Li, Y.; Mo, G.; Wu, Z. Preparation of Polyimide Composites Reinforced with Oxygen Doped Boron Nitride Nano-Sheet as Multifunctional Materials. Mater. Des. 2019, 180, 107963. [Google Scholar] [CrossRef]
- Li, Y.; Lv, L.; Huang, W.; Zhu, Y.; Long, F.; Zheng, W.; Qu, Q.; Zheng, H. In Situ Polymerized and Imidized Si@Polyimide Microcapsules with Flexible Solid-Electrolyte Interphase and Enhanced Electrochemical Activity for Li-Storage. ChemElectroChem 2022, 9, e202101409. [Google Scholar] [CrossRef]
- Zhu, J.; Lim, J.; Lee, C.-H.; Joh, H.-I.; Kim, H.C.; Park, B.; You, N.-H.; Lee, S. Multifunctional Polyimide/Graphene Oxide Composites Via in Situ Polymerization. J. Appl. Polym. Sci. 2014, 9, 131. [Google Scholar]
- Guo, Y.; Xu, G.; Yang, X.; Ruan, K.; Ma, T.; Zhang, Q.; Gu, J.; Wu, Y.; Liu, H.; Guo, Z. Significantly Enhanced and Precisely Modeled Thermal Conductivity in Polyimide Nanocomposites with Chemically Modified Graphene Via in Situ Polymerization and Electrospinning-Hot Press Technology. J. Mater. Chem. C 2018, 6, 34–315. [Google Scholar] [CrossRef]
- Chen, B.; Li, X.; Li, X.; Jia, Y.; Yang, J.; Yang, G.; Li, C. Friction and Wear Properties of Polyimide-Based Composites with a Multiscale Carbon Fiber-Carbon Nanotube Hybrid. Tribol. Lett. 2017, 65, 111. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, J.; Lee, J.; Han, P.; Park, D.; Han, H. Fabrication of Polyimide Composite Films Based on Carbon Black for High-Temperature Resistance. Polym. Compos. 2014, 35, 2214–2220. [Google Scholar] [CrossRef]
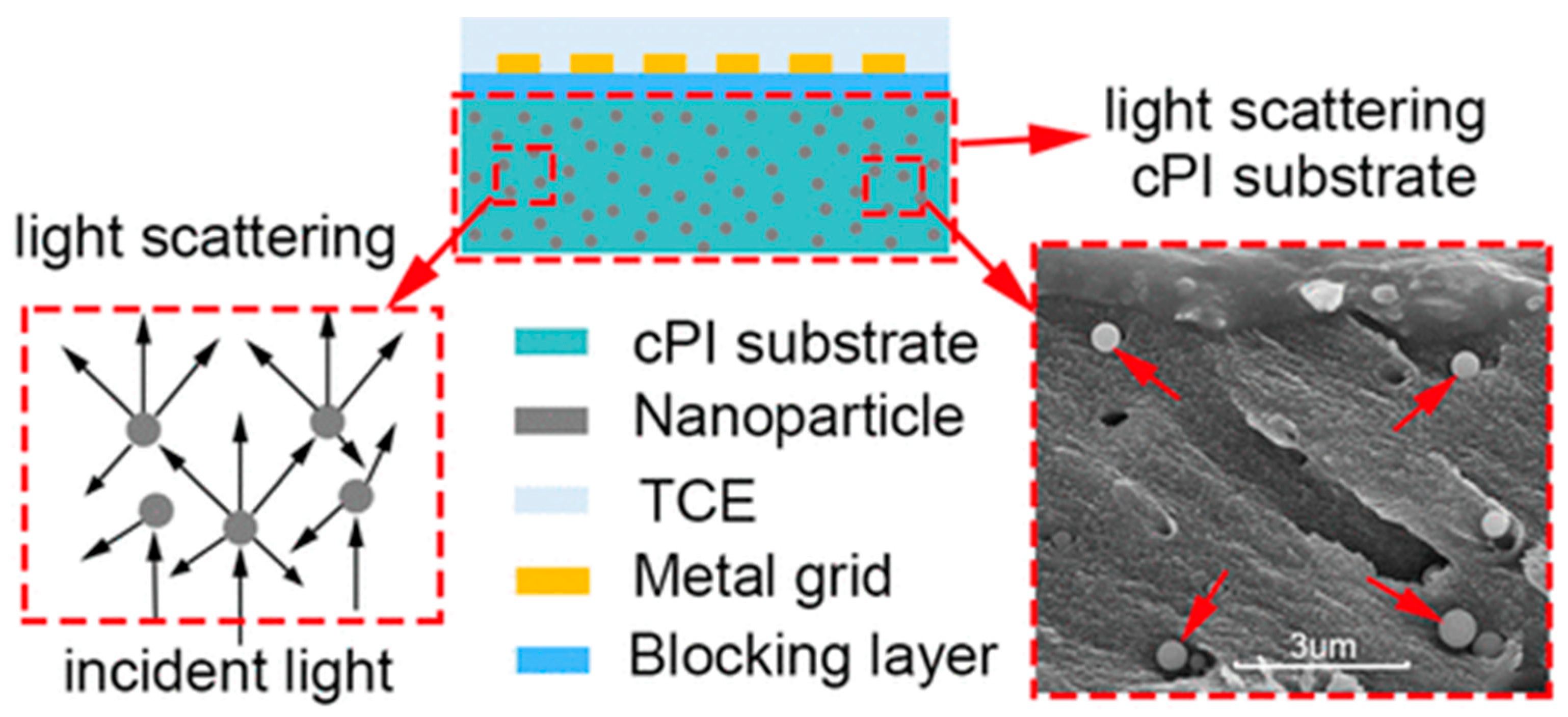
- Shen, J.; Li, F.; Cao, Z.; Barat, D.; Tu, G. Light Scattering in Nanoparticle Doped Transparent Polyimide Substrates. ACS Appl. Mater. Interfaces 2017, 9, 14990–14997. [Google Scholar] [CrossRef]
- Huang, J.-W.; Wen, Y.-L.; Kang, C.-C.; Yeh, M.-Y. Preparation of Polyimide-Silica Nanocomposites from Nanoscale Colloidal Silica. Polym. J. 2007, 39, 654–658. [Google Scholar] [CrossRef]
- Liu, J.; Tian, G.; Qi, S.; Wu, Z.; Wu, D. Enhanced Dielectric Permittivity of a Flexible Three-Phase Polyimide–Graphene–BaTiO3 Composite Material. Mater. Lett. 2014, 124, 117–119. [Google Scholar] [CrossRef]
- Pei, J.-Y.; Zha, J.-W.; Zhou, W.-Y.; Wang, S.-J.; Zhong, S.-L.; Yin, L.-J.; Zheng, M.-S.; Cai, H.-W.; Dang, Z.-M. Enhancement of Breakdown Strength of Multilayer Polymer Film through Electric Field Redistribution and Defect Modification. Appl. Phys. Lett. 2019, 114, 103702. [Google Scholar] [CrossRef]
- Liu, W.-D.; Zhu, B.-K.; Zhang, J.; Xu, Y.-Y. Preparation and Dielectric Properties of Polyimide/Silica Nanocomposite Films Prepared from Sol-Gel and Blending Process. Polym. Adv. Technol. 2007, 18, 522–528. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, Z.; Fu, Q.; Deng, H. Multi-Layered Boron Nitride/Polyimide High-Temperature Capacitor Dielectric Film. Mater. Today Energy 2022, 29, 101093. [Google Scholar] [CrossRef]
- Liu, X.-J.; Zheng, M.-S.; Chen, G.; Dang, Z.-M.; Zha, J.-W. High-Temperature Polyimide Dielectric Materials for Energy Storage: Theory, Design, Preparation and Properties. Energy Environ. Sci. 2022, 15, 56–81. [Google Scholar] [CrossRef]
- Hsiao, Y.-S.; Chang-Jian, C.-W.; Huang, T.-Y.; Chen, Y.-L.; Huang, C.-W.; Huang, J.-H.; Wu, N.-J.; Hsu, S.-C.; Chen, C.-P. Lightweight Flexible Polyimide-Derived Laser-Induced Graphenes for High-Performance Thermal Management Applications. Chem. Eng. J. 2023, 451, 138656. [Google Scholar] [CrossRef]
- Park, J.; Kim, W.; Aggawal, Y.; Shin, K.; Choi, E.H.; Park, B. Highly Efficient and Stable Organic Light-Emitting Diodes with Inner Passivating Hole-Transfer Interlayers of Poly(Amic Acid)-Polyimide Copolymer. Adv. Sci. 2022, 9, 2105851. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Ding, X.; Yu, D. Multilayer-Structured Ni-Co-Fe-P/Polyaniline/Polyimide Composite Fabric for Robust Electromagnetic Shielding with Low Reflection Characteristic. Chem. Eng. J. 2020, 380, 122553. [Google Scholar] [CrossRef]
- Ishida, A.; Sato, M. Ti–Ni–Cu Shape-Memory Alloy Thin Film Formed on Polyimide Substrate. Thin Solid Films 2008, 516, 7836–7839. [Google Scholar] [CrossRef]
- Liu, D.; Chi, H.; Ma, C.; Song, M.; Zhang, P.; Dai, P. Improving in-Plane and out-of-Plane Thermal Conductivity of Polyimide/Boron Nitride Film with Reduced Graphene Oxide by a Moving Magnetic Field Induction. Compos. Sci. Technol. 2022, 220, 109292. [Google Scholar] [CrossRef]
- Strack, G.; AitElAoud, Y.; Osgood, R.M.; Akyurtlu, A. Magnetic Nanoarrays on Flexible Substrates. MRS Adv. 2022, 7, 410–414. [Google Scholar] [CrossRef]
- Doan, H.N.; Tagami, S.; Vo, P.P.; Negoro, M.; Sakai, W.; Tsutsumi, N.; Kanamori, K.; Kinashi, K. Scalable Fabrication of Cross-Linked Porous Centrifugally Spun Polyimide Fibers for Thermal Insulation Application. Eur. Polym. J. 2022, 169, 111123. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Ding, Y.; Zhao, S.; Ma, Z.; Zhang, H.; He, X. Multifunctional Carbon Fiber@Nico/Polyimide Films with Outstanding Electromagnetic Interference Shielding Performance. Chem. Eng. J. 2022, 427, 131937. [Google Scholar] [CrossRef]
- Deng, J.; Cao, D.; Yang, X.; Zhang, G. Cross-Linked Cellulose/Carboxylated Polyimide Nanofiber Separator for Lithium-Ion Battery Application. Chem. Eng. J. 2022, 433, 133934. [Google Scholar] [CrossRef]
- Zheng, S.; Jiang, L.; Zhang, C.; Ma, N.; Liu, X. Facile and Environment-Friendly Preparation of High-Performance Polyimide Aerogels Using Water as the Only Solvent. Polym. Chem. 2022, 13, 2375–2382. [Google Scholar] [CrossRef]
- Tafreshi, O.A.; Ghaffari-Mosanenzadeh, S.; Karamikamkar, S.; Saadatnia, Z.; Kiddell, S.; Park, C.B.; Naguib, H.E. Novel, Flexible, and Transparent Thin Film Polyimide Aerogels with Enhanced Thermal Insulation and High Service Temperature. J. Mater. Chem. C 2022, 1, 5088–5108. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.; Zheng, Y.; Zhai, Y.; Liu, X.; Liu, C.; Mi, L.; Guo, Z.; Shen, C. Highly Compressible and Robust Polyimide/Carbon Nanotube Composite Aerogel for High-Performance Wearable Pressure Sensor. ACS Appl. Mater. Interfaces 2019, 11, 42594–42606. [Google Scholar] [CrossRef]
- Lin, J.; Liu, Y.; Yang, W.; Xie, Z.; Zhang, P.; Li, X.; Lin, H.; Chen, G.; Lei, Q. Structure and Mechanical Properties of the Hybrid Films of Well Dispersed SiO2 Nanoparticle in Polyimide (PI/SiO2) Prepared by Sol–Gel Process. J. Polym. Res. 2014, 21, 531. [Google Scholar] [CrossRef]
- Lei, Y.; Huo, J.; Liao, H. Fabrication and Catalytic Mechanism Study of CeO2-Fe2O3-ZnO Mixed Oxides on Double Surfaces of Polyimide Substrate Using Ion-Exchange Technique. Mater. Sci. Semicond. Process. 2018, 74, 154–164. [Google Scholar] [CrossRef]
- Luo, M.; Liu, Z.; Wang, Q.; Liu, R.; Xu, Y.; Wang, K.; Shi, X.; Ye, S. Surface Engineering on Polyimide–Silver Films in Low-Cost, Flexible Humidity Sensors. ACS Appl. Mater. Interfaces 2022, 14, 16621–16630. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, J.; Li, N.; Ding, L.; Li, D. Conductive Silver Coatings with Ultra-Low Silver Consumption on Polyimide Film: Via a Mild Surface Ion Exchange Self-Metallization Method. J. Mater. Chem. C 2017, 5, 10630–10637. [Google Scholar] [CrossRef]
- Pan, C.; Chen, S.-J.; Huang, Y.-H.; Wang, L.; Luo, J.-L.; Fu, X.-Z. A Facile Method to Fabricate Lightweight Copper Coated Polyimide Film Current Collectors for Lithium-Ion Batteries. J. Power Sources 2022, 528, 231207. [Google Scholar] [CrossRef]
- Chen, L.; Liang, B.; Lv, J.; Chen, M.; Hu, J.; Zeng, K.; Yang, G. Route to a Porous Carbon Nanofiber Membrane Containing Fexcy/Fe by Facile in Situ Ion-Exchange Functionalization of the Paa Carboxyl Group: Exemplified by a Supercapacitor. ACS Appl. Energy Mater. 2022, 5, 1580–1594. [Google Scholar] [CrossRef]
- Tu, H.-Y.; Chang, T.-C.; Tsao, Y.-C.; Tai, M.-C.; Zheng, Y.-Z.; Tu, Y.-F.; Kuo, C.-W.; Wu, C.-C.; Tsai, Y.-L.; Tsai, T.-M.; et al. Abnormal Two-Stage Degradation on P-Type Low-Temperature Polycrystalline-Silicon Thin-Film Transistor under Hot Carrier Conditions. IEEE Electron Device Lett. 2022, 43, 721–724. [Google Scholar] [CrossRef]
- Jin, Y.; Ao, W.; Liu, B.; Lu, Y.; Li, L.; Xi, W.; Jiang, J.; Ma, M.; Hu, F.; Fu, D.; et al. Systematic Investigation on Anode Etching Residue Widely Generated in Manufacturing of Low-Temperature Polycrystalline-Si Active Matrix Organic Light-Emitting Diode. J. Soc. Inf. Disp. 2022, 30, 271–291. [Google Scholar] [CrossRef]
- Ji, D.; Li, T.; Hu, W.; Fuchs, H. Recent Progress in Aromatic Polyimide Dielectrics for Organic Electronic Devices and Circuits. Adv. Mater. 2019, 31, 1806070. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Huang, Z.X.; Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Highly Thermally Conductive Flexible Copper Clad Laminates Based on Sea-Island Structured Boron Nitride/Polyimide Composites. Compos. Sci. Technol. 2021, 230, 109087. [Google Scholar] [CrossRef]
- Ding, D.; Zou, M.; Wang, X.; Qin, G.; Zhang, S.; Chan, S.Y.; Meng, Q.; Liu, Z.; Zhang, Q.; Chen, Y. Thermal Conductivity of Polydisperse Hexagonal Bn/Polyimide Composites: Iterative Emt Model and Machine Learning Based on First Principles Investigation. Chem. Eng. J. 2022, 437, 135438. [Google Scholar] [CrossRef]
- Feng, C.-P.; Wei, F.; Sun, K.-Y.; Wang, Y.; Lan, H.-B.; Shang, H.-J.; Ding, F.-Z.; Bai, L.; Yang, J.; Yang, W. Emerging Flexible Thermally Conductive Films: Mechanism, Fabrication, Application. Nano-Micro Lett. 2022, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-B.; Liu, J.-D.; Du, X.-Y.; Wang, Z.-Y.; Zhang, C.; Ming, S.-F. Fabrication of Silver Nanoparticles/Copper Nanoparticles Jointly Decorated Nitride Flakes to Improve the Thermal Conductivity of Polymer Composites. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128104. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, K.; Ma, S.; Wang, C.; Fan, X.; Jia, T.; Zhang, Z.; Xu, H.; Xing, H.; Wang, D.; et al. A Triple-Layer Structure Flexible Sensor Based on Nano-Sintered Silver for Power Electronics with High Temperature Resistance and High Thermal Conductivity. Chem. Eng. J. 2022, 432, 134431. [Google Scholar] [CrossRef]
- Weng, M.; Luo, X.; Jian, L.; Liang, J.; Hu, J.; Liu, Y.; Zhang, J.; Feng, X.; Min, Y. Lutidine Catalyzed Highly Thermal Conductive Graphite Polyimide Films Via Controlling Grain Size. Appl. Surf. Sci. 2022, 578, 152029. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Z.; Liu, S.; Chi, Z.; Chen, X.; Zhang, Y.; Xu, J. Ultrahigh Thermal and Electric Conductive Graphite Films Prepared by G-C3n4 Catalyzed Graphitization of Polyimide Films. Chem. Eng. J. 2022, 430, 132530. [Google Scholar] [CrossRef]
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-Temperature-Resistant and Colorless Polyimide: Preparations, Properties, and Applications. Sol. Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- An, L.; Yang, Z.; Zeng, X.; Hu, W.; Yu, Y.; Zhang, J.; Wang, Q. Flexible and Quasi-Isotropically Thermoconductive Polyimide Films by Guided Assembly of Boron Nitride Nanoplate/Boron Nitride Flakes for Microelectronic Application. Chem. Eng. J. 2022, 431, 133740. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Liu, F.; Wu, X.; Xu, J.; Cui, H.; Wu, Y.; Xue, R.; Tian, C.; Zheng, B.; et al. Flexible Printed Circuit Board Based on Graphene/Polyimide Composites with Excellent Thermal Conductivity and Sandwich Structure. Compos. Part A Appl. Sci. Manuf. 2020, 138, 106075. [Google Scholar] [CrossRef]
- Wu, X.; Li, H.; Cheng, K.; Qiu, H.; Yang, J. Modified Graphene/Polyimide Composite Films with Strongly Enhanced Thermal Conductivity. Nanoscale 2019, 11, 8219–8225. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Liu, J.; Jia, P.; Qi, N.; Chen, Z.; Xu, S.; Li, N. Synergistic Effect of Thermal Crosslinking and Thermal Rearrangement on Free Volume and Gas Separation Properties of 6fda Based Polyimide Membranes Studied by Positron Annihilation. J. Membr. Sci. 2022, 645, 120163. [Google Scholar] [CrossRef]
- Wang, C.; Cai, Z.; Xie, W.; Jiao, Y.; Liu, L.; Gong, L.; Zhang, Q.-W.; Ma, X.; Zhang, H.; Luo, S. Finely Tuning the Microporosity in Dual Thermally Crosslinked Polyimide Membranes for Plasticization Resistance Gas Separations. J. Membr. Sci. 2022, 659, 120769. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Shi, Y.; Zhu, S.; Zhang, Y.; Jin, J. Synergistic Design of Enhanced Π–Π Interaction and Decarboxylation Cross-Linking of Polyimide Membranes for Natural Gas Separation. Macromolecules 2022, 55, 2970–2982. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Ebadi Amooghin, A.; Bandehali, S.; Moghadassi, A.; Matsuura, T.; Van der Bruggen, B. Polyimides in Membrane Gas Separation: Monomer’s Molecular Design and Structural Engineering. Prog. Polym. Sci. 2019, 91, 80–125. [Google Scholar] [CrossRef]
- Tong, X.; Wang, S.; Dai, J.; Wang, S.; Zhao, X.; Wang, D.; Chen, C. Synthesis and Gas Separation Properties of Aromatic Polyimides Containing Noncoplanar Rigid Sites. ACS Appl. Polym. Mater. 2022, 4, 6265–6275. [Google Scholar] [CrossRef]
- Cornelius, C.J.; Marand, E. Hybrid Silica-Polyimide Composite Membranes: Gas Transport Properties. J. Membr. Sci. 2002, 202, 97–118. [Google Scholar] [CrossRef]
- Kusakabe, K.; Ichiki, K.; Hayashi, J.-i.; Maeda, H.; Morooka, S. Preparation and Characterization of Silica—Polyimide Composite Membranes Coated on Porous Tubes for CO2 Separation. J. Membr. Sci. 1996, 115, 65–75. [Google Scholar] [CrossRef]
- Lua, A.C.; Shen, Y. Preparation and Characterization of Polyimide–Silica Composite Membranes and Their Derived Carbon–Silica Composite Membranes for Gas Separation. Chem. Eng. J. 2013, 220, 441–451. [Google Scholar] [CrossRef]
- Hou, M.; Li, L.; Song, J.; Xu, R.; He, Z.; Lu, Y.; Pan, Z.; Song, C.; Wang, T. Polyimide-Derived Carbon Molecular Sieve Membranes for High-Efficient Hydrogen Purification: The Development of a Novel Phthalide-Containing Polyimide Precursor. Sep. Purif. Technol. 2022, 301, 121982. [Google Scholar] [CrossRef]
- Ning, X.; Koros, W.J. Carbon Molecular Sieve Membranes Derived from Matrimid® Polyimide for Nitrogen/Methane Separation. Carbon 2014, 66, 511–522. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, H.; Zhang, S.; Zhang, F.; Jin, J. Carbon Molecular Sieve Membranes Derived from Tröger’s Base-Based Microporous Polyimide for Gas Separation. ChemSusChem 2018, 11, 916–923. [Google Scholar] [CrossRef]
- Swaidan, R.; Ma, X.; Litwiller, E.; Pinnau, I. High Pressure Pure- and Mixed-Gas Separation of CO2/CH4 by Thermally-Rearranged and Carbon Molecular Sieve Membranes Derived from a Polyimide of Intrinsic Microporosity. J. Membr. Sci. 2013, 447, 387–394. [Google Scholar] [CrossRef]
- Reddy, M.R. Effect of Low Earth Orbit Atomic Oxygen on Spacecraft Materials. J. Mater. Sci. 1995, 30, 281–307. [Google Scholar] [CrossRef]
- Packirisamy, S.; Schwam, D.; Litt, M.H. Atomic Oxygen Resistant Coatings for Low Earth Orbit Space Structures. J. Mater. Sci. 1995, 30, 308–320. [Google Scholar] [CrossRef]
- Wang, R.; Dong, N.; Tian, G.; Liu, G.; Zhou, B.; Qi, S.; Wu, D. Enhanced Atomic-Oxygen Resistance of Surface-Siliconized Polyimide Film Via an in-Situ Precursor Network at the Interface. Appl. Surf. Sci. 2022, 596, 153679. [Google Scholar] [CrossRef]
- Lachance, J.; Coïa, C.; Fozza, A.C.; Czeremuszkin, G.; Houdayer, A.; Wertheimer, M.R. Radiation-Induced Degradation of Polymeric Spacecraft Materials under Protective Oxide Coatings. Nucl. Instrum. Methods Phys. Res. 2001, 185, 328–335. [Google Scholar] [CrossRef]
- Cooper, R.; Upadhyaya, H.P.; Minton, T.K.; Berman, M.R.; Du, X.; George, S.M. Protection of Polymer from Atomic-Oxygen Erosion Using Al2O3 Atomic Layer Deposition Coatings. Thin Solid Films 2008, 516, 4036–4039. [Google Scholar] [CrossRef]
- Gotlib-Vainstein, K.; Gouzman, I.; Girshevitz, O.; Bolker, A.; Atar, N.; Grossman, E.; Sukenik, C.N. Liquid Phase Deposition of a Space-Durable, Antistatic SnO2 Coating on Kapton. ACS Appl. Mater. Interfaces 2015, 7, 3539–3546. [Google Scholar] [CrossRef] [PubMed]
- Gouzman, I.; Gershevitz, O.; Grossman, E.; Eliaz, N.; Sukenik, C.N. Novel Approach to Space-Survivable Polyimides: Liquid Phase Deposition of Titania Coating on Kapton. AIP Conf. Proc. 2009, 1087, 391. [Google Scholar]
- Gouzman, I.; Girshevitz, O.; Grossman, E.; Eliaz, N.; Sukenik, C.N. Thin Film Oxide Barrier Layers: Protection of Kapton from Space Environment by Liquid Phase Deposition of Titanium Oxide. ACS Appl. Mater. Interfaces 2010, 2, 1835–1843. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Li, S.; Zhang, X.; Wang, T.; Wang, Q. Fully Closed-Loop Recyclable Thermosetting Shape Memory Polyimide. ACS Sustain. Chem. Eng. 2020, 8, 18869–18878. [Google Scholar] [CrossRef]
- Xiao, X.; Kong, D.; Qiu, X.; Zhang, W.; Zhang, F.; Liu, L.; Liu, Y.; Zhang, S.; Hu, Y.; Leng, J. Shape-Memory Polymers with Adjustable High Glass Transition Temperatures. Macromolecules 2015, 48, 3582–3589. [Google Scholar] [CrossRef]
- Narayane, D.; Taiwade, R.V.; Sahu, K. Review on Development and Performance of Shape Memory Alloy/Polyimide Thin-Film Composites. Mater. Manuf. Process. 2022, 38, 245–259. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Liu, Y.; Leng, J. Active and Deformable Organic Electronic Devices Based on Conductive Shape Memory Polyimide. ACS Appl. Mater. Interfaces 2020, 12, 23236–23243. [Google Scholar] [CrossRef]
- Yoonessi, M.; Shi, Y.; Scheiman, D.A.; Lebron-Colon, M.; Tigelaar, D.M.; Weiss, R.A.; Meador, M.A. Graphene Polyimide Nanocomposites; Thermal, Mechanical, and High-Temperature Shape Memory Effects. ACS Nano 2012, 6, 7644–7655. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Zhang, Z.; Kong, D.; Ao, X.; Xiao, X. Electroactive High-Temperature Shape Memory Polymers with High Recovery Stress Induced by Ground Carbon Fibers. Macromol. Chem. Phys. 2019, 220, 1900164. [Google Scholar] [CrossRef]
- Kong, D.; Li, J.; Guo, A.; Xiao, X. High Temperature Electromagnetic Shielding Shape Memory Polymer Composite. Chem. Eng. J. 2021, 408, 127365. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, J.H.; Jin, R.; Dong, J.Y. Effect of Content and Layer Thickness on the Corona-Resistance of PI/TiO2 Nanocomposite Films. Appl. Mech. Mater. 2013, 395, 133–137. [Google Scholar]
- Yao, L.; Chen, L.; Chen, Y.; Sun, X. Effect of SiO2 Nanoparticles on the Thermal Properties of Dielectric Composite Films. Int. J. Manuf. Technol. Manag. 2016, 30, 410–421. [Google Scholar] [CrossRef]
- Rui, J.M. Preparation of Novel Corona-Resistance Polyimide/Spherical SiO2 Hybrid Films. Adv. Mat. Res. 2013, 716, 172–176. [Google Scholar]
- Hu, W.; Du, B.; Li, J.; Liu, Y.; Liu, M. Electrical and Mechanical Characteristics of Polyimide Nanocomposite Films for Wind Generator. In Proceedings of the IEEE PES Innovative Smart Grid Technologies, Tianjin, China, 21–24 May 2012. [Google Scholar]
- Wang, X.; Fan, Y.; Chen, H.; Yang, R.; Zhao, W. Electrical, Mechanical, and Thermal Properties of Mg(OH)2/PI Nanocomposite Films. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1778–1786. [Google Scholar] [CrossRef]
- Li, J.-Y.; Jiang, X.; Lin, L.; Zhou, J.-J.; Xu, G.-S.; Yuan, Y.-P. Improving the Photocatalytic Performance of Polyimide by Constructing an Inorganic-Organic Hybrid Zno-Polyimide Core–Shell Structure. J. Mol. Catal. A Chem. 2015, 406, 46–50. [Google Scholar] [CrossRef]
- Sheng, W.; Shi, J.-L.; Hao, H.; Li, X.; Lang, X. Polyimide-TiO2 Hybrid Photocatalysis: Visible Light-Promoted Selective Aerobic Oxidation of Amines. Chem. Eng. J. 2020, 379, 122399. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Seid, M.G.; Lee, W.; Kim, C.U.; Kim, E.-J.; Hong, S.W.; Choi, K.J. Preparation, Characterization, and Application of TiO2-Patterned Polyimide Film as a Photocatalyst for Oxidation of Organic Contaminants. J. Hazard. Mater. 2017, 340, 300–308. [Google Scholar] [CrossRef]
- Chu, S.; Pan, Y.; Wang, Y.; Zhang, H.; Xiao, R.; Zou, Z. Polyimide-Based Photocatalysts: Rational Design for Energy and Environmental Applications. J. Mater. Chem. A 2020, 8, 14441–14462. [Google Scholar] [CrossRef]
- Ma, C.; Zhu, H.; Zhou, J.; Cui, Z.; Liu, T.; Wang, Y.; Wang, Y.; Zou, Z. Confinement Effect of Monolayer MoS2 Quantum Dots on Conjugated Polyimide and Promotion of Solar-Driven Photocatalytic Hydrogen Generation. Dalton Trans. 2017, 46, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhou, J.; Cui, Z.; Wang, Y.; Zou, Z. In Situ Growth MoO3 Nanoflake on Conjugated Polymer: An Advanced Photocatalyst for Hydrogen Evolution from Water Solution under Solar Light. Sol. Energy Mater. Sol. Cells 2016, 150, 102–111. [Google Scholar] [CrossRef]
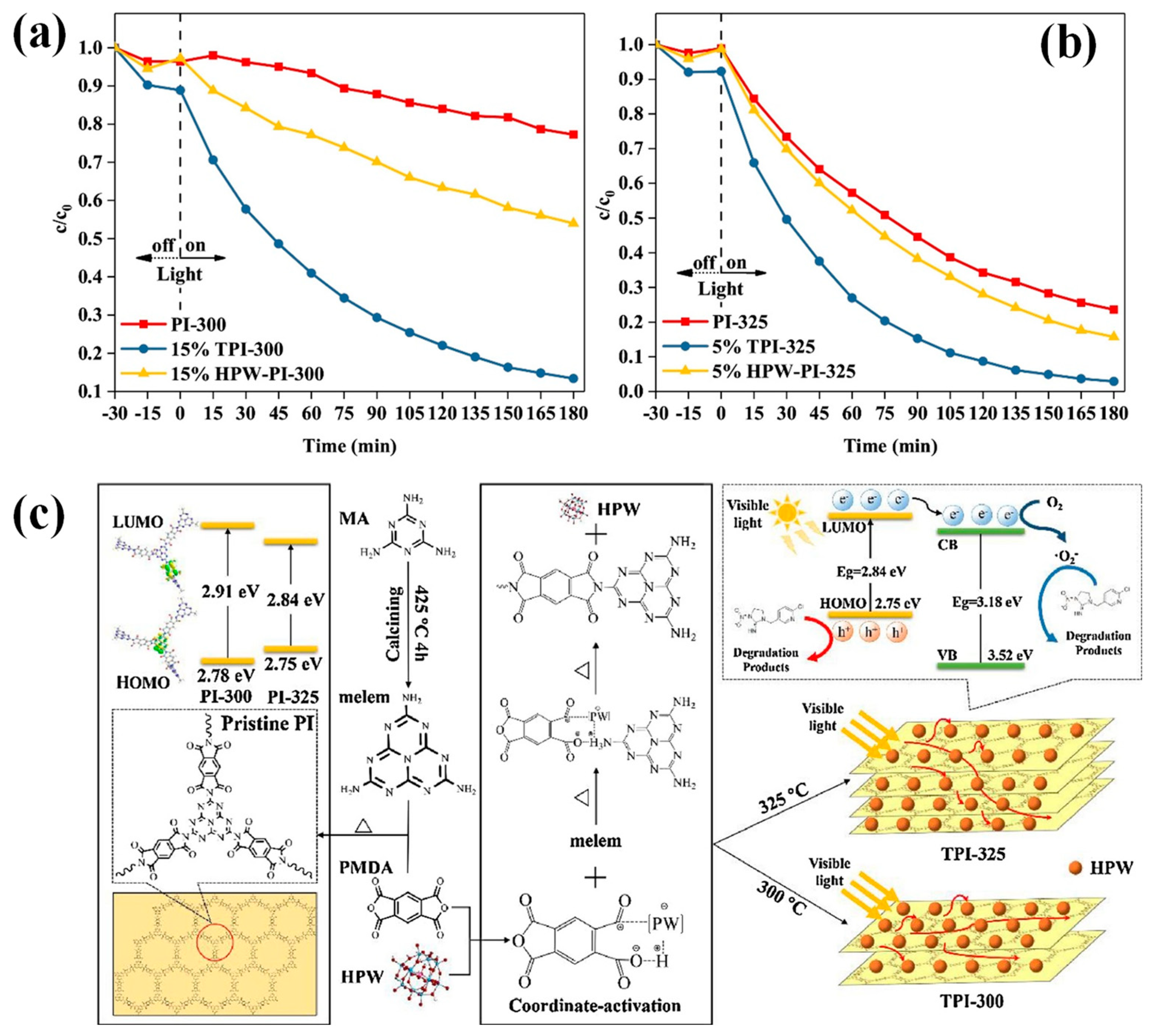
- Zhou, L.; Kamyab, H.; Surendar, A.; Maseleno, A.; Ibatova, A.Z.; Chelliapan, S.; Karachi, N.; Parsaee, Z. Novel Z-Scheme Composite Ag2CrO4/Ng/Polyimide as High Performance Nano Catalyst for Photoreduction of CO2: Design, Fabrication, Characterization and Mechanism. J. Photochem. Photobiol. A 2019, 368, 30–40. [Google Scholar] [CrossRef]
- Hu, Y.; Hao, X.; Cui, Z.; Zhou, J.; Chu, S.; Wang, Y.; Zou, Z. Enhanced Photocarrier Separation in Conjugated Polymer Engineered Cds for Direct Z-Scheme Photocatalytic Hydrogen Evolution. Appl. Catal. B 2020, 260, 118131. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, B.; Zhang, H.; Zhao, X.; Zhu, C. Graphene Oxide Enwrapped Polyimide Composites with Efficient Photocatalytic Activity for 2,4-Dichlorophenol Degradation under Visible Light Irradiation. Mater. Res. Bull. 2019, 112, 115–123. [Google Scholar] [CrossRef]
- Lei, Y.; Huo, J. Enhanced Visible-Light Photoelectrochemical and Photoelectrocatalytic Activity of Nano-TiO2/Polyimide/Ni Foam Photoanode. Res. Chem. Intermed. 2018, 44, 6401–6418. [Google Scholar] [CrossRef]
- Guo, Q.; Li, H.; Zhang, Q.; Zhang, Y. Fabrication, Characterization and Mechanism of a Novel Z-Scheme Ag3PO4/Ng/Polyimide Composite Photocatalyst for Microcystin-Lr Degradation. Appl. Catal. B 2018, 229, 192–203. [Google Scholar] [CrossRef]
- Meng, P.; Heng, H.; Sun, Y.; Huang, J.; Yang, J.; Liu, X. Positive Effects of Phosphotungstic Acid on the in-Situ Solid-State Polymerization and Visible Light Photocatalytic Activity of Polyimide-Based Photocatalyst. Appl. Catal. B 2018, 226, 487–498. [Google Scholar] [CrossRef]
- Ma, C.; Zhou, J.; Zhu, H.; Yang, W.; Liu, J.; Wang, Y.; Zou, Z. Constructing a High-Efficiency MoO3/Polyimide Hybrid Photocatalyst Based on Strong Interfacial Interaction. ACS Appl. Mater. Interfaces 2015, 7, 14636–14637. [Google Scholar] [CrossRef]
- Li, Y.; Fu, M.; Lu, P.; Hu, X.; Wang, R.; Bai, J.; He, Y. Visible Light Photocatalytic Abatement of Tetracycline over Unique Z-Scheme Zns/PI Composites. Appl. Surf. Sci. 2022, 575, 151798. [Google Scholar] [CrossRef]
- Pumera, M.; Sánchez, S.; Ichinose, I.; Tang, J. Electrochemical Nanobiosensors. Sens. Actuator B Chem. 2007, 123, 1195–1205. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical Advanced Oxidation Processes: Today and Tomorrow. A Review. Environ. Sci. Pollut. Res. Int. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, J. A Review of Electrode Materials for Electrochemical Supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Jia, L.; Wang, C. MoO2 Nanoparticles on Reduced Graphene Oxide/Polyimide-Carbon Nanotube Film as Efficient Hydrogen Evolution Electrocatalyst. J. Power Sources 2016, 304, 146–154. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Wang, C. An Advanced Flower-Like Co-Ni/PI-CNT Film Electrocatalyst for Oxygen Evolution Reaction. J. Alloys Compd. 2017, 729, 19–26. [Google Scholar] [CrossRef]
- Shen, X.; Xia, X.; Ye, W.; Du, Y.; Wang, C. Hexagram-Like Cos-MoS2 Composites with Enhanced Activity for Hydrogen Evolution Reaction. J. Solid State Electrochem. 2017, 21, 409–417. [Google Scholar] [CrossRef]
- Kothuru, A.; Hanumanth Rao, C.; Puneeth, S.B.; Salve, M.; Amreen, K.; Goel, S. Laser-Induced Flexible Electronics (Life) for Resistive, Capacitive and Electrochemical Sensing Applications. IEEE Sens. J. 2020, 20, 7392–7399. [Google Scholar] [CrossRef]
- Kim, H.-U.; Kim, H.Y.; Seok, H.; Kanade, V.; Yoo, H.; Park, K.-Y.; Lee, J.-H.; Lee, M.-H.; Kim, T. Flexible MoS2–Polyimide Electrode for Electrochemical Biosensors and Their Applications for the Highly Sensitive Quantification of Endocrine Hormones: Pth, T3, and T4. Anal. Chem. 2020, 92, 6327–6333. [Google Scholar] [CrossRef]
- Huang, T.-C.; Yeh, L.-C.; Huang, H.-Y.; Nian, Z.-Y.; Yeh, Y.-C.; Chou, Y.-C.; Yeh, J.-M.; Tsai, M.-H. The Use of a Carbon Paste Electrode Mixed with Multiwalled Carbon Nanotube/Electroactive Polyimide Composites as an Electrode for Sensing Ascorbic Acid. Polym. Chem. 2014, 5, 630–637. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, S.; Li, J.; Jia, L.; Wang, C. Improvement of Sensitive Ni(OH)2 Nonenzymatic Glucose Sensor Based on Carbon Nanotube/Polyimide Membrane. Carbon 2013, 63, 367–375. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Ye, W.; Wang, C. Ni(OH)2/MoSX Nanocomposite Electrodeposited on a Flexible CNT/PI Membrane as an Electrochemical Glucose Sensor: The Synergistic Effect of Ni(OH)2 and MoSX. J. Solid State Electrochem. 2016, 20, 133–142. [Google Scholar] [CrossRef]
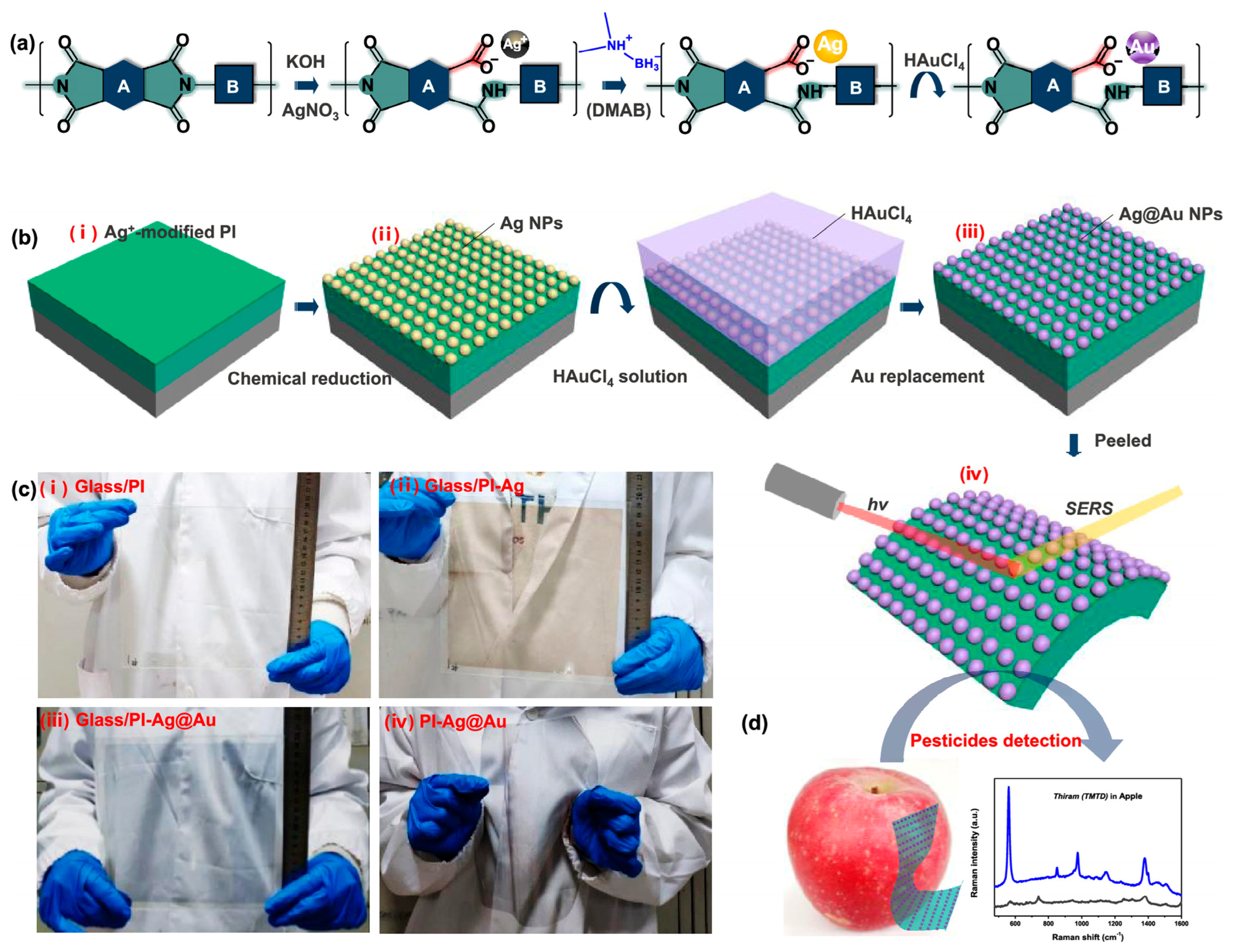
- Liu, X.; Ma, J.; Jiang, P.; Shen, J.; Wang, R.; Wang, Y.; Tu, G. Large-Scale Flexible Surface-Enhanced Raman Scattering (SERS) Sensors with High Stability and Signal Homogeneity. ACS Appl. Mater. Interfaces 2020, 12, 45332–45341. [Google Scholar] [CrossRef] [PubMed]





| Company | Country | Product Name | Transmission (%) | Resin | Tg (°C) |
|---|---|---|---|---|---|
| Mitsubishi Gas Chemical | Japan | Neopulim® | 89–90 | PI | 300–489 |
| DuPont Toray | USA | Colorless Kapton® | 87 | PI | >300 |
| Kolon | South Korea | / | 89 | PI | 330–350 |
| Japan Synthetic Rubber | Japan | Lucera® | 88 | / | 280 |
| Toyobo | Japan | HM type | 91 | Polymamideimide (PAI) | 225 |
| Nippon Steel Chemical | Japan | Sillplus® | 91–92 | Resin + glass | / |
| Toray | Japan | Aramid® | / | Polyamide (PA) | 315 |
| Sumitomo Bakelite | Japan | Sumilite®FS-1300 | 89 | Polyethersulfone (PES) | 223 |
| Showa Electricity | Japan | Shorayal® | 92 | / | 250 |
| Tosoh | Japan | OPS film | 93 | Polysulfone (PS) | 220 |
| Kurabo | Japan | Examid® | / | Polyamide (PA) | 220 |
| Monomers | Chemical Structure | References |
|---|---|---|
| 1,4-Diaminocyclohexane |  | [62] |
| 2,2′-Bis(trifluoromethyl)-4,4′-diaminobiphenyl (TFMB) |  | [63] |
| 1,2,4-Cyclohexane tricarboxylic dianhydride (HTA) |  | [64] |
| 2,3,5-Tricarboxycyclopentylacetic dianhydride (TCA-AH) | 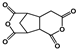 | [65] |
| 1,2,3,4-Cyclobutane tetracarboxylic dianhydride (CBDA) | 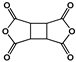 | [66] |
| 3,3′,4,4′-Bicyclohexyl tetracarboxylic dianhydride (HBPDA) | 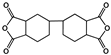 | [67] |
| 1R,2S,4S,5R-cyclobutane tetracarboxylic dianhydride (CBDA) | 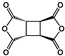 | [68] |
| 1S,2R,4S,5R-cyclohexane tetracarboxylic dianhydride (HPMDA) | 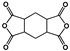 | [69] |
| 2,2′-Bis(3,4-dicarboxyphenyl)hexafluoro-propane dianhydride (6FDA) | 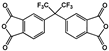 | [70] |
| 3,4-Dicarboxy-1,2,3,4-tetrahydro-1-naphthalene succinic dianhydride(TDA) | 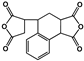 | [71] |
| 3,4-Dicarboxy-1,2,3,4-tetrahydro-6-fluoro-1-naphthalene succinicdianhydride (FTDA) | 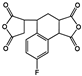 | [72] |
| 3,4-Dicarboxy-1,2,3,4-tetrahydro-6-chloro-methyl-1-naphthalenesuccinic dianhydride (CMTDA) | 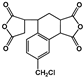 | [73] |
| (5,11-difluoro-5,11-bis (trifluoromethyl)-5,11-dihydro-1 H, 3H-anthraceno [2,3- c: 6, 7-c’] difuran 1,3,7,9-tetraone) (8FDA) |  | Our work [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Liu, X.; Wang, R.; Lu, C.; Wen, X.; Tu, G. Research Progress and Application of Polyimide-Based Nanocomposites. Nanomaterials 2023, 13, 656. https://doi.org/10.3390/nano13040656
Ma J, Liu X, Wang R, Lu C, Wen X, Tu G. Research Progress and Application of Polyimide-Based Nanocomposites. Nanomaterials. 2023; 13(4):656. https://doi.org/10.3390/nano13040656
Chicago/Turabian StyleMa, Jinming, Xiangfu Liu, Rongwen Wang, Chengxu Lu, Xueqin Wen, and Guoli Tu. 2023. "Research Progress and Application of Polyimide-Based Nanocomposites" Nanomaterials 13, no. 4: 656. https://doi.org/10.3390/nano13040656
APA StyleMa, J., Liu, X., Wang, R., Lu, C., Wen, X., & Tu, G. (2023). Research Progress and Application of Polyimide-Based Nanocomposites. Nanomaterials, 13(4), 656. https://doi.org/10.3390/nano13040656