Diatomite-like KFeS2 for Use in High-Performance Electrodes for Energy Storage and Oxygen Evolution
Abstract
:1. Introduction
2. Materials and Methods
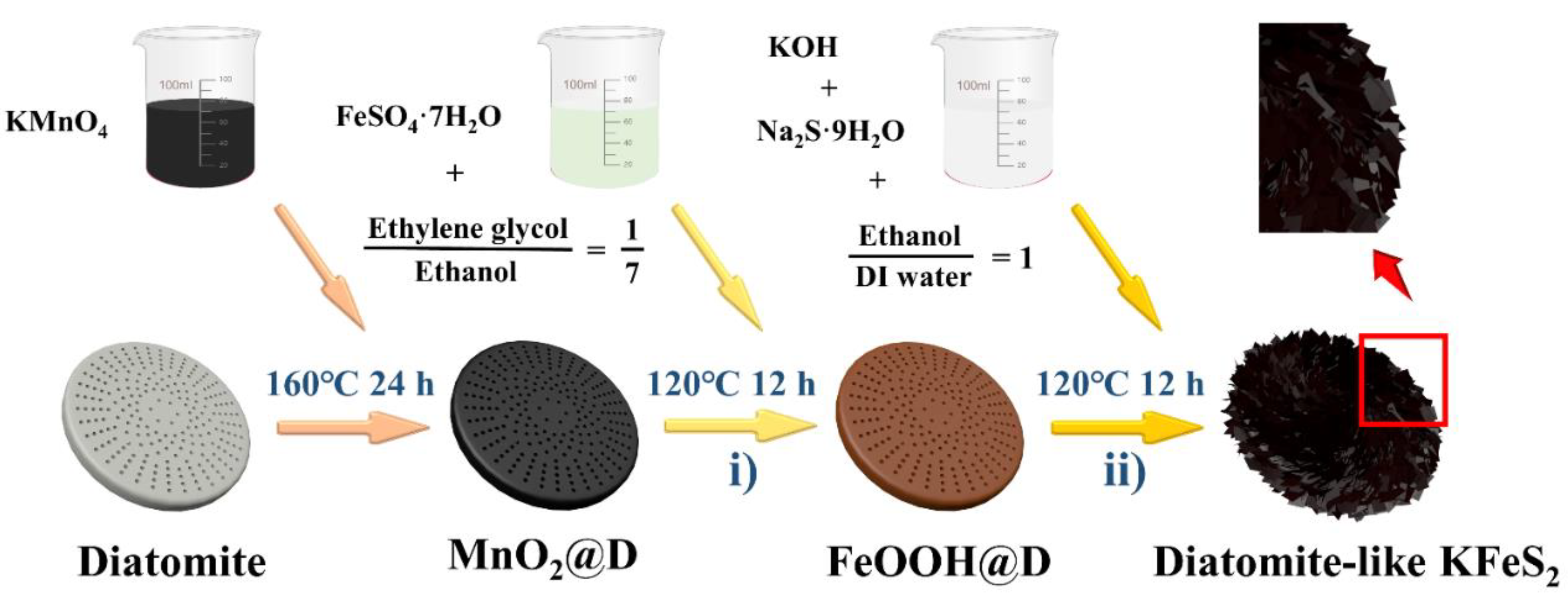
2.1. Synthesis of FeOOH@D Nanorods on Diatomite (D)
2.2. Synthesis of Diatomite (D)-like KFeS2
2.3. Synthesis of FeS2-Modified Diatomite
2.4. Synthesis of KFeS2
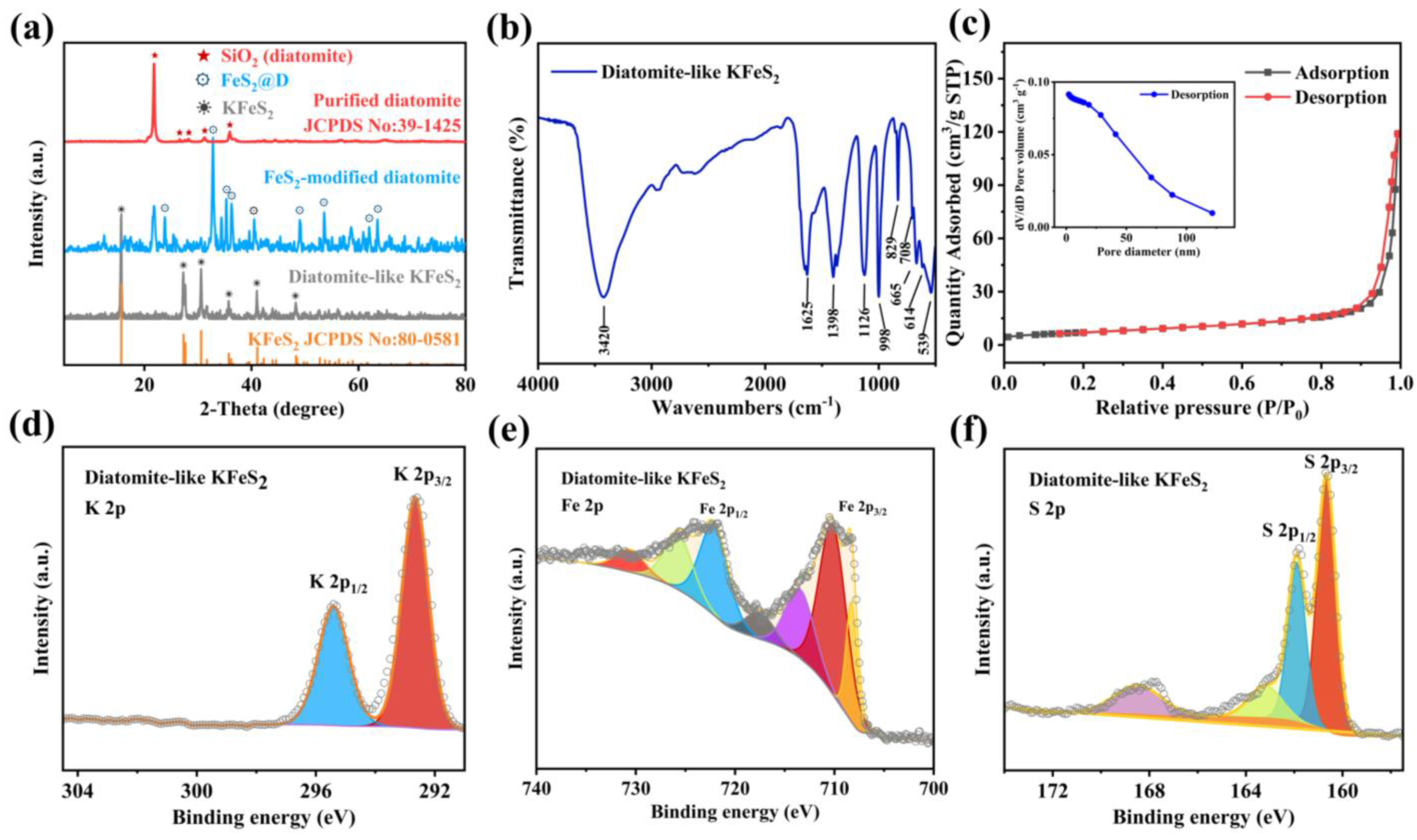
2.5. Characterization of Materials
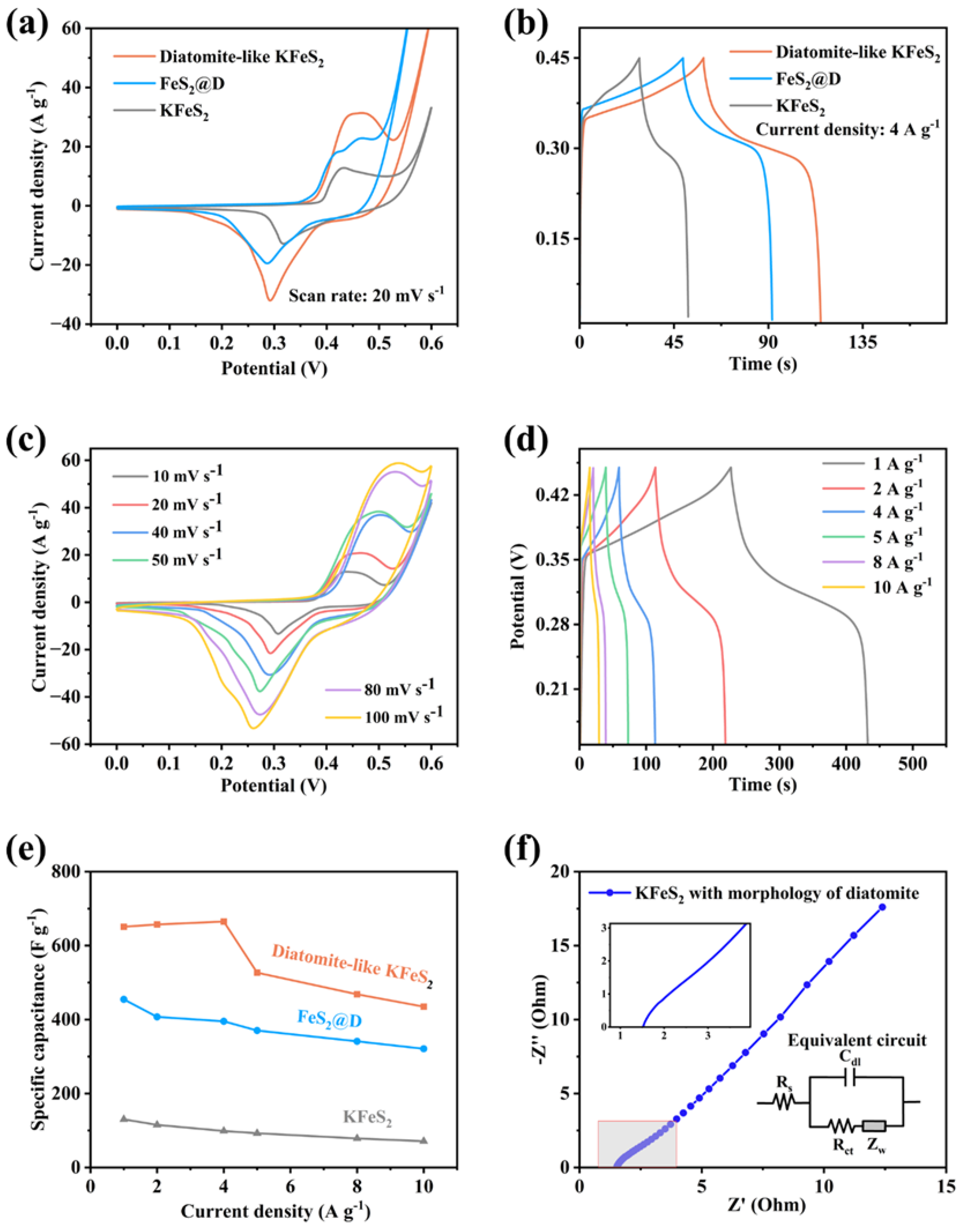
2.6. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Lee, J.; Liu, C.; Pandey, S.; Joo, S.W.; Son, N.; Kang, M. Achieving a long-term stability by self-redox property between Fe and Mn ions in the iron-manganese spinel structured electrode in oxygen evolution reaction. Appl. Surf. Sci. 2021, 546, 149124. [Google Scholar] [CrossRef]
- Liu, J.H.; He, X.; Wang, Y.Y.; Sun, Z.J.; Liu, Y.S.; Liu, B.S.; Li, H.D.; Guo, F.; Li, X. Deep reconstruction of highly disordered iron/nickel nitrate hydroxide nanoplates for high-performance oxygen evolution reaction in alkaline media. J. Alloy. Compd. 2022, 927, 167060. [Google Scholar] [CrossRef]
- Zhang, G.G.; Yuan, J.Y.; Liu, Y.; Lu, W.; Fu, N.Q.; Li, W.F.; Huang, H.T. Boosting the oxygen evolution reaction in non-precious catalysts by structural and electronic engineering. J. Mater. Chem. A 2018, 6, 10253–10263. [Google Scholar] [CrossRef]
- Wang, C.H.; Li, C.M.; Liu, J.L.; Guo, C.X. Engineering transition metal-based nanomaterials for high-performance electrocatalysis. Mater. Rep. Energy 2021, 1, 100006. [Google Scholar] [CrossRef]
- Chen, W.; Rakhi, R.B.; Hu, L.B.; Xie, X.; Cui, Y.; Alshareef, H.N. High-Performance Nanostructured Supercapacitors on a Sponge. Nano Lett. 2011, 11, 5165–5172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Huang, M.; Li, F.; Wang, X.L.; Wen, Z.Q. One-pot synthesis of hierarchical MnO2-modified diatomites for electrochemical capacitor electrodes. J. Power Sources 2014, 246, 449–456. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yu, M.; Meng, Y.; Fang, P.; Lu, X.; Tong, Y. Iron-Based Supercapacitor Electrodes: Advances and Challenges. Adv. Energy Mater. 2016, 6, 1601053. [Google Scholar] [CrossRef]
- Owusu, K.A.; Qu, L.; Li, J.; Wang, Z.; Zhao, K.; Yang, C.; Hercule, K.M.; Lin, C.; Shi, C.; Wei, Q.; et al. Low-crystalline iron oxide hydroxide nanoparticle anode for high-performance supercapacitors. Nat. Commun. 2017, 8, 14264. [Google Scholar] [CrossRef]
- Bu, R.R.; Deng, Y.; Wang, Y.L.; Zhao, Y.; Shi, Q.Q.; Zhang, Q.; Xiao, Z.Y.; Li, Y.Y.; Sun, W.; Wang, L. Bucket Effect: A Metal-Organic Framework Derived High-Performance FeS2/Fe2O3@S-rGO Negative Material for Enhanced Overall Supercapacitor Capacitance. ACS Appl. Energ. Mater. 2021, 4, 11004–11013. [Google Scholar] [CrossRef]
- Chen, X.L.; Shi, T.; Zhong, K.L.; Wu, G.L.; Lu, Y. Capacitive behavior of MoS2 decorated with FeS2@carbon nanospheres. Chem. Eng. J. 2020, 379, 122240. [Google Scholar] [CrossRef]
- Durga, I.K.; Rao, S.S.; Kalla, R.M.N.; Ahn, J.W.; Kim, H.J. Facile synthesis of FeS2/PVP composite as high-performance electrodes for supercapacitors. J. Energy Storage 2020, 28, 101216. [Google Scholar] [CrossRef]
- Pham, D.T.; Baboo, J.P.; Song, J.; Kim, S.; Mathew, V.; Alfaruqi, M.H.; Sambandam, B.; Kim, J. Facile synthesis of pyrite (FeS2/C) nanoparticles as an electrode material for non-aqueous hybrid electrochemical capacitors. Nanoscale 2018, 10, 5938–5949. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.Q.; Du, H.T.; Kong, Y.; Qu, F.L.; Lu, L.M. A novel FeS-NiS hybrid nanoarray: An efficient and durable electrocatalyst for alkaline water oxidation. Chem. Commun. 2019, 55, 7335–7338. [Google Scholar] [CrossRef]
- Khan, N.A.; Rashid, N.; Ahmad, I.; Zahidullah; Zairov, R.; Rehman, H.U.; Nazar, M.F.; Jabeen, U. An efficient Fe2O3/FeS heterostructures water oxidation catalyst. Int. J. Hydrog. Energy 2022, 47, 22340–22347. [Google Scholar] [CrossRef]
- Guo, W.H.; Zhang, Q.; Fu, H.C.; Yang, Y.X.; Chen, X.H.; Luo, H.Q.; Li, N.B. Semi-sacrificial template growth of FeS/Fe2O3 heterogeneous nanosheets on iron foam for efficient oxygen evolution reaction. J. Alloy. Compd. 2022, 909, 164670. [Google Scholar] [CrossRef]
- Xue, Y.R.; Liu, M.Y.; Qin, Y.Y.X.; Zhang, Y.F.; Zhang, X.J.; Fang, J.J.; Zhang, X.; Zhu, W.; Zhuang, Z.B. Ultrathin NiFeS nanosheets as highly active electrocatalysts for oxygen evolution reaction. Chin. Chem. Lett. 2022, 33, 3916–3920. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, X.; Sun, Y.; Ma, T. Surface-defective FeS2 for electrochemical NH3 production under ambient conditions. Nano Mater. Sci. 2020, 2, 132–139. [Google Scholar] [CrossRef]
- Huang, F.; Wu, R.; Li, J.; Sun, Y.F.; Jian, J.K. Synthesis of NaFeS2 Nanorods by Salvothermal Technique, International Forum on Mechanical and Material Engineering (IFMME 2013); Trans Tech Publications Ltd.: Guangzhou, China, 2013; pp. 603–608. [Google Scholar]
- Huang, Y.; Zhao, H.; Bao, S.; Yin, Y.; Zhang, Y.; Lu, J. Hollow FeS2 nanospheres encapsulated in N/S co-doped carbon nanofibers as electrode material for electrochemical energy storage. J. Alloy. Compd. 2022, 905, 164184. [Google Scholar] [CrossRef]
- Li, L.X.; Gao, T.Y.; Ge, Y.S.; Zhang, Q.; Wang, J.Y.; Ma, Z.P.; Guo, W.F.; Yu, S.X.; Fan, Y.Q. Ultra-long KFeS2 nanowires grown on Fe foam as a high-performance anode for aqueous solid-state energy storage. J. Mater. Chem. A 2021, 9, 27727–27735. [Google Scholar] [CrossRef]
- Liang, D.X.; Ji, M.H.; Zhu, S.Y.; Chen, Y.; Wang, Z.H.; Liu, Y.W.; Khan, A.H.R.; Ri, Y.H.; Yu, H.B.; Huo, M.X. A novel Fe recycling method from pickling wastewater producing a KFeS2 whisker for electroplating wastewater treatment. Environ. Sci. Wat. Res. Technol. 2021, 7, 1480–1491. [Google Scholar] [CrossRef]
- Tufa, L.T.; Gicha, B.B.; Wu, H.; Lee, J. Fe-Based Mesoporous Nanostructures for Electrochemical Conversion and Storage of Energy. Batter. Supercaps 2021, 4, 429–444. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.W.; Wang, Z.X.; Wang, Z.; Zhang, Q.C.; He, B.; Zhang, T.; Gong, W.B.; Chen, M.X.; Qi, M.; et al. High-Capacity Iron-Based Anodes for Aqueous Secondary Nickel-Iron Batteries: Recent Progress and Prospects. Chem. Electro. Chem. 2021, 8, 274–290. [Google Scholar]
- Fang, Y.J.; Chen, Z.X.; Xiao, L.F.; Ai, X.P.; Cao, Y.L.; Yang, H.X. Recent Progress in Iron-Based Electrode Materials for Grid-Scale Sodium-Ion Batteries. Small 2018, 14, 1703116. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Pang, H.; Xue, H.G. Fe-based phosphate nanostructures for supercapacitors. Chin. Chem. Lett. 2021, 32, 885–889. [Google Scholar] [CrossRef]
- Wu, X.Y.; Markir, A.; Xu, Y.K.; Zhang, C.; Leonard, D.P.; Shin, W.; Ji, X.L. A Rechargeable Battery with an Iron Metal Anode. Adv. Funct. Mater. 2019, 29, 1900911. [Google Scholar] [CrossRef]
- Jayathilake, B.S.; Plichta, E.J.; Hendrickson, M.A.; Narayanan, S.R. Improvements to the Coulombic Efficiency of the Iron Electrode for an All-Iron Redox-Flow Battery. J. Electrochem. Soc. 2018, 165, A1630–A1638. [Google Scholar] [CrossRef]
- Liu, W.W.; Zhu, M.H.; Liu, J.H.; Li, X.; Liu, J. Flexible asymmetric supercapacitor with high energy density based on optimized MnO2 cathode and Fe2O3 anode. Chin. Chem. Lett. 2019, 30, 750–756. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Sun, Y.; Tan, Y.-Z.; Yang, S.; Feng, X.; Muellen, K. Three-Dimensional Graphene-Based Macro- and Mesoporous Frameworks for High-Performance Electrochemical Capacitive Energy Storage. J. Am. Chem. Soc. 2012, 134, 19532–19535. [Google Scholar] [CrossRef]
- Yu, Y.; Addai-Mensah, J.; Losic, D. Synthesis of Self-Supporting Gold Microstructures with Three-Dimensional Morphologies by Direct Replication of Diatom Templates. Langmuir 2010, 26, 14068–14072. [Google Scholar] [CrossRef]
- Dai, X.; Rao, J.; Bao, Z.; Li, K.; Feng, L.; Song, D.; Zhao, L.; Li, W.; Liu, X.; Yi, S.; et al. Magnetic double-core@shell MnO2@NiFe@DE as a multifunctional scavenger for efficient removal of tetracycline, anionic and cationic dyes. J. Colloid Interface Sci. 2022, 628, 769–783. [Google Scholar] [CrossRef]
- Li, K.; Teng, H.; Sun, Q.; Li, Y.; Wu, X.; Dai, X.; Wang, Y.; Wang, S.; Zhang, Y.; Yao, K.; et al. Engineering active sites on nitrogen-doped carbon nanotubes/cobaltosic oxide heterostructure embedded in biotemplate for high-performance supercapacitors. J. Energy Storage 2022, 53, 105094. [Google Scholar] [CrossRef]
- Philias, J.M.; Marsan, B. FTIR spectroscopic study and thermal and electrical properties of polymer electrolytes containing a cesium thiolate/disulfide redox couple. Electrochim. Acta 1999, 44, 2351–2363. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Gadzala-Kopciuch, R.; Nowak, K.; Buszewski, B. Removal of zearalenone toxin from synthetics gastric and body fluids using talc and diatomite: A batch kinetic study. Colloids Surf. B 2021, 94, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.F.; Sun, M.L.; Li, L. Highly Efficient Sodium Storage in Iron Oxide Nanotube Arrays Enabled by Built-In Electric Field. Adv. Mater. 2019, 31, 1902603. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.X.; Wang, L.S.; Yue, G.H.; Wang, X.; Yan, P.X.; Peng, D.L. Single crystal of CuFeS2 nanowires synthesized through solventothermal process. Mater. Chem. Phys. 2009, 115, 147–150. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.C.; Wang, Z.X.; Wang, Z.; Kang, L.X.; Qi, M.; Chen, M.X.; Liu, W.; Gong, W.B.; Lu, W.B.; et al. Rational Construction of Self-Standing Sulfur-Doped Fe2O3 Anodes with Promoted Energy Storage Capability for Wearable Aqueous Rechargeable NiCo-Fe Batteries. Adv. Energy Mater. 2020, 10, 2001064. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Wu, Y.; Zhao, S.; Wang, Z.; Wang, S. Millisecond photo-thermal process on significant improvement of supercapacitor’s performance. Appl. Therm. Eng. 2016, 109, 186–195. [Google Scholar] [CrossRef]
- Liu, X.X.; Deng, W.Z.; Liu, L.M.; Wang, Y.F.; Huang, C.F.; Wang, Z.J. Passion fruit-like microspheres of FeS2 wrapped with carbon as an excellent fast charging material for supercapacitors. New J. Chem. 2022, 46, 11212–11219. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Q.Q.; Ni, Y.L.; Yang, Y.; Zhu, X.F.; Song, Y. N-Doped FeS2 Achieved by Thermal Annealing of Anodized Fe in Ammonia and Sulfur Atmosphere: Applications for Supercapacitors. J. Electrochem. Soc. 2021, 168, 080522. [Google Scholar] [CrossRef]
- Cai, J.; Lai, C.L.; Sun, P.X.; Cui, B.L.; Yang, C. 3 electrons donator-FeS/C composite anode for supercapacitor. J. Mater. Sci. Mater. Electron. 2022, 33, 26966–26979. [Google Scholar] [CrossRef]
- Shao, X.X.; Zhu, Z.Q.; Zhao, C.J.; Zhao, C.H.; Qian, X.Z. Hierarchical FeS/RGO/FeS@Fe foil as high-performance negative electrode for asymmetric supercapacitors. Inorg. Chem. Front. 2018, 5, 1912–1922. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Nguyen, T.; Silva, T.M.; Carmezim, M.J.; Montemor, M.F. Pseudocapacitive behaviour of FeSx grown on stainless steel up to 1.8 V in aqueous electrolyte. J. Energy Storage 2019, 26, 100949. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, R. Advanced Transition Metal-Based OER Electrocatalysts: Current Status, Opportunities, and Challenges. Small 2021, 17, 2100129. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wei, C.; Huang, Z.-F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef]
- Chen, Q.W.; Zhang, X.Y.; Dong, Y.W.; Guo, B.Y.; Ma, Y.; Wang, L.; Lv, R.Q.; Zhou, Y.L.; Chai, Y.M.; Dong, B. Ultrafast surface modification of FeS nanosheet arrays with Fe-Ni bimetallic hydroxides for efficient oxygen evolution. J. Alloy. Compd. 2020, 835, 155298. [Google Scholar] [CrossRef]
- Liu, H.J.; Yu, N.; Yuan, X.Q.; Zhao, H.Y.; Zhang, X.Y.; Chai, Y.M.; Dong, B. 0D-2D Schottky heterostructure coupling of FeS nanosheets and Co9S8 nanoparticles for long-term industrial-level water oxidation. Nano Res. 2022. [Google Scholar] [CrossRef]
- Shankar, A.; Elakkiya, R.; Maduraiveeran, G. Self-supported fabrication and electrochemical water splitting study of transition-metal sulphide nanostructured electrodes. New J. Chem. 2020, 44, 5071–5078. [Google Scholar] [CrossRef]
- Wang, W.B.; Xu, R.D.; Yu, B.H.; Wang, X.B.; Feng, S.Y. Electrochemical fabrication of FeSx films with high catalytic activity for oxygen evolution. Rsc Adv. 2019, 9, 31979–31987. [Google Scholar] [CrossRef]
- Xie, Y.Y.; Yu, H.Z.; Deng, L.M.; Amin, R.S.; Yu, D.S.; Fetohi, A.E.; Maximov, M.Y.; Li, L.L.; El-Khatib, K.M.; Peng, S.J. Anchoring stable FeS2 nanoparticles on MXene nanosheets via interface engineering for efficient water splitting. Inorg. Chem. Front. 2022, 9, 662–669. [Google Scholar] [CrossRef]


| No. | Materials | Morphology | Electrolyte | Specific Capacitance | Reference |
|---|---|---|---|---|---|
| 1 | Diatomite-like KFeS2 | Diatomite-like | 6 M KOH | 651 F g−1 at 1 A g−1 | This work |
| 2 | FeS2 CNFs | Nanosphere | 6 M KOH | 203.4 F g−1 at 1 A g−1 | [20] |
| 3 | Fruit-like FeS2@Carbon microspheres | Microsphere | 1 M KOH | 278.4 F g−1 at 1 A g−1 | [40] |
| 4 | N-doped FeS2 nanosphere | Nanosphere | 0.5 M NaOH | 238.2 mF g−1 at 3 mA g−1 | [41] |
| 5 | 50% FeS2/3DPC (FeS2 content at 50% weight percent) | Nanoparticles | 1 M KOH | 304 F g−1 at 2 A g−1 | [30] |
| 6 | Donator-FeS/C | Nanoparticles | 2 M KOH | 275.65 F g−1 at 30 mA cm−2 | [42] |
| 7 | FeS2/PVP/NF | Nanoparticles | 3 M KOH | 526.08 F g−1 at 1 A g−1 | [12] |
| 8 | FeSx grown on stainless steel | Cuboidal-like | 1 M Na2SO4 | 730 mF g−1 at 1 mA g−1 | [43] |
| 9 | Hierarchical FeS/RGO/FeS@Fe foil | Nanosheets | 2 M KOH | 206.25 F g−1 at 20 mA cm−2 | [44] |
| No. | Materials | Morphology | Tafel Slope (mV dec−1) | Overpotential (mV) | Reference |
|---|---|---|---|---|---|
| 1 | Diatomite-like KFeS2 | Diatomite-like | 48.4 | 254@10 mA cm−2 | This work |
| 2 | FeNi(OH)x/FeS/IF | Nanosheets | 53 | 273@10 mA cm−2 | [47] |
| 3 | FeS/Fe2O3 heterogeneous nanosheets | Nanosheets | 51.71 | 266.5@10 mA cm−2 | [16] |
| 4 | Fe2O3/FeS | Nanorods | 90 | 370@40 mA cm−2 | [15] |
| 5 | Hybrid nanoarray | Nanoarray | 80 | 260@10 mA cm−2 | [14] |
| 6 | FeS-Co9S8/IF | Heterostructure | 50.3 | 332@500 mA cm−2 | [48] |
| 7 | FeS | - | 69 | 320@10 mA cm−2 | [49] |
| 8 | FeSx/CF | Heterostructure | 105 | 340@10 mA cm−2 | [50] |
| 9 | FeS2@MXene | Nanoparticles | 58.6 | 240@10 mA cm−2 | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Li, K.; Sun, Q.; Zhu, S.; Zhang, C.; Zhang, Y.; Shi, Z.; Hu, Y.; Zhang, Y. Diatomite-like KFeS2 for Use in High-Performance Electrodes for Energy Storage and Oxygen Evolution. Nanomaterials 2023, 13, 643. https://doi.org/10.3390/nano13040643
Wang C, Li K, Sun Q, Zhu S, Zhang C, Zhang Y, Shi Z, Hu Y, Zhang Y. Diatomite-like KFeS2 for Use in High-Performance Electrodes for Energy Storage and Oxygen Evolution. Nanomaterials. 2023; 13(4):643. https://doi.org/10.3390/nano13040643
Chicago/Turabian StyleWang, Can, Kailin Li, Qing Sun, Shijin Zhu, Chenzhi Zhang, Yunhao Zhang, Zhongyi Shi, Youzhong Hu, and Yuxin Zhang. 2023. "Diatomite-like KFeS2 for Use in High-Performance Electrodes for Energy Storage and Oxygen Evolution" Nanomaterials 13, no. 4: 643. https://doi.org/10.3390/nano13040643
APA StyleWang, C., Li, K., Sun, Q., Zhu, S., Zhang, C., Zhang, Y., Shi, Z., Hu, Y., & Zhang, Y. (2023). Diatomite-like KFeS2 for Use in High-Performance Electrodes for Energy Storage and Oxygen Evolution. Nanomaterials, 13(4), 643. https://doi.org/10.3390/nano13040643







