Enhancing the Electrochemical Performance of High Voltage LiNi0.5Mn1.5O4 Cathode Materials by Surface Modification with Li1.3Al0.3Ti1.7(PO4)3/C
Abstract
1. Introduction
2. Experimental Section
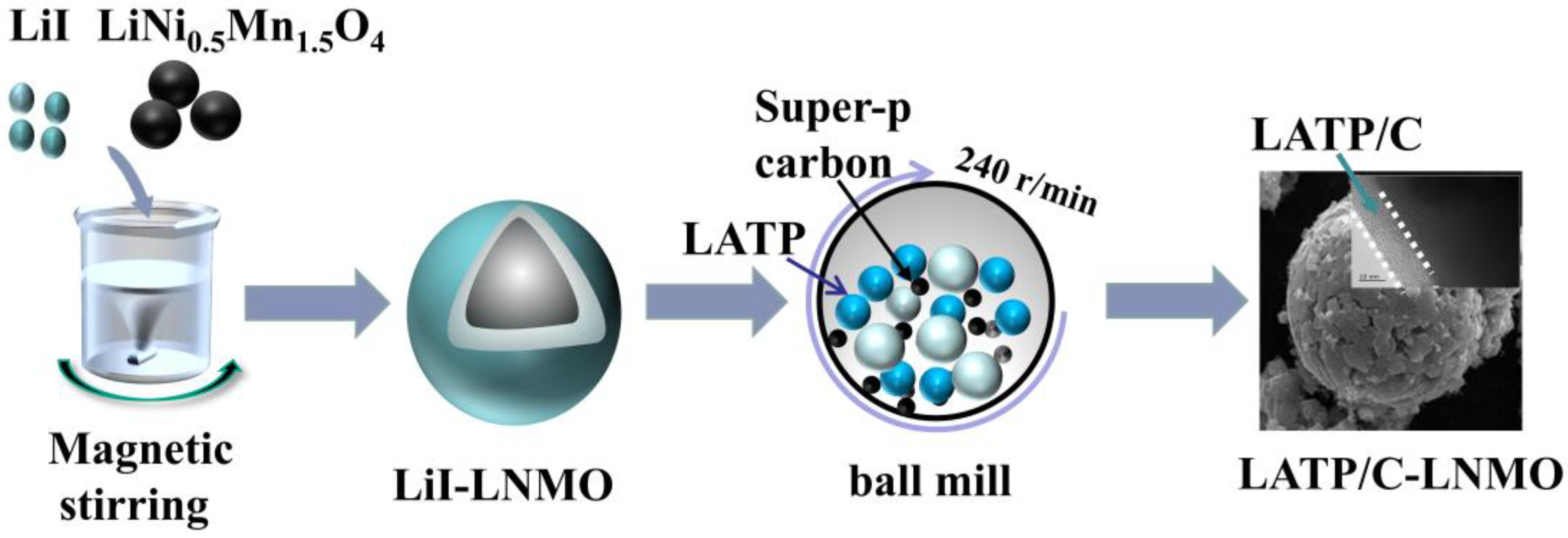
2.1. Preparation of LATP and LATP/C-LNMO Composite Materials
2.2. Materials Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
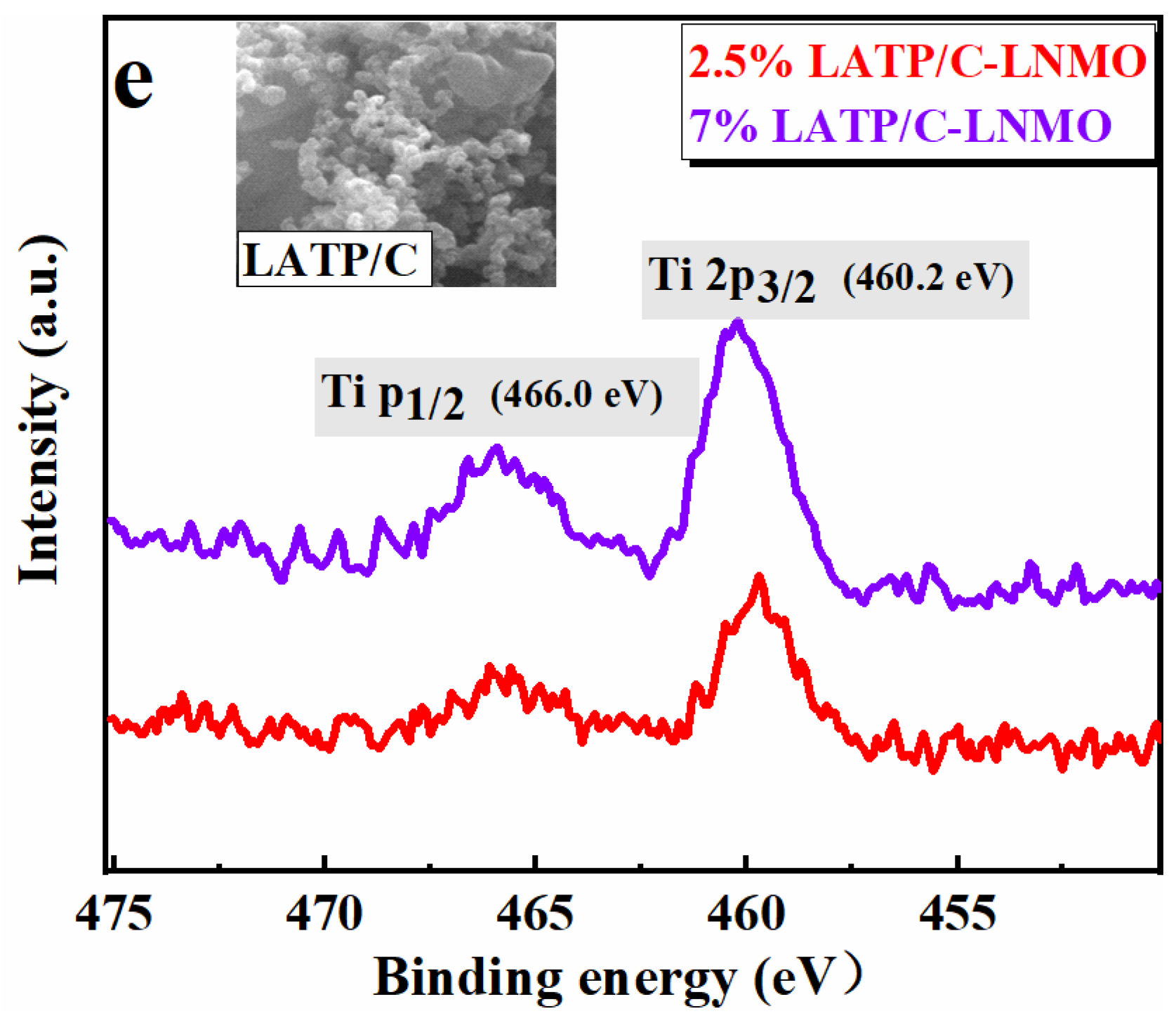
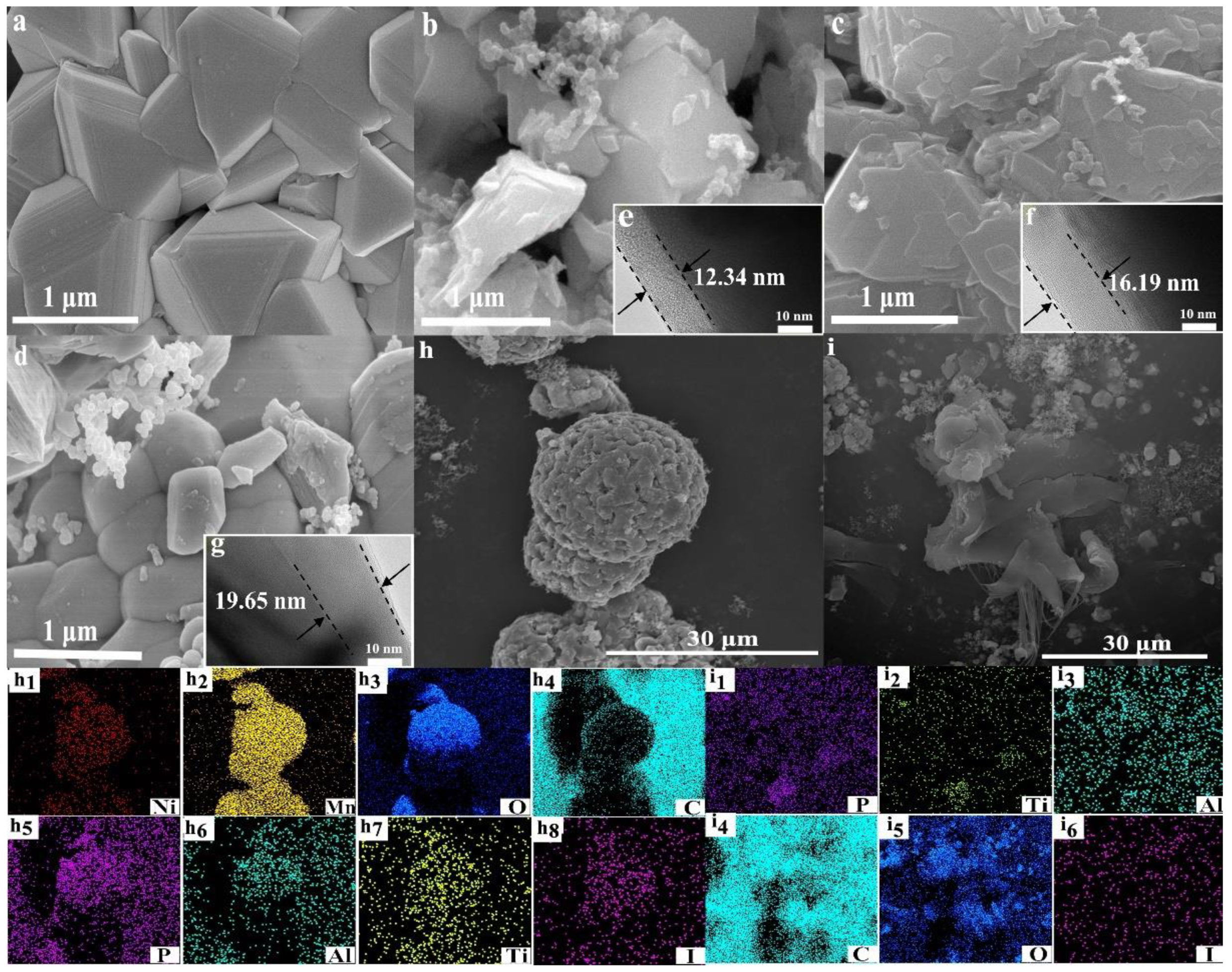
3.1. Crystal Structure and Surface Morphology of the Composite Cathodes
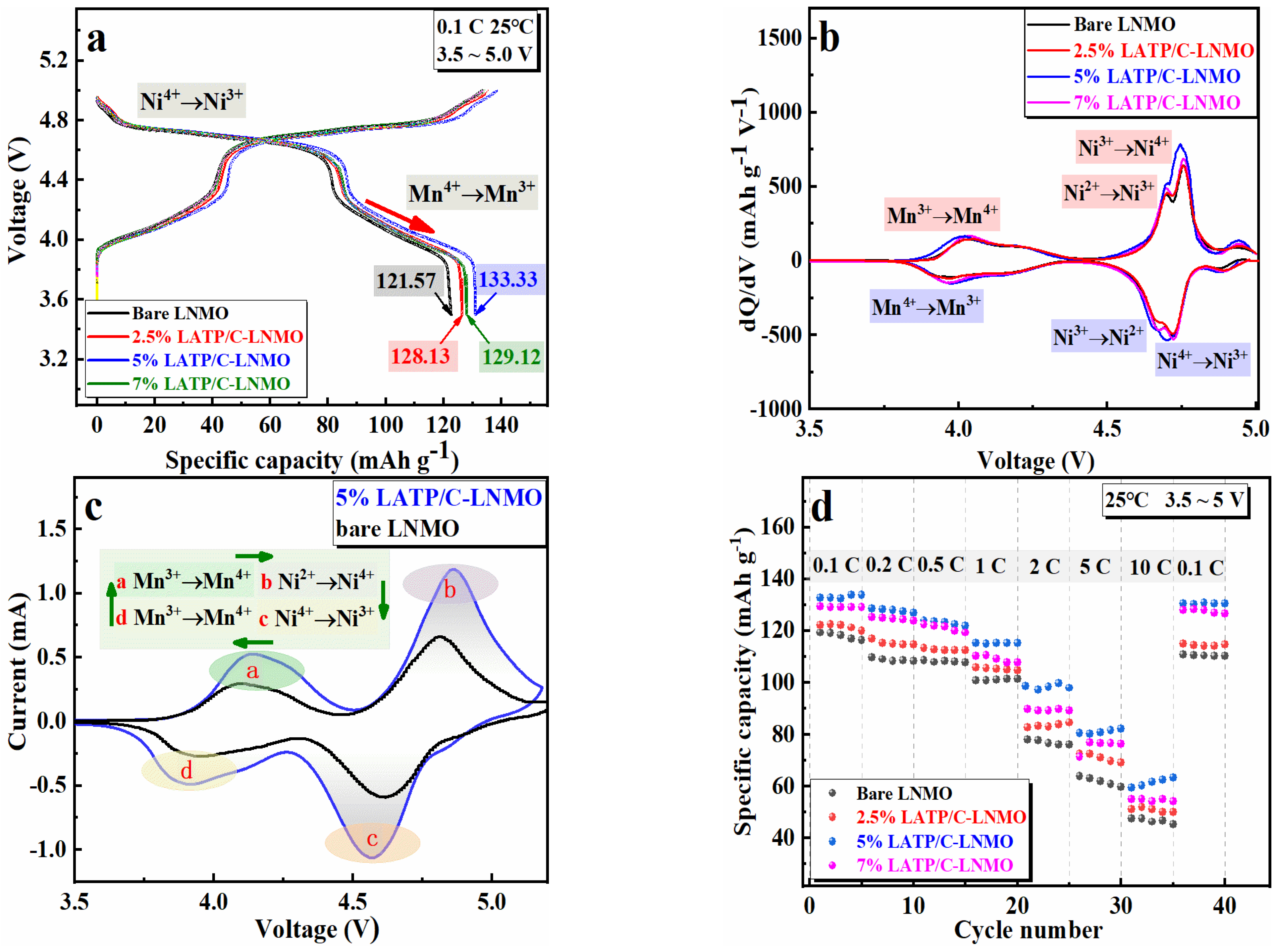
3.2. Electrochemical Properties
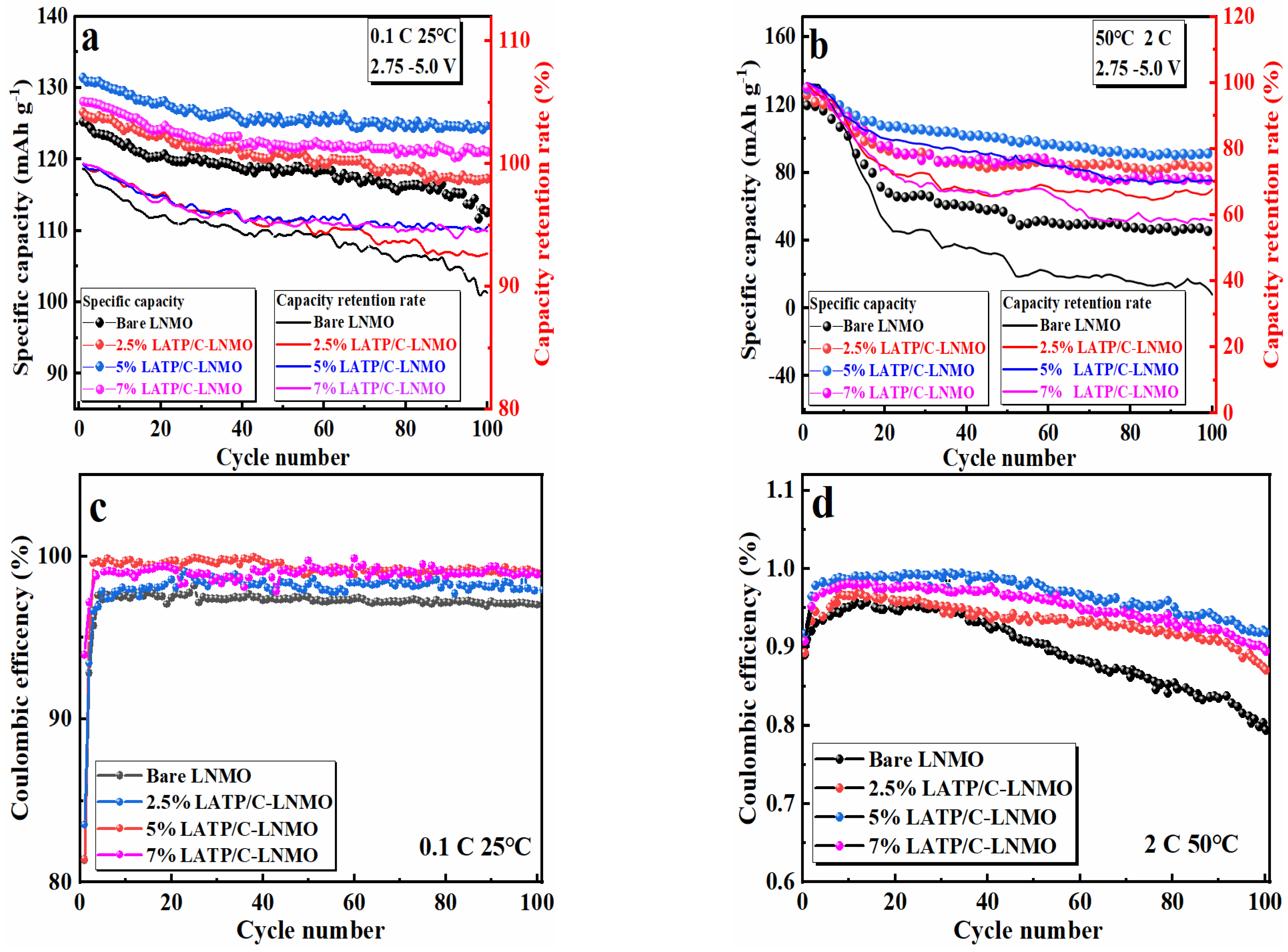
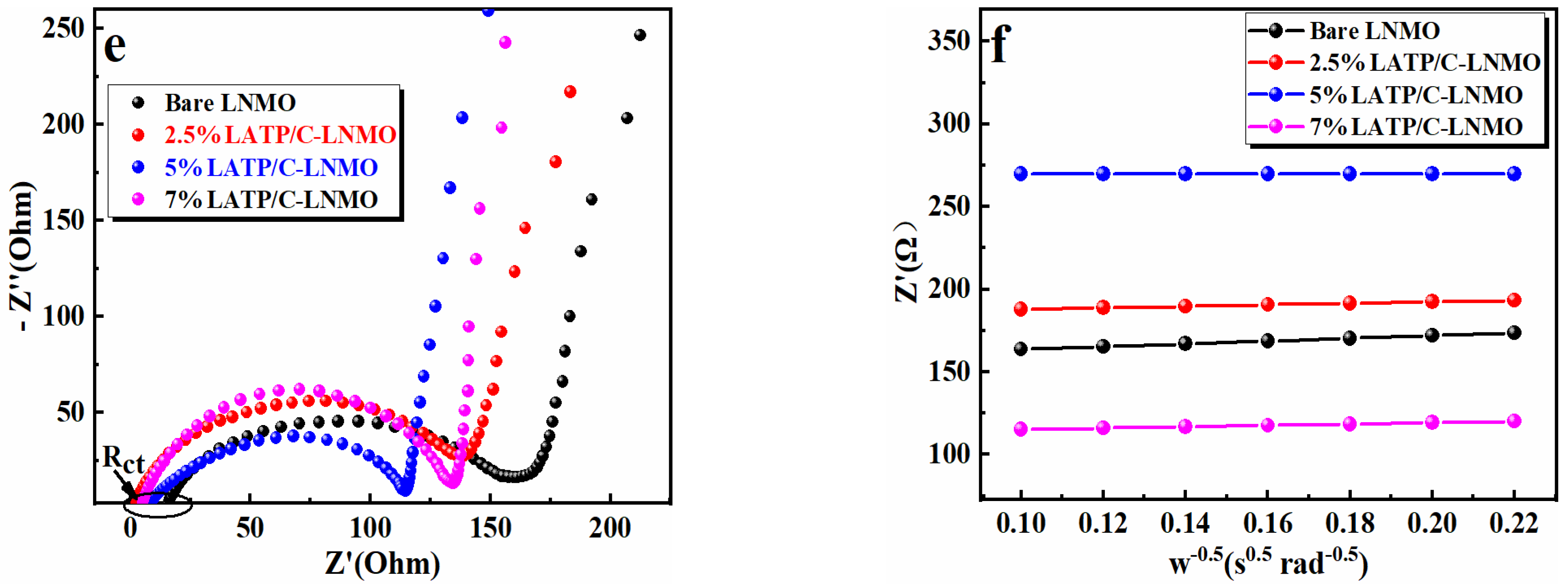
3.3. Cyclic Behaviors and Kinetic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Whittingham, M.S. Materials Challenges Facing Electrical Energy Storage. MRS Bull. 2008, 33, 411–419. [Google Scholar] [CrossRef]
- Choi, N.; Chen, Z.; Freunberger, S.A.; Ji, X.; Sun, Y.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges Facing Lithium Batteries and Electrical Double-Layer Capacitors. Angew. Chem. Int. Ed. 2012, 51, 9994–10024. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.X.; Deng, Y.F.; Wan, L.N.; Xu, H.J.; Qin, X.S.; Chen, G.H. Investigation of the Effect of Extra Lithium Addition and Postannealing on the Electrochemical Performance of High-Voltage Spinel LiNi0.5Mn1.5O4 Cathode Material. J. Phys. Chem. C 2014, 18, 15581–15589. [Google Scholar] [CrossRef]
- Haridas, A.K.; Sharma, C.S.; Rao, T.N. Caterpillar-like sub-micron LiNi0.5Mn1.5O4 structures with site disorder and excess Mn3+ as high performance cathode material for lithium ion batteries. Electrochim. Acta 2016, 212, 500–509. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, D.; Yan, H.; Li, W. Precise preparation of high performance spherical hierarchical LiNi0.5Mn1.5O4 for 5 V lithium ion secondary batteries. J. Mater. Chem. A 2013, 1, 5492–5496. [Google Scholar] [CrossRef]
- Lim, G.; Shin, D.K.; Chae, K.H.; Cho, M.K.; Kim, C.; Sohn, S.S.; Lee, M.; Hong, J. Regulating dynamic electrochemical interface of LiNi0.5Mn1.5O4 spinel cathode for realizing simultaneous mn and ni redox in rechargeable lithium batteries. Adv. Energy Mater. 2022, 12, 2049. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Minakshi, M.; Bahri, P.A.; Paul, S.; Kumari, P.; Divakaran, A.M.; Manjunatha, K.N. Rational design on materials for developing next generation lithium-ion secondary battery. Prog. Solid State Chem. 2020, 62, 100298. [Google Scholar] [CrossRef]
- Minakshi, M.; Ralph, D. A novel Sodium-Ion rechargeable battery. ECS Trans. 2013, 45, 95–102. [Google Scholar] [CrossRef]
- Yang, L.; Ravdel, B.; Lucht, B.L. Electrolyte Reactions with the Surface of High Voltage LiNi0.5Mn1.5O4 Cathodes for Lithium-Ion Batteries. Electrochem. Solid-State Lett. 2010, 13, A95. [Google Scholar] [CrossRef]
- Kim, J.; Pieczonka, N.P.W.; Li, Z.; Wu, Y.; Harris, S.; Powell, B.R. Understanding the capacity fading mechanism in LiNi0.5Mn1.5O4/graphite Li-ion batteries. Electrochim. Acta 2013, 90, 556–562. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Wang, Z.; Guo, H.; Yue, P. Capacity fading reason of LiNi0.5Mn1.5O4 with commercial electrolyte. Ionics 2013, 19, 379–383. [Google Scholar] [CrossRef]
- Hsu, S.C.; Hsiao, Y.S.; Lu, C.Z.; Chiang, H.H.; Kuo, C.L.; Wu, N.J.; Huang, J.H.; Chang-Jian, C.W.; Weng, H.C.; Chen, C.P. The effect of dual-doping on the electrochemical performance of LiNi0.5Mn1.5O4 and its application in full-cell lithium-ion batteries. Ceram. Int. 2022, 48, 14778–14788. [Google Scholar] [CrossRef]
- Ma, C.Z.; Wen, Y.H.; Qiao, Q.; He, P.; Ren, S.Q.; Li, M.; Zhao, P.C.; Qiu, J.Y.; Tang, G.S. Improving Electrochemical Performance of High-Voltage Spinel LiNi0.5Mn1.5O4 Cathodes by Silicon Oxide Surface Modification. ACS Appl. Energy Mater. 2022, 48, 14778–14788. [Google Scholar] [CrossRef]
- Fehse, M.; Etxebarria, N.; Otaegui, L.; Cabello, M.; Martín-Fuentes, S.; Cabañero, M.A.; Monterrubio, I.; Elkjær, C.F.; Fabelo, O.; Enkubari, N.A.; et al. Influence of Transition-Metal order on the reaction mechanism of LNMO cathode spinel: An operando x-ray absorption spectroscopy study. Chem. Mater. 2022, 34, 6529–6540. [Google Scholar] [CrossRef]
- Pieczonka, N.P.W.; Liu, Z.; Lu, P.; Olson, K.L.; Moote, J.; Powell, B.R.; Kim, J. Understanding Transition-Metal Dissolution Behavior in LiNi0.5Mn1.5O4 High-Voltage Spinel for Lithium Ion Batteries. J. Phys. Chem. C 2013, 117, 15947–15957. [Google Scholar] [CrossRef]
- Liang, G.; Wu, Z.; Didier, C.; Zhang, W.; Cuan, J.; Li, B.; Ko, K.Y.; Hung, P.Y.; Lu, C.Z.; Chen, Y.; et al. A Long Cycle-Life High-Voltage Spinel Lithium-Ion Battery Electrode Achieved by Site-Selective Doping. Angew. Chem. Int. Ed. Engl. 2020, 59, 10594–10602. [Google Scholar] [CrossRef] [PubMed]
- Nisar, U.; Al-Hail, S.; Petla, R.K.; Shakoor, R.A.; Essehli, R.; Kahraman, R.; AlQaradawi, S.Y.; Kim, D.K.; Belharouak, I.; Amin, M.R. Understanding the Origin of the Ultrahigh Rate Performance of a SiO2-Modified LiNi0.5Mn1.5O4 Cathode for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 7263–7271. [Google Scholar] [CrossRef]
- Huang, X.K.; Chen, K.; Liu, Y.Z. Enhancement of LiNi0.5Mn1.5O4 Cathode Materials through Interfacial Modification of Amorphous Al2O3 in Lithium Ion Batteries. J. Electrochem. Soc. 2018, 166, A5081–A5089. [Google Scholar] [CrossRef]
- Song, C.L.; Lu, J.N.; Liu, Y.; Yuan, Q.; Yang, J.G.; He, H.; Liu, D.Y.; Wang, Q. Enhanced electrochemical performance of spinel LiNi0.5Mn1.5O4 for Li-ion batteries with moderate Mn3+ concentration and nanosized thin Al2O3 coating. J. Mater. Sci. Mater. Electron. 2020, 31, 4815–4821. [Google Scholar] [CrossRef]
- Wu, H.M.; Belharouak, I.; Abouimrane, A.; Sun, Y.; Amine, K. Surface modification of LiNi0.5Mn1.5O4 by ZrP2O7 and ZrO2 for lithium-ion batteries. J. Power Sources 2010, 195, 2909–2913. [Google Scholar] [CrossRef]
- Luo, H.; Chen, H.X.; Luo, X.L.; Liu, Z.Z.; Zhou, H.M. Effects of in situ-converted Li3PO4 coating on electrochemical performance of MOF-assisted LiNi0.5Mn1.5O4 cathode materials for lithium-ion batteries. J. Mater. Sci. Mater. Electron. 2022, 33, 6872–6887. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P.; Sharma, N.; Blackford, M.; Ionescu, M. Lithium Extraction−Insertion from/into LiCoPO4 in Aqueous Batteries. Ind. Eng. Chem. Res. 2011, 50, 1899–1905. [Google Scholar] [CrossRef]
- Minakshi, M.; Sharma, N.; Ralph, D.; Appadoo, D.; Nallathamby, K. Synthesis and characterization of Li(Co0.5Ni0.5)PO4 cathode for Li-Ion aqueous battery applications. Electrochem. Solid-State Lett. 2011, 14, A86. [Google Scholar] [CrossRef]
- Gao, X.W.; Deng, Y.F.; Wexler, D.; Chen, G.H.; Chou, S.L.; Liu, H.K.; Shi, Z.C.; Wang, J.Z. Improving the electrochemical performance of the LiNi0.5Mn1.5O4 spinel by polypyrrole coating as a cathode material for the lithium-ion battery. J. Mater. Chem. A 2015, 3, 404–411. [Google Scholar] [CrossRef]
- Chen, S.; He, T.; Su, Y.; Lu, Y.; Bao, L.; Chen, L.; Zhang, Q.Y.; Wang, J.; Chen, R.J.; Wu, F. Ni-Rich LiNi0.8Co0.1Mn0.1O2Oxide Coated by Dual-Conductive Layers as High Performance Cathode Material for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 29732–29743. [Google Scholar] [CrossRef]
- Bei, Y.Y.; Zhang, Y.; Li, Y.Y.; Song, Y.; Liu, L.; Ma, J.J.; Liu, J. Controlled preparation of nano-Al2O3/PPy composite coatings to compensate surface structural defects of lithium-rich layered oxides. J. Alloy. Compd. 2022, 928, 167140. [Google Scholar] [CrossRef]
- Lin, W. Improving the Electrochemical Performance of LiNi0.5Mn1.5O4 Cathode Materials by Surface Coating with Cyclized Polyacrylonitrile for Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2017, 12, 12047–12059. [Google Scholar] [CrossRef]
- Miao, X.Y.; Qin, X.; Huang, S.Y.; Wei, T.Y.; Lei, C.R. Hollow spherical LiNi0.5Mn1.5O4 synthesized by a glucose-assisted hydrothermal method. Mater. Lett. 2021, 289, 129417. [Google Scholar] [CrossRef]
- Salian, G.D.; Lebouin, C.; Galeyeva, M.; Kurbatov, A.P.; Djenizian, T. Electrodeposition of polymer electrolyte into porous LiNi0.5Mn1.5O4 for high performance All-Solid-State microbatteries. Front. Chem. 2019, 6, 675. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.Y.; Zhang, Y.J.; Zhang, S.Q.; Tang, P.P.; Xiao, X.L.; Ma, M.Y.; Zhang, H.S.; Yin, Y.H.; Wang, D.; Yang, S.T. Improved high temperature performance of a spinel LiNi0.5Mn1.5O4 cathode for High-Voltage Lithium-Ion batteries by surface modification of a flexible conductive nanolayer. ACS Omega 2019, 4, 185–194. [Google Scholar] [CrossRef]
- Sim, S.; Lee, S.; Jin, B.; Kim, H. Use of carbon coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced performances of lithium-ion batteries. Sci. Rep. Sci. Rep. 2020, 10, 11114. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, Y.; Li, H.; Wang, Z.; Chen, L. Porous Li4Ti5O12 Coated with N-Doped Carbon from Ionic Liquids for Li-Ion Batteries. Adv. Mater. 2011, 23, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Q.; Jiang, Z.J. Effects of carbon content on the electrochemical performance of LiFePO4/C core/shell nanocomposites fabricated using FePO4/polyaniline as an iron source. J. Alloy. Compd. 2012, 537, 308–317. [Google Scholar] [CrossRef]
- Ku, B.J.; Lee, J.H.; Lee, S.J.; Koo, M.; Lee, B.J. Effects of carbon coating on LiNi0.5Mn1.5O4 cathode material for lithium ion batteries using an atmospheric microwave plasma torch. Surf. Coat. Tech. 2019, 376, 25–30. [Google Scholar] [CrossRef]
- Hwang, T.; Lee, J.K.; Mun, J.; Choi, W. Surface-modified carbon nanotube coating on high-voltage LiNi0.5Mn1.5O4 cathodes for lithium ion batteries. J. Power Sources 2016, 322, 40–48. [Google Scholar] [CrossRef]
- Wang, H.L.; Shi, Z.Q.; Li, J.W.; Yang, S.; Ren, R.B.; Cui, J.Y.; Xiao, J.L.; Zhang, B. Direct carbon coating at high temperature on LiNi0.5Mn1.5O4 cathode: Unexpected influence on crystal structure and electrochemical performances. J. Power Sources 2015, 288, 206–213. [Google Scholar] [CrossRef]
- Waetzig, K.; Rost, A.; Heubner, C.; Coeler, M.; Nikolowski, K.; Wolter, M.; Schilm, J. Synthesis and sintering of Li1.3Al0.3Ti1.7(PO4)3 (LATP) electrolyte for ceramics with improved Li+ conductivity. J. Alloy. Compd. 2020, 818, 153237. [Google Scholar] [CrossRef]
- Bai, H.; Hu, J.; Duan, Y.; Kozawa, T.; Naito, M.; Zhang, J.; Dong, S. Surface modification of Li1.3Al0.3Ti1.7(PO4)3 ceramic electrolyte by Al2O3-doped ZnO coating to enable dendrites-free all-solid-state lithium-metal batteries. Ceram. Int. 2019, 45, 14663–14668. [Google Scholar] [CrossRef]
- Bi, K.; Zhao, S.; Huang, C.; Nan, C. Improving low-temperature performance of spinel LiNi0.5Mn1.5O4 electrode and LiNi0.5Mn1.5O4/Li4Ti5O12 full-cell by coating solid-state electrolyte for Li-Al-Ti-P-O. J. Power Sources 2018, 38, 240–248. [Google Scholar] [CrossRef]
- Kang, H.; Bui, T.T.; Yun, B.; Ho, V.; Lee, D.; Mun, J.; Kim, M. Phosphazene based LATP precursor for a CEI coating layer on high voltage LiNi0.5Mn1.5O4 cathode with improved cycling durability. Mater. Chem. Phys. 2022, 290, 126492. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, S.; Xu, Y.; Nan, C. Effect of temperature of Li2O-Al2O3-TiO2-P2O5 solid-state electrolyte coating process on the performance of LiNi0.5Mn1.5O4 cathode materials. J. Power Sources 2015, 296, 261–267. [Google Scholar] [CrossRef]
- Qu, X.; Yu, Z.; Ruan, D.; Dou, A.; Su, M.; Zhou, Y.; Liu, Y.; Chu, D. Enhanced Electrochemical Performance of Ni-Rich Cathode Materials with Li1.3Al0.3Ti1.7(PO4)3 Coating. ACS Sustain. Chem. Eng. 2020, 8, 5819–5830. [Google Scholar] [CrossRef]
- Kim, M.Y.; Song, Y.W.; Lim, J.; Park, S.J.; Kang, B.S.; Hong, Y.; Kim, H.S.; Han, J.H. LATP-coated NCM-811 for high-temperature operation of all-solid lithium battery. Mater. Chem. Phys. 2022, 290, 126644. [Google Scholar] [CrossRef]
- Nie, Y.; Xiao, W.; Miao, C.; Fang, R.; Kou, Z.; Wang, D.; Xu, M.B.; Wang, C.J. Boosting the electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode materials in-situ modified with Li1.3Al0.3Ti1.7(PO4)3 fast ion conductor for lithium-ion batteries. Electrochim. Acta 2020, 353, 136477. [Google Scholar] [CrossRef]
- Morimoto, H.; Someno, Y.; Arai, Y.; Tobishima, S. Preparation of High Lithium Ion Conductive Glass-ceramics Solid Electrolytes in the LiTi2(PO4)3 System by the Mechanochemical Method and Their Application as Coating Materials of LiCoO2. Electroceram. Jpn. Xi 2009, 388, 77–80. [Google Scholar] [CrossRef]
- Li, L.; Zhao, R.; Xu, T.; Wang, D.; Pan, D.; Zhang, K.; Yu, C.; Lu, X.; He, G.; Bai, Y. Stabilizing a high-voltage LiNi0.5Mn1.5O4 cathode towards all solid state batteries: A Li–Al–Ti–P–O solid electrolyte nano-shell with a host material. Nanoscale 2019, 11, 8967–8977. [Google Scholar] [CrossRef]
- Xu, M.; Yang, M.; Chen, M.F.; Gu, L.H.; Luo, L.S.; Chen, S.Y.; Chen, J.Z.; Liu, B.; Han, X. Enabling structural and interfacial stability of 5 V spinel LiNi0.5Mn1.5O4 cathode by a coherent interface. J. Energy Chem. 2023, 76, 266–276. [Google Scholar] [CrossRef]
- Yang, C.C.; Jiang, J.R.; Karuppiah, C.; Jang, J.H.; Wu, Z.H.; Jose, R.; Lue, S.J. LATP ionic conductor and in-situ graphene hybrid-layer coating on LiFePO4 cathode material at different temperatures. J. Alloy. Compd. 2018, 765, 800–811. [Google Scholar] [CrossRef]
- Nguyen, T.; Karuppiah, C.; Chien, W.C.; Wu, S.H.; Jose, R.; Lue, S.J.; Yang, C.C. Mechanical alloy coating of LATP decorated porous carbon on LiFe1/3Mn1/3Co1/3PO4/C composite cathode for high-voltage Li-ion battery. Electrochim. Acta 2020, 359, 136980. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.; Huang, X.; Liu, D.; Dou, A.; Su, M.; Chu, D. Electrochemical performance of Li1.2Ni0.2Mn0.6O2 coated with a facilely synthesized Li1.3Al0.3Ti1.7(PO4)3. J. Power Sources 2018, 403, 27–37. [Google Scholar] [CrossRef]
- Kobylianska, S.; Demchuk, D.; Khomenko, V.; Barsukov, V.; Belous, A. Surface Modification of the LiNi0.5Co0.2Mn0.3O2 Cathode by a Protective Interface Layer of Li1.3Ti1.7Al0.3(PO4)3. J. Electrochem. Soc. 2019, 166, A1920–A1925. [Google Scholar] [CrossRef]
- Zhong, G.B.; Wang, Y.Y.; Yu, Y.Q.; Chen, C.H. Electrochemical investigations of the LiNi0.45M0.10Mn1.45O4 (M=Fe, Co, Cr) 5V cathode materials for lithium ion batteries. J. Power Sources 2012, 205, 385–393. [Google Scholar] [CrossRef]
- Zhou, S.; Cui, Z.; Mei, T.; Wang, X.; Qian, Y. Hierarchical desert-waves-like LiNi0.5Mn1.5O4 as advanced cathodes with superior rate capability and cycling stability. Mater. Today Energy 2019, 14, 100363. [Google Scholar] [CrossRef]
- Lan, J.; Zheng, Q.; Zhou, H.; Li, J.; Xing, L.; Xu, K.; Fan, W.; Yu, L.; Li, W. Stabilizing a High-Voltage Lithium-Rich Layered Oxide Cathode with a Novel Electrolyte Additive. ACS Appl. Mater. Inter. 2019, 11, 28841–28850. [Google Scholar] [CrossRef] [PubMed]


| a | b | c | V(Å3) | |
|---|---|---|---|---|
| LNMO | 8.1666 | 8.1666 | 8.1666 | 544.67 |
| 2.5% LATP/C-LNMO | 8.1714 | 8.1714 | 8.1714 | 545.62 |
| 5% LATP/C-LNMO | 8.1885 | 8.1885 | 8.1885 | 549.05 |
| 7% LATP/C-LNMO | 8.1891 | 8.1891 | 8.1891 | 549.17 |
| Bare LNMO | 2.5% LATP/C-LNMO | 5% LATP/C-LNMO | 7% LATP/C-LNMO | |
|---|---|---|---|---|
| Rs [Ω] | 15.35 | 2.17 | 8.04 | 5.14 |
| Rct [Ω] | 142.45 | 135.43 | 106.84 | 129.41 |
| DLi+ [cm2 s−1] | 3.51×10−12 | 4.90 × 10−12 | 3.04 × 10−11 | 1.55 × 10−11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Chin, C.-T.; Cheng, C.-H.; Zhao, J. Enhancing the Electrochemical Performance of High Voltage LiNi0.5Mn1.5O4 Cathode Materials by Surface Modification with Li1.3Al0.3Ti1.7(PO4)3/C. Nanomaterials 2023, 13, 628. https://doi.org/10.3390/nano13040628
Yang T, Chin C-T, Cheng C-H, Zhao J. Enhancing the Electrochemical Performance of High Voltage LiNi0.5Mn1.5O4 Cathode Materials by Surface Modification with Li1.3Al0.3Ti1.7(PO4)3/C. Nanomaterials. 2023; 13(4):628. https://doi.org/10.3390/nano13040628
Chicago/Turabian StyleYang, Tingting, Chi-Te Chin, Ching-Hsiang Cheng, and Jinsheng Zhao. 2023. "Enhancing the Electrochemical Performance of High Voltage LiNi0.5Mn1.5O4 Cathode Materials by Surface Modification with Li1.3Al0.3Ti1.7(PO4)3/C" Nanomaterials 13, no. 4: 628. https://doi.org/10.3390/nano13040628
APA StyleYang, T., Chin, C.-T., Cheng, C.-H., & Zhao, J. (2023). Enhancing the Electrochemical Performance of High Voltage LiNi0.5Mn1.5O4 Cathode Materials by Surface Modification with Li1.3Al0.3Ti1.7(PO4)3/C. Nanomaterials, 13(4), 628. https://doi.org/10.3390/nano13040628






