Recent Progress of Nanodiamond Film in Controllable Fabrication and Field Emission Properties
Abstract
1. Introduction
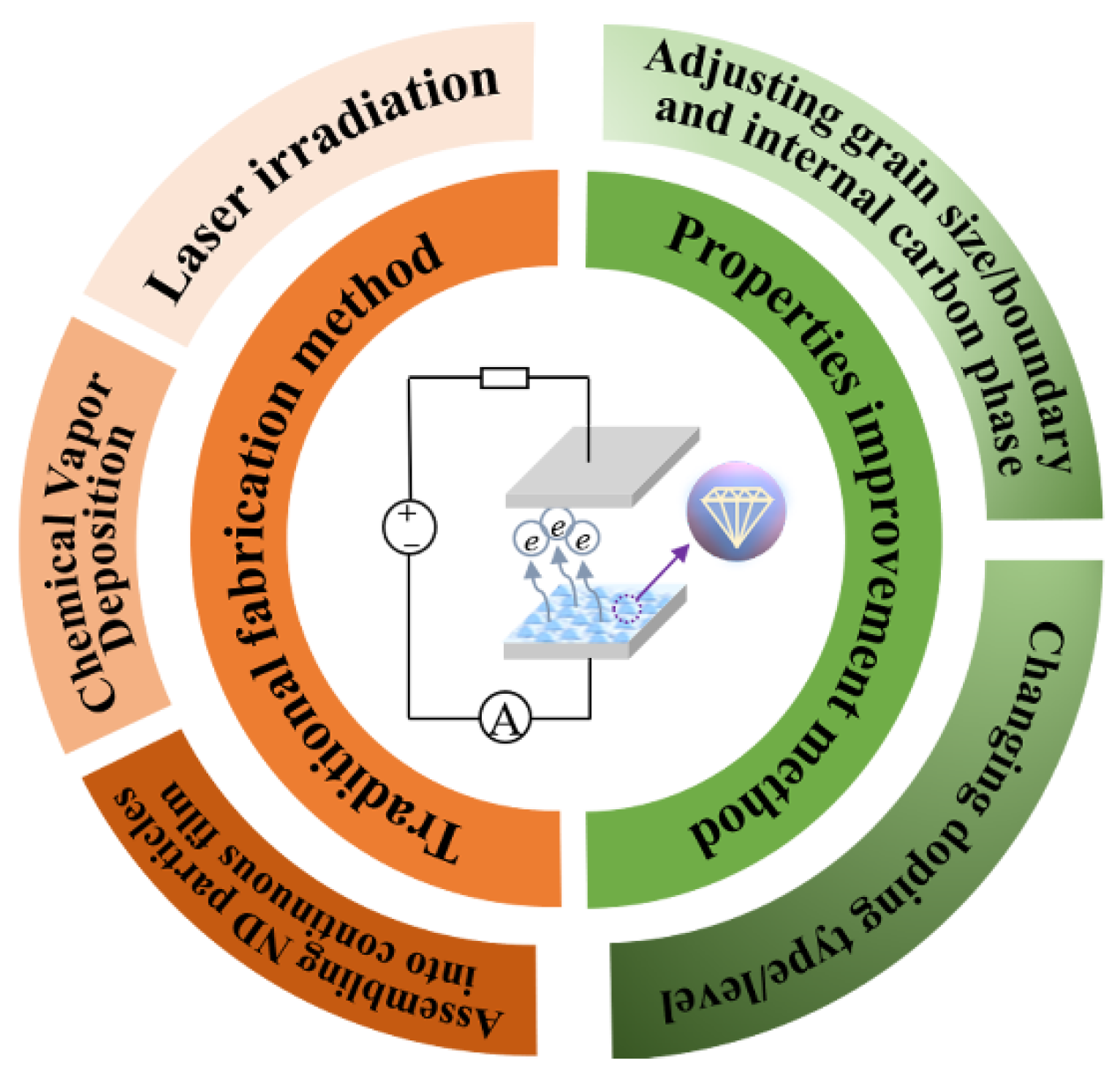
2. Fabrication Method and Nucleation Process of Nanodiamond Film
2.1. Chemical Composition and Structure of Nanodiamonds
2.2. Chemical Vapor Deposition (CVD)
2.2.1. Diamond Seeding Process
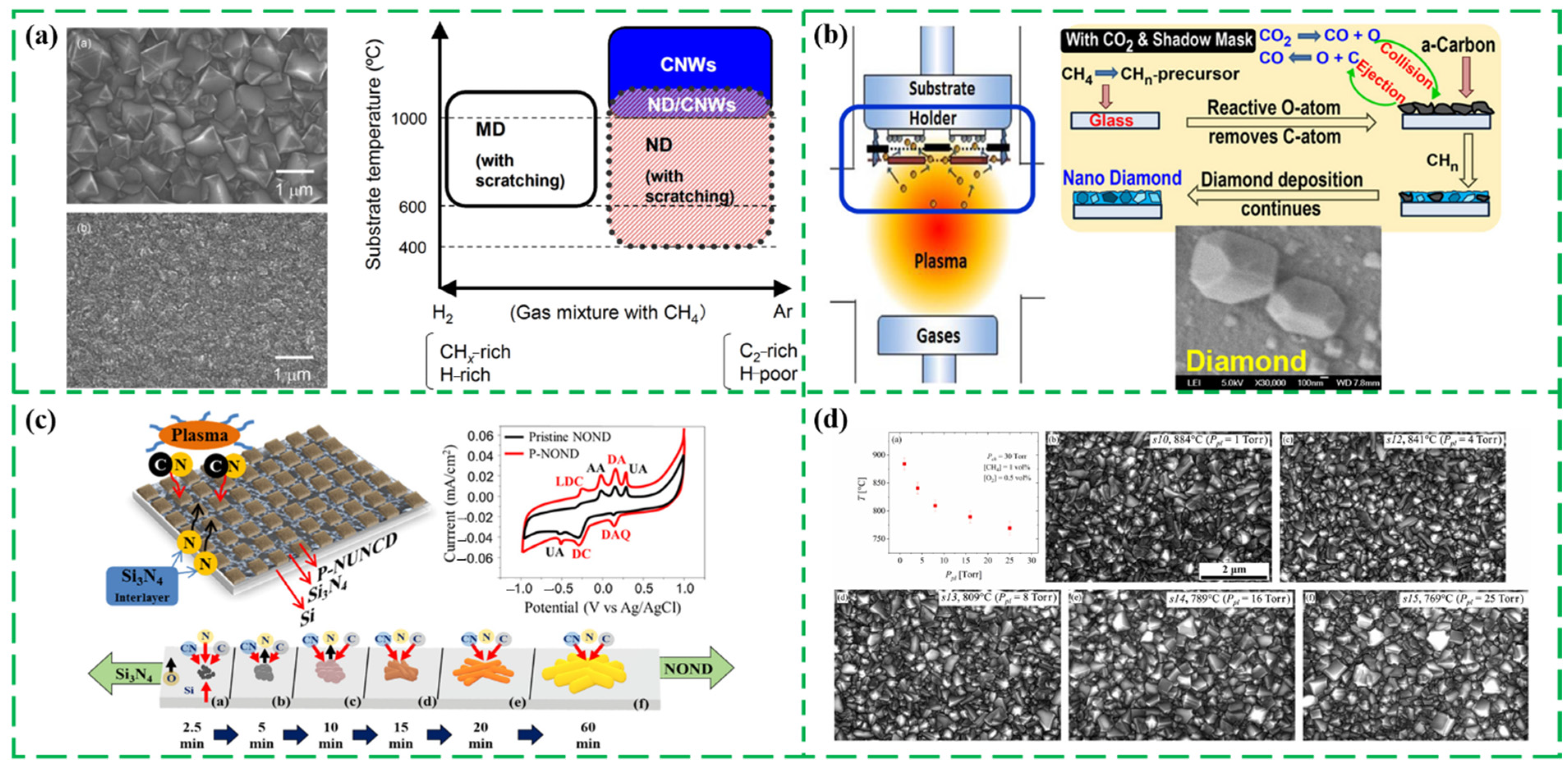
2.2.2. CVD with Different Energy Sources
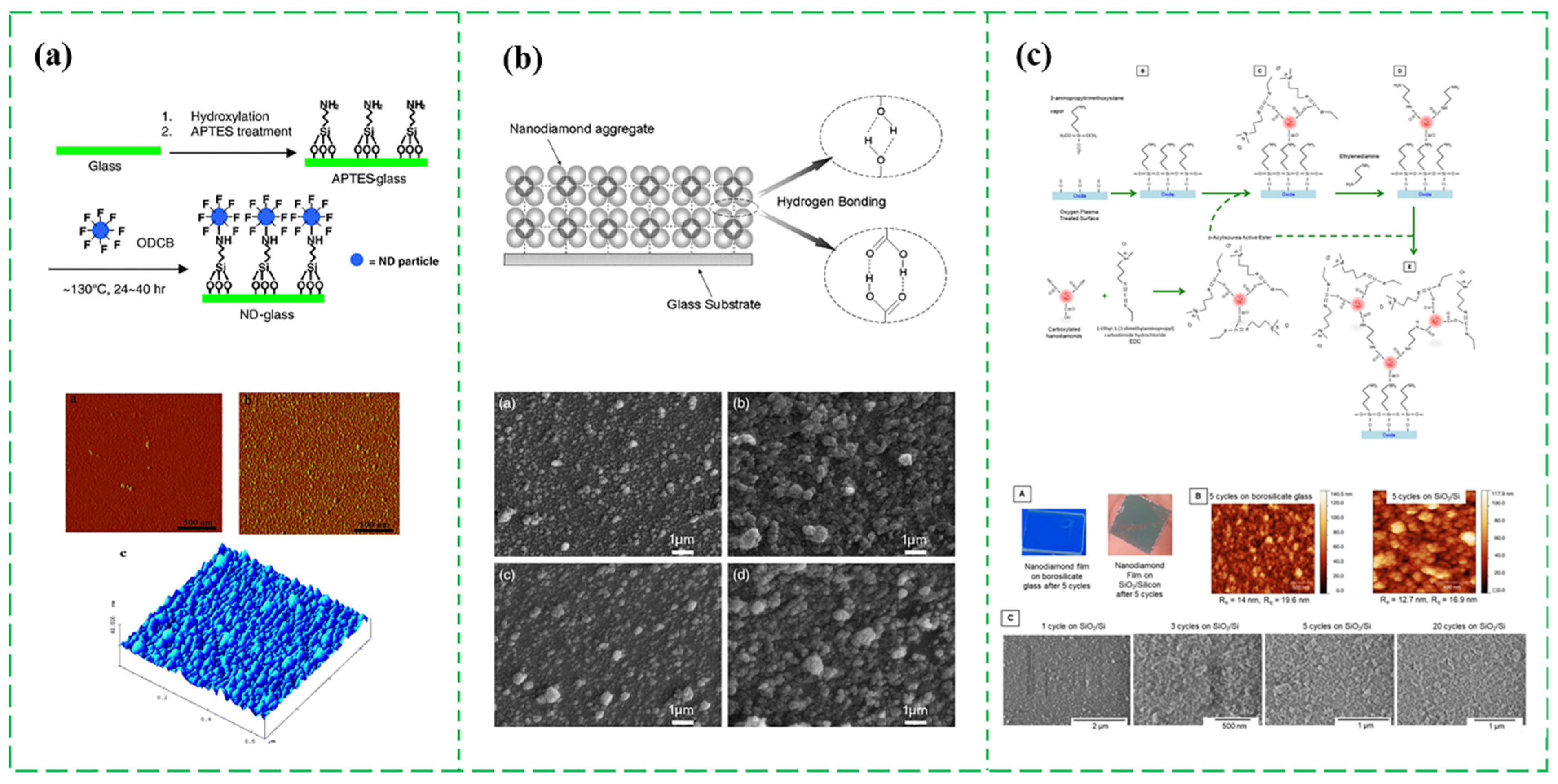
2.2.3. Assembling ND Particles into Continuous Films
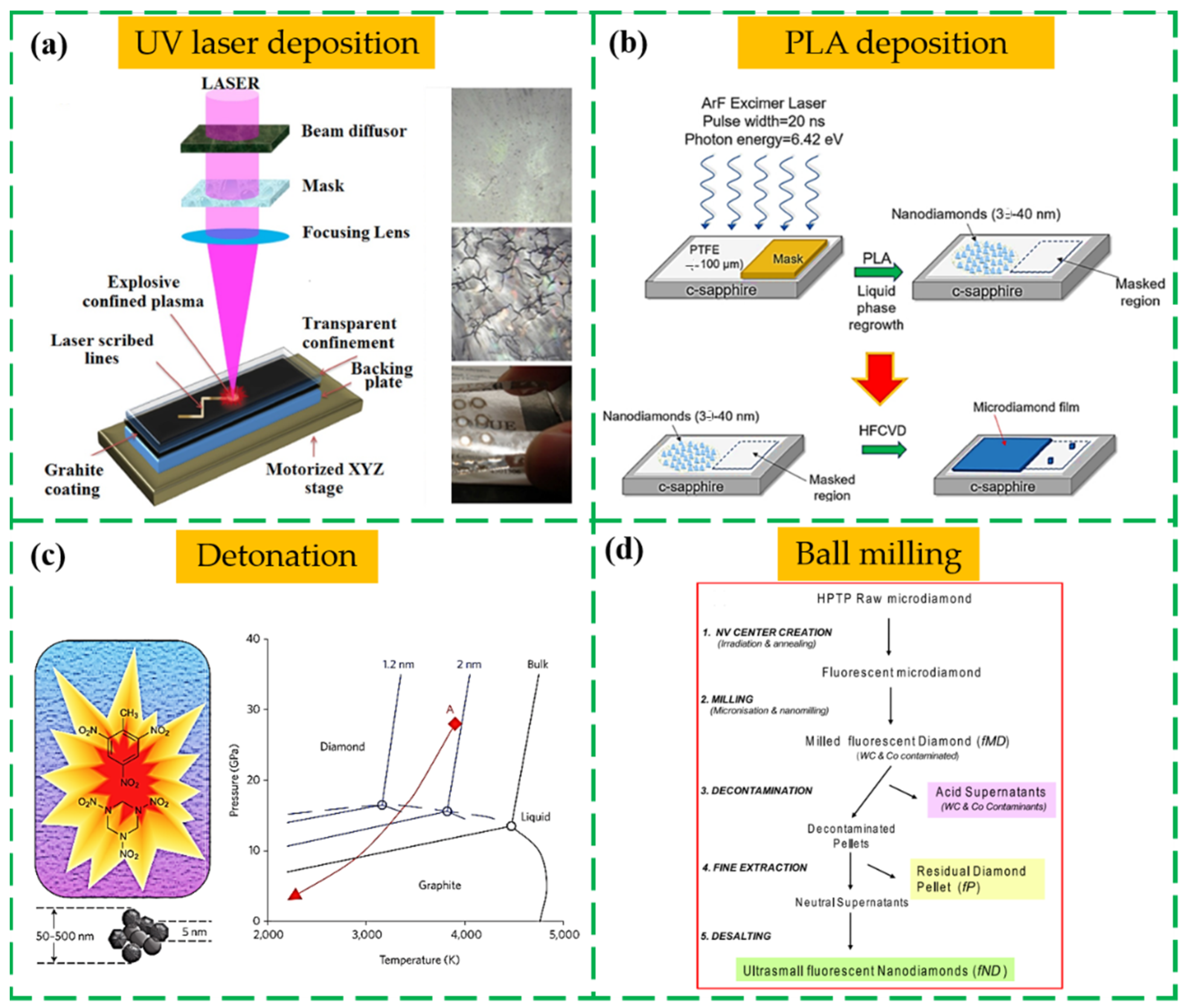
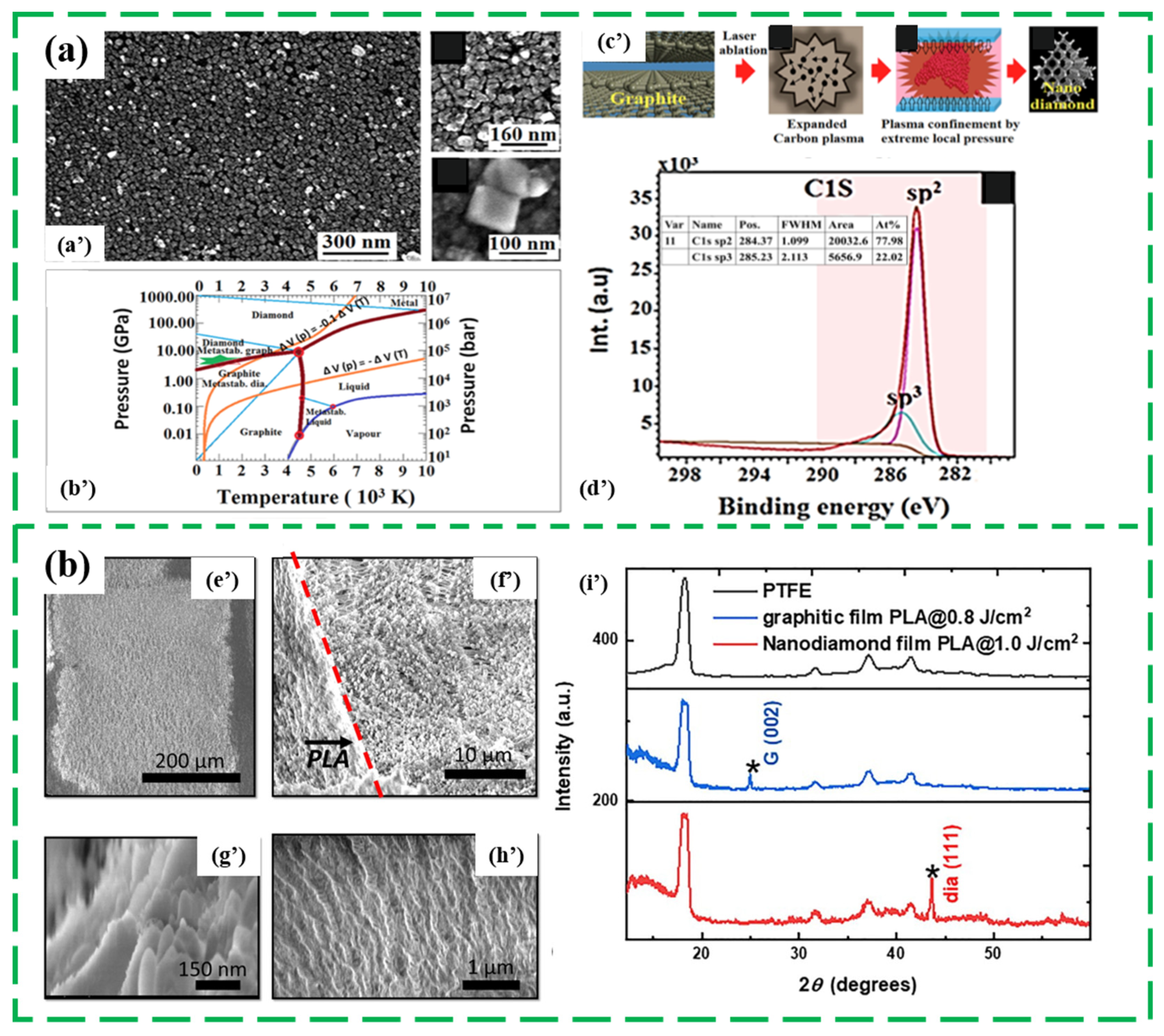
2.3. Laser Irradiation
3. Field Emission Properties of ND Film
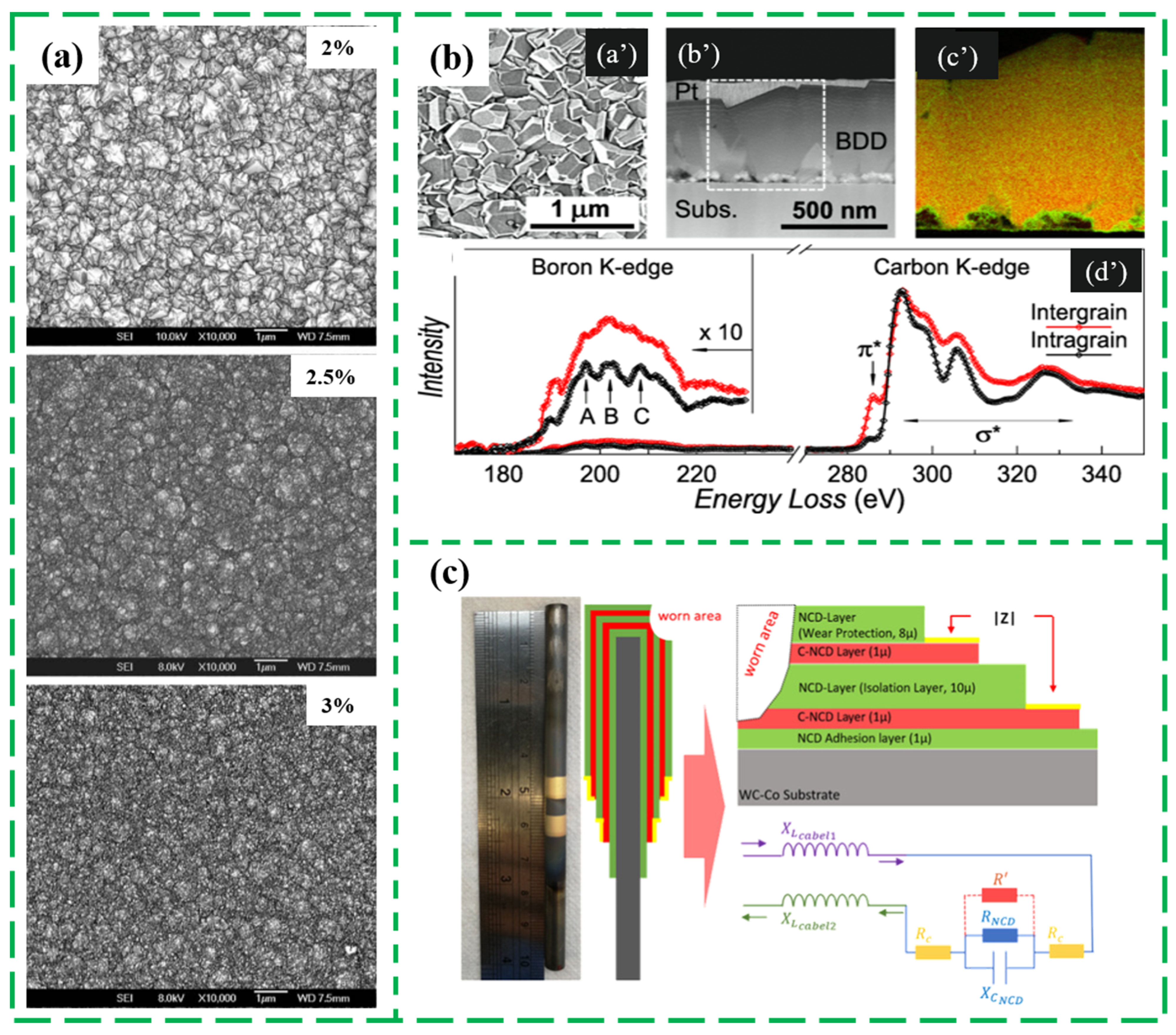
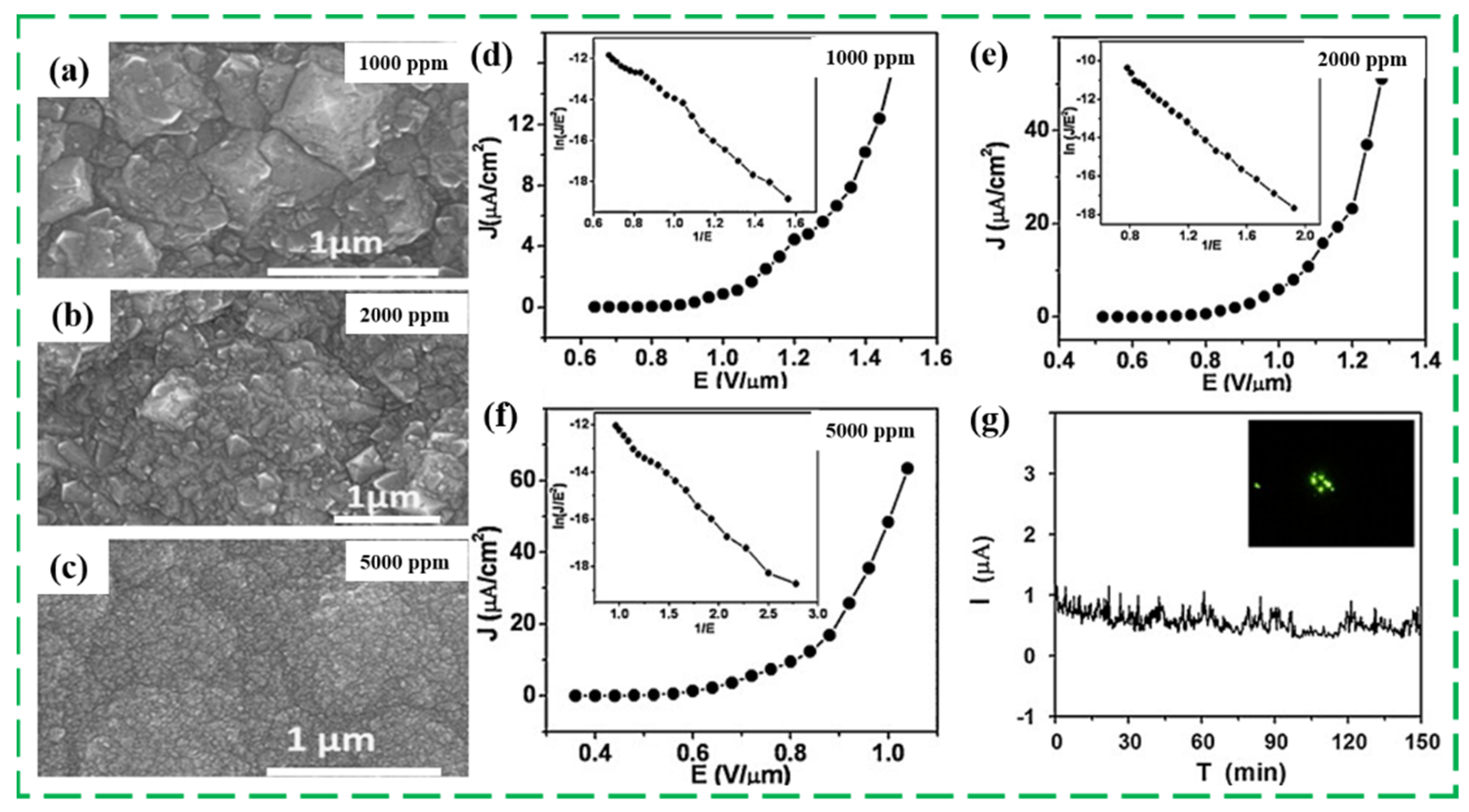
3.1. Adjusting Grain Size/Boundary and Internal Carbon Phase
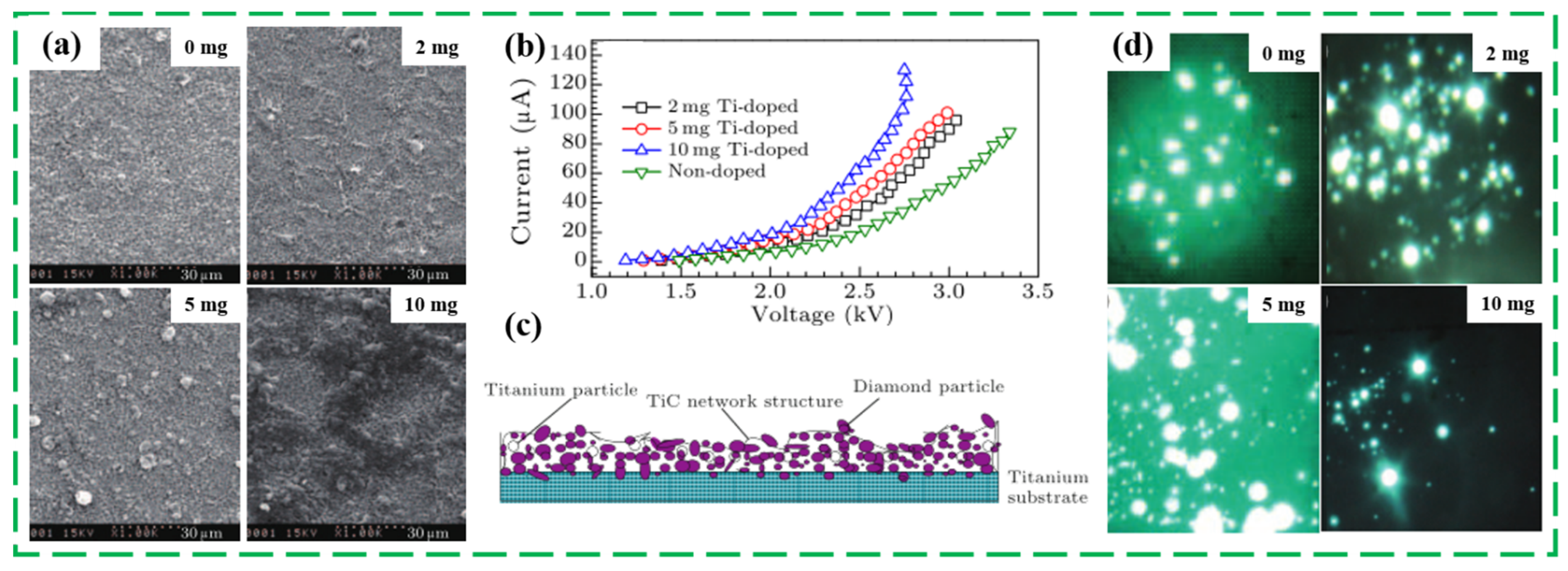
3.2. Changing Doping Type/Level
4. Conclusions and Prospect
Author Contributions
Funding
Conflicts of Interest
References
- Philip, J.; Hess, P.; Feygelson, T.; Butler, J.E.; Chattopadhyay, S.; Chen, K.H.; Chen, L.C. Elastic, mechanical, and thermal properties of nanocrystalline diamond films. J. Appl. Phys. 2003, 93, 2164–2171. [Google Scholar] [CrossRef]
- Erdemir, A.; Fenske, G.; Krauss, A.; Gruen, D.; McCauley, T.; Csencsits, R. Tribological properties of nanocrystalline diamond films. Surf. Coat. Technol. 1999, 120, 565–572. [Google Scholar] [CrossRef]
- Arenal, R.; Montagnac, G.; Bruno, P.; Gruen, D.M. Multiwavelength Raman spectroscopy of diamond nanowires present in n-type ultrananocrystalline films. Phys. Rev. B 2022, 49, 22–32. [Google Scholar] [CrossRef]
- Woerner, E.; Wild, C.; Mueller-Sebert, W.; Koidl, P. CVD-diamond optical lenses. Diam. Relat. Mater. 2001, 10, 557–560. [Google Scholar] [CrossRef]
- Whitfield, M.D.; Audic, B.; Flannery, C.M.; Kehoe, L.P.; Crean, G.M.; Johnston, C.; Chalker, P.R.; Jackman, R.B. Polycrystalline diamond films for acoustic wave devices. Diam. Relat. Mater. 1998, 7, 533–539. [Google Scholar] [CrossRef]
- Grausova, L.; Kromka, A.; Burdikova, Z.; Eckhardt, A.; Rezek, B.; Vacik, J.; Haenen, K.; Lisa, V.; Bacakova, L. Enhanced growth and osteogenic differentiation of human osteoblast-like cells on boron-doped nanocrystalline diamond thin films. PLoS ONE 2011, 6, e20943. [Google Scholar] [CrossRef]
- Shabani, M.; Abreu, C.; Gomes, J.; Silva, R.; Oliveira, F. Effect of relative humidity and temperature on the tribology of multilayer micro/nanocrystalline CVD diamond coatings. Diam. Relat. Mater. 2017, 73, 190–198. [Google Scholar]
- Okano, K.; Koizumi, S.; Silva, S.R.P.; Amaratunga, G.A.J. Low-threshold cold cathodes made of nitrogen-doped chemical-vapour-deposited diamond. Nature 1996, 381, 140. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Churochkin, D. Polarization dependent asymmetric magneto-resistance features in nanocrystalline diamond films. Appl. Phys. Lett. 2014, 105, 073111. [Google Scholar] [CrossRef]
- Terranova, M.L.; Orlanducci, S.; Rossi, M.; Tamburri, E. Nanodiamonds for field emission: State of the art. Nanoscale 2015, 7, 5094–5114. [Google Scholar] [CrossRef]
- Chubenko, O.; Baturin, S.S.; Kovi, K.K.; Sumant, A.V.; Baryshev, S.V. Locally resolved electron emission area and unified view of field emission from ultrananocrystalline diamond films. ACS Appl. Mater. Interfaces 2017, 9, 33229–33237. [Google Scholar] [CrossRef] [PubMed]
- Kolekar, S.K.; Godbole, R.V.; Godbole, V.P.; Dharmadhikari, C.V. Electron transport across nanocrystalline diamond films: Field emission and conducting atomic force microscopic investigations. AIP Adv. 2020, 10, 045129. [Google Scholar] [CrossRef]
- Yu, H.Y.; Gao, N.; Li, H.D.; Huang, X.R.; Duan, D.F.; Bao, K.; Zhu, M.F.; Liu, B.B.; Cui, T. Structural model of substitutional sulfur in diamond. Chin. Phys. B 2019, 28, 088102. [Google Scholar] [CrossRef]
- Tse, K.Y.; Nichols, B.M.; Yang, W.; Butler, J.E.; Russell, J.J.N.; Hamers, R.J. Electrical properties of diamond surfaces functionalized with molecular monolayers. J. Phys. Chem. B 2005, 109, 8523. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Wang, X.; Xi, X.; Gu, Y.; Liu, Q.; Li, Y.; Li, J. Nitrogen-doped nanodiamond films grown just by heating solid precursor thin layers for field emission application. J. Phys. D Appl. Phys. 2020, 53, 015101. [Google Scholar] [CrossRef]
- Ficek, M.; Sankaran, K.J.; Ryl, J.; Bogdanowicz, R.; Lin, I.N.; Haenen, K.; Darowicki, K. Ellipsometric investigation of nitrogen doped diamond thin films grown in microwave CH4/H2/N2 plasma enhanced chemical vapor deposition. Appl. Phys. Lett. 2016, 108, 241906. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lin, S.J.; Lin, I.N.; Cheng, H.F. Effect of boron doping on the electron-field-emission properties of nanodiamond films. J. Appl. Phys. 2005, 97, 054310. [Google Scholar] [CrossRef]
- Lee, Y.C.; Pradhan, D.; Lin, S.J.; Chia, C.T.; Cheng, H.F.; Lin, I.N. Effect of surface treatment on the electron field emission property of nano-diamond films. Diam. Relat. Mater. 2005, 14, 2055–2058. [Google Scholar] [CrossRef]
- Dong, C.L.; Chen, S.S.; Chiou, J.W.; Chen, Y.Y.; Guo, J.H.; Cheng, H.F.; Li, I.N.; Chang, C.L. Effect of surface treatments on the electronic properties of ultra-nanocrystalline diamond films. Diam. Relat. Mater. 2008, 17, 1150–1153. [Google Scholar] [CrossRef]
- Subramanian, K.; Kang, W.P.; Davidson, J.L.; Jarvis, J.D.; Hofmeister, W.H.; Choi, B.K.; Howell, M. Geometrical field enhancement on micropatterned nanodiamond film for electron emissions. Diam. Relat. Mater. 2006, 15, 417–425. [Google Scholar] [CrossRef]
- Hao, T.; Li, W.; Liu, Z.; Sun, Y.; Jin, L.; Li, J.; Gu, C. Low turn-on field nanodiamond conic field emitter. Diam. Relat. Mater. 2017, 75, 91–95. [Google Scholar] [CrossRef]
- Vul, A.; Reich, K.; Eidelman, E.; Terranova, M.L.; Ciorba, A.; Orlanducci, S.; Sessa, V.; Rossi, M. A model of field emission from carbon nanotubes decorated by nanodiamonds. Adv. Sci. Lett. 2010, 3, 110–116. [Google Scholar] [CrossRef]
- Varshney, D.; Weiner, B.R.; Morell, G. Growth and field emission study of a monolithic carbon nanotube/diamond composite. Carbon 2010, 48, 3353–3358. [Google Scholar] [CrossRef]
- Butler, J.E.; Sumant, A.V. The CVD of nanodiamond materials. Chem. Vapor Depos. 2008, 14, 145–160. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhee, K.Y.; Hui, D.; Park, S.J. A critical review of nanodiamond based nanocomposites: Synthesis, properties and applications. Compos. Part B Eng. 2018, 143, 19–27. [Google Scholar] [CrossRef]
- Hong, S.P.; Lee, K.I.; You, H.J.; Jang, S.O.; Choi, Y.S. Scanning deposition method for large-area diamond film synthesis using multiple microwave mlasma mources. Nanomaterials 2022, 12, 1959. [Google Scholar] [CrossRef]
- Nian, Q.; Wang, Y.; Yang, Y.; Li, J.; Zhang, M.Y.; Shao, J.; Tang, L.; Cheng, G.J. Direct Laser Writing of Nanodiamond Films from Graphite under Ambient Conditions. Sci. Rep. 2014, 4, 6612. [Google Scholar] [CrossRef]
- Joshi, P.; Gupta, S.; Riley, P.R.; Narayan, R.J.; Narayan, J. Liquid phase regrowth of <110> nanodiamond film by UV laser annealing of PTFE to generate dense CVD microdiamond film. Diam. Relat. Mater. 2021, 117, 108481. [Google Scholar]
- Sharda, T.; Soga, T.; Jimbo, T.; Umeno, M. Highly stressed carbon film coatings on silicon: Potential applications. Appl. Phys. Lett. 2002, 80, 2880. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, S.; Xue, Y.; Hei, H.; Wu, Y.; Shen, Y. Effect of metal nanoparticle doping concentration on surface morphology and field emission properties of nano-diamond films. Chin. Phys. B 2021, 30, 68101. [Google Scholar] [CrossRef]
- Baidakova, M. New prospects and frontiers of nanodiamond clusters. J. Phys. D Appl. Phys. 2007, 40, 6300. [Google Scholar] [CrossRef]
- Kuznetsov, V.L.; Butenko Yu, V. Ultra-Nanocrystalline Diamond: Syntheses, Properties and Applications; Shenderova, O., Gruen, D., Eds.; William Andrew: New York, NY, USA, 2006; pp. 405–463. [Google Scholar]
- Krueger, A. Diamond nanoparticles: Jewels for chemistry and physics. Adv. Mater. 2008, 20, 2445–2449. [Google Scholar] [CrossRef]
- Arenal, R. EELS Studies on Nanodiamonds and amorphous diamond-like carbon materials. Microsc. Microanal. 2017, 23, 2274–2275. [Google Scholar] [CrossRef]
- Panich, A.M. Nuclear magnetic resonance studies of nanodiamonds. Crit. Rev. Solid State Mater. Sci. 2012, 37, 276–303. [Google Scholar] [CrossRef]
- Johnson, D.F.; Mullin, J.M.; Mattson, W.D. High-velocity collisions of nanodiamond. J. Phys. Chem. C 2017, 121, 1140–1145. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.H. Nanodiamonds: Emerging face of future nanotechnology. Carbon 2019, 143, 678–699. [Google Scholar] [CrossRef]
- Afandi, A.; Howkins, A.; Boyd, I.W.; Jackman, R.B. Nanodiamonds for device applications: An investigation of the properties of boron-doped detonation nanodiamonds. Sci. Rep. 2018, 8, 3270. [Google Scholar] [CrossRef]
- Knowles, H.S.; Kara, D.M.; Atatüre, M. Observing bulk diamond spin coherence in high-purity nanodiamonds. Nat. Mater. 2014, 13, 21–25. [Google Scholar] [CrossRef]
- Belobrov, P.I.; Bursill, L.A.; Maslakov, K.I.; Dementjev, A.P. Electron spectroscopy of nanodiamond surface states. Appl. Surf. Sci. 2003, 215, 169–177. [Google Scholar] [CrossRef]
- Kaciulis, S.; Mezzi, A.; Calvani, P.; Trucchi, D.M. Electron spectroscopy of the main allotropes of carbon. Surf. Interface Anal. 2014, 46, 966–969. [Google Scholar] [CrossRef]
- Santos, M.; Campos, R.A.; Azevedo, A.F.; Baldan, M.R.; Ferreira, N.G. Nanocrystalline diamond films prepared with different diamond seeding processes of 4 nm and 0.25 mm diamond powders. Mater. Sci. Forum 2014, 802, 146–151. [Google Scholar] [CrossRef]
- Smith, E.J.W.; Piracha, A.H.; Field, D.; Pomeroy, J.W.; Mackenzie, G.R.; Abdallah, Z.; Massabuau, F.C.P.; Hinz, A.M.; Wallis, D.J.; Oliver, R.A.; et al. Mixed-size diamond seeding for low-thermal-barrier growth of CVD diamond onto GaN and AlN. Carbon 2020, 167, 620–626. [Google Scholar] [CrossRef]
- Pasternak, D.G.; Dai, J.; Kalashnikov, D.A.; Sedov, V.S.; Martyanov, A.K.; Ralchenko, V.G.; Krivitsky, L.A.; Vlasov, I.I. Low-Temperature Silicon-Vacancy Luminescence of Individual Chemical Vapor Deposition Nanodiamonds Grown by Seeding and Spontaneous Nucleation. Phys. Status Solidi A 2021, 218, 2000274. [Google Scholar] [CrossRef]
- Yarbrough, W.A. A chemical perspective on the nucleation and growth of diamond from hydrocarbons. In Proceedings Applications of Diamond Films and Related Materials; Elsevier: Amsterdam, The Netherlands, 1991; pp. 25–34. [Google Scholar]
- Ralchenko, V.G.; Korotoushenko, K.G.; Smolin, A.A.; Konov, V.I. Patterning of diamond films by direct laser writing: Selective-area deposition, chemical etching and surface smoothing. In Advances in New Diamond Science and Technology; MY: Tokyo, Japan, 1994; pp. 493–496. [Google Scholar]
- Higuchi, K.; Noda, S. Selected area diamond deposition by control the nucleation sites. Diam. Relat. Mater. 1992, 1, 220–229. [Google Scholar] [CrossRef]
- Smolin, A.A.; Ralchenko, V.G.; Pimenov, S.M.; Kononenko, T.V.; Loubnin, E.N. Optical monitoring of nucleation and growth of diamond films. Appl. Phys. Lett. 1993, 62, 3449–3451. [Google Scholar] [CrossRef]
- Arnault, J.C.; Girard, H.A. Diamond Nucleation and Seeding Techniques: Two Complementary Strategies for the Growth of Ultra-thin Diamond Films. In Nanodiamond (RSC Nanoscience and Nanotechnology 31); Williams, O.A., Ed.; Royal Society of Chemistry: London, UK, 2014; p. 221. [Google Scholar]
- Gupta, S.; Sachan, R.; Narayan, J. Scale-up of Q-carbon and nanodiamonds by pulsed laser annealing. Diam. Relat. Mater. 2019, 99, 107531. [Google Scholar] [CrossRef]
- Danilenko, V.V. Nanocarbon phase diagram and conditions for detonation nanodiamond formation. Synthesis, Properties and applications of ultrananocrystalline diamond. In Proceedings of NATO Advanced Research Workshop; Springer: Dordrecht, The Netherlands, 2005; pp. 181–198. [Google Scholar]
- Pobedinskas, P.; Degutis, G.; Dexters, W.; D’Haen, J.; Van Bael, M.K.; Haenen, K. Nanodiamond seeding on plasma-treated tantalum thin films and the role of surface contamination. Appl. Surf. Sci. 2021, 538, 148016. [Google Scholar] [CrossRef]
- Boudou, J.P.; Curmi, P.A.; Jelezko, F.; Wrachtrup, J.; Aubert, P.; Sennour, M.; Balasubramanian, G.; Reuter, R.; Thore, A.; Gaffet, E. High yield fabrication of fluorescent nanodiamonds. Nanotechnology 2009, 20, 235602. [Google Scholar] [CrossRef]
- Stacey, A.; Aharonovich, I.; Prawer, S.; Butler, J.E. Controlled synthesis of high quality micro/nano-diamonds by microwave plasma chemical vapor deposition. Diam. Relat. Mater. 2009, 18, 51–55. [Google Scholar] [CrossRef]
- Gogotsi, Y.G.; Nickel, K.G.; Bahloul-Hourlier, D.; Merle-Mejean, T.; Khomenko, G.E.; Skjerlie, K.P. Structure of carbon produced by hydrothermal treatment of β-SiC powder. J. Mater. Chem. 1996, 6, 595–604. [Google Scholar] [CrossRef]
- Piazza, F.; Solá, F.; Resto, O.; Fonseca, L.F.; Morell, G. Synthesis of diamond nanocrystals on polyimide film. Diam. Relat. Mater. 2009, 18, 113–116. [Google Scholar] [CrossRef]
- Ong, T.P.; Chiou, W.A.; Chen, F.R.; Chang, R.P.H. Preparation of nanocrystalline diamond films for optical coating applications using a pulsed microwave plasma CVD method. Carbon 1990, 28, 799. [Google Scholar] [CrossRef]
- Fendrych, F.; Taylor, A.; Peksa, L.; Kratochvilova, I.; Vlcek, J.; Rezacova, V.; Petrak, V.; Kluiber, Z.; Fekete, L.; Liehr, M.; et al. Growth and characterization of nanodiamond layers prepared using the plasma-enhanced linear antennas microwave CVD system. J. Phys. D Appl. Phys. 2010, 43, 374018. [Google Scholar] [CrossRef]
- Heiman, A.; Gouzman, I.; Christiansen, S.H.; Strunk, H.P.; Hoffman, A. Nano-diamond films deposited by direct current glow discharge assisted chemical vapor deposition. Diam. Relat. Mater. 2000, 9, 866–871. [Google Scholar] [CrossRef]
- Hoffman, A. Mechanism and Properties of Nanodiamond Films Deposited by the DC-GD-CVD Process. Synthesis, Properties and Applications of Ultrananocrystalline Diamond; Springer: Dordrecht, The Netherlands, 2005; pp. 125–144. [Google Scholar]
- Ikenaga, N.; Sakudo, N.; Awazu, K.; Yasui, H.; Hasegawa, Y. Study on hybrid nano-diamond films formed by plasma chemical vapor deposition (CVD). Vacuum 2006, 80, 810–813. [Google Scholar] [CrossRef]
- Zuo, Y.G.; Li, J.J.; Bai, Y.; Liu, H.; Yuan, H.W.; Chen, G.C. Growth of nanocrystalline diamond by dual radio frequency inductively coupled plasma jet CVD. Diam. Relat. Mater. 2017, 73, 67–71. [Google Scholar] [CrossRef]
- Su, Q.; Shi, W.; Li, D.; Lai, J.; Jiang, L.; Wang, L.; Ding, W.; Xia, Y. Effects of carbon concentration on properties of nano-diamond films. Appl. Surf. Sci. 2012, 258, 4645–4648. [Google Scholar] [CrossRef]
- KC, A.; Saha, R.; Anderson, J.; Ayala, A.; Engdahl, C.; Piner, E.L.; Holtz, M.W. Effect of seeding density on the growth of diamond films by hot-filament chemical vapor deposition from sparse to dense range. J. Appl. Phys. 2021, 130, 225302. [Google Scholar]
- Sharda, T.; Rahaman, M.M.; Nukaya, Y.; Soga, T.; Jimbo, T.; Umeno, M. Structural and optical properties of diamond and nano-diamond films grown by microwave plasma chemical vapor deposition. Diam. Relat. Mater. 2001, 10, 561–567. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. Nanocrystalline diamond films grown by microwave plasma chemical vapor deposition and its biocompatible property. Adv. Mater. Phys. Chem. 2018, 8, 157–176. [Google Scholar] [CrossRef]
- Raina, S.; LeQuan, X.A.C.; Kang, W.P.; Davidson, J.L. Effect of Nitrogen Concentration on Nanodiamond Film Characteristics for Electrode Application. ECS Trans. 2009, 19, 23. [Google Scholar] [CrossRef]
- Liu, Y.; Tzeng, Y.K.; Lin, D.; Pei, A.; Lu, H.; Melosh, N.A.; Shen, Z.X.; Chu, S.; Cui, Y. An ultrastrong double-layer nanodiamond interface for stable lithium metal anodes. Joule 2018, 2, 1595–1609. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Teii, K. Control of the growth regimes of nanodiamond and nanographite in microwave plasmas. IEEE Trans. Plasma Sci. 2012, 40, 1783–1788. [Google Scholar] [CrossRef]
- Das, D.; Roy, A. Growth of nanostructured diamond films on glass substrates by low-temperature microwave plasma-enhanced chemical vapor deposition for applications in nanotribology. ACS Appl. Nano Mater. 2022, 5, 3558–3571. [Google Scholar] [CrossRef]
- Chang, C.; Lee, C.Y.; Tai, N.H. Nitrogen-incorporated ovoid-shaped nanodiamond films for dopamine detection. ACS Appl. Nano Mater. 2020, 3, 11970–11978. [Google Scholar] [CrossRef]
- Giussani, A.; Janssens, S.D.; Vázquez-Cortés, D.; Fried, E. Evolution of nanodiamond seeds during the chemical vapor deposition of diamond on silicon substrates in oxygen-rich plasmas. Appl. Surf. Sci. 2022, 581, 152103. [Google Scholar] [CrossRef]
- Tang, Y.H.; Zhou, X.T.; Hu, Y.F.; Lee, C.S.; Lee, S.T.; Sham, T.K. A soft X-ray absorption study of nanodiamond films prepared by hot-filament chemical vapor deposition. Chem. Phys. Lett. 2003, 372, 320–324. [Google Scholar]
- Zhang, G.; Zhou, Y.; Korneychuk, S.; Samuely, T.; Liu, L.; May, P.W.; Xu, Z.; Onufriienko, O.; Zhang, X.; Verbeeck, J.; et al. Superconductor-insulator transition driven by pressure-tuned intergrain coupling in nanodiamond films. Phys. Rev. Mater. 2019, 3, 034801. [Google Scholar]
- Chen, X.; Mohr, M.; Brühne, K.; Mertens, M.; Gluche, P.; Garrn, I.; Fecht, H.J. Smart wear sensor device based on nanodiamond multilayers. Micro Nano Eng. 2022, 16, 100151. [Google Scholar] [CrossRef]
- Liu, Y.; Khabashesku, V.N.; Halas, N.J. Fluorinated nanodiamond as a wet chemistry precursor for diamond coatings covalently bonded to glass surface. J. Am. Chem. Soc. 2005, 127, 3712–3713. [Google Scholar]
- Huang, H.; Dai, L.; Wang, D.H.; Tan, L.S.; Osawa, E. Large-scale self-assembly of dispersed nanodiamonds. J. Mater. Chem. 2008, 18, 1347–1352. [Google Scholar] [CrossRef]
- Wang, H.D.; Yang, Q.; Niu, C.H. Preparation of films of nanodiamonds by step-by-step deposition approach through hydrogen bonding. Diam. Relat. Mater. 2012, 25, 73–79. [Google Scholar] [CrossRef]
- Patoary, N.H.; Rai, A.; Patel, K.P.; Rebecca, A.; Zhang, W.; Ulrich, A.J.; Galib, M.; Desai, T.; Zivanovic, S.; Yousufuddin, M.; et al. Directed covalent assembly of nanodiamonds into thin films. Diam. Relat. Mater. 2020, 101, 107605. [Google Scholar] [CrossRef]
- Desai, T.; Patoary, N.H.; Moore, A.L.; Radadia, A.D. Effect of pH variation and annealing on covalently assembled nanodiamond films. Appl. Surf. Sci. 2021, 565, 150585. [Google Scholar] [CrossRef]
- Gupta, S.; Narayan, J. Direct conversion of Teflon into nanodiamond films. Mater. Res. Lett. 2020, 8, 408–416. [Google Scholar] [CrossRef]
- Joshi, P.; Riley, P.; Gupta, S.; Narayan, R.J.; Narayan, J. Advances in laser-assisted conversion of polymeric and graphitic carbon into nanodiamond films. Nanotechnology 2021, 32, 432001. [Google Scholar] [CrossRef]
- Zhou, D.; Krauss, A.R.; Qin, L.C.; McCauley, T.G.; Gruen, D.M.; Corrigan, T.D.; Chang, R.P.H.; Gnaser, H. Synthesis and electron field emission of nanocrystalline diamond thin films grown from N2/CH4 microwave plasmas. J. Appl. Phys. 1997, 82, 4546–4550. [Google Scholar] [CrossRef]
- Tian, S.; Li, Y.; Xia, X.; Gu, C.; Li, J. Highly efficient field emission from nanodiamond films treated by fast reactive ion etching process. Phys. E Low Dimens. Syst. Nanostruct. 2011, 43, 1902–1905. [Google Scholar] [CrossRef]
- Wu, K.; Wang, E.G.; Cao, Z.X.; Wang, Z.L.; Jiang, X. Microstructure and its effect on field electron emission of grain-size-controlled nanocrystalline diamond films. J. Appl. Phys. 2000, 88, 2967–2974. [Google Scholar] [CrossRef]
- Wang, S.G.; Zhang, Q.; Yoon, S.F.; Ahn, J.; Zhou, Q.; Wang, Q.; Yang, D.J.; Li, J.Q.; Shanyong, S.Z. Electron field emission enhancement effects of nano-diamond films. Surf. Coat. Technol. 2003, 167, 143–147. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lin, S.J.; Chia, C.T.; Cheng, H.F.; Lin, I.N. Synthesis and electron field emission properties of nanodiamond films. Diam. Relat. Mater. 2004, 13, 2100–2104. [Google Scholar] [CrossRef]
- Long, H.; Li, S.; Luo, H.; Wang, Y.; Wei, Q.P.; Yu, Z.M. The effect of periodic magnetic field on the fabrication and field emission properties of nanocrystalline diamond films. Appl. Surf. Sci. 2015, 353, 548–552. [Google Scholar] [CrossRef]
- LeQuan, X.C.; Kang, W.P.; Davidson, J.L.; Choi, B.K.; Wong, Y.M.; Barbosa, R.; Lu, W. Effect of rearranging sp2/sp3 hybridized-bonding on the field emission characteristics of nano-crystalline diamond films. Diam. Relat. Mater. 2009, 18, 200–205. [Google Scholar] [CrossRef]
- Koinkar, P.M.; Patil, S.S.; Kim, T.G.; Yonekura, D.; More, M.A.; Joag, D.S.; Murakami, R.I. Enhanced field emission characteristics of boron doped diamond films grown by microwave plasma assisted chemical vapor deposition. Appl. Surf. Sci. 2011, 257, 1854–1858. [Google Scholar] [CrossRef]
- Ivanov, O.A.; Bogdanov, S.A.; Vikharev, A.L.; Luchinin, V.V.; Golubkov, V.A.; Ivanov, A.S.; Ilyin, V.A. Emission properties of undoped and boron-doped nanocrystalline diamond films coated silicon carbide field emitter arrays. J. Vac. Sci. Technol. B 2018, 36, 021204. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Zhang, F.; Dong, J.; Zhao, W.; Zhai, C.X.; Zhang, W.H. The field emission characteristics of titanium-doped nano-diamonds. Chin. Phys. Lett. 2012, 29, 018103. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Zhang, Z.; Zhai, C.; Liu, Q. Field Emission Characteristics of Metal-doped Nano-diamond Cathode on Titanium Substrate. Rare Metal Mater. Eng. 2017, 46, 617–621. [Google Scholar] [CrossRef]

| Gas Mixture | CVD | Growth Temperature (°C) | Growth Duration | Substrate Pretreatment | References |
|---|---|---|---|---|---|
| 91%H2–9%CH4 | DCCVD | 800–950 | 30 min | Bias-enhanced nucleation (BEN) | [59] |
| 90~98%H2–2~10%CH4 | RFCVD | 726.85–826.85 | 6–17 h | Scratched and seeding | [62] |
| 98%~97%H2–2%~3% C3H6O | HFCVD | Filament: 2100; Substrate: 600–700 | - | BEN | [64] |
| 69%Ar–30%N2–1%CH4 | MWCVD | 400–1200 | 120 min | Scratched and seeding | [69] |
| 7.5sccmH2–0~8sccmCO2–7.5sccmCH4 | MWCVD | 300 | - | none | [70] |
| 98.5%H2–0.5%O2–1%CH4 | MWCVD | 769–884 | 0–30 h | seeding | [72] |
| 98.5%H2–1.5%CH4 | HFCVD | Filament: 2100; Substrate: 800. | 7 h | Bias-enhanced nucleation (BEN) | [73] |
| 99.4%H2–0.6%CH4 (B2H6/CH4 ratio of 5%) | HFCVD | Filament: 2200; Substrate: 800. | 40 min | seeding | [74] |
| 93%,97%H2–7%,3%CH4 | HFCVD | - | - | Electrochemically treated and seeding | [75] |
| Gas Mixture (Method) | Sample | Grain Size | Turn-On /Threshold Electron Field | Maximum Current Density | References | ||
|---|---|---|---|---|---|---|---|
| E | J | Jmax | E | ||||
| 4%CH4/ 96%N2 (CVD) | N-doped ND film | 10–30 nm | 3.2 V/μm | 4 μA/cm2 | 400 μA/cm2 | 6 V/μm | [83] |
| 20%CH4/ 80%H2 (CVD) | ND film | 10 nm | 2.5 V/μm | 10 μA/cm2 | 150 μA/cm2 | ~3.75 V/μm | [84] |
| 1%CH4/ 99%H2 (CVD) | ND film | 15–20 nm | 4.0 V/μm | 1 μA/cm2 | 560 μA/cm2 | 7.2 V/μm | [86] |
| 1%CH4/ 4%H2 /95%N2 (CVD) | N-doped ND film | 15–20 nm | 2.2 V/μm | 1 μA/cm2 | 720 μA/cm2 | 6.4 V/μm | [86] |
| 5%CH4/ 95%H2 (CVD) | ND film | 20 nm | 8.5 V/μm | 10 μA/cm2 | 500 μA/cm2 | 20 V/μm | [87] |
| 1%CH4/ 99%H2 (CVD) | ND film | ~ | 2.9 V/μm | 1 μA/cm2 | 32.7 μA/cm2 | 6.5 V/μm | [88] |
| 9.1%CH4/ 81.8% H2/9.1%N2 (CVD) | N-doped ND film | 10–20 nm | 3.5 V/μm | 1 μA/cm2 | - | - | [89] |
| 5%CH4/ 94.5%H2/0.5%B(OCH3)3 (CVD) | B-doped ND film | 20 nm | 18 V/μm | 10 μA/cm2 | 700 μA/cm2 | 30 V/μm | [17] |
| 19.9%CH4/ 79.6%H2/0.5%B2O3 (CVD) | B-doped ND film | <30 nm | 0.8 V/μm | 1 μA/cm2 | ~60 μA/cm2 | ~1 V/μm | [91] |
| 1.92%CH4/ 98%H2/0.08%B(OCH3)3 (CVD) | B-doped ND film coated 6H-SiC FEA | - | 9 V/μm | 1 μA/cm2 | ~50 μA/cm2 | ~16.2 V/μm | [92] |
| Glucose@urea solid layer (heating precursor) | N-doped ND film | 20~100 nm | 3.6 V/μm | 10 μA/cm2 | 1000 μA/cm2 | 6.0 V/μm | [15] |
| ND powder/Ti powder (EPD @annealing) | Ti-doped ND coating | - | 5.95 V/μm | 1 μA/cm2 | 130 μA/cm2 | 13.8 V/μm | [93] |
| ND powder/Ni nano powder (EPD @annealing) | Ni-doped ND film | - | 1.38 V/μm | 1 μA/cm2 | 1323 μA/cm2 | 2.94 V/μm | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; You, Y.; Bao, A.; Jia, P.; Xiong, J.; Li, J. Recent Progress of Nanodiamond Film in Controllable Fabrication and Field Emission Properties. Nanomaterials 2023, 13, 577. https://doi.org/10.3390/nano13030577
Guo X, You Y, Bao A, Jia P, Xiong J, Li J. Recent Progress of Nanodiamond Film in Controllable Fabrication and Field Emission Properties. Nanomaterials. 2023; 13(3):577. https://doi.org/10.3390/nano13030577
Chicago/Turabian StyleGuo, Xin, Yajun You, Aida Bao, Pinggang Jia, Jijun Xiong, and Junshuai Li. 2023. "Recent Progress of Nanodiamond Film in Controllable Fabrication and Field Emission Properties" Nanomaterials 13, no. 3: 577. https://doi.org/10.3390/nano13030577
APA StyleGuo, X., You, Y., Bao, A., Jia, P., Xiong, J., & Li, J. (2023). Recent Progress of Nanodiamond Film in Controllable Fabrication and Field Emission Properties. Nanomaterials, 13(3), 577. https://doi.org/10.3390/nano13030577







