Defining Quality Criteria for Nanoplastic Hazard Evaluation: The Case of Polystyrene Nanoplastics and Aquatic Invertebrate Daphnia spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Collection
2.2. Data Extraction and Organisation in Matrix Table
2.3. Study Evaluation, Refinement of Quality Criteria, and Scoring System
3. Results and Discussion
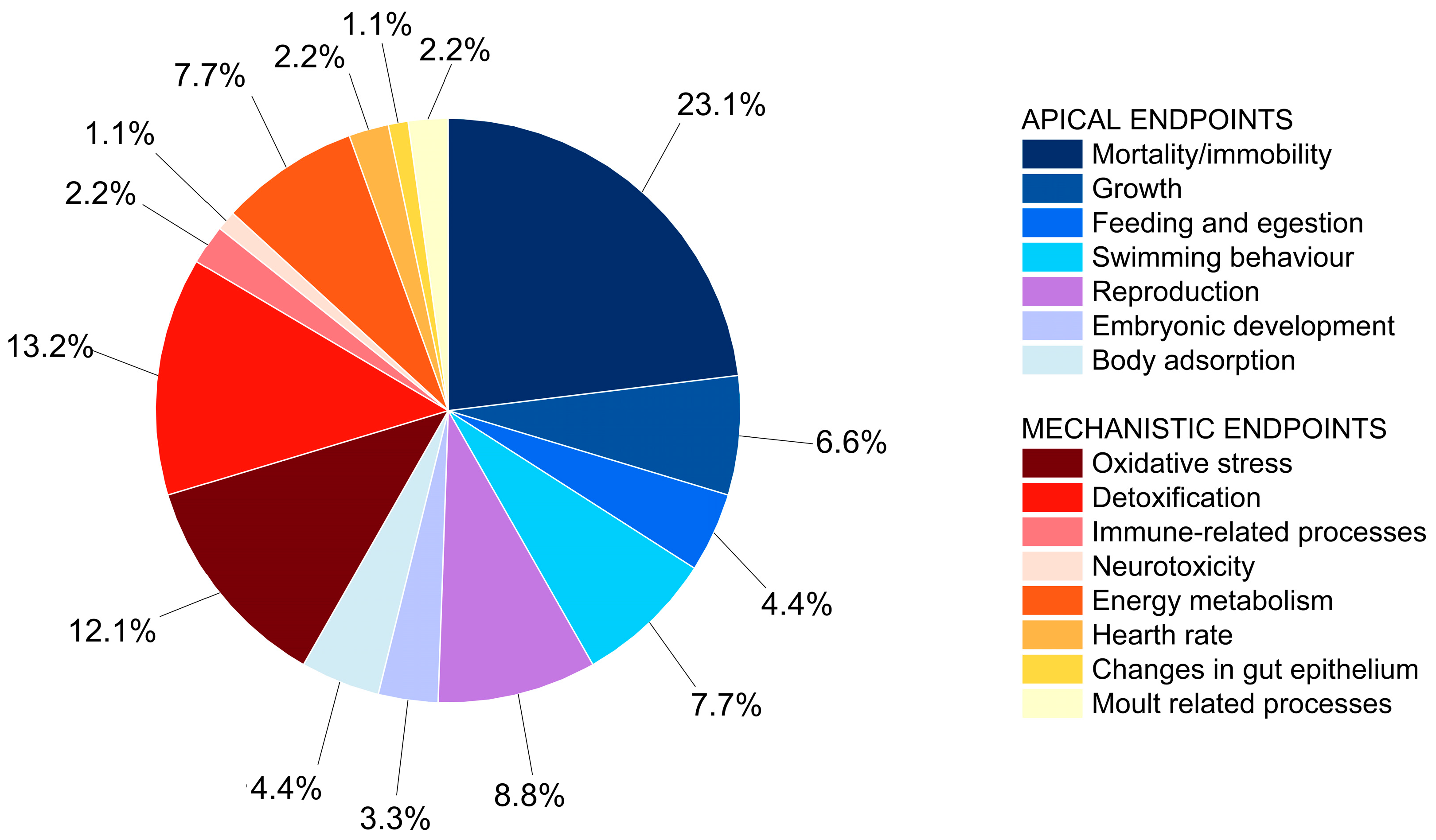
3.1. Description of Studies Retrieved and Organization in a Matrix Table
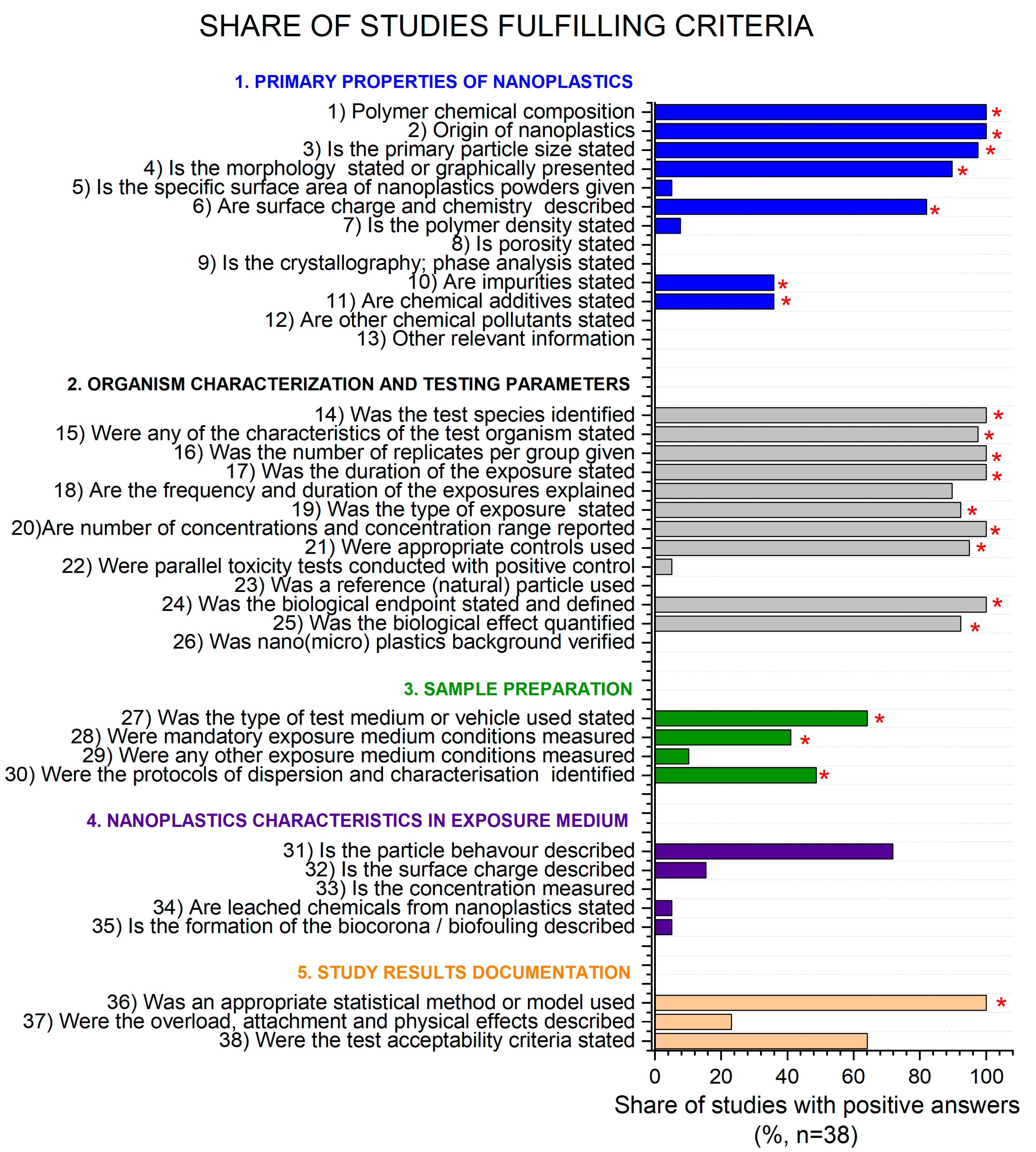
3.2. Refinement of Study Quality Criteria for Hazard Evaluation of NanoPS with Daphnia sp.
- (1)
- The initial criteria needed additional explanation in order to avoid misinterpretation by the different evaluators. Detailed explanations for the existing criteria elaborated in this study are given in Table 1.
- (2)
- Some criteria needed nanoPS property-specific clarifications. NanoPS are usually sold as suspensions, hence some physico-properties cannot be provided (e.g., surface area) or are less commonly provided in general for nanoplastics (density, porosity, crystallography, radical production capacity, magnetic properties, surface charge, and concentration of particles prior/after the test). A common property for nanoPS commercial suspensions is the addition of NaN3, which acts as an antimicrobial additive. Specific attention was thus given to define criteria related to additives and impurities.
- (3)
- Authors commonly refer to standard guidelines being followed for daphnids, but this was often not true in all aspects. Specifically, when the authors referred to the use of a modified standard, the study should be thoroughly checked for all methodological details. For example, the type of medium was rarely reported, but a reference to the standard was made. However, the standard itself does not define which type of test medium should be used but provides several options. Hence, each study should specify the exact type of medium used. Additionally, the standards specifically define that some media parameters, such as pH and dissolved oxygen, should be measured, but this was rarely reported. When it comes to the use of a reference chemical in the tests, this criterion should be reported in studies regardless of the standard mentioned. This is because the standards do not define the reference chemical that should be tested in each experiment.
- (4)
- Searching for specific data in the manuscripts was not user friendly. We used the automatic built-in search tool in the pdf viewer whenever possible, but this was commonly not sufficient. The information in manuscripts was scattered and did not following a uniform nomenclature. Accordingly, this might have introduced a bias into the evaluation, as some criteria might have been met, but the information was not found by the evaluator. Additionally, data were available in the supplementary materials, which are not commonly the main focus of readers.
- (5)
- The authors did not commonly provide all data related to the nanoplastics tested or the test methodology but rather referred to previously published work. This led to additional work to search for previously published papers and these data might be missing for proper interpretation of the toxicity data provided. Hence, a minimum set of physico-chemical properties of nanoplastics and methodological details should be available in each study. Transparency, openness, and reproducibility of research are already recognized as vital features of scientific research and data reuse aligned with FAIR principles [63]. The minimum reporting information (MRI) as identified in our study covered fundamental aspects that should be included already in research planning and later in research reporting [64]. Only more complete and transparent reporting could facilitate data search and reuse as well as study quality evaluation [65]. We suggest an MRI, as presented in Supplementary information (Table S2).
3.3. Classification of Criteria Importance and Threshold Development
3.4. Results of Study Quality Evaluation of NanoPS Toxicity Studies with Daphnia spp.
3.5. Hazard Evaluation based on Accepted Studies
4. Conclusions
- Refining the existing criteria and providing an unambiguous description of each criterion for the selected case study served the purpose of re-evaluating data in scientific contexts (here: evaluation of polystyrene nanoplastic hazard);
- A scoring system and threshold values for acceptance of studies/data for the purpose of hazard evaluation were developed;
- A data reporting template for future studies, providing a list of criteria that authors can follow, was created that facilitated the retrieval of relevant information for data quality evaluation (Table S2).
- No consensus on the hazard potential of nanoPS for Daphnia spp. was determined, even among the high-quality studies.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qiao, R.; Mortimer, M.; Richter, J.; Rani-Borges, B.; Yu, Z.; Heinlaan, M.; Lin, S.; Ivask, A. Hazard of polystyrene micro-and nanospheres to selected aquatic and terrestrial organisms. Sci. Total. Environ. 2022, 853, 158560. [Google Scholar] [CrossRef] [PubMed]
- Kelpsiene, E.; Ekvall, M.T.; Lundqvist, M.; Torstensson, O.; Hua, J.; Cedervall, T. Review of ecotoxicological studies of widely used polystyrene nanoparticles. Environ. Sci. Process. Impacts 2021, 24, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Gaylarde, C.C.; Baptista Neto, J.A.; da Fonseca, E.M. Nanoplastics in aquatic systems—Are they more hazardous than micro-plastics? Environ. Pollut. 2021, 272, 115950. [Google Scholar] [CrossRef] [PubMed]
- Schröter, L.; Ventura, N. Nanoplastic Toxicity: Insights and Challenges from Experimental Model Systems. Small 2022, 18, 2201680. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Nowack, B. A Meta-analysis of Ecotoxicological Hazard Data for Nanoplastics in Marine and Freshwater Systems. Environ. Toxicol. Chem. 2020, 39, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Heinlaan, M.; Kasemets, K.; Aruoja, V.; Blinova, I.; Bondarenko, O.; Lukjanova, A.; Khosrovyan, A.; Kurvet, I.; Pullerits, M.; Sihtmäe, M.; et al. Hazard evaluation of polystyrene nanoplastic with nine bioassays did not show particle-specific acute toxicity. Sci. Total. Environ. 2019, 707, 136073. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Stiles, C.R.; Hagen, N.A.; Biondo, P.D.; Cummings, G.G. Assessment of study quality for systematic reviews: A comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: Methodological research. J. Eval. Clin. Pr. 2010, 18, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Kokalj, A.J.; Hartmann, N.B.; Drobne, D.; Potthoff, A.; Kühnel, D. Quality of nanoplastics and microplastics ecotoxicity studies: Refining quality criteria for nanomaterial studies. J. Hazard. Mater. 2021, 415, 125751. [Google Scholar] [CrossRef]
- Pikuda, O.; Xu, E.G.; Berk, D.; Tufenkji, N. Toxicity Assessments of Micro- and Nanoplastics Can Be Confounded by Preservatives in Commercial Formulations. Environ. Sci. Technol. Lett. 2018, 6, 21–25. [Google Scholar] [CrossRef]
- Loos, C.; Syrovets, T.; Musyanovych, A.; Mailänder, V.; Landfester, K.; Nienhaus, G.U.; Simmet, T. Functionalized polystyrene nanoparticles as a platform for studying bio–nano interactions. Beilstein J. Nanotechnol. 2014, 5, 2403–2412. [Google Scholar] [CrossRef]
- Fernández-Cruz, M.L.; Hernández-Moreno, D.; Catalán, J.; Cross, R.K.; Stockmann-Juvala, H.; Cabellos, J.; Lopes, V.R.; Matzke, M.; Ferraz, N.; Izquierdo, J.J.; et al. Quality evaluation of human and environmental toxicity studies performed with nanomaterials—The GUIDEnano approach. Environ. Sci. Nano 2017, 5, 381–397. [Google Scholar] [CrossRef]
- Nau, K.; Bohmer, N.; Kühnel, D.; Marquardt, C.; Paul, F.; Steinbach, C.; Krug, H.F. The Dana2.0 Knowledge Base on Nanomaterials—Communicating Current Nanosafety Research Based on Evaluated Literature Data. J. Mater. Educ. 2016, 38, 93–109. [Google Scholar]
- Cui, R.; Kim, S.W.; An, Y.-J. Polystyrene nanoplastics inhibit reproduction and induce abnormal embryonic development in the freshwater crustacean Daphnia galeata. Sci. Rep. 2017, 7, 12095. [Google Scholar] [CrossRef] [PubMed]
- Fadare, O.O.; Wan, B.; Guo, L.-H.; Xin, Y.; Qin, W.; Yang, Y. Humic acid alleviates the toxicity of polystyrene nanoplastic particles to Daphnia magna. Environ. Sci. Nano 2019, 6, 1466–1477. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, M.; Yu, P.; Chen, M.; Wu, D.; Zhang, M.; Zhao, Y. Age-dependent survival, stress defense, and AMPK in Daphnia pulex after short-term exposure to a polystyrene nanoplastic. Aquat. Toxicol. 2018, 204, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, P.; Cai, M.; Wu, D.; Zhang, M.; Huang, Y.; Zhao, Y. Polystyrene nanoplastic exposure induces immobilization, reproduction, and stress defense in the freshwater cladoceran Daphnia pulex. Chemosphere 2018, 215, 74–81. [Google Scholar] [CrossRef]
- Nasser, F.; Lynch, I. Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna. J. Proteom. 2016, 137, 45–51. [Google Scholar] [CrossRef]
- Rist, S.; Baun, A.; Hartmann, N.B. Ingestion of micro- and nanoplastics in Daphnia magna—Quantification of body burdens and assessment of feeding rates and reproduction. Environ. Pollut. 2017, 228, 398–407. [Google Scholar] [CrossRef]
- Kelpsiene, E.; Torstensson, O.; Ekvall, M.T.; Hansson, L.-A.; Cedervall, T. Long-term exposure to nanoplastics reduces life-time in Daphnia magna. Sci. Rep. 2020, 10, 5979. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Jiao, Y.; Chen, Q.; Wu, D.; Yu, P.; Li, Y.; Cai, M.; Zhao, Y. Polystyrene nanoplastic induces ROS production and affects the MAPK-HIF-1/NFkB-mediated antioxidant system in Daphnia pulex. Aquat. Toxicol. 2020, 220, 105420. [Google Scholar] [CrossRef]
- Xu, E.G.; Cheong, R.S.; Liu, L.; Hernandez, L.M.; Azimzada, A.; Bayen, S.; Tufenkji, N. Primary and Secondary Plastic Particles Exhibit Limited Acute Toxicity but Chronic Effects on Daphnia magna. Environ. Sci. Technol. 2020, 54, 6859–6868. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, D.S.; Nogueira, D.J.; Melegari, S.P.; Arl, M.; Köerich, J.S.; Cruz, L.; Justino, N.M.; Oscar, B.V.; Puerari, R.C.; da Silva, M.L.N.; et al. Toxicological Evaluation and Quantification of Ingested Metal-Core Nanoplastic by Daphnia magna Through Fluorescence and Inductively Coupled Plasma-Mass Spectrometric Methods. Environ. Toxicol. Chem. 2019, 38, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Z.; Wang, S.; Fang, H.; Wang, D. Aquatic behavior and toxicity of polystyrene nanoplastic particles with different functional groups: Complex roles of pH, dissolved organic carbon and divalent cations. Chemosphere 2019, 228, 195–203. [Google Scholar] [CrossRef]
- Lin, W.; Jiang, R.; Hu, S.; Xiao, X.; Wu, J.; Wei, S.; Xiong, Y.; Ouyang, G. Investigating the toxicities of different functionalized polystyrene nanoplastics on Daphnia magna. Ecotoxicol. Environ. Saf. 2019, 180, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Wang, B.; Lürling, M.; Koelmans, A.A. Correction to Nanoplastic Affects Growth of S. obliquus and Reproduction of D. magna. Environ. Sci. Technol. 2014, 48, 14065. [Google Scholar] [CrossRef]
- Chae, Y.; Kim, D.; Kim, S.W.; An, Y.-J. Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain. Sci. Rep. 2018, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Pérez, E.; Jiang, Q.; Chen, Q.; Jiao, Y.; Huang, Y.; Yang, Y.; Zhao, Y. Polystyrene nanoplastic induces oxidative stress, immune defense, and glycometabolism change in Daphnia pulex: Application of transcriptome profiling in risk assessment of nanoplastics. J. Hazard. Mater. 2020, 402, 123778. [Google Scholar] [CrossRef]
- Vaz, V.P.; Nogueira, D.J.; Vicentini, D.S.; Matias, W.G. Can the sonication of polystyrene nanoparticles alter the acute toxicity and swimming behavior results for Daphnia magna? Environ. Sci. Pollut. Res. 2021, 28, 14192–14198. [Google Scholar] [CrossRef]
- Reynolds, A.; Giltrap, M.; Chambers, G. Evaluation of non-invasive toxicological analysis of nano-polystyrene in relative in vivo conditions to D. magna. Environ. Sci. Nano 2019, 6, 2832–2849. [Google Scholar] [CrossRef]
- Lin, W.; Jiang, R.; Xiong, Y.; Wu, J.; Xu, J.; Zheng, J.; Zhu, F.; Ouyang, G. Quantification of the combined toxic effect of polychlorinated biphenyls and nano-sized polystyrene on Daphnia magna. J. Hazard. Mater. 2018, 364, 531–536. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Sepúlveda, M.S.; Jiang, Q.; Jiao, Y.; Chen, Q.; Huang, Y.; Tian, J.; Zhao, Y. Development of an adverse outcome pathway for nanoplastic toxicity in Daphnia pulex using proteomics. Sci. Total. Environ. 2020, 766, 144249. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiang, R.; Lin, W.; Ouyang, G. Effect of salinity and humic acid on the aggregation and toxicity of polystyrene nanoplastics with different functional groups and charges. Environ. Pollut. 2018, 245, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Grintzalis, K.; Lawson, T.N.; Nasser, F.; Lynch, I.; Viant, M.R. Metabolomic method to detect a metabolite corona on amino-functionalized polystyrene nanoparticles. Nanotoxicology 2019, 13, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Z.; Tang, S.; Li, D.; Jiang, Q.; Zhang, T. Transcriptional response provides insights into the effect of chronic polystyrene nanoplastic exposure on Daphnia pulex. Chemosphere 2019, 238, 124563. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Z.; Cai, M.; Jiao, Y.; Li, Y.; Chen, Q.; Zhao, Y. Molecular characterisation of cytochrome P450 enzymes in waterflea (Daphnia pulex) and their expression regulation by polystyrene nanoplastics. Aquat. Toxicol. 2019, 217, 105350. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, M.; Wu, D.; Yu, P.; Jiao, Y.; Jiang, Q.; Zhao, Y. Effects of nanoplastics at predicted environmental concentration on Daphnia pulex after exposure through multiple generations. Environ. Pollut. 2019, 256, 113506. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Chen, Q.; Li, Y.; Tian, J.; Huang, Y.; Cai, M.; Wu, D.; Zhao, Y. Two sigma and two mu class genes of glutathione S-transferase in the waterflea Daphnia pulex: Molecular characterization and transcriptional response to nanoplastic exposure. Chemosphere 2020, 248, 126065. [Google Scholar] [CrossRef]
- Frankel, R.; Ekvall, M.T.; Kelpsiene, E.; Hansson, L.-A.; Cedervall, T. Controlled protein mediated aggregation of polystyrene nanoplastics does not reduce toxicity towards Daphnia magna. Environ. Sci. Nano 2020, 7, 1518–1524. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, Z.; Lu, G.; Ji, Y. Single and combined effects of microplastics and roxithromycin on Daphnia magna. Environ. Sci. Pollut. Res. 2019, 26, 17010–17020. [Google Scholar] [CrossRef]
- Fadare, O.O.; Wan, B.; Liu, K.; Yang, Y.; Zhao, L.; Guo, L.-H. Eco-Corona vs Protein Corona: Effects of Humic Substances on Corona Formation and Nanoplastic Particle Toxicity in Daphnia magna. Environ. Sci. Technol. 2020, 54, 8001–8009. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Song, L.; Fang, H.; Wang, D.-G. Aquatic toxicity of iron-oxide-doped microplastics to Chlorella pyrenoidosa and Daphnia magna. Environ. Pollut. 2020, 257, 113451. [Google Scholar] [CrossRef] [PubMed]
- De Felice, B.; Sabatini, V.; Antenucci, S.; Gattoni, G.; Santo, N.; Bacchetta, R.; Ortenzi, M.A.; Parolini, M. Polystyrene microplastics ingestion induced behavioral effects to the cladoceran Daphnia magna. Chemosphere 2019, 231, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, J.; Stoll, S.; Slaveykova, V.I. Influence of nanoplastic surface charge on eco-corona formation, aggregation and toxicity to freshwater zooplankton. Environ. Pollut. 2019, 252, 715–722. [Google Scholar] [CrossRef]
- De Felice, B.; Sugni, M.; Casati, L.; Parolini, M. Molecular, biochemical and behavioral responses of Daphnia magna under long-term exposure to poly-styrene nanoplastics. Environ. Int. 2022, 164, 107264. [Google Scholar] [CrossRef]
- Ma, C.; Liu, X.; Zuo, D. Cloning and characterization of AMP-activated protein kinase genes in Daphnia pulex: Modulation of AMPK gene expression in response to polystyrene nanoparticles. Biochem. Biophys. Res. Commun. 2021, 583, 114–120. [Google Scholar] [CrossRef]
- Nogueira, D.J.; Silva, A.C.d.O.d.; da Silva, M.L.N.; Vicentini, D.S.; Matias, W.G. Individual and combined multigenerational effects induced by polystyrene nanoplastic and glyphosate in Daphnia magna (Strauss, 1820). Sci. Total. Environ. 2022, 811, 151360. [Google Scholar] [CrossRef] [PubMed]
- Pochelon, A.; Stoll, S.; Slaveykova, V.I. Polystyrene Nanoplastic Behavior and Toxicity on Crustacean Daphnia magna: Media Composition, Size, and Surface Charge Effects. Environments 2021, 8, 101. [Google Scholar] [CrossRef]
- Verdú, I.; Amariei, G.; Plaza-Bolaños, P.; Agüera, A.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Polystyrene nanoplastics and wastewater displayed antagonistic toxic effects due to the sorption of wastewater micropollutants. Sci. Total. Environ. 2022, 819, 153063. [Google Scholar] [CrossRef]
- Saei, A.; Yazdani, M.; Lohse, S.E.; Bakhtiary, Z.; Serpooshan, V.; Ghavami, M.; Asadian, M.; Mashaghi, S.; Dreaden, E.; Mashaghi, A.; et al. Nanoparticle Surface Functionality Dictates Cellular and Systemic Toxicity. Chem. Mater. 2017, 29, 6578–6595. [Google Scholar] [CrossRef]
- Jeong, J.; Choi, J. Adverse outcome pathways potentially related to hazard identification of microplastics based on toxicity mechanisms. Chemosphere 2019, 231, 249–255. [Google Scholar] [CrossRef]
- Botha, T.L.; Boodhia, K.; Wepener, W. Adsorption, uptake and distribution of gold nanoparticles in Daphnia magna fol-lowing long term exposure. Aquat. Toxicol. 2016, 170, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Novak, S.; Kokalj, A.J.; Hočevar, M.; Godec, M.; Drobne, D. The significance of nanomaterial post-exposure responses in Daphnia magna standard acute immobilisation assay: Example with testing TiO2 nanoparticles. Ecotoxicol. Environ. Saf. 2018, 152, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, G.A.; Torelli, M.D.; Buchman, J.T.; Haynes, C.L.; Hamers, R.J.; Klaper, R.D. Size dependent oxidative stress response of the gut of Daphnia magna to functionalized nanodiamond particles. Environ. Res. 2018, 167, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Palić, D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef]
- Li, M.; Czymmek, K.; Huang, C. Responses of Ceriodaphnia dubia to TiO2 and Al2O3 nanoparticles: A dynamic nano-toxicity assessment of energy budget distribution. J. Hazard. Mater. 2011, 187, 502–508. [Google Scholar] [CrossRef]
- Bahrndorff, S.; Michaelsen, T.Y.; Jensen, A.; Marcussen, L.F.; Nielsen, M.E.; Roslev, P. Automated swimming activity monitor for examining temporal patterns of toxicant effects on individual Daphnia magna. J. Appl. Toxicol. 2015, 36, 896–902. [Google Scholar] [CrossRef]
- Nikitin, O.V.; Nasyrova, E.I.; Nuriakhmetova, V.R.; Stepanova, N.Y.; Danilova, N.V.; Latypova, V.Z. Toxicity assessment of polluted sediments using swimming behavior alteration test with Daphnia magna. IOP Conf. Ser. Earth Environ. Sci. 2018, 107, 012068. [Google Scholar] [CrossRef]
- Ishii, T.; Kamaya, M.; Nagashima, K. A Monitoring System of Feeding Rate in Daphnia Magna for Toxicity Test. J. Environ. Chem. 2006, 16, 369–379. [Google Scholar] [CrossRef]
- Kamaya, M.; Oka, Y.; Fujito, S.; Ginatullina, E. Evolution of Method for Acute Toxicity Using Fluorescent Measurement in D. Magna Feeding Suppression Bioassay in the Case of Potassium Dichromate. Int. J. Emerg. Technol. Adv. Eng. 2017, 7, 6–10. [Google Scholar]
- Park, S.; Jo, A.; Choi, J.; Kim, J.; Zoh, K.-D.; Choi, K. Rapid screening for ecotoxicity of plating and semiconductor wastewater employing the heartbeat of Daphnia magna. Ecotoxicol. Environ. Saf. 2019, 186, 109721. [Google Scholar] [CrossRef]
- Zitova, A.; Cross, M.; Hernan, R.; Davenport, J.; Papkovsky, D.B. Respirometric acute toxicity screening assay using Daphnia magna. Chem. Ecol. 2009, 25, 217–227. [Google Scholar] [CrossRef]
- Jemec, A.; Tišler, T.; Drobne, D.; Sepčić, K.; Jamnik, P.; Roš, M. Biochemical biomarkers in chronically metal-stressed daphnids. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 147, 61–68. [Google Scholar] [CrossRef]
- Jeliazkova, N.; Apostolova, M.D.; Andreoli, C.; Barone, F.; Barrick, A.; Battistelli, C.; Bossa, C.; Botea-Petcu, A.; Châtel, A.; De Angelis, I.; et al. Towards FAIR nanosafety data. Nat. Nanotechnol. 2021, 16, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Higman, R.; Bangert, D.; Jones, S. Three camps, one destination: The intersections of research data management, FAIR and Open. Insights 2019, 32, 1. [Google Scholar] [CrossRef]
- Elberskirch, L.; Binder, K.; Riefler, N.; Sofranko, A.; Liebing, J.; Minella, C.B.; Mädler, L.; Razum, M.; van Thriel, C.; Unfried, K.; et al. Digital research data: From analysis of existing standards to a scientific foundation for a modular metadata schema in nanosafety. Part. Fibre Toxicol. 2022, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Ogonowski, M.; Schür, C.; Jarsén, Å.; Gorokhova, E. The Effects of Natural and Anthropogenic Microparticles on Individual Fitness in Daphnia magna. PLoS ONE 2016, 11, e0155063. [Google Scholar] [CrossRef] [PubMed]
- Bogdanowicz, A.; Zubrowska-Sudol, M.; Krasinski, A.; Sudol, M. Cross-Contamination as a Problem in Collection and Analysis of Environmental Samples Containing Microplastics—A Review. Sustainability 2021, 13, 12123. [Google Scholar] [CrossRef]
| No. | Criteria | Specific Guidance for Evaluator- Example for NanoPS | Relevance |
|---|---|---|---|
| 1. PrimaryProperties of Nanoplastics | |||
| 1 | Polymer chemical composition (CAS No) | This refers to the type of polymer, e.g., polystyrene (PS). | Mandatory |
| 2 | Origin of nanoplastics (commercial supply, laboratory production) including protocol of nanoplastic production and collection if relevant | Protocol for field collection is not relevant for primary nanoPS. | Mandatory |
| 3 | Is the primary particle size stated? | This refers to the diameter of particles. | Mandatory |
| 4 | Is the morphology (shape) stated or graphically presented? | This criterion is also valid if a microscopy image is provided. Most nanoPS are spheres and hence commonly not explicitly mentioned in studies. | Mandatory |
| 5 | Is the specific surface area of nanoplastic powders (e.g., BET surface) given? | This criterion is not valid for nanoPS in suspensions, because BET is measured on powders. | Desirable |
| 6 | Are surface charge and chemistry (any of the following: unlabeled, fluorescent label, functionalization, hydrophobic, hydrophilic, …)/coatings/modifications described? | Special attention should be paid to information regarding labeling. If information on labeling is provided, this is considered as information on particle modification. This criterion is also valid if the study provides information that PS NPs are unlabelled. | Mandatory |
| 7 | Is the polymer density stated? | This is not commonly reported for nanoPS. | Voluntary |
| 8 | Is porosity stated? | This is not commonly reported for nanoPS. | Voluntary |
| 9 | Is the crystallography (crystalline or amorphous phase) and phase analysis (pure or mixed) stated? | This is not commonly reported for nanoPS. | Voluntary |
| 10 | Is the concentration and/or identity of chemical impurities stated to the suspension? Or have any other steps (e.g., dialysis) been taken to remove impurities? | Impurities are chemicals that are present in plastics and/or plastic suspension but were not added intentionally. They might originate from synthesis. If the authors report that no impurities were present, this is a valid information. This criterion is also fulfilled if any step to remove impurities (e.g., dialysis, ultracentrifugation) has been undertaken. | Mandatory |
| 11 | Is the concentration and/or identity of chemical additives stated for the suspension? Or have any other steps (e.g., dialysis; ultracentrifugation) been taken to remove additives? | Additives are chemicals added to plastics and/or plastic suspension to improve their processability, properties, and performance. They are, thus, an essential part of the formulation. The most common additive of nanoPS suspension is sodium azide (NaN3). If the authors report that no additives were present, this criterion is also fulfilled. | Mandatory |
| 12 | Is the concentration and identity of other chemical pollutants stated? | This refers to pollutants that might sorb to nanoPS during use and disposal and is relevant only for secondary nanoPS. | Voluntary |
| 13 | Other relevant information (i.e., radical production capacity, magnetic properties for composite nanoplastics particles with magnetic substances) | This is not commonly reported for nanoPS. | Voluntary |
| 2. Organism Characterization and Testing Parameters | |||
| 14 | Was the test species identified? | Specific species of Daphnia are not required by the standard guidelines. Hence, different species can be used (D. magna, D. pulex, D. galeata, etc.) | Mandatory |
| 15 | Were any of the characteristics of the test organism (strain, sex, length, body weight, age, growth stage) stated? | Any of these characteristics should be reported, preferably at least age. | Mandatory |
| 16 | Was the number of replicates per group given? | If the test was done exactly according to the standard, it is assumed that this information was provided by referring to the standard. If use of the Daphtox kit is referenced, it is assumed that this information is provided | Mandatory |
| 17 | Was the duration of the exposure stated (e.g., 48 or 96 h)? | Mandatory | |
| 18 | Are the frequency and duration of the exposures, as well as the time-points of the observation explained? | This applies to additional observations during the time of exposure. | Desirable |
| 19 | Was the type of exposure (e.g., static, flow through, diet) stated? | This is usually not specifically defined, but if medium is not changed during the exposure, this refers to a static test. In chronic tests, the reference to the standard implies feeding the daphnids. | Mandatory |
| 20 | Are the number of concentrations and concentration range tested reported? | Mandatory | |
| 21 | Were appropriate controls (e.g., solvent or negative control) used? | Solvent control is usually not used with nanoPS, but negative control is. | Mandatory |
| 22 | Were parallel toxicity tests conducted with reference chemical or positive control (to ensure performance of the test system)? | This criterion is fulfilled if the authors report on the use of reference chemical. Referring to standard guideline is not sufficient, because standards do not define that the reference chemical should be tested in each experiment. | Desirable |
| 23 | Was a reference (natural) particle used (to improve interpretation of environmental relevance)? | This is not commonly reported for nanoPS. | Desirable |
| 24 | Was the biological endpoint of the toxicity study under evaluation stated and defined? | Definition of endpoint is most important when mechanistic endpoints are studied. | Mandatory |
| 25 | Was the biological effect quantified (e.g., LC50, EC50, NOEC, LOEC, 25% effect, BCF, BAF) or statistical significance determined (for mechanistic endpoints)? | Mandatory | |
| 26 | Was nano(micro) plastic background verified? | This refers to background in the laboratory not in the organism. | Desirable |
| 3. Sample Preparation (Dispersion of as Prepared or Delivered Nanoplastic In Media Used for Biological Experiments) | |||
| 27 | Was the type of test medium or vehicle used stated? | Although the reference to the standard method is made, the type of test medium (M4, M7, Elendt, tap water) should be reported because the standard does not specifically define the type of test medium. | Mandatory |
| 28 | For ecotoxicity studies, were mandatory exposure medium conditions (at least pH and oxygen) measured? Or is this implied by mentioning the test method used, e.g., USEPA, OECD, ASTM? | Standards specifically define that some properties, pH and dissolved oxygen, should be measured. This criterion is valid if the authors mention the use of the standard guideline. The test should be done exactly according to the standard. If the standard was modified, this criterion should be checked specifically in the paper. | Mandatory |
| 29 | For ecotoxicity studies, were any other exposure medium conditions measured? | This includes hardness, conductivity, etc. | Desirable |
| 30 | Were the protocols of dispersion and characterization in the exposure medium identified? Or, were the protocols of preparation of exposure medium stated? | This includes sonication, vortexing, mixing, etc. | Mandatory |
| 4. Nanoplastic Characteristics in Exposure Medium | |||
| 31 | Is the particle behavior before or during the exposure described (i.e., agglomeration / aggregation / sedimentation / floating)? | This includes any type of measurement to describe the behavior of particles in exposure medium, such as DLS, nanoparticle tracking analysis, visual observation of sedimentation | Desirable |
| 32 | Is the surface charge before or during the exposure described? | This is not commonly reported for nanoPS. | Voluntary |
| 33 | Is the concentration before or during the exposure measured? | This is not commonly reported for nanoPS. | Voluntary |
| 34 | Are the concentration and identity of leached chemicals from nanoplastics under experimental conditions stated? | This is not commonly reported for nanoPS. | Desirable |
| 35 | Is the formation of the biocorona / biofouling described? | This is not commonly reported for nanoPS. | Voluntary |
| 5. Study Results Documentation | |||
| 36 | Was an appropriate statistical method or model used to determine toxicity? | This refers to the method used to calculate effect values or statistical difference in comparison to control. The method should take into consideration the normality and homoscedascity of data. | Mandatory |
| 37 | Were the potential overload, attachment, and physical effects in organisms described? | Usually the authors report the adsorption of particles or physical attachment. | Desirable |
| 38 | Were the test acceptability criteria stated (e.g., mortality in control must not exceed a certain percentage)? OR, were test acceptability criteria implied by mention of the test method used (e.g., USEPA, OECD, ASTM, etc)? | Standards specifically define the validity criteria. This criterion is valid if the authors mention the use of the standard guideline. The test should be performed exactly according to the standard. If the standard was modified, this criterion should be checked specifically in the paper. | Desirable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jemec Kokalj, A.; Heinlaan, M.; Novak, S.; Drobne, D.; Kühnel, D. Defining Quality Criteria for Nanoplastic Hazard Evaluation: The Case of Polystyrene Nanoplastics and Aquatic Invertebrate Daphnia spp. Nanomaterials 2023, 13, 536. https://doi.org/10.3390/nano13030536
Jemec Kokalj A, Heinlaan M, Novak S, Drobne D, Kühnel D. Defining Quality Criteria for Nanoplastic Hazard Evaluation: The Case of Polystyrene Nanoplastics and Aquatic Invertebrate Daphnia spp. Nanomaterials. 2023; 13(3):536. https://doi.org/10.3390/nano13030536
Chicago/Turabian StyleJemec Kokalj, Anita, Margit Heinlaan, Sara Novak, Damjana Drobne, and Dana Kühnel. 2023. "Defining Quality Criteria for Nanoplastic Hazard Evaluation: The Case of Polystyrene Nanoplastics and Aquatic Invertebrate Daphnia spp." Nanomaterials 13, no. 3: 536. https://doi.org/10.3390/nano13030536
APA StyleJemec Kokalj, A., Heinlaan, M., Novak, S., Drobne, D., & Kühnel, D. (2023). Defining Quality Criteria for Nanoplastic Hazard Evaluation: The Case of Polystyrene Nanoplastics and Aquatic Invertebrate Daphnia spp. Nanomaterials, 13(3), 536. https://doi.org/10.3390/nano13030536







