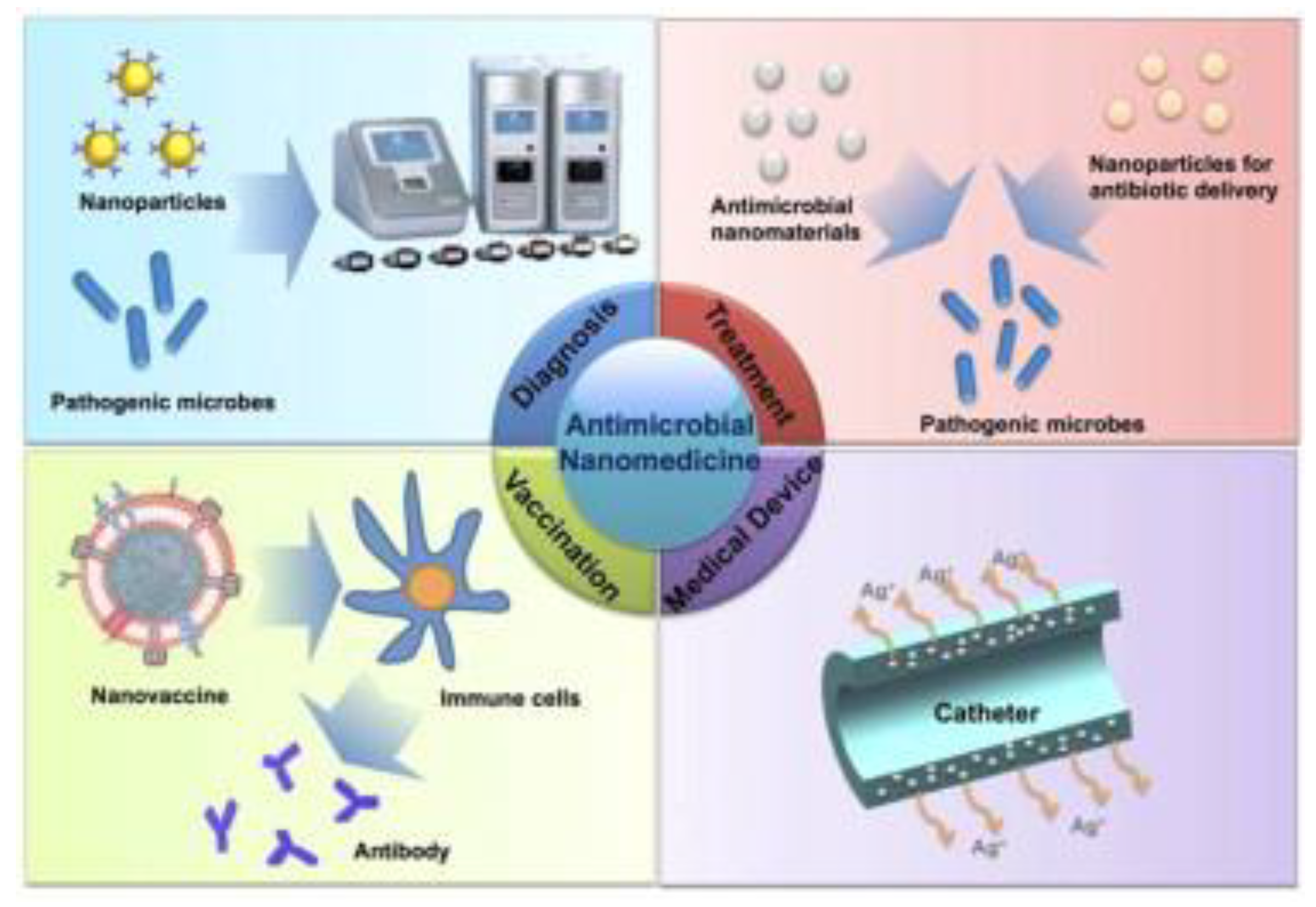
Nanomedicine: New Frontiers in Fighting Microbial Infections
Abstract
1. Introduction
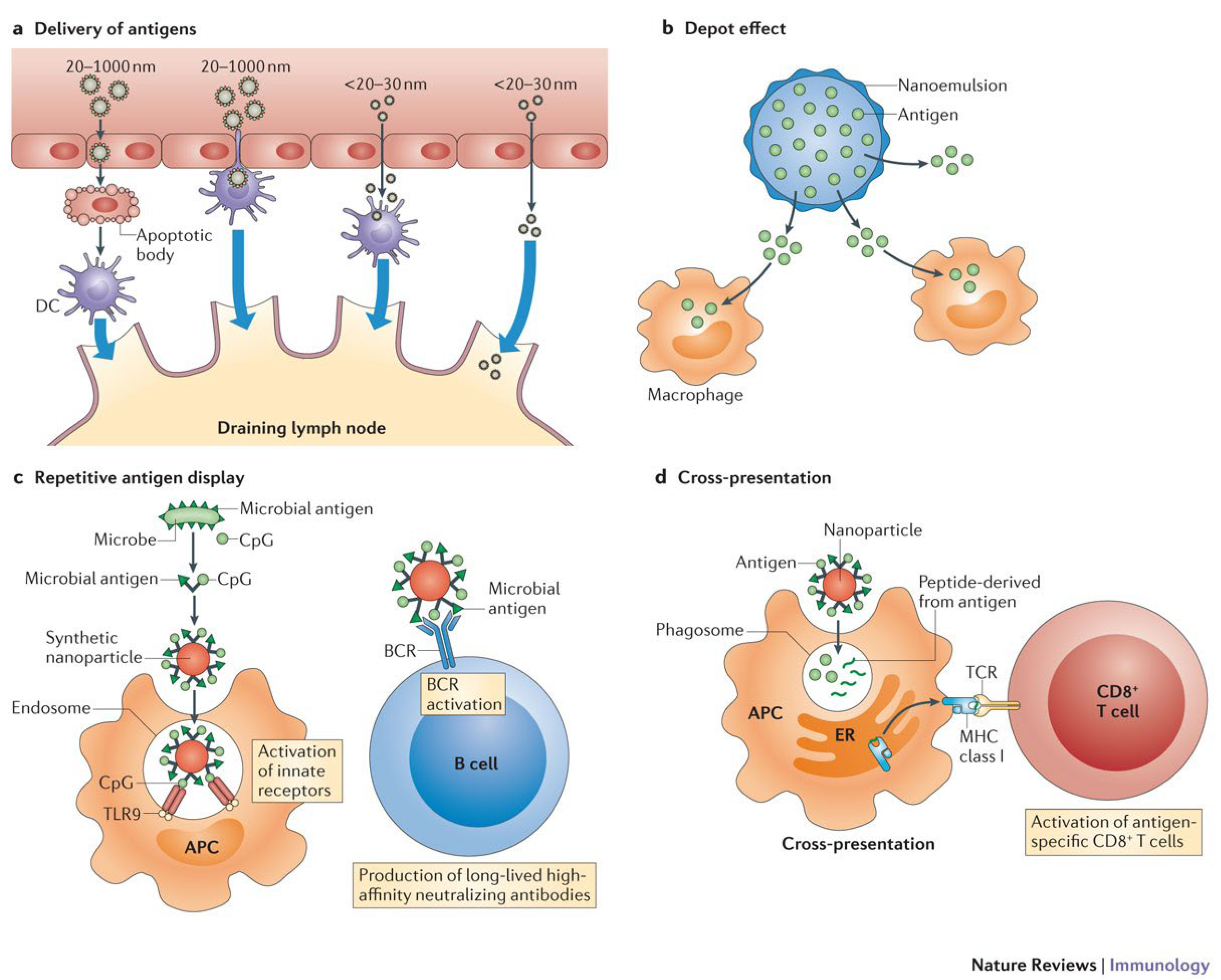
2. Vaccination
2.1. Adjuvant
2.2. Vaccine Delivery
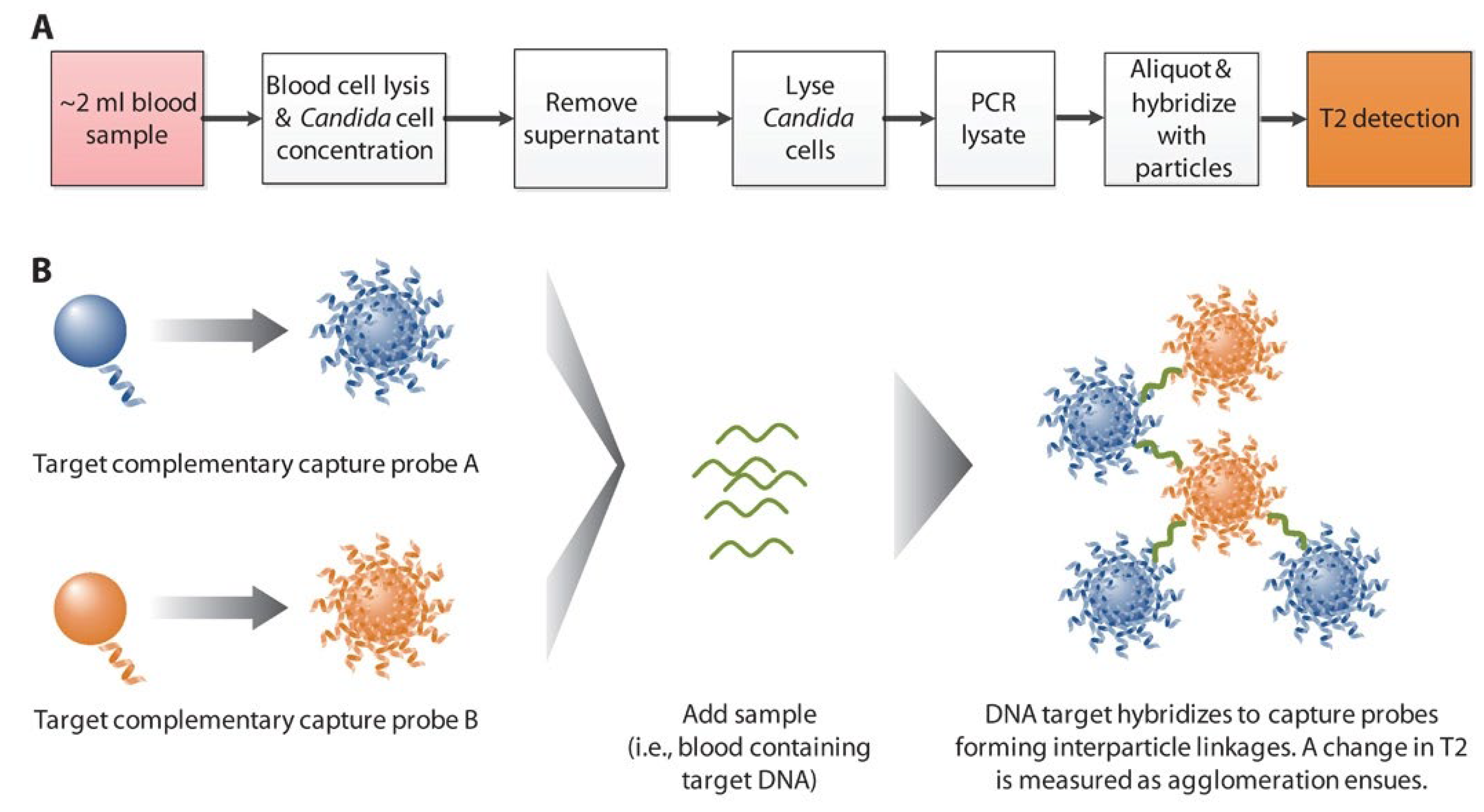
3. Diagnosis
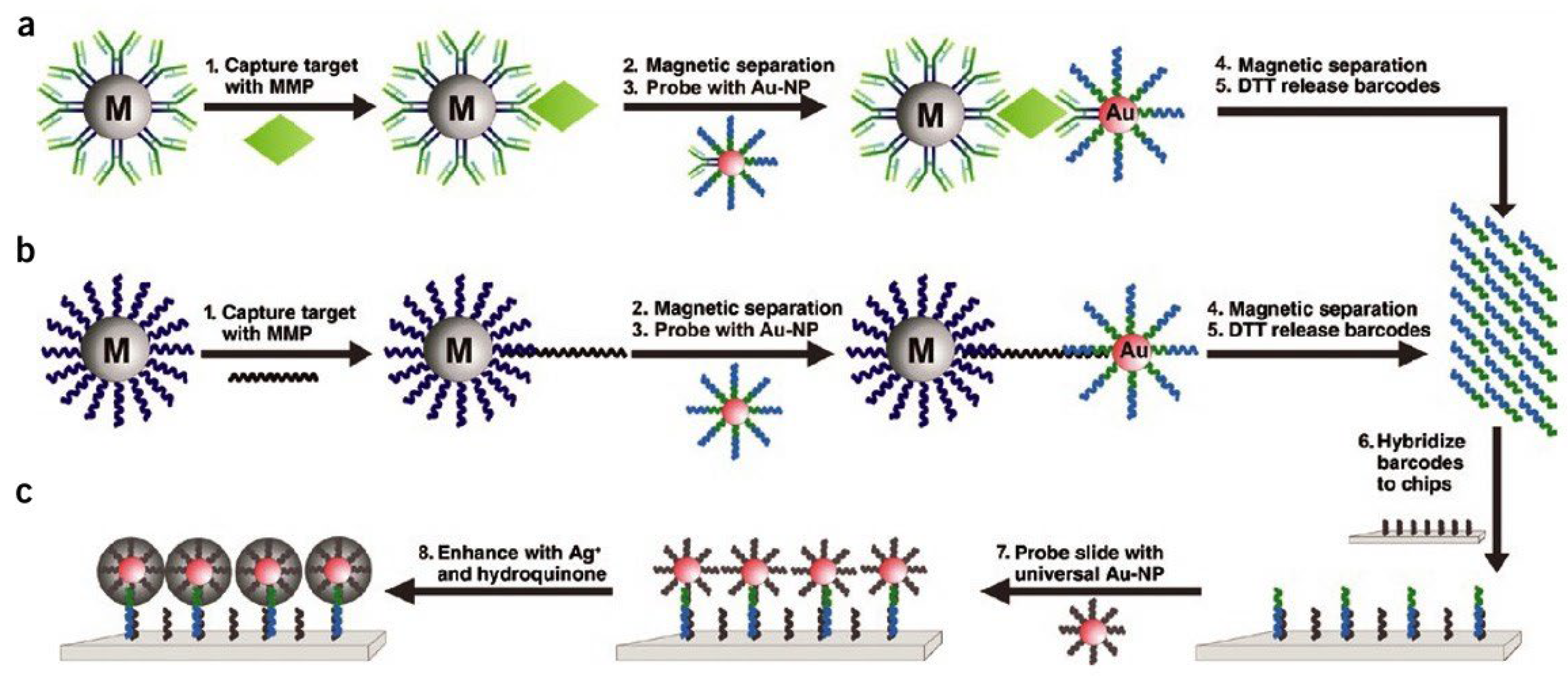
3.1. Magnetic Nanoparticles
3.2. Au Nanoparticles
3.3. Fluorescent Nanoparticles
4. Treatment
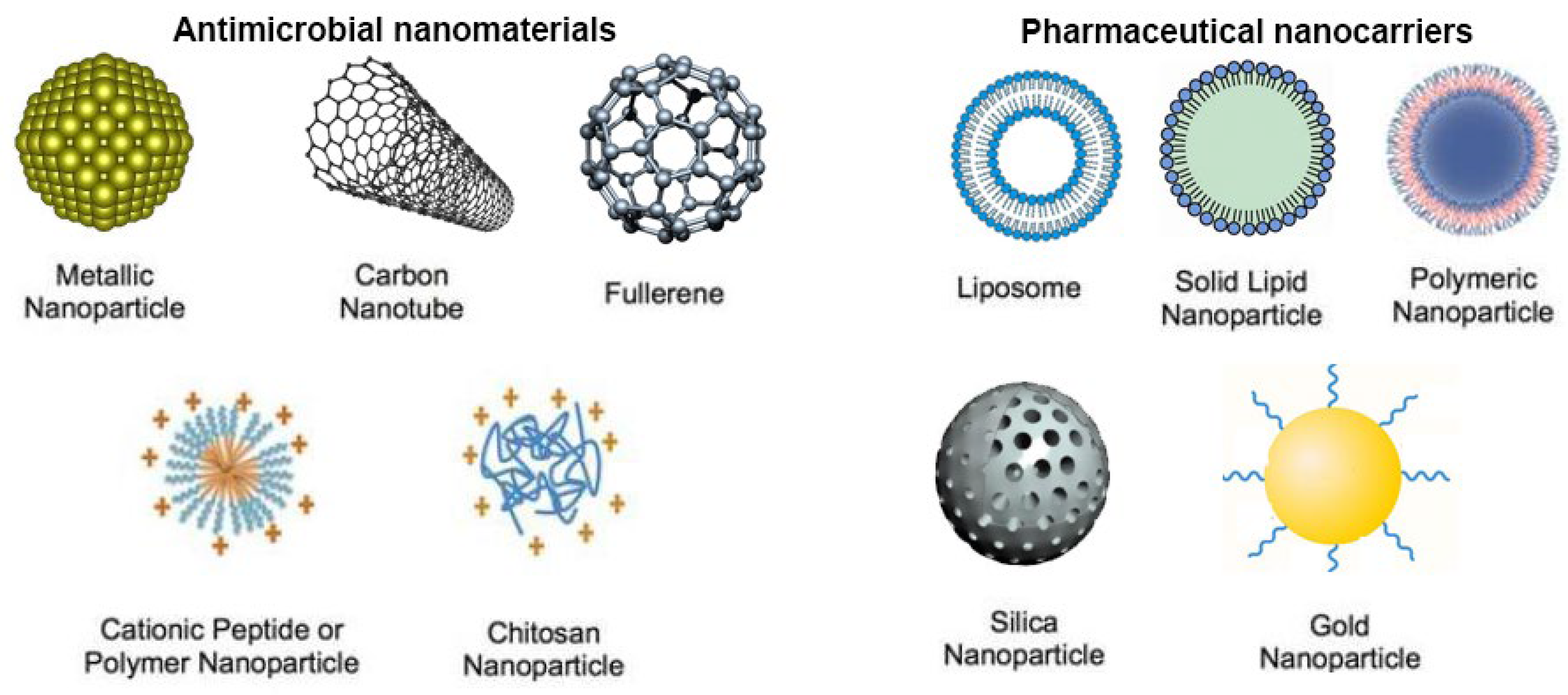
4.1. Antimicrobial Nanomaterials
4.1.1. Inorganic Nanoparticles
4.1.2. Peptide- and Polymer-based Nanoparticles
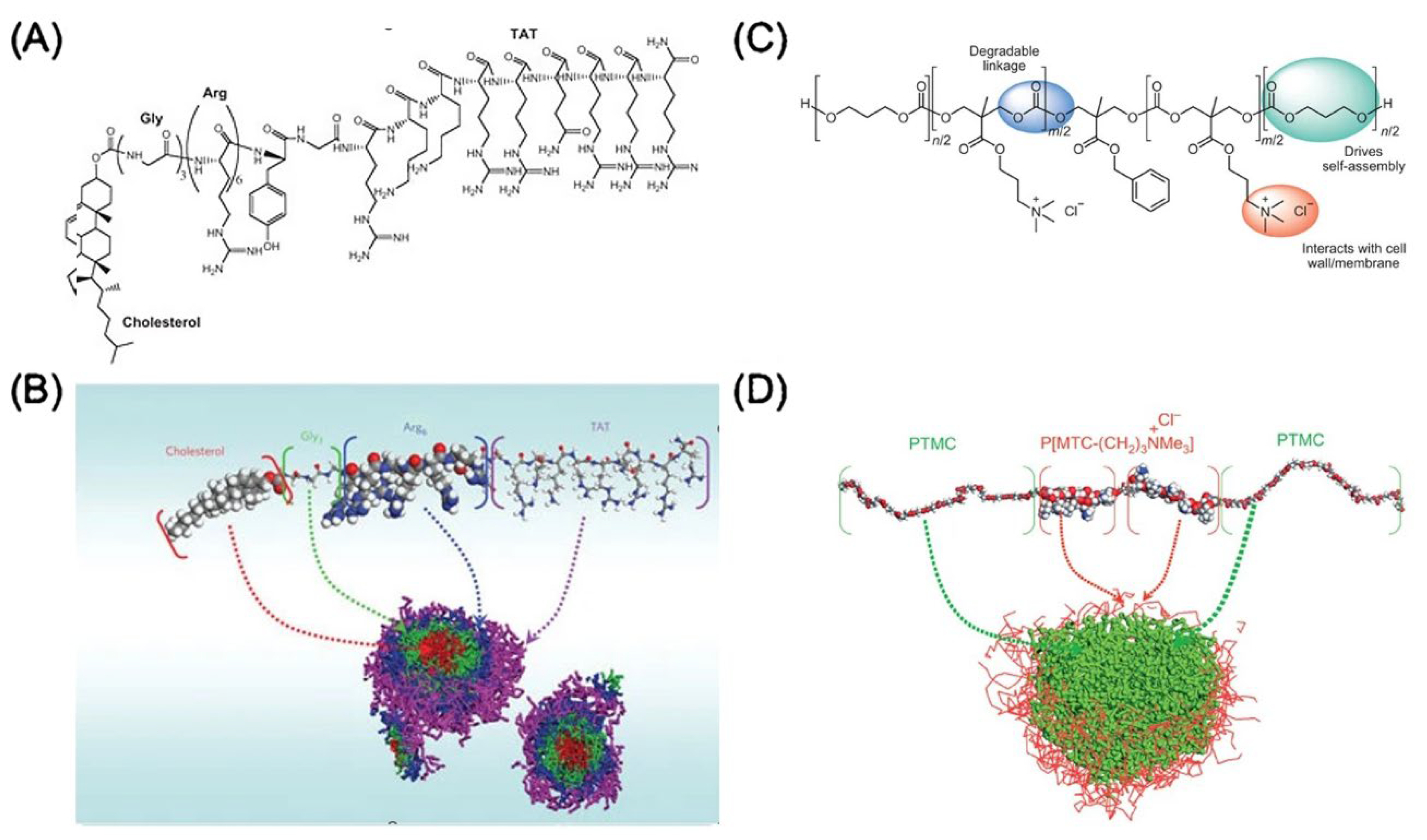
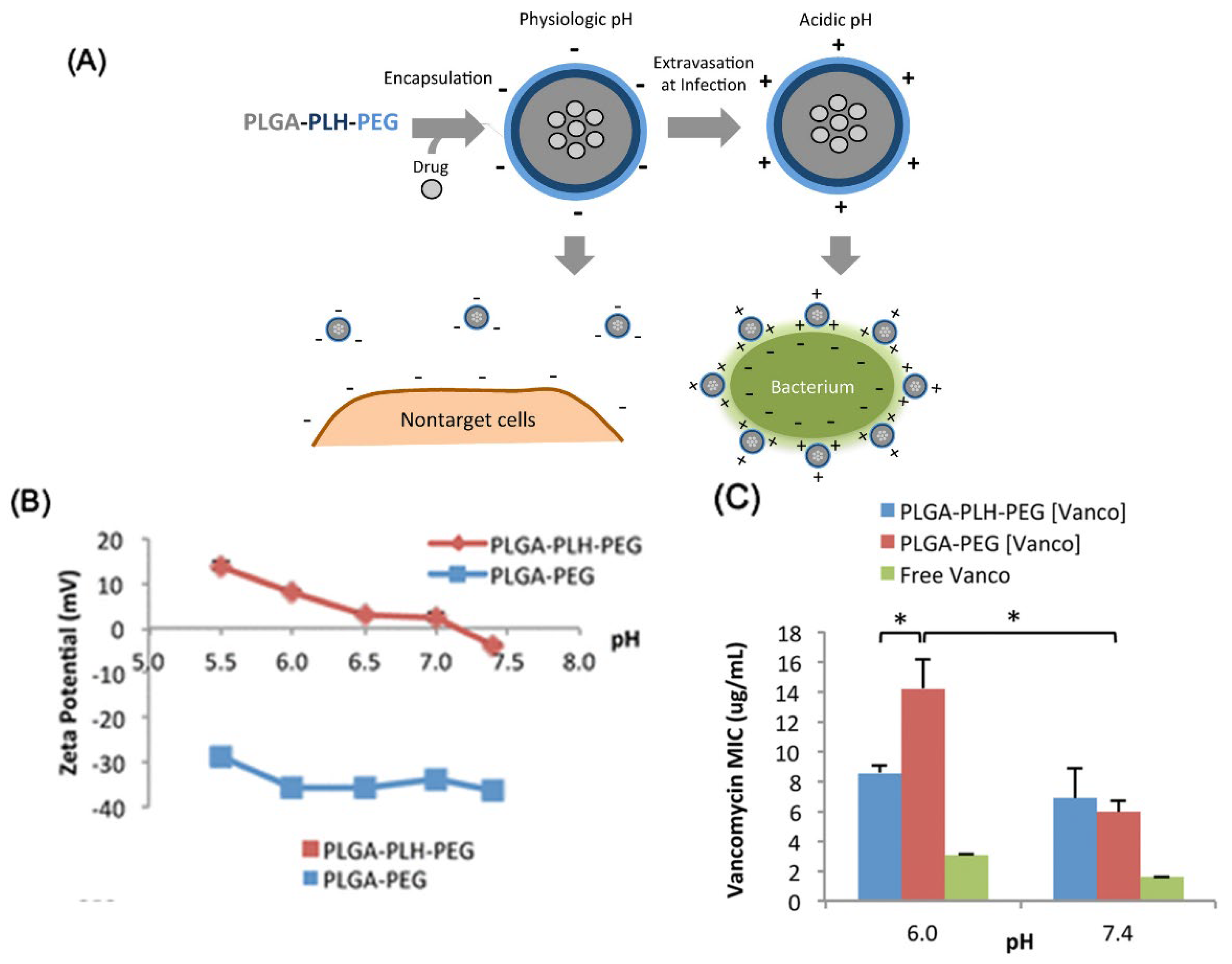
4.2. Drug Delivery
5. Preclinical and Clinical Translation
5.1. Preclinical Translation: Animal-Tested Antimicrobial Nanoparticles
5.1.1. Skin and Subcutaneous Region Infection
5.1.2. Pulmonary Infection
5.1.3. Gastrointestinal (GI) Infection
5.1.4. The Other Infection Sites
5.2. Clinical Trials
| Antimicrobial | Trial Phase | Application | Ref. |
|---|---|---|---|
| Abelcet | Marketed | Fungal infection | [207] |
| AmBisome | Marketed | Fungal infection | [208] |
| Amphotec | Marketed | Fungal infection | [209] |
| Fungisome | Marketed | Fungal infection | [210] |
| Ciprofloxacin | Phase 1 | Pseudomonas aeruginosa | [211] |
| Ciprofloxacin | Phase 2a | Pseudomonas aeruginosa | [211] |
| Ciprofloxacin | Phase 3 | Bronchiectasis and Chronic P. Aeruginosa Infection | [197] |
| Ciprofloxacin | Phase 3 | Non-cystic fibrosis bronchiectasis (NCFB) | [212] |
| Amikacin | Phase 2 | Mycobacterium Infections, Nontuberculous | [199] |
| Amikacin | Phase 3 | Cystic Fibrosis Patients with Chronic Pseudomonas aeruginosa Infection | [202] |
| Amikacin | Phase 2 | Mycobacterium Infections, Nontuberculous Mycobacteria, Atypical | [201] |
| Amikacin | Phase 3 | Mycobacterium Infections, Nontuberculous | [213] |
| Amikacin | Phase 2 | Cystic Fibrosis | [200] |
| Biological: CAL02 | Phase 3 | Severe community-acquired pneumonia | [205] |
| Biological: GS-CDA1 Biological: MDX-1388 | Phase 2 | Clostridium Difficile Associated Disease | [214] |
| Novacta biosystems (NVB-302) | Phase 1 | Clostridium difficile | [215] |
| Human lactoferrin (hlf1-11) | Phase 2 | Infection following transplantation | [216] |
| (a potent cyclic lipodepsipeptides antibiotic) Wap-8294A2 | Phase 2 | Gm+ve bacteria (VRE and MRSA) | [217] |
| The specifically targeted antimicrobialpeptide (C16G2) | Phase 2 | Streptococcus mutans | [218] |
| Antimicrobial Peptide (DPK-060) | Phase 2 | Acute external otitis | [219] |
| LTX-109 (Lytixar) | Phase 2 | Nasal decolonization of MRSA Impetigo | [220] |
| p2TA (AB 103) | Phase 3 | Necrotizing soft tissue infections | [198] |
| Surotomycin | Phase 3 | Clostridium difficile | [221] |
| Ramoplanin (NTI-851) | Phase 2 | Clostridium difficile | [222] |
6. Concluding Remarks
Funding
Data Availability Statement
Conflicts of Interest
References
- Armstrong, G.L.; Conn, L.A.; Pinner, R.W. Trends in Infectious Disease Mortality in the United States During the 20th Century. JAMA 1999, 281, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.L. Changing patterns of infectious disease. Nature 2000, 406, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Jadidi, A.; Salahinejad, E.; Sharifi, E.; Tayebi, L. Drug-delivery Ca-Mg silicate scaffolds encapsulated in PLGA. Int. J. Pharm. 2020, 589, 119855. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Health Estimates 2016: Disease Burden by Cause, Age, Sex, by Country and by region, 2000–2016. 2018. Available online: https://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html (accessed on 28 October 2020).
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef]
- Zunt, J.R.; Kassebaum, N.J.; Blake, N.; Glennie, L.; Wright, C.; Nichols, E.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Adamu, A.A. Global, regional, and national burden of meningitis, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 1061–1082. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Kåhrström, C.T. Entering a post-antibiotic era? Nat. Rev. Genet. 2013, 11, 146. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, J.; Yang, D.; Shao, J.; Wang, W.; Zhang, Q.; Dong, X. Recent advances in pH-responsive nanomaterials for anti-infective therapy. J. Mater. Chem. B 2020, 8, 10700–10711. [Google Scholar] [CrossRef]
- Alekshun, M.N.; Levy, S.B. Molecular Mechanisms of Antibacterial Multidrug Resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef]
- Limbago, B.M.; Kallen, A.J.; Zhu, W.; Eggers, P.; McDougal, L.K.; Albrecht, V.S. Report of the 13th vancomycin-resistant Staph-ylococcus aureus isolate from the United States. J. Clin. Microbiol. 2014, 52, 998–1002. [Google Scholar] [CrossRef]
- Schäberle, T.F.; Hack, I.M. Overcoming the current deadlock in antibiotic research. Trends Microbiol. 2014, 22, 165–167. [Google Scholar] [CrossRef]
- Taubes, G. The Bacteria Fight Back; American Association for the Advancement of Science: Washington, DC, USA, 2008. [Google Scholar]
- Boucher, H.W.; Talbot, G.H.; Benjamin, D.K., Jr.; Bradley, J.; Guidos, R.J.; Jones, R.N.; Murray, B.E.; Bonomo, R.A.; Gilbert, D. 10 × ‘20 Progress—Development of New Drugs Active Against Gram-Negative Bacilli: An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1685–1694. [Google Scholar] [CrossRef]
- Kashkooli, F.M.; Soltani, M.; Souri, M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: Static and dynamic targeting strategies. J. Control. Release 2020, 327, 316–349. [Google Scholar] [CrossRef]
- Kashkooli, F.M.; Soltani, M.; Souri, M.; Meaney, C.; Kohandel, M. Nexus between in silico and in vivo models to enhance clinical translation of nanomedicine. Nano Today 2021, 36, 101057. [Google Scholar] [CrossRef]
- Souri, M.; Soltani, M.; Kashkooli, F.M.; Shahvandi, M.K.; Chiani, M.; Shariati, F.S.; Mehrabi, M.R.; Munn, L.L. Towards principled design of cancer nanomedicine to accelerate clinical translation. Mater. Today Bio 2022, 13, 100208. [Google Scholar] [CrossRef]
- Souri, M.; Soltani, M.; Kashkooli, F.M.; Shahvandi, M.K. Engineered strategies to enhance tumor penetration of drug-loaded nanoparticles. J. Control. Release 2022, 341, 227–246. [Google Scholar] [CrossRef]
- Soltani, M.; Kashkooli, F.M.; Souri, M.; Harofte, S.Z.; Harati, T.; Khadem, A.; Pour, M.H.; Raahemifar, K. Enhancing Clinical Translation of Cancer Using Nanoinformatics. Cancers 2021, 13, 2481. [Google Scholar] [CrossRef]
- Souri, M.; Chiani, M.; Farhangi, A.; Mehrabi, M.R.; Nourouzian, D.; Raahemifar, K.; Soltani, M. Anti-COVID-19 Nanomaterials: Directions to Improve Prevention, Diagnosis, and Treatment. Nanomaterials 2022, 12, 783. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; Lv, X.; Xu, Y.; Xie, Y.; Yuwen, L.; Song, Y.; Li, S.; Shao, J.; Yang, D. Stimuli-responsive therapeutic systems for the treatment of diabetic infected wounds. Nanoscale 2022, 14, 12967–12983. [Google Scholar] [CrossRef]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Radovic-Moreno, A.F.; Wu, J.; Langer, R.; Shi, J. Nanomedicine in the management of microbial infection—Overview and perspectives. Nano Today 2014, 9, 478–498. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Vaccines: Past, present and future. Nat. Med. 2005, 11, S5–S11. [Google Scholar] [CrossRef]
- Choh, L.-C.; Ong, G.-H.; Vellasamy, K.M.; Kalaiselvam, K.; Kang, W.-T.; Al-Maleki, A.R.; Mariappan, V.; Vadivelu, J. Burkholderia vaccines: Are we moving forward? Front. Cell. Infect. Microbiol. 2013, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Curtiss, R. Bacterial infectious disease control by vaccine development. J. Clin. Investig. 2002, 110, 1061–1066. [Google Scholar] [CrossRef]
- Carleton, H.A. Combating Evolving Pathogens: Pathogenic Bacteria as Vaccine Vectors: Teaching Old Bugs New Tricks. Yale J. Biol. Med. 2010, 83, 217. [Google Scholar]
- Peek, L.J.; Middaugh, C.R.; Berkland, C. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 2008, 60, 915–928. [Google Scholar] [CrossRef]
- Smith, D.M.; Simon, J.K.; Baker, J.R., Jr. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef]
- Reddy, S.T.; van der Vlies, A.J.; Simeoni, E.; Angeli, V.; Randolph, G.J.; O’Neil, C.P.; Lee, L.K.; Swartz, M.A.; Hubbell, J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007, 25, 1159–1164. [Google Scholar] [CrossRef]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef]
- Neutra, M.R.; Pringault, E.; Kraehenbuhl, J.-P. Antigen Sampling Across Epithelial Barriers and Induction of Mucosal Immune Responses. Annu. Rev. Immunol. 1996, 14, 275–300. [Google Scholar] [CrossRef]
- Kammona, O.; Kiparissides, C. Recent advances in nanocarrier-based mucosal delivery of biomolecules. J. Control. Release 2012, 161, 781–794. [Google Scholar] [CrossRef]
- Manocha, M.; Pal, P.C.; Chitralekha, K.; Thomas, B.E.; Tripathi, V.; Gupta, S.D.; Paranjape, R.; Kulkarni, S.; Rao, D.N. Enhanced mucosal and systemic immune response with intranasal immunization of mice with HIV peptides entrapped in PLG mi-croparticles in combination with Ulex europaeus-I lectin as M cell target. Vaccine 2005, 23, 5599–5617. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, T.; Myc, A.; Donovan, B.; Shih, A.Y.; Reuter, J.D.; Baker, J.R. A novel surfactant nanoemulsion with a unique non-irritant topical antimicrobial activity against bacteria, enveloped viruses and fungi. Microbiol. Res. 2001, 156, 1–7. [Google Scholar] [CrossRef]
- Bielinska, A.U.; Janczak, K.W.; Landers, J.J.; Makidon, P.; Sower, L.E.; Peterson, J.W.; Baker, J.R., Jr. Mucosal immunization with a novel nanoemulsion-based recombinant anthrax protective antigen vaccine protects against Bacillus anthracis spore chal-lenge. Infect. Immun. 2007, 75, 4020–4029. [Google Scholar] [CrossRef]
- Makidon, P.E.; Knowlton, J.; Groom, J.V.; Blanco, L.P.; Lipuma, J.J.; Bielinska, A.U.; Baker, J.R. Induction of immune response to the 17 kDa OMPA Burkholderia cenocepacia polypeptide and protection against pulmonary infection in mice after nasal vaccination with an OMP nanoemulsion-based vaccine. Med. Microbiol. Immunol. 2010, 199, 81–92. [Google Scholar] [CrossRef]
- Martel, C.J.-M.; Agger, E.M.; Poulsen, J.J.; Jensen, T.H.; Andresen, L.; Christensen, D.; Nielsen, L.P.; Blixenkrone-Møller, M.; Andersen, P.; Aasted, B. CAF01 Potentiates Immune Responses and Efficacy of an Inactivated Influenza Vaccine in Ferrets. PLoS ONE 2011, 6, e22891. [Google Scholar] [CrossRef]
- Kamath, A.T.; Rochat, A.-F.; Christensen, D.; Agger, E.M.; Andersen, P.; Lambert, P.-H.; Siegrist, C.-A. A liposome-based my-cobacterial vaccine induces potent adult and neonatal multifunctional T cells through the exquisite targeting of dendritic cells. PLoS ONE 2009, 4, e5771. [Google Scholar] [CrossRef]
- Henderson, A.; Propst, K.; Kedl, R.; Dow, S. Mucosal immunization with liposome-nucleic acid adjuvants generates effective humoral and cellular immunity. Vaccine 2011, 29, 5304–5312. [Google Scholar] [CrossRef] [PubMed]
- Blecher, K.; Nasir, A.; Friedman, A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2, 395–401. [Google Scholar] [CrossRef]
- Fairley, S.J.; Singh, S.R.; Yilma, A.N.; Waffo, A.B.; Subbarayan, P.; Dixit, S.; Taha, M.A.; Cambridge, C.D.; Dennis, V.A. Chlamydia trachomatis recombinant MOMP encapsulated in PLGA nanoparticles triggers primarily T helper 1 cellular and antibody immune responses in mice: A desirable candidate nanovaccine. Int. J. Nanomed. 2013, 8, 2085–2099. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Fang, R.H.; Luk, B.T.; Zhang, L. Nanoparticle-detained toxins for safe and effective vaccination. Nat. Nanotechnol. 2013, 8, 933–938. [Google Scholar] [CrossRef]
- Kong, I.G.; Sato, A.; Yuki, Y.; Nochi, T.; Takahashi, H.; Sawada, S.; Mejima, M.; Kurokawa, S.; Okada, K.; Sato, S.; et al. Nanogel-Based PspA Intranasal Vaccine Prevents Invasive Disease and Nasal Colonization by Streptococcus pneumoniae. Infect. Immun. 2013, 81, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Cambridge, C.D.; Singh, S.R.; Waffo, A.B.; Fairley, S.J.; Dennis, V.A. Formulation, characterization, and expression of a re-combinant MOMP Chlamydia trachomatis DNA vaccine encapsulated in chitosan nanoparticles. Int. J. Nanomed. 2013, 8, 1759–1771. [Google Scholar]
- Florindo, H.; Pandit, S.; Lacerda, L.; Gonçalves, L.; Alpar, H.; Almeida, A. The enhancement of the immune response against S. equi antigens through the intranasal administration of poly-ɛ-caprolactone-based nanoparticles. Biomaterials 2009, 30, 879–891. [Google Scholar] [CrossRef]
- Schroeder, U.; Graff, A.; Buchmeier, S.; Rigler, P.; Silvan, U.; Tropel, D.; Jockusch, B.M.; Aebi, U.; Burkhard, P.; Schoenenberger, C.-A. Peptide Nanoparticles Serve as a Powerful Platform for the Immunogenic Display of Poorly Antigenic Actin Determinants. J. Mol. Biol. 2009, 386, 1368–1381. [Google Scholar] [CrossRef]
- Kaba, S.A.; Brando, C.; Guo, Q.; Mittelholzer, C.; Raman, S.; Tropel, D.; Aebi, U.; Burkhard, P.; Lanar, D.E. A nonadjuvanted pol-ypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria. J. Immunol. 2009, 183, 7268–7277. [Google Scholar] [CrossRef]
- Sun, H.-X.; Xie, Y.; Ye, Y.-P. ISCOMs and ISCOMATRIX™. Vaccine 2009, 27, 4388–4401. [Google Scholar] [CrossRef]
- Hu, K.-F.; Lövgren-Bengtsson, K.; Morein, B. Immunostimulating complexes (ISCOMs) for nasal vaccination. Adv. Drug Deliv. Rev. 2001, 51, 149–159. [Google Scholar] [CrossRef]
- Salyers, A.A.; Whitt, D.D.; Whitt, D.D. Bacterial Pathogenesis: A Molecular Approach; ASM Press: Washington, DC, USA, 1994. [Google Scholar]
- Allegranzi, B.; Nejad, S.B.; Combescure, C.; Graafmans, W.; Attar, H.; Donaldson, L.; Pittet, D. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet 2011, 377, 228–241. [Google Scholar] [CrossRef]
- Kaittanis, C.; Santra, S.; Perez, J.M. Emerging nanotechnology-based strategies for the identification of microbial pathogenesis. Adv. Drug Deliv. Rev. 2010, 62, 408–423. [Google Scholar] [CrossRef]
- Xie, J.; Liu, G.; Eden, H.S.; Ai, H.; Chen, X. Surface-Engineered Magnetic Nanoparticle Platforms for Cancer Imaging and Therapy. Acc. Chem. Res. 2011, 44, 883–892. [Google Scholar] [CrossRef]
- Souri, M.; Kashkooli, F.M.; Soltani, M. Analysis of Magneto-Hyperthermia Duration in Nano-sized Drug Delivery System to Solid Tumors Using Intravascular-Triggered Thermosensitive-Liposome. Pharm. Res. 2022, 39, 753–765. [Google Scholar] [CrossRef]
- Souri, M.; Soltani, M.; Kashkooli, F.M. Computational modeling of thermal combination therapies by magneto-ultrasonic heating to enhance drug delivery to solid tumors. Sci. Rep. 2021, 11, 19539. [Google Scholar] [CrossRef]
- Chow, E.K.-H.; Ho, D. Cancer Nanomedicine: From Drug Delivery to Imaging. Sci. Transl. Med. 2013, 5, 216rv4. [Google Scholar] [CrossRef]
- Neely, L.A.; Audeh, M.; Phung, N.A.; Min, M.; Suchocki, A.; Plourde, D.; Blanco, M.; Demas, V.; Skewis, L.R.; Anagnostou, T.; et al. T2 Magnetic Resonance Enables Nanoparticle-Mediated Rapid Detection of Candidemia in Whole Blood. Sci. Transl. Med. 2013, 5, 182ra54. [Google Scholar] [CrossRef]
- Bizzini, A.; Durussel, C.; Bille, J.; Greub, G.; Prod’Hom, G. Performance of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Identification of Bacterial Strains Routinely Isolated in a Clinical Microbiology Laboratory. J. Clin. Microbiol. 2010, 48, 1549–1554. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Tsai, P.-J.; Weng, M.-F.; Chen, Y.-C. Affinity Capture Using Vancomycin-Bound Magnetic Nanoparticles for the MALDI-MS Analysis of Bacteria. Anal. Chem. 2005, 77, 1753–1760. [Google Scholar] [CrossRef]
- Lee, J.-J.; Jeong, K.J.; Hashimoto, M.; Kwon, A.H.; Rwei, A.; Shankarappa, S.A.; Tsui, J.H.; Kohane, D.S. Synthetic Ligand-Coated Magnetic Nanoparticles for Microfluidic Bacterial Separation from Blood. Nano Lett. 2014, 14, 1–5. [Google Scholar] [CrossRef]
- Kaittanis, C.; Nath, S.; Perez, J.M. Rapid Nanoparticle-Mediated Monitoring of Bacterial Metabolic Activity and Assessment of Antimicrobial Susceptibility in Blood with Magnetic Relaxation. PLoS ONE 2008, 3, e3253. [Google Scholar] [CrossRef]
- Uehara, N. Polymer-functionalized Gold Nanoparticles as Versatile Sensing Materials. Anal. Sci. 2010, 26, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective Colorimetric Detection of Polynucleotides Based on the Distance-Dependent Optical Properties of Gold Nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef]
- Storhoff, J.J.; Lucas, A.D.; Garimella, V.; Bao, Y.P.; Müller, U.R. Homogeneous detection of unamplified genomic DNA sequences based on colorimetric scatter of gold nanoparticle probes. Nat. Biotechnol. 2004, 22, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.C.; Jin, R.; Mirkin, C.A. Nanoparticles with Raman Spectroscopic Fingerprints for DNA and RNA Detection. Science 2002, 297, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.D.; Mirkin, C.A. The bio-barcode assay for the detection of protein and nucleic acid targets using DTT-induced ligand exchange. Nat. Protoc. 2006, 1, 324–336. [Google Scholar] [CrossRef]
- Scott, L.J. Verigene® Gram-Positive Blood Culture Nucleic Acid Test. Mol. Diagn. Ther. 2013, 17, 117–122. [Google Scholar] [CrossRef]
- Chan, P.-H.; Wong, S.-Y.; Lin, S.-H.; Chen, Y.-C. Lysozyme-encapsulated gold nanocluster-based affinity mass spectrometry for pathogenic bacteria. Rapid Commun. Mass Spectrom. 2013, 27, 2143–2148. [Google Scholar] [CrossRef]
- Chan, P.-H.; Chen, Y.-C. Human serum albumin stabilized gold nanoclusters as selective luminescent probes for Staphylo-coccus aureus and methicillin-resistant Staphylococcus aureus. Anal. Chem. 2012, 84, 8952–8956. [Google Scholar] [CrossRef]
- Nath, S.; Kaittanis, C.; Tinkham, A.; Perez, J.M. Dextran-coated gold nanoparticles for the assessment of antimicrobial suscep-tibility. Anal. Chem. 2008, 80, 1033–1038. [Google Scholar] [CrossRef]
- Zhao, X.; Hilliard, L.R.; Mechery, S.J.; Wang, Y.; Bagwe, R.P.; Jin, S.; Tan, W. A rapid bioassay for single bacterial cell quantitation using bioconjugated nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 15027–15032. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, W.; O’Donoghu, M.B.; Tan, W. Fluorescent Nanoparticles for Multiplexed Bacteria Monitoring. Bioconjugate Chem. 2007, 18, 297–301. [Google Scholar] [CrossRef]
- Zrazhevskiy, P.; Sena, M.; Gao, X. Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chem. Soc. Rev. 2010, 39, 4326–4354. [Google Scholar] [CrossRef]
- Tully, E.; Hearty, S.; Leonard, P.; O’Kennedy, R. The development of rapid fluorescence-based immunoassays, using quantum dot-labelled antibodies for the detection of Listeria monocytogenes cell surface proteins. Int. J. Biol. Macromol. 2006, 39, 127–134. [Google Scholar] [CrossRef]
- Jayaraman, R. Antibiotic resistance: An overview of mechanisms and a paradigm shift. Curr. Sci. 2009, 96, 1475–1484. [Google Scholar]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Ray, K.; Marteyn, B.; Sansonetti, P.J.; Tang, C.M. Life on the inside: The intracellular lifestyle of cytosolic bacteria. Nat. Rev. Genet. 2009, 7, 333–340. [Google Scholar] [CrossRef]
- Lv, X.; Wang, L.; Mei, A.; Xu, Y.; Ruan, X.; Wang, W.; Shao, J.; Yang, D.; Dong, X. Recent Nanotechnologies to Overcome the Bacterial Biofilm Matrix Barriers. Small 2022, 2206220. [Google Scholar] [CrossRef]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Hu, Y.; Ruan, X.; Lv, X.; Xu, Y.; Wang, W.; Cai, Y.; Ding, M.; Dong, H.; Shao, J.; Yang, D.; et al. Biofilm microenvironment-responsive nanoparticles for the treatment of bacterial infection. Nano Today 2022, 46, 101602. [Google Scholar] [CrossRef]
- Huang, L.; Dai, T.; Xuan, Y.; Tegos, G.P.; Hamblin, M.R. Synergistic Combination of Chitosan Acetate with Nanoparticle Silver as a Topical Antimicrobial: Efficacy against Bacterial Burn Infections. Antimicrob. Agents Chemother. 2011, 55, 3432–3438. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Tran, P. Nanomaterial-Based Treatments for Medical Device-Associated Infections. ChemPhysChem 2012, 13, 2481–2494. [Google Scholar] [CrossRef]
- Makvandi, P.; Wang, C.Y.; Zare, E.N.; Borzacchiello, A.; Niu, L.N.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio Silver: Its Interactions with Peptides and Bacteria, and Its Uses in Medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef] [PubMed]
- Yougbaré, S.; Chou, H.-L.; Yang, C.-H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Kuo, T.-R. Facet-dependent gold nanocrystals for effective photothermal killing of bacteria. J. Hazard. Mater. 2021, 407, 124617. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Núnez, N.V.; del Carmen Ixtepan Turrent, L.; Rodríguez Padilla, C. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Knetsch, M.L.W.; Koole, L.H. New Strategies in the Development of Antimicrobial Coatings: The Example of Increasing Usage of Silver and Silver Nanoparticles. Polymers 2011, 3, 340–366. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- Veerapandian, M.; Lim, S.K.; Nam, H.M.; Kuppannan, G.; Yun, K.S. Glucosamine-functionalized silver glyconanoparticles: Characterization and antibacterial activity. Anal. Bioanal. Chem. 2010, 398, 867–876. [Google Scholar] [CrossRef]
- Zare, B.; Faramarzi, M.A.; Sepehrizadeh, Z.; Shakibaie, M.; Rezaie, S.; Shahverdi, A.R. Biosynthesis and recovery of rod-shaped tellurium nanoparticles and their bactericidal activities. Mater. Res. Bull. 2012, 47, 3719–3725. [Google Scholar] [CrossRef]
- Webster, T.; Wang, Q.; Perez, J.M. Inhibited growth of Pseudomonas aeruginosa by dextran- and polyacrylic acid-coated ceria nanoparticles. Int. J. Nanomed. 2013, 8, 3395–3399. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.; Lin, M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, X.; Yan, D.; Yin, G.; Liao, X.; Kang, Y.; Yao, Y.; Huang, D.; Hao, B. Toxicological Effect of ZnO Nanoparticles Based on Bacteria. Langmuir 2008, 24, 4140–4144. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Kim, S.H.; Kim, S.S. Hybrid organic/inorganic reverse osmosis (RO) membrane for bactericidal anti-fouling. 1. Preparation and characterization of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane. Environ. Sci. Technol. 2001, 35, 2388–2394. [Google Scholar] [CrossRef]
- Hernandez-Delgadillo, R.; Velasco-Arias, D.; Diaz, D.; Arevalo-Niño, K.; Garza-Enriquez, M.; De la Garza-Ramos, M.A.; Cabral-Romero, C. Zerovalent bismuth nanoparticles inhibit Streptococcus mutans growth and formation of biofilm. Int. J. Nanomed. 2012, 7, 2109–2113. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Lankveld, D.P.K.; Oomen, A.G.; Krystek, P.; Neigh, A.; De Jong, A.T.; Noorlander, C.W.; Van Eijkeren, J.; Geertsma, R.E.; De Jong, W.H. The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials 2010, 31, 8350–8361. [Google Scholar] [CrossRef]
- Qiu, Z.; Yu, Y.; Chen, Z.; Jin, M.; Yang, D.; Zhao, Z.; Wang, J.; Shen, Z.; Wang, X.; Qian, D.; et al. Nanoalumina promotes the horizontal transfer of multiresistance genes mediated by plasmids across genera. Proc. Natl. Acad. Sci. USA 2012, 109, 4944–4949. [Google Scholar] [CrossRef]
- Veerapandian, M.; Yun, K. Functionalization of biomolecules on nanoparticles: Specialized for antibacterial applications. Appl. Microbiol. Biotechnol. 2011, 90, 1655–1667. [Google Scholar] [CrossRef]
- Kotagiri, N.; Lee, J.S.; Kim, J.-W. Selective pathogen targeting and macrophage evading carbon nanotubes through dextran sulfate coating and PEGylation for photothermal theranostics. J. Biomed. Nanotechnol. 2013, 9, 1008–1016. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.R.; Monteiro-Riviere, N.A.; Riviere, J.E. Intrinsic biological property of colloidal fullerene nanoparticles (nC60): Lack of lethality after high dose exposure to human epidermal and bacterial cells. Toxicol. Lett. 2010, 197, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, S.; Koshi, E.; Philip, K.; Mohan, A. Antimicrobial photodynamic therapy: An overview. J. Indian Soc. Periodontol. 2011, 15, 323–327. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Peschel, A.; Sahl, H.-G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Genet. 2006, 4, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Eby, D.M.; Farrington, K.E.; Johnson, G.R. Synthesis of Bioinorganic Antimicrobial Peptide Nanoparticles with Potential Therapeutic Properties. Biomacromolecules 2008, 9, 2487–2494. [Google Scholar] [CrossRef]
- Blin, T.; Purohit, V.; Leprince, J.; Jouenne, T.; Glinel, K. Bactericidal Microparticles Decorated by an Antimicrobial Peptide for the Easy Disinfection of Sensitive Aqueous Solutions. Biomacromolecules 2011, 12, 1259–1264. [Google Scholar] [CrossRef]
- Liu, L.; Xu, K.; Wang, H.; Jeremy Tan, P.; Fan, W.; Venkatraman, S.S.; Li, L.; Yang, Y.-Y. Self-assembled cationic peptide nanopar-ticles as an efficient antimicrobial agent. Nat. Nanotechnol. 2009, 4, 457–463. [Google Scholar] [CrossRef]
- Makovitzki, A.; Baram, J.; Shai, Y. Antimicrobial lipopolypeptides composed of palmitoyl di- and tricationic peptides: In vitro and in vivo activities, self-assembly to nanostructures, and a plausible mode of action. Biochemistry 2008, 47, 10630–10636. [Google Scholar] [CrossRef]
- Khara, J.S.; Wang, Y.; Ke, X.-Y.; Liu, S.; Newton, S.M.; Langford, P.R.; Yang, Y.Y.; Ee, P.L.R. Anti-mycobacterial activities of synthetic cationic α-helical peptides and their synergism with rifampicin. Biomaterials 2014, 35, 2032–2038. [Google Scholar] [CrossRef]
- Engler, A.C.; Wiradharma, N.; Ong, Z.Y.; Coady, D.J.; Hedrick, J.L.; Y. -Yang, Y. Emerging trends in macromolecular antimi-crobials to fight multi-drug-resistant infections. Nano Today 2012, 7, 201–222. [Google Scholar] [CrossRef]
- Song, J.; Kang, H.; Lee, C.; Hwang, S.H.; Jang, J. Aqueous Synthesis of Silver Nanoparticle Embedded Cationic Polymer Nanofibers and Their Antibacterial Activity. ACS Appl. Mater. Interfaces 2012, 4, 460–465. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Friedman, A.J.; Phan, J.; Schairer, D.O.; Champer, J.; Qin, M.; Pirouz, A.; Blecher-Paz, K.; Oren, A.; Liu, P.T.; Modlin, R.L. Antimi-crobial and anti-inflammatory activity of chitosan–alginate nanoparticles: A targeted therapy for cutaneous pathogens. J. Investig. Dermatol. 2013, 133, 1231–1239. [Google Scholar] [CrossRef]
- Potara, M.; Jakab, E.; Damert, A.; Popescu, O.; Canpean, V.; Astilean, S. Synergistic antibacterial activity of chitosan–silver nanocomposites on Staphylococcus aureus. Nanotechnology 2011, 22, 135101. [Google Scholar] [CrossRef]
- Hosseinkhani, H.; Hong, P.-D.; Yu, D.-S. Self-Assembled Proteins and Peptides for Regenerative Medicine. Chem. Rev. 2013, 113, 4837–4861. [Google Scholar] [CrossRef]
- Elsabahy, M.; Heo, G.S.; Lim, S.-M.; Sun, G.; Wooley, K.L. Polymeric Nanostructures for Imaging and Therapy. Chem. Rev. 2015, 115, 10967–11011. [Google Scholar] [CrossRef]
- Jadidi, A.; Davoodian, F.; Salahinejad, E. Effect of poly lactic-co-glycolic acid encapsulation on drug delivery kinetics from vancomycin-impregnated Ca-Mg silicate scaffolds. Prog. Org. Coat. 2020, 149, 105970. [Google Scholar] [CrossRef]
- Jadidi, A.; Shokrgozar, M.A.; Sardari, S.; Maadani, A.M. Gefitinib-loaded polydopamine-coated hollow mesoporous silica nanoparticle for gastric cancer application. Int. J. Pharm. 2022, 629, 122342. [Google Scholar] [CrossRef] [PubMed]
- Kashkooli, F.M.; Soltani, M.; Momeni, M.M.; Rahmim, A. Enhanced Drug Delivery to Solid Tumors via Drug-Loaded Nanocarriers: An Image-Based Computational Framework. Front. Oncol. 2021, 11, 655781. [Google Scholar] [CrossRef]
- Kashkooli, F.M.; Rezaeian, M.; Soltani, M. Drug delivery through nanoparticles in solid tumors: A mechanistic understanding. Nanomedicine 2022, 17, 695–716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.-M.; Huang, C.-M. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Mechanisms of bacterial biocide and antibiotic resistance. J. Appl. Microbiol. 2002, 92, 55S–64S. [Google Scholar] [CrossRef]
- Goharshadi, E.K.; Goharshadi, K.; Moghayedi, M. The use of nanotechnology in the fight against viruses: A critical review. Co-Ord. Chem. Rev. 2022, 464, 214559. [Google Scholar] [CrossRef]
- De Siqueira, L.B.D.O.; Matos, A.P.D.S.; da Silva, M.R.M.; Pinto, S.R.; Santos-Oliveira, R.; Ricci-Júnior, E. Pharmaceutical nanotechnology applied to phthalocyanines for the promotion of antimicrobial photodynamic therapy: A literature review. Photodiagnosis Photodyn. Ther. 2022, 39, 102896. [Google Scholar] [CrossRef]
- Nicolosi, D.; Scalia, M.; Nicolosi, V.M.; Pignatello, R. Encapsulation in fusogenic liposomes broadens the spectrum of action of vancomycin against Gram-negative bacteria. Int. J. Antimicrob. Agents 2010, 35, 553–558. [Google Scholar] [CrossRef]
- Cheow, W.S.; Chang, M.W.; Hadinoto, K. The roles of lipid in anti-biofilm efficacy of lipid–polymer hybrid nanoparticles encapsulating antibiotics. Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 158–165. [Google Scholar] [CrossRef]
- Sanderson, N.M.; Guo, B.; Jacob, A.E.; Handley, P.S.; Cunniffe, J.G.; Jones, M.N. The interaction of cationic liposomes with the skin-associated bacterium Staphylococcus epidermidis: Effects of ionic strength and temperature. Biochim. Biophys. Acta Biomembr. 1996, 1283, 207–214. [Google Scholar] [CrossRef]
- Abed, N.; Couvreur, P. Nanocarriers for antibiotics: A promising solution to treat intracellular bacterial infections. Int. J. Antimicrob. Agents 2014, 43, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Clemens, D.L.; Lee, B.-Y.; Xue, M.; Thomas, C.R.; Meng, H.; Ferris, D.; Nel, A.E.; Zink, J.I.; Horwitz, M.A. Targeted Intracellular Delivery of Antituberculosis Drugs to Mycobacterium tuberculosis-Infected Macrophages via Functionalized Mesoporous Silica Nanoparticles. Antimicrob. Agents Chemother. 2012, 56, 2535–2545. [Google Scholar] [CrossRef] [PubMed]
- Forier, K.; Raemdonck, K.; De Smedt, S.C.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control. Release 2014, 190, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Pissuwan, D.; Cortie, C.H.; Valenzuela, S.M.; Cortie, M.B. Functionalised gold nanoparticles for controlling pathogenic bacteria. Trends Biotechnol. 2010, 28, 207–213. [Google Scholar] [CrossRef]
- Gu, H.; Ho, P.L.; Tong, E.; Wang, L.; Xu, B. Presenting Vancomycin on Nanoparticles to Enhance Antimicrobial Activities. Nano Lett. 2003, 3, 1261–1263. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, Y.; Cui, Y.; Liu, W.; Ma, W.; Jiang, X. Small Molecule-Capped Gold Nanoparticles as Potent Antibacterial Agents That Target Gram-Negative Bacteria. J. Am. Chem. Soc. 2010, 132, 12349–12356. [Google Scholar] [CrossRef]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef]
- Privett, B.J.; Broadnax, A.D.; Bauman, S.J.; Riccio, D.A.; Schoenfisch, M.H. Examination of bacterial resistance to exogenous nitric oxide. Nitric Oxide 2012, 26, 169–173. [Google Scholar] [CrossRef]
- Pinto, R.V.; Carvalho, S.; Antunes, F.; Pires, J.; Pinto, M.L. Emerging Nitric Oxide and Hydrogen Sulfide Releasing Carriers for Skin Wound Healing Therapy. ChemMedChem 2021, 17, e202100429. [Google Scholar] [CrossRef]
- Afshari, A.R.; Sanati, M.; Mollazadeh, H.; Kesharwani, P.; Johnston, T.P.; Sahebkar, A. Nanoparticle-based drug delivery systems in cancer: A focus on inflammatory pathways. Semin. Cancer Biol. 2022, 86, 860–872. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Shin, J.H.; Stasko, N.A.; Johnson, C.B.; Wespe, D.A.; Holmuhamedov, E.; Schoenfisch, M.H. Bactericidal Efficacy of Nitric Oxide-Releasing Silica Nanoparticles. ACS Nano 2008, 2, 235–246. [Google Scholar] [CrossRef]
- Lu, Y.; Slomberg, D.L.; Schoenfisch, M.H. Nitric oxide-releasing chitosan oligosaccharides as antibacterial agents. Biomaterials 2014, 35, 1716–1724. [Google Scholar] [CrossRef]
- Han, G.; Martinez, L.R.; Mihu, M.R.; Friedman, A.J.; Friedman, J.M.; Nosanchuk, J.D. Nitric Oxide Releasing Nanoparticles Are Therapeutic for Staphylococcus aureus Abscesses in a Murine Model of Infection. PLoS ONE 2009, 4, e7804. [Google Scholar] [CrossRef]
- Friedman, A.J.; Han, G.; Navati, M.S.; Chacko, M.; Gunther, L.; Alfieri, A.; Friedman, J.M. Sustained release nitric oxide releasing nanoparticles: Characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide 2008, 19, 12–20. [Google Scholar] [CrossRef]
- Martinez, L.R.; Han, G.; Chacko, M.; Mihu, M.R.; Jacobson, M.; Gialanella, P.; Friedman, A.J.; Nosanchuk, J.D.; Friedman, J.M. Antimicrobial and Healing Efficacy of Sustained Release Nitric Oxide Nanoparticles Against Staphylococcus aureus Skin Infection. J. Investig. Dermatol. 2009, 129, 2463–2469. [Google Scholar] [CrossRef]
- Chow, J.W.; Victor, L.Y. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: A commentary. Int. J. Antimicrob. Agents 1999, 11, 7–12. [Google Scholar] [CrossRef]
- Toti, U.S.; Guru, B.R.; Hali, M.; McPharlin, C.M.; Wykes, S.M.; Panyam, J.; Whittum-Hudson, J.A. Targeted delivery of antibiotics to intracellular chlamydial infections using PLGA nanoparticles. Biomaterials 2011, 32, 6606–6613. [Google Scholar] [CrossRef]
- Carmona, D.; Lalueza, P.; Balas, F.; Arruebo, M.; Santamaría, J. Mesoporous silica loaded with peracetic acid and silver nano-particles as a dual-effect, highly efficient bactericidal agent. Microporous Mesoporous Mater. 2012, 161, 84–90. [Google Scholar] [CrossRef]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef]
- Chono, S.; Tanino, T.; Seki, T.; Morimoto, K. Efficient drug targeting to rat alveolar macrophages by pulmonary administration of ciprofloxacin incorporated into mannosylated liposomes for treatment of respiratory intracellular parasitic infections. J. Control. Release 2008, 127, 50–58. [Google Scholar] [CrossRef]
- Soltani, M.; Souri, M.; Moradi Kashkooli, F. Effects of hypoxia and nanocarrier size on pH-responsive nano-delivery system to solid tumors. Sci. Rep. 2021, 11, 19350. [Google Scholar] [CrossRef] [PubMed]
- Radovic-Moreno, A.F.; Lu, T.K.; Puscasu, V.A.; Yoon, C.J.; Langer, R.; Farokhzad, O.C. Surface charge-switching polymeric na-noparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano 2012, 6, 4279–4287. [Google Scholar] [CrossRef] [PubMed]
- Pornpattananangkul, D.; Olson, S.; Aryal, S.; Sartor, M.; Huang, C.-M.; Vecchio, K.; Zhang, L. Stimuli-Responsive Liposome Fusion Mediated by Gold Nanoparticles. ACS Nano 2010, 4, 1935–1942. [Google Scholar] [CrossRef]
- Gao, W.; Vecchio, D.; Li, J.; Zhu, J.; Zhang, Q.; Fu, V.; Li, J.; Thamphiwatana, S.; Lu, D.; Zhang, L. Hydrogel containing nanoparti-cle-stabilized liposomes for topical antimicrobial delivery. Acs Nano 2014, 8, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Pornpattananangkul, D.; Zhang, L.; Olson, S.; Aryal, S.; Obonyo, M.; Vecchio, K.; Huang, C.-M.; Zhang, L. Bacterial Toxin-Triggered Drug Release from Gold Nanoparticle-Stabilized Liposomes for the Treatment of Bacterial Infection. J. Am. Chem. Soc. 2011, 133, 4132–4139. [Google Scholar] [CrossRef]
- Xiong, M.-H.; Bao, Y.; Yang, X.-Z.; Wang, Y.-C.; Sun, B.; Wang, J. Lipase-sensitive polymeric triple-layered nanogel for “on-demand” drug delivery. J. Am. Chem. Soc. 2012, 134, 4355–4362. [Google Scholar] [CrossRef]
- Xiong, M.-H.; Li, Y.-J.; Bao, Y.; Yang, X.-Z.; Hu, B.; Wang, J. Bacteria-Responsive Multifunctional Nanogel for Targeted Antibiotic Delivery. Adv. Mater. 2012, 24, 6175–6180. [Google Scholar] [CrossRef]
- Shi, B.; Leung, D.Y.; Taylor, P.A.; Li, H. MRSA colonization is associated with decreased skin commensal bacteria in atopic dermatitis. J. Investig. Dermatol. 2018, 138, 1668. [Google Scholar] [CrossRef]
- Lu, B.; Ye, H.; Shang, S.; Xiong, Q.; Yu, K.; Li, Q.; Xiao, Y.; Dai, F.; Lan, G. Novel wound dressing with chitosan gold nanoparticles capped with a small molecule for effective treatment of multiantibiotic-resistant bacterial infections. Nanotechnology 2018, 29, 425603. [Google Scholar] [CrossRef]
- Liu, M.; He, D.; Yang, T.; Liu, W.; Mao, L.; Zhu, Y.; Wu, J.; Luo, G.; Deng, J. An efficient antimicrobial depot for infectious site-targeted chemo-photothermal therapy. J. Nanobiotechnol. 2018, 16, 23. [Google Scholar] [CrossRef]
- Alfatemi, S.H.; Rad, M.S.; Iriti, M. Antimicrobial synergic effect of allicin and silver nanoparticles on skin infection caused by methicillin-resistant Staphylococcus aureus spp. Ann. Med. Heath Sci. Res. 2014, 4, 863–868. [Google Scholar] [CrossRef]
- Ran, X.; Du, Y.; Wang, Z.; Wang, H.; Pu, F.; Ren, J.; Qu, X. Hyaluronic Acid-Templated Ag Nanoparticles/Graphene Oxide Composites for Synergistic Therapy of Bacteria Infection. ACS Appl. Mater. Interfaces 2017, 9, 19717–19724. [Google Scholar] [CrossRef]
- Ribeiro, K.L.; Frías, I.A.; Franco, O.L.; Dias, S.C.; Sousa-Junior, A.A.; Silva, O.N.; Bakuzis, A.F.; Oliveira, M.D.; Andrade, C.A. Clavanin A-bioconjugated Fe3O4/Silane core-shell nanoparticles for thermal ablation of bacterial biofilms. Colloids Surf. B Biointerfaces 2018, 169, 72–81. [Google Scholar] [CrossRef]
- Francolini, I.; Giansanti, L.; Piozzi, A.; Altieri, B.; Mauceri, A.; Mancini, G. Glucosylated liposomes as drug delivery systems of usnic acid to address bacterial infections. Colloids Surf. B Biointerfaces 2019, 181, 632–638. [Google Scholar] [CrossRef]
- Szaciłowski, K.; Macyk, W.; Stochel, G. Synthesis, structure and photoelectrochemical properties of the TiO2–Prussian blue nanocomposite. J. Mater. Chem. 2006, 16, 4603–4611. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; Permana, A.D.; Rodgers, A.M.; Donnelly, R.F.; Rehman, A.U. Enhancement in site-specific delivery of car-vacrol against methicillin resistant Staphylococcus aureus induced skin infections using enzyme responsive nanoparticles: A proof of concept study. Pharmaceutics 2019, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Papalia, T. Antimicrobial Activity of Carvacrol: Current Progress and Future Prospectives. Recent Pat. Anti-Infect. Drug Discov. 2012, 7, 28–35. [Google Scholar] [CrossRef]
- Lange-Asschenfeldt, B.; Marenbach, D.; Lang, C.; Patzelt, A.; Ulrich, M.; Maltusch, A.; Terhorst, D.; Stockfleth, E.; Sterry, W.; Lademann, J. Distribution of Bacteria in the Epidermal Layers and Hair Follicles of the Human Skin. Ski. Pharmacol. Physiol. 2011, 24, 305–311. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Yang, S.-C.; Sung, C.T.; Weng, Y.-H.; Fang, J.-Y. Anti-MRSA malleable liposomes carrying chloramphenicol for ameliorating hair follicle targeting. Int. J. Nanomed. 2017, 12, 8227–8238. [Google Scholar] [CrossRef]
- Alalaiwe, A.; Wang, P.-W.; Lu, P.-L.; Chen, Y.-P.; Fang, J.-Y.; Yang, S.-C. Synergistic Anti-MRSA Activity of Cationic Nanostructured Lipid Carriers in Combination with Oxacillin for Cutaneous Application. Front. Microbiol. 2018, 9, 1493. [Google Scholar] [CrossRef]
- Yang, S.; Han, X.; Yang, Y.; Qiao, H.; Yu, Z.; Liu, Y.; Wang, J.; Tang, T. Bacteria-targeting nanoparticles with microenviron-ment-responsive antibiotic release to eliminate intracellular Staphylococcus aureus and associated infection. ACS Appl. Mater. Interfaces 2018, 10, 14299–14311. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Aljuffali, I.A.; Sung, C.T.; Lin, C.-F.; Fang, J.-Y. Antimicrobial activity of topically-applied soyaethyl morpholinium ethosulfate micelles against Staphylococcus species. Nanomedicine 2016, 11, 657–671. [Google Scholar] [CrossRef]
- Kang, X.-Q.; Shu, G.-F.; Jiang, S.-P.; Xu, X.-L.; Qi, J.; Jin, F.-Y.; Liu, D.; Xiao, Y.-H.; Lu, X.-Y.; Du, Y.-Z. Effective targeted therapy for drug-resistant infection by ICAM-1 antibody-conjugated TPGS modified β-Ga2O3: Cr3+ nanoparticles. Theranostics 2019, 9, 2739. [Google Scholar] [CrossRef]
- Wang, X.-S.; Situ, J.-Q.; Ying, X.-Y.; Chen, H.; Pan, H.-F.; Jin, Y.; Du, Y.-Z. β-Ga2O3:Cr3+ nanoparticle: A new platform with near infrared photoluminescence for drug targeting delivery and bio-imaging simultaneously. Acta Biomater. 2015, 22, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Kłodzińska, S.N.; Priemel, P.A.; Rades, T.; Mørck Nielsen, H. Inhalable antimicrobials for treatment of bacterial bio-film-associated sinusitis in cystic fibrosis patients: Challenges and drug delivery approaches. Int. J. Mol. Sci. 2016, 17, 1688. [Google Scholar] [CrossRef]
- Deacon, J.; Abdelghany, S.M.; Quinn, D.J.; Schmid, D.; Megaw, J.; Donnelly, R.F.; Jones, D.S.; Kissenpfennig, A.; Elborn, J.S.; Gilmore, B.F.; et al. Antimicrobial efficacy of tobramycin polymeric nanoparticles for Pseudomonas aeruginosa infections in cystic fibrosis: Formulation, characterisation and functionalisation with dornase alfa (DNase). J. Control. Release 2015, 198, 55–61. [Google Scholar] [CrossRef]
- Casciaro, B.; D’Angelo, I.; Zhang, X.; Loffredo, M.R.; Conte, G.; Cappiello, F.; Quaglia, F.; Di, Y.-P.P.; Ungaro, F.; Mangoni, M.L. Poly(lactide-co-glycolide) Nanoparticles for Prolonged Therapeutic Efficacy of Esculentin-1a-Derived Antimicrobial Peptides against Pseudomonas aeruginosa Lung Infection: In Vitro and in Vivo Studies. Biomacromolecules 2019, 20, 1876–1888. [Google Scholar] [CrossRef]
- Chen, M.; Xie, S.; Wei, J.; Song, X.; Ding, Z.; Li, X. Antibacterial micelles with vancomycin-mediated targeting and pH/lipase-triggered release of antibiotics. ACS Appl. Mater. Interfaces 2018, 10, 36814–36823. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Q.; Feng, W.; Pu, W.; Ding, J.; Zhang, H.; Li, X.; Yang, B.; Dai, Q.; Cheng, L.; et al. Targeted delivery of antibiotics to the infected pulmonary tissues using ROS-responsive nanoparticles. J. Nanobiotechnol. 2019, 17, 1–16. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Sung, C.T.; Aljuffali, I.A.; Chen, C.-H.; Hu, K.-Y.; Fang, J.-Y. Intravenous anti-MRSA phosphatiosomes mediate en-hanced affinity to pulmonary surfactants for effective treatment of infectious pneumonia. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 215–225. [Google Scholar] [CrossRef]
- Tenland, E.; Pochert, A.; Krishnan, N.; Umashankar Rao, K.; Kalsum, S.; Braun, K.; Glegola-Madejska, I.; Lerm, M.; Robertson, B.D.; Lindén, M. Effective delivery of the anti-mycobacterial peptide NZX in mesoporous silica nanoparticles. PLoS ONE 2019, 14, e0212858. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.-G.; Kim, D.; Mann, A.P.; Mölder, T.; Teesalu, T. Antibiotic-loaded nano-particles targeted to the site of infection enhance antibacterial efficacy. Nat. Biomed. Eng. 2018, 2, 95–103. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, Y.; He, Y.; Chen, F.; Gong, Y.; Chen, S.; Xu, Y.; Su, Y.; Wang, C.; Wang, J. Succinylated casein-coated pep-tide-mesoporous silica nanoparticles as an antibiotic against intestinal bacterial infection. Biomater. Sci. 2019, 7, 2440–2451. [Google Scholar] [CrossRef]
- Calabrese, I.; Cavallaro, G.; Scialabba, C.; Licciardi, M.; Merli, M.; Sciascia, L.; Liveri, M.L.T. Montmorillonite nanodevices for the colon metronidazole delivery. Int. J. Pharm. 2013, 457, 224–236. [Google Scholar] [CrossRef]
- Ping, Y.; Hu, X.; Yao, Q.; Hu, Q.; Amini, S.; Miserez, A.; Tang, G. Engineering bioinspired bacteria-adhesive clay nanoparticles with a membrane-disruptive property for the treatment of Helicobacter pylori infection. Nanoscale 2016, 8, 16486–16498. [Google Scholar] [CrossRef]
- Angsantikul, P.; Thamphiwatana, S.; Zhang, Q.; Spiekermann, K.; Zhuang, J.; Fang, R.H.; Gao, W.; Obonyo, M.; Zhang, L. Coating Nanoparticles with Gastric Epithelial Cell Membrane for Targeted Antibiotic Delivery against Helicobacter pylori Infection. Adv. Ther. 2018, 1, 1800016. [Google Scholar] [CrossRef]
- Huttunen, R.; Aittoniemi, J. New concepts in the pathogenesis, diagnosis and treatment of bacteremia and sepsis. J. Infect. 2011, 63, 407–419. [Google Scholar] [CrossRef]
- Rai, A.; Pinto, S.; Velho, T.R.; Ferreira, A.F.; Moita, C.; Trivedi, U.; Evangelista, M.; Comune, M.; Rumbaugh, K.P.; Simões, P.N.; et al. One-step synthesis of high-density peptide-conjugated gold nanoparticles with antimicrobial efficacy in a systemic infection model. Biomaterials 2016, 85, 99–110. [Google Scholar] [CrossRef]
- Qadri, S.; Haik, Y.; Mensah-Brown, E.; Bashir, G.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. Metallic nanoparticles to eradicate bacterial bone infection. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2241–2250. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-Inhibiting Coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef]
- Fang, C.-H.; Tsai, P.-I.; Huang, S.-W.; Sun, J.-S.; Chang, J.Z.-C.; Shen, H.-H.; Chen, S.-Y.; Lin, F.H.; Hsu, L.-T.; Chen, Y.-C. Magnetic hyperthermia enhance the treatment efficacy of peri-implant osteomyelitis. BMC Infect. Dis. 2017, 17, 516. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Bai, X.; Li, L.; Liu, H.; Tan, L.; Liu, T.; Meng, X. Solvothermal Synthesis of ZnO Nanoparticles and Anti-Infection Application in Vivo. ACS Appl. Mater. Interfaces 2015, 7, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Haworth, C.S.; Bilton, D.; Chalmers, J.D.; Davis, A.M.; Froehlich, J.; Gonda, I.; Thompson, B.; Wanner, A.; O’Donnell, A.E. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): Two phase 3, randomised controlled trials. Lancet Respir. Med. 2019, 7, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Liposomal Amikacin for Inhalation (LAI) for Nontuberculous Mycobacteria. Available online: https://clinicaltrials.gov/ct2/show/NCT01315236 (accessed on 4 November 2019).
- ClinicalTrials.gov. Study of Dose Escalation of Liposomal Amikacin for Inhalation (ARIKAYCE™)—Extension Phase. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03905642 (accessed on 18 November 2019).
- ClinicalTrials.gov. Liposomal Amikacin for Inhalation (LAI) in the Treatment of Mycobacterium Abscessus Lung Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT03038178 (accessed on 4 November 2019).
- ClinicalTrials.gov. Extension Study of Liposomal Amikacin for Inhalation in Cystic Fibrosis (CF) Patients with Chronic Pseudomonas Aeruginosa (Pa) Infection. Available online: https://clinicaltrials.gov/ct2/show/NCT01316276 (accessed on 12 November 2019).
- Mullard, A. FDA approves antitoxin antibody. Nat. Rev. Drug Discov. 2016, 15, 811–812. [Google Scholar] [CrossRef]
- Azeredo da Silveira, S.; Perez, A. Improving the fate of severely infected patients: The promise of anti-toxin treatments and superiority trials. Expert Rev. Anti-Infect. Ther. 2017, 15, 973–975. [Google Scholar] [CrossRef]
- Laterre, P.-F.; Colin, G.; Dequin, P.-F.; Dugernier, T.; Boulain, T.; da Silveira, S.A.; Lajaunias, F.; Perez, A.; François, B. CAL02, a novel antitoxin liposomal agent, in severe pneumococcal pneumonia: A first-in-human, double-blind, placebo-controlled, ran-domised trial. Lancet Infect. Dis. 2019, 19, 620–630. [Google Scholar] [CrossRef]
- Molchanova, N.; Hansen, P.R.; Franzyk, H. Advances in Development of Antimicrobial Peptidomimetics as Potential Drugs. Molecules 2017, 22, 1430. [Google Scholar] [CrossRef]
- Stevens, D.A. Overview of amphotericin B colloidal dispersion (Amphocil). J. Infect. 1994, 28, 45–49. [Google Scholar] [CrossRef]
- Boswell, G.W.; Buell, D.; Bekersky, I. AmBisome (liposomal amphotericin B): A comparative review. J. Clin. Pharmacol. 1998, 38, 583–592. [Google Scholar] [CrossRef]
- Paterson, D.L.; David, K.; Mrsic, M.; Cetkovsky, P.; Weng, X.-H.; Sterba, J.; Krivan, G.; Boskovic, D.; Lu, M.; Zhu, L.-P. Pre-medication practices and incidence of infusion-related reactions in patients receiving AMPHOTEC®: Data from the Patient Registry of Amphotericin B Cholesteryl Sulfate Complex for Injection Clinical Tolerability (PRoACT) registry. J. Antimicrob. Chemother. 2008, 62, 1392–1400. [Google Scholar] [CrossRef]
- Jadhav, M.; Bamba, A.; Shinde, V.; Gogtay, N.; Kshirsagar, N.; Bichile, L.; Mathai, D.; Sharma, A.; Varma, S.; Digumarathi, R. Liposomal amphotericin B (Fungisome TM) for the treatment of cryptococcal meningitis in HIV/AIDS patients in India: A mul-ticentric, randomized controlled trial. J. Postgrad. Med. 2010, 56, 71. [Google Scholar] [CrossRef]
- Bruinenberg, P.; Blanchard, J.D.; Cipolla, D.C.; Dayton, F.; Mudumba, S.; Gonda, I. Inhaled liposomal ciprofloxacin: Once a day management of respiratory infections. In Respiratory Drug Delivery; Davis Healthcare International Publishing: Orlando, FL, USA, 2010; pp. 73–82. [Google Scholar]
- ClinicalTrials.gov. Phase 3 Study with Ciprofloxacin Dispersion for Inhalation in Non-CF Bronchiectasis (ORBIT-3). Available online: https://clinicaltrials.gov/ct2/show/NCT01515007 (accessed on 21 November 2019).
- ClinicalTrials.gov. Study to Evaluate Efficacy of LAI When Added to Multi-drug Regimen Compared to Multi-drug Regimen Alone (CONVERT). Available online: https://clinicaltrials.gov/ct2/show/NCT02344004 (accessed on 25 October 2019).
- ClinicalTrials.gov. Study of the Clinical Effectiveness of a Human Monoclonal Antibody to C. Difficile Toxin A and Toxin B in Patients with Clostridium Difficile Associated Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT00350298?term=anti+toxin&draw=3&rank=13 (accessed on 16 November 2019).
- Crowther, G.S.; Baines, S.D.; Todhunter, S.L.; Freeman, J.; Chilton, C.H.; Wilcox, M.H. Evaluation of NVB302 versus vancomycin activity in an in vitro human gut model of Clostridium difficile infection. J. Antimicrob. Chemother. 2013, 68, 168–176. [Google Scholar] [CrossRef]
- Van der Velden, W.J.; van Iersel, T.M.; Blijlevens, N.; Donnelly, J.P. Safety and tolerability of the antimicrobial peptide human lactoferrin 1-11 (hLF1-11). BMC Med. 2009, 7, 44. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Qian, G.; Wang, Y.; Chen, H.; Li, Y.-Z.; Liu, F.; Shen, Y.; Du, L. Identification and characterization of the an-ti-methicillin-resistant Staphylococcus aureus WAP-8294A2 biosynthetic gene cluster from Lysobacter enzymogenes OH11. Antimicrob. Agents Chemother. 2011, 55, 5581–5589. [Google Scholar] [CrossRef]
- Kaplan, C.W.; Sim, J.H.; Shah, K.R.; Kolesnikova-Kaplan, A.; Shi, W.; Eckert, R. Selective Membrane Disruption: Mode of Action of C16G2, a Specifically Targeted Antimicrobial Peptide. Antimicrob. Agents Chemother. 2011, 55, 3446–3452. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Study of DPK-060 to Investigate Clinical Safety and Efficacy in Patients with Acute External Otitis. Available online: https://clinicaltrials.gov/ct2/show/NCT01447017 (accessed on 10 December 2019).
- Nilsson, A.C.; Janson, H.; Wold, H.; Fugelli, A.; Andersson, K.; Håkangård, C.; Olsson, P.; Olsen, W.M. LTX-109 Is a Novel Agent for Nasal Decolonization of Methicillin-Resistant and -Sensitive Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 145–151. [Google Scholar] [CrossRef]
- Knight-Connoni, V.; Mascio, C.; Chesnel, L.; Silverman, J. Discovery and development of surotomycin for the treatment of Clostridium difficile. J. Ind. Microbiol. Biotechnol. 2016, 43, 195–204. [Google Scholar] [CrossRef]
- Stiefel, U.; Pultz, N.J.; Helfand, M.S.; Donskey, C.J. Efficacy of Oral Ramoplanin for Inhibition of Intestinal Colonization by Vancomycin-Resistant Enterococci in Mice. Antimicrob. Agents Chemother. 2004, 48, 2144–2148. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehrabi, M.R.; Soltani, M.; Chiani, M.; Raahemifar, K.; Farhangi, A. Nanomedicine: New Frontiers in Fighting Microbial Infections. Nanomaterials 2023, 13, 483. https://doi.org/10.3390/nano13030483
Mehrabi MR, Soltani M, Chiani M, Raahemifar K, Farhangi A. Nanomedicine: New Frontiers in Fighting Microbial Infections. Nanomaterials. 2023; 13(3):483. https://doi.org/10.3390/nano13030483
Chicago/Turabian StyleMehrabi, Mohammad Reza, Madjid Soltani, Mohsen Chiani, Kaamran Raahemifar, and Ali Farhangi. 2023. "Nanomedicine: New Frontiers in Fighting Microbial Infections" Nanomaterials 13, no. 3: 483. https://doi.org/10.3390/nano13030483
APA StyleMehrabi, M. R., Soltani, M., Chiani, M., Raahemifar, K., & Farhangi, A. (2023). Nanomedicine: New Frontiers in Fighting Microbial Infections. Nanomaterials, 13(3), 483. https://doi.org/10.3390/nano13030483







