In Tandem Control of La-Doping and CuO-Heterojunction on SrTiO3 Perovskite by Double-Nozzle Flame Spray Pyrolysis: Selective H2 vs. CH4 Photocatalytic Production from H2O/CH3OH
Abstract
1. Introduction
2. Materials and Methods
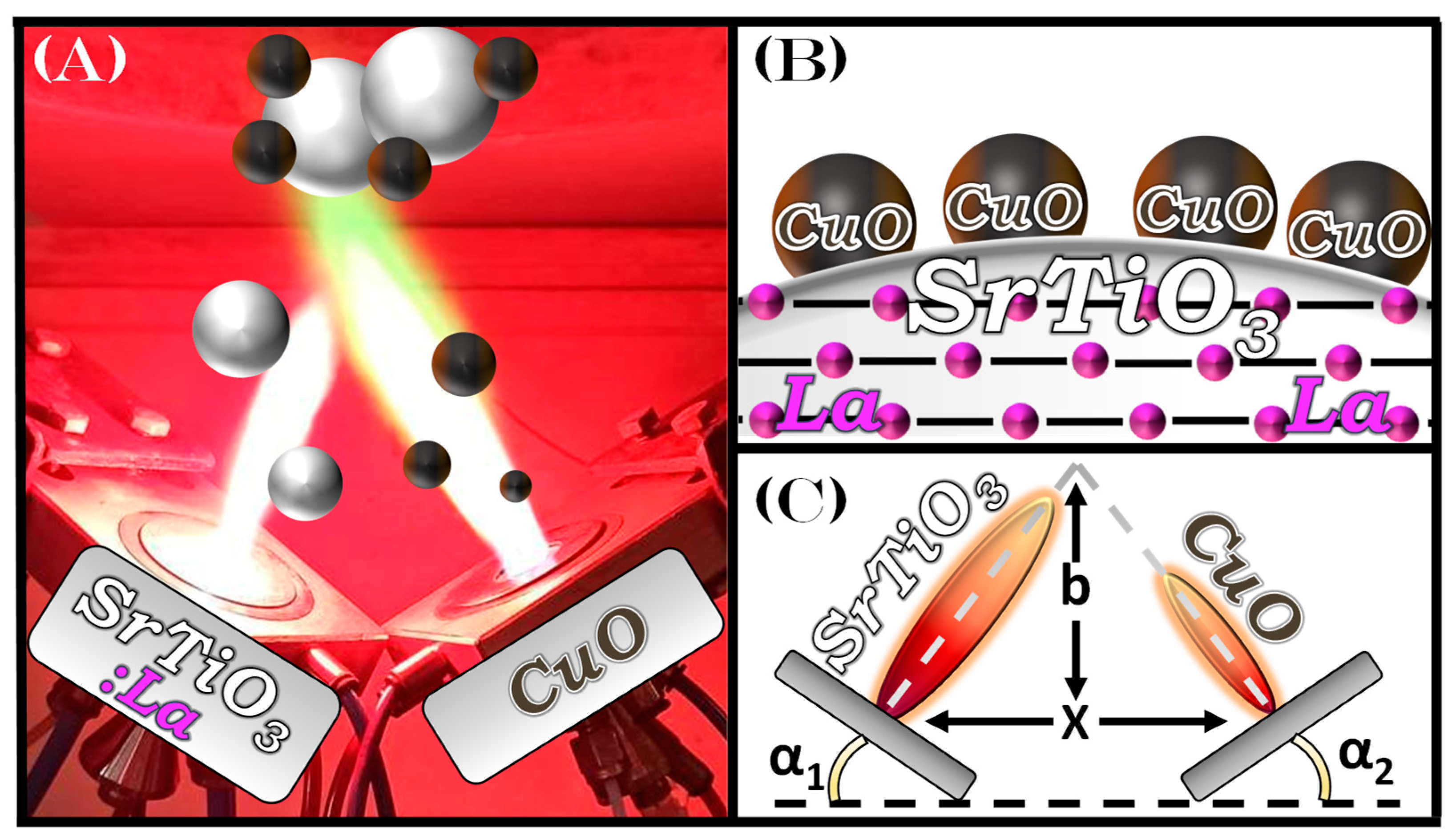
2.1. Flame Spray Pyrolysis (FSP) Synthesis of SrTiO3 Nanoparticles
2.2. Photocatalytic Evaluation
2.3. Characterization of Materials
3. Results
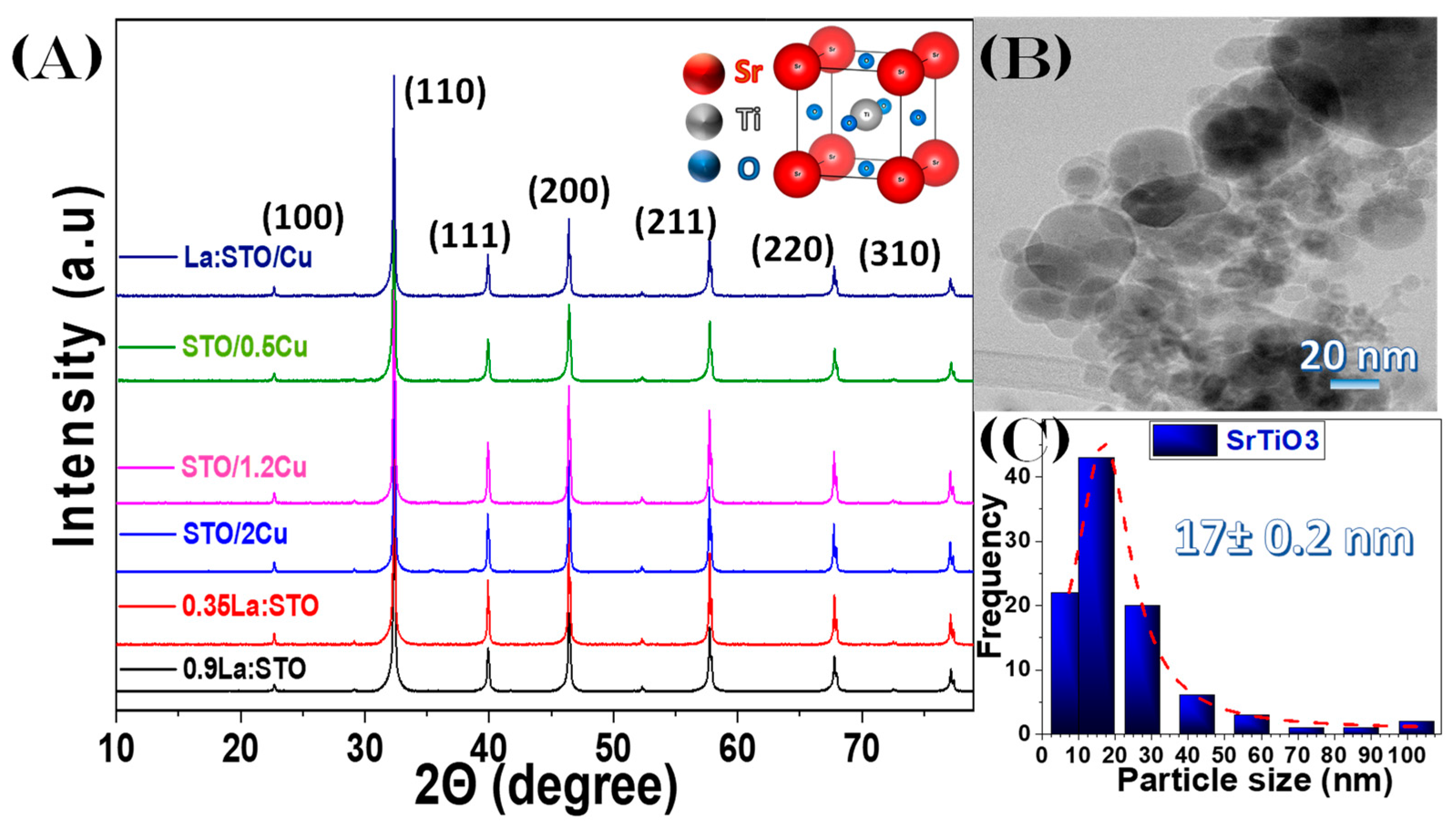
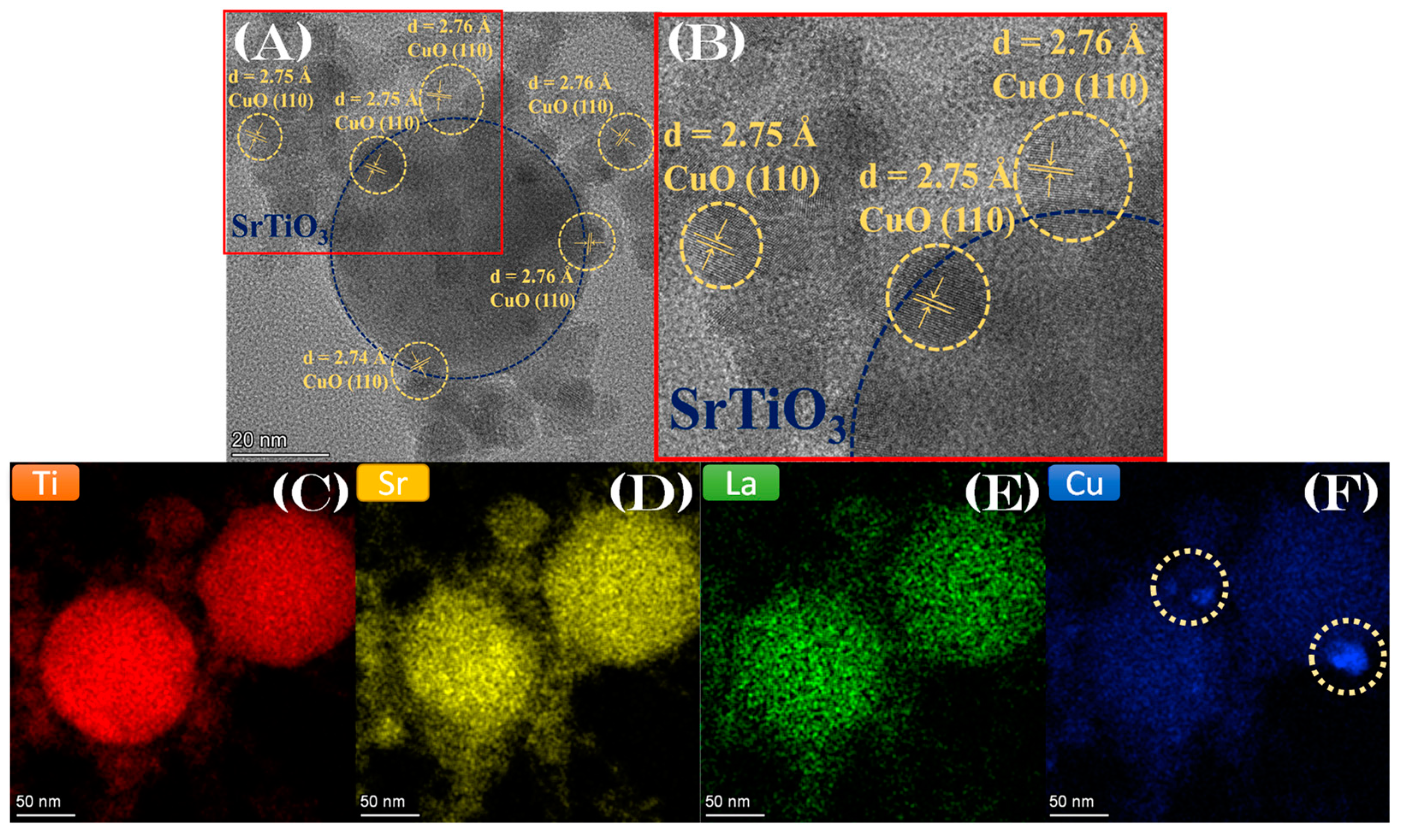
3.1. SrTiO3-Based Perovskite Nanoparticle Synthesis by FSP
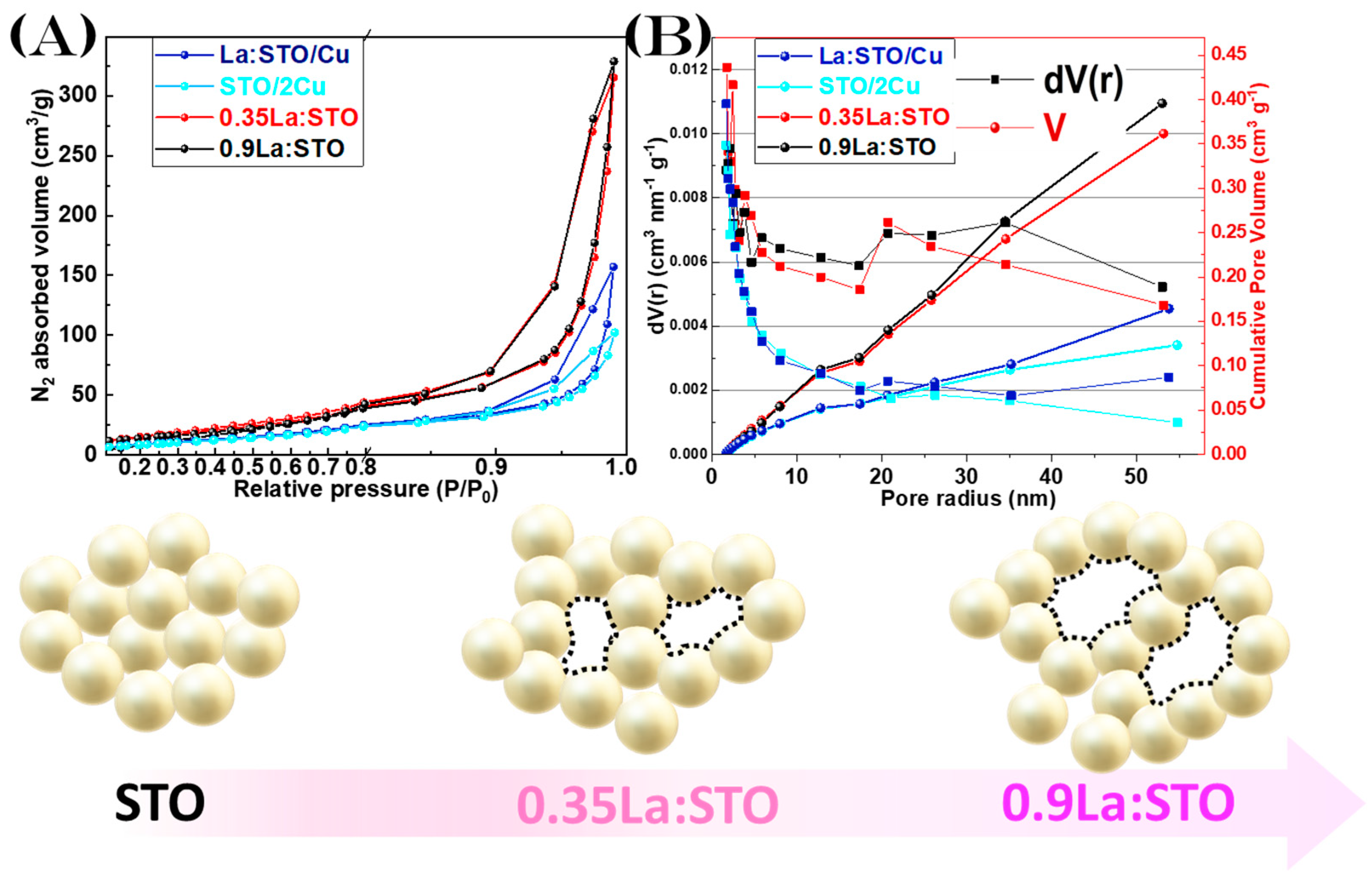
| Nanomaterial 1 | La-, Cu-Content XRF Analysis (%wt) | dXRD (nm) (±4) | SSA (m2 g−1) (± 0.5) | Total Pore Volume (cm3 g−1) (±0.02 × 10−2) | Band Gap Eg (eV) (± 0.1) |
|---|---|---|---|---|---|
| Single-Nozzle FSP | |||||
| Pristine SrTiO3 | La:0 /Cu:0 | 45 | 32.3 | 0.14 × 10−2 | 3.2 |
| 0.9La:STO | La:0.93 ± 0.05 /Cu:0 | 47 | 53.1 | 0.39 × 10−2 | 3.2 |
| 0.35La:STO | La:0.36 ± 0.05 /Cu:0 | 55 | 57.5 | 0.36 × 10−2 | 3.2 |
| Double-Nozzle FSP | |||||
| STO/2Cu | La:0.05 ± 0.05 /Cu:2 ± 0.1 | 53 | 34.9 | 0.12 × 10−2 | 3.2 |
| STO/1.2Cu | La:0/Cu:1.2 ± 0.1 | 49 | 32.1 | 0.11 × 10−2 | 3.2 |
| STO/0.5Cu | La: 0.05 ± 0.05 /Cu:0.5 ± 0.1 | 45 | 32.0 | 0.11 × 10−2 | 3.2 |
| La:STO/Cu | La: 0.26 ± 0.05 /Cu:0.5 ± 0.1 | 52 | 37.3 | 0.16 × 10−2 | 3.2 |
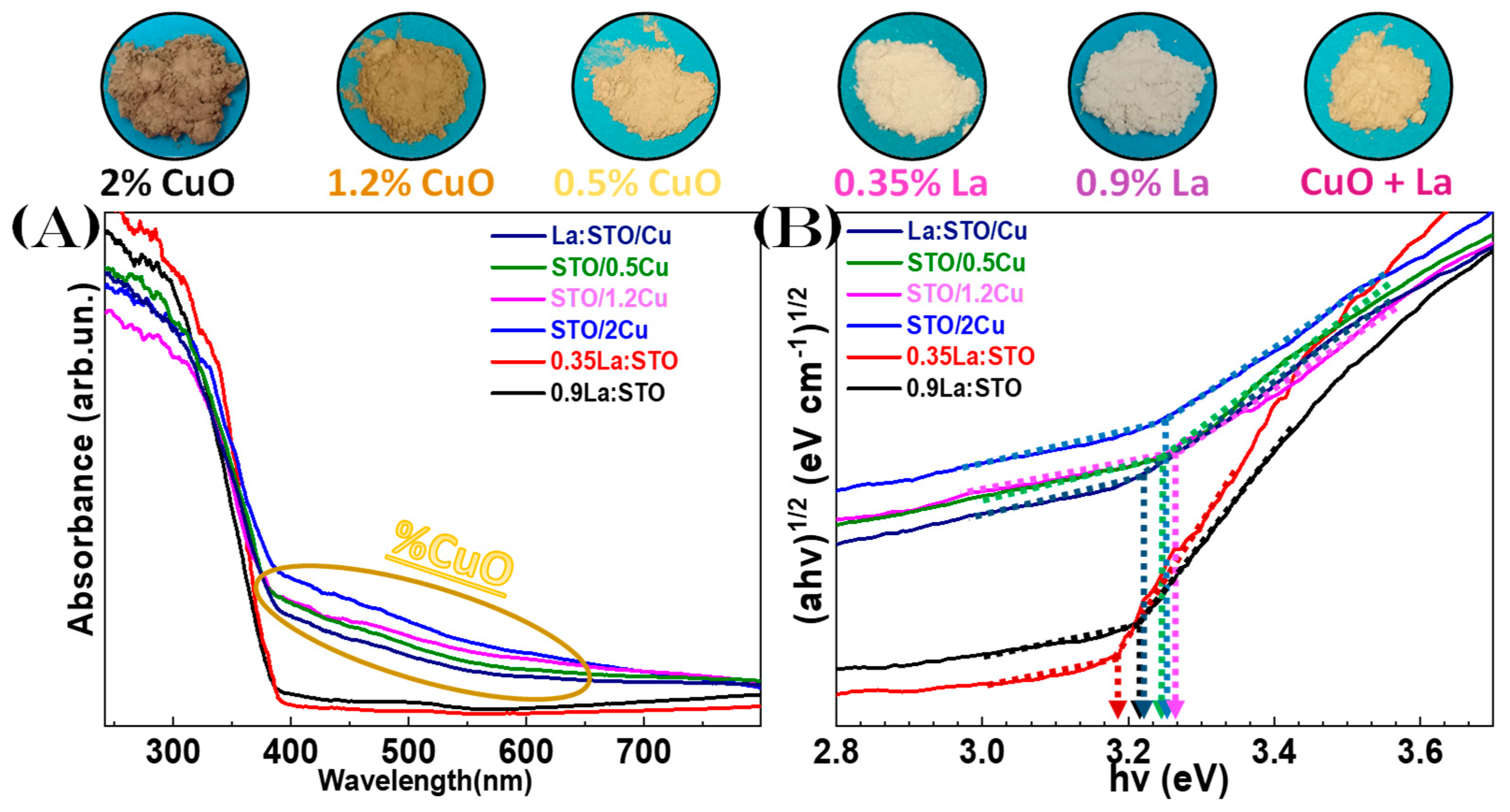
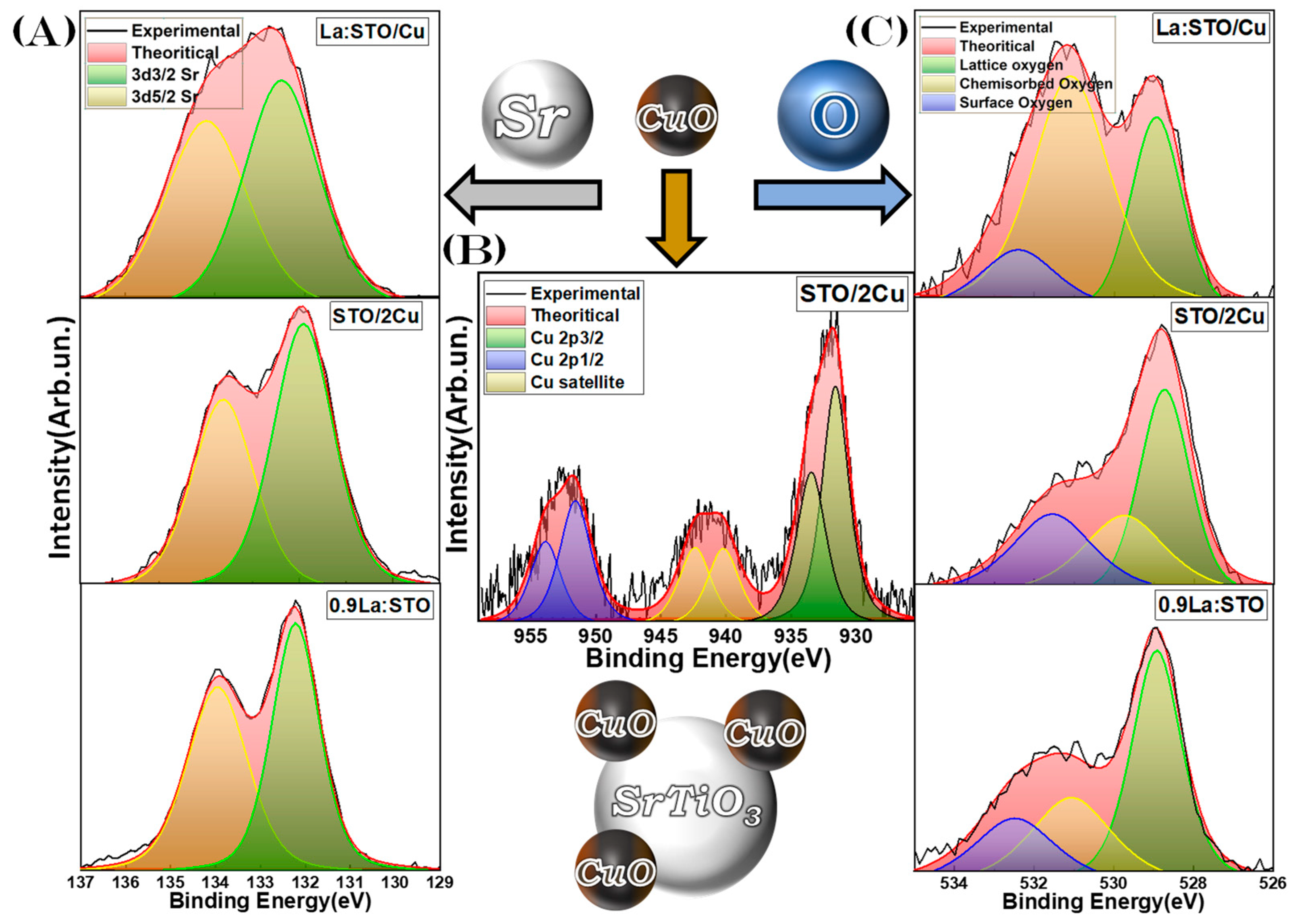
3.2. Spectroscopic Characterization
3.2.1. Diffuse Reflectance UV-Vis Spectroscopy
3.2.2. X-ray Photoelectron Spectroscopy
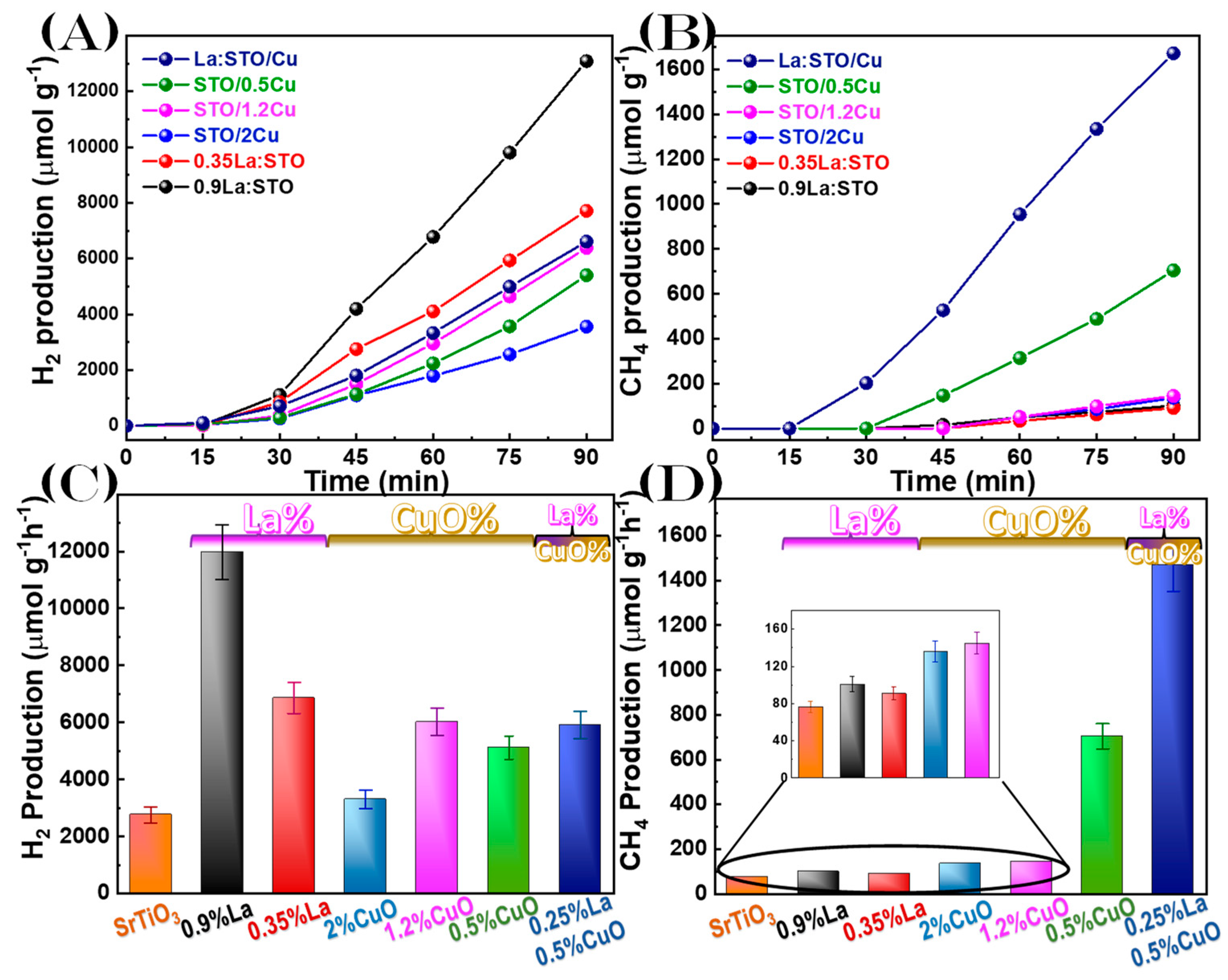
3.3. Photocatalytic Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrog. Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef] [PubMed]
- Phoon, B.L.; Lai, C.W.; Juan, J.C.; Show, P.; Chen, W. A Review of Synthesis and Morphology of SrTiO3 for Energy and Other Applications. Int. J. Energy Res. 2019, 43, 5151–5174. [Google Scholar] [CrossRef]
- Suárez-Vázquez, S.I.; Gil, S.; García-Vargas, J.M.; Cruz-López, A.; Giroir-Fendler, A. Catalytic Oxidation of Toluene by SrTi1-XBXO3 (B = Cu and Mn) with Dendritic Morphology Synthesized by One Pot Hydrothermal Route. Appl. Catal. B Environ. 2018, 223, 201–208. [Google Scholar] [CrossRef]
- Moos, R.; Hardtl, K.H. Defect Chemistry of Donor-Doped and Undoped Strontium Titanate Ceramics between 1000° and 1400 °C. J. Am. Ceram. Soc. 2005, 80, 2549–2562. [Google Scholar] [CrossRef]
- Opoku, F.; Govender, K.K.; van Sittert, C.G.C.E.; Govender, P.P. Enhancing Charge Separation and Photocatalytic Activity of Cubic SrTiO3 with Perovskite-Type Materials MTaO3 (M=Na, K) for Environmental Remediation: A First-Principles Study. ChemistrySelect 2017, 2, 6304–6316. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, N.; Xu, S.; Li, Z.; Liu, X.; Cheng, T.; Han, A.; Lv, H.; Sun, W.; Hou, Y. Towards High Visible Light Photocatalytic Activity in Rare Earth and N Co-Doped SrTiO3: A First Principles Evaluation and Prediction. RSC Adv. 2017, 7, 16282–16289. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Cui, H.; Wang, W.; Shang, Q.; Shi, X.; Cui, G.; Tang, B. Photocatalytic Overall Water Splitting by SrTiO3 with Surface Oxygen Vacancies. Nanomaterials 2020, 10, 2572. [Google Scholar] [CrossRef]
- Chen, H.-C.; Huang, C.-W.; Wu, J.C.S.; Lin, S.-T. Theoretical Investigation of the Metal-Doped SrTiO3 Photocatalysts for Water Splitting. J. Phys. Chem. C 2012, 116, 7897–7903. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, Z.; Wang, H.; Wang, G.; Zhang, Y.; Hu, P.; Li, Y.; Gao, D.; Pu, H.; Wang, B.; et al. Synthesis of Nanocrystalline Strontium Titanate by a Sol–Gel Assisted Solid Phase Method and Its Formation Mechanism and Photocatalytic Activity. CrystEngComm 2019, 21, 3982–3992. [Google Scholar] [CrossRef]
- Kakihana, M.; Okubo, T.; Arima, M.; Nakamura, Y.; Yashima, M.; Yoshimura, M. Polymerized Complex Route to the Synthesis of Pure SrTiO3 at Reduced Temperatures: Implication for Formation of Sr-Ti Heterometallic Citric Acid Complex. J. Sol-Gel Sci. Technol. 1998, 12, 95–109. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef]
- Madler, L.; Kammler, H.K.; Mueller, R.; Pratsinis, S.E. Controlled Synthesis of Nanostructured Particles by Ame Spray Pyrolysis. Aerosol. Sci. 2002, 33, 369–389. [Google Scholar] [CrossRef]
- Mädler, L.; Stark, W.J.; Pratsinis, S.E. Flame-Made Ceria Nanoparticles. J. Mater. Res. 2002, 17, 1356–1362. [Google Scholar] [CrossRef]
- Mueller, R.; Mädler, L.; Pratsinis, S.E. Nanoparticle Synthesis at High Production Rates by Flame Spray Pyrolysis. Chem. Eng. Sci. 2003, 58, 1969–1976. [Google Scholar] [CrossRef]
- Shih, S.-J.; Tzeng, W.-L. Manipulation of Morphology of Strontium Titanate Particles by Spray Pyrolysis. Powder Technol. 2014, 264, 291–297. [Google Scholar] [CrossRef]
- Kang, H.W.; Park, S.B. Doping of Fluorine into SrTiO3 by Spray Pyrolysis for H2 Evolution under Visible Light Irradiation. Chem. Eng. Sci. 2013, 100, 384–391. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Mädler, L. Flame Spray Pyrolysis: An Enabling Technology for Nanoparticles Design and Fabrication. Nanoscale 2010, 2, 1324. [Google Scholar] [CrossRef]
- Gröhn, A.J.; Pratsinis, S.E.; Sánchez-Ferrer, A.; Mezzenga, R.; Wegner, K. Scale-up of Nanoparticle Synthesis by Flame Spray Pyrolysis: The High-Temperature Particle Residence Time. Ind. Eng. Chem. Res. 2014, 53, 10734–10742. [Google Scholar] [CrossRef]
- Moularas, C.; Psathas, P.; Deligiannakis, Y. Electron Paramagnetic Resonance Study of Photo-Induced Hole/Electron Pairs in NaTaO3 Nanoparticles. Chem. Phys. Lett. 2021, 782, 139031. [Google Scholar] [CrossRef]
- Psathas, P.; Solakidou, M.; Mantzanis, A.; Deligiannakis, Y. Flame Spray Pyrolysis Engineering of Nanosized Mullite-Bi2Fe4O9 and Perovskite-BiFeO3 as Highly Efficient Photocatalysts for O2 Production from H2O Splitting. Energies 2021, 14, 5235. [Google Scholar] [CrossRef]
- Psathas, P.; Georgiou, Y.; Moularas, C.; Armatas, G.S.; Deligiannakis, Y. Controlled-Phase Synthesis of Bi2Fe4O9 & BiFeO3 by Flame Spray Pyrolysis and Their Evaluation as Non-Noble Metal Catalysts for Efficient Reduction of 4-Nitrophenol. Powder Technol. 2020, 368, 268–277. [Google Scholar] [CrossRef]
- Stathi, P.; Solakidou, M.; Deligiannakis, Y. Lattice Defects Engineering in W-, Zr-Doped BiVO4 by Flame Spray Pyrolysis: Enhancing Photocatalytic O2 Evolution. Nanomaterials 2021, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Kho, Y.K.; Teoh, W.Y.; Iwase, A.; Mädler, L.; Kudo, A.; Amal, R. Flame Preparation of Visible-Light-Responsive BiVO4 Oxygen Evolution Photocatalysts with Subsequent Activation via Aqueous Route. ACS Appl. Mater. Interfaces 2011, 3, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Laine, R.M. Photocatalytic Plate-like La2Ti2O7 Nanoparticles Synthesized via Liquid-feed Flame Spray Pyrolysis (LF-FSP) of Metallo-organic Precursors. J. Am. Ceram. Soc. 2020, 103, 4832–4839. [Google Scholar] [CrossRef]
- Yuan, X.; Meng, L.; Zheng, C.; Zhao, H. Deep Insight into the Mechanism of Catalytic Combustion of CO and CH4 over SrTi1– xBxO3 (B = Co, Fe, Mn, Ni, and Cu) Perovskite via Flame Spray Pyrolysis. ACS Appl. Mater. Interfaces 2021, 13, 52571–52587. [Google Scholar] [CrossRef]
- Yuan, X.; Meng, L.; Xu, Z.; Zheng, C.; Zhao, H. CuO Quantum Dots Supported by SrTiO3 Perovskite Using the Flame Spray Pyrolysis Method: Enhanced Activity and Excellent Thermal Resistance for Catalytic Combustion of CO and CH4. Environ. Sci. Technol. 2021, 55, 14080–14086. [Google Scholar] [CrossRef]
- Ahmed, A.J.; Hossain, M.S.A.; Kazi Nazrul Islam, S.M.; Yun, F.; Yang, G.; Hossain, R.; Khan, A.; Na, J.; Eguchi, M.; Yamauchi, Y.; et al. Significant Improvement in Electrical Conductivity and Figure of Merit of Nanoarchitectured Porous SrTiO3 by La Doping Optimization. ACS Appl. Mater. Interfaces 2020, 12, 28057–28064. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, H.; Lei, W.; Sinclair, D.C.; Reaney, I.M. High-Figure-of-Merit Thermoelectric La-Doped A-Site-Deficient SrTiO3 Ceramics. Chem. Mater. 2016, 28, 925–935. [Google Scholar] [CrossRef]
- Jia, Y.; Shen, S.; Wang, D.; Wang, X.; Shi, J.; Zhang, F.; Han, H.; Li, C. Composite Sr2TiO4/SrTiO3(La,Cr) Heterojunction Based Photocatalyst for Hydrogen Production under Visible Light Irradiation. J. Mater. Chem. A 2013, 1, 7905. [Google Scholar] [CrossRef]
- Abdi, M.; Mahdikhah, V.; Sheibani, S. Visible Light Photocatalytic Performance of La-Fe Co-Doped SrTiO3 Perovskite Powder. Opt. Mater. 2020, 102, 109803. [Google Scholar] [CrossRef]
- Wang, Q.; Warnan, J.; Rodríguez-Jiménez, S.; Leung, J.J.; Kalathil, S.; Andrei, V.; Domen, K.; Reisner, E. Molecularly Engineered Photocatalyst Sheet for Scalable Solar Formate Production from Carbon Dioxide and Water. Nat. Energy 2020, 5, 703–710. [Google Scholar] [CrossRef]
- Moss, B.; Wang, Q.; Butler, K.T.; Grau-Crespo, R.; Selim, S.; Regoutz, A.; Hisatomi, T.; Godin, R.; Payne, D.J.; Kafizas, A.; et al. Linking in Situ Charge Accumulation to Electronic Structure in Doped SrTiO3 Reveals Design Principles for Hydrogen-Evolving Photocatalysts. Nat. Mater. 2021, 20, 511–517. [Google Scholar] [CrossRef]
- Wang, Q.; Hisatomi, T.; Suzuki, Y.; Pan, Z.; Seo, J.; Katayama, M.; Minegishi, T.; Nishiyama, H.; Takata, T.; Seki, K.; et al. Particulate Photocatalyst Sheets Based on Carbon Conductor Layer for Efficient Z-Scheme Pure-Water Splitting at Ambient Pressure. J. Am. Chem. Soc. 2017, 139, 1675–1683. [Google Scholar] [CrossRef]
- Iwashina, K.; Kudo, A. Rh-Doped SrTiO3 Photocatalyst Electrode Showing Cathodic Photocurrent for Water Splitting under Visible-Light Irradiation. J. Am. Chem. Soc. 2011, 133, 13272–13275. [Google Scholar] [CrossRef]
- Tse, J.; Aziz, A.; Flitcroft, J.M.; Skelton, J.M.; Gillie, L.J.; Parker, S.C.; Cooke, D.J.; Molinari, M. Unraveling the Impact of Graphene Addition to Thermoelectric SrTiO3 and La-Doped SrTiO3 Materials: A Density Functional Theory Study. ACS Appl. Mater. Interfaces 2021, 13, 41303–41314. [Google Scholar] [CrossRef]
- Wang, B.; Shen, S.; Guo, L. SrTiO3 single crystals enclosed with high-indexed {0 2 3} facets and {0 0 1} facets for photocatalytic hydrogen and oxygen evolution. Appl. Catal. B Environ. 2015, 166, 320–326. [Google Scholar] [CrossRef]
- Niishiro, R.; Tanaka, S.; Kudo, A. Hydrothermal-Synthesized SrTiO3 Photocatalyst Codoped with Rhodium and Antimony with Visible-Light Response for Sacrificial H2 and O2 Evolution and Application to Overall Water Splitting. Appl. Catal. B Environ. 2014, 150–151, 187–196. [Google Scholar] [CrossRef]
- Ali, S.; Razzaq, A.; Kim, H.; In, S.-I. Activity, Selectivity, and Stability of Earth-Abundant CuO/Cu2O/Cu0-Based Photocatalysts toward CO2 Reduction. Chem. Eng. J. 2022, 429, 131579. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, E.; Tang, J. Insight on Reaction Pathways of Photocatalytic CO2 Conversion. ACS Catal. 2022, 12, 7300–7316. [Google Scholar] [CrossRef]
- Chen, W.-T.; Jovic, V.; Sun-Waterhouse, D.; Idriss, H.; Waterhouse, G.I.N. The Role of CuO in Promoting Photocatalytic Hydrogen Production over TiO2. Int. J. Hydrog. Energy 2013, 38, 15036–15048. [Google Scholar] [CrossRef]
- Yu, J.; Hai, Y.; Jaroniec, M. Photocatalytic Hydrogen Production over CuO-Modified Titania. J. Colloid Interface Sci. 2011, 357, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, K.; Sadanandam, G.; Kumari, V.D.; Subrahmanyam, M.; Sreedhar, B.; Hebalkar, N.Y. Highly Stabilized and Finely Dispersed Cu2O/TiO2: A Promising Visible Sensitive Photocatalyst for Continuous Production of Hydrogen from Glycerol:Water Mixtures. J. Phys. Chem. C 2010, 114, 22181–22189. [Google Scholar] [CrossRef]
- Chen, J.-L.; Liu, M.-M.; Xie, S.-Y.; Yue, L.-J.; Gong, F.-L.; Chai, K.-M.; Zhang, Y.-H. Cu2O-Loaded TiO2 Heterojunction Composites for Enhanced Photocatalytic H2 Production. J. Mol. Struct. 2022, 1247, 131294. [Google Scholar] [CrossRef]
- Strobel, R.; Mädler, L.; Piacentini, M.; Maciejewski, M.; Baiker, A.; Pratsinis, S.E. Two-Nozzle Flame Synthesis of Pt/Ba/Al2O3 for NOx Storage. Chem. Mater. 2006, 18, 2532–2537. [Google Scholar] [CrossRef]
- Lovell, E.C.; Großman, H.; Horlyck, J.; Scott, J.; Mädler, L.; Amal, R. Asymmetrical Double Flame Spray Pyrolysis-Designed SiO2/Ce0.7Zr0.3O2 for the Dry Reforming of Methane. ACS Appl. Mater. Interfaces 2019, 11, 25766–25777. [Google Scholar] [CrossRef]
- Solakidou, M.; Georgiou, Y.; Deligiannakis, Y. Double-Nozzle Flame Spray Pyrolysis as a Potent Technology to Engineer Noble Metal-TiO2 Nanophotocatalysts for Efficient H2 Production. Energies 2021, 14, 817. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.; Badreldin, A.; Chava, R.; Do, J.; Kang, M.; Abdel-Wahab, A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Khan, M.A.; Nayan, N.; Shadiullah; Ahmad, M.K.; Soon, C.F. Surface Study of CuO Nanopetals by Advanced Nanocharacterization Techniques with Enhanced Optical and Catalytic Properties. Nanomaterials 2020, 10, 1298. [Google Scholar] [CrossRef]
- Baba-Ahmed, I.; Ghercă, D.; Iordan, A.-R.; Palamaru, M.N.; Mita, C.; Baghdad, R.; Ababei, G.; Lupu, N.; Benamar, M.A.; Abderrahmane, A.; et al. Sequential Synthesis Methodology Yielding Well-Defined Porous 75%SrTiO3/25%NiFe2O4 Nanocomposite. Nanomaterials 2021, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- López-Suárez, F.E.; Parres-Esclapez, S.; Bueno-López, A.; Illán-Gómez, M.J.; Ura, B.; Trawczynski, J. Role of Surface and Lattice Copper Species in Copper-Containing (Mg/Sr)TiO3 Perovskite Catalysts for Soot Combustion. Appl. Catal. B Environ. 2009, 93, 82–89. [Google Scholar] [CrossRef]
- van Benthem, K.; Elsässer, C.; French, R.H. Bulk Electronic Structure of SrTiO3: Experiment and Theory. J. Appl. Phys. 2001, 90, 6156–6164. [Google Scholar] [CrossRef]
- Maček Kržmanc, M.; Daneu, N.; Čontala, A.; Santra, S.; Kamal, K.M.; Likozar, B.; Spreitzer, M. SrTiO3/Bi4Ti3O12 nanoheterostructural platelets synthesized by topotactic epitaxy as effective noble-metal-free photocatalysts for pH-neutral hydrogen evolution. ACS Appl. Mater. Interfaces 2020, 13, 370–381. [Google Scholar] [CrossRef]
- Mai, X.T.; Bui, D.N.; Pham, V.K.; Nguyen, T.H.L.; Nguyen, T.T.L.; Chau, H.D.; Tran, T.K.N. Effect of CuO Loading on the Photocatalytic Activity of SrTiO3 for Hydrogen Evolution. Inorganics 2022, 10, 130. [Google Scholar] [CrossRef]
- Xia, Y.; He, Z.; Hu, K.; Tang, B.; Su, J.; Liu, Y.; Li, X. Fabrication of N-SrTiO3/p-Cu2O Heterojunction Composites with Enhanced Photocatalytic Performance. J. Alloy. Compd. 2018, 753, 356–363. [Google Scholar] [CrossRef]
- Li, W.; Liu, S.; Wang, S.; Guo, Q.; Guo, J. The Roles of Reduced Ti Cations and Oxygen Vacancies in Water Adsorption and Dissociation on SrTiO3 (110). J. Phys. Chem. C 2014, 118, 2469–2474. [Google Scholar] [CrossRef]
- Ura, B.; Trawczyński, J.; Kotarba, A.; Bieniasz, W.; Illán-Gómez, M.J.; Bueno-López, A.; López-Suárez, F.E. Effect of Potassium Addition on Catalytic Activity of SrTiO3 Catalyst for Diesel Soot Combustion. Appl. Catal. B Environ. 2011, 101, 169–175. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Z.; Zhu, W.; Coker, E.N.; Li, B.; Zheng, M.; Yu, W.; Fan, H.; Sun, Z. Oxygen Vacancy Enhanced Photocatalytic Activity of Pervoskite SrTiO3. ACS Appl. Mater. Interfaces 2014, 6, 19184–19190. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Sanchez, J.E.; Camposeco, R.; Lee, S.-W.; Rodríguez-González, V. Sustainable Synthesis of AgNPs/Strontium-Titanate-Perovskite-like Catalysts for the Photocatalytic Production of Hydrogen. Catal. Today 2020, 341, 112–119. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, L.; Li, Y.; Yang, R.; Qu, J.; Li, Y.; Li, X. Synthesis and High Photocatalytic Hydrogen Production of SrTiO3 Nanoparticles from Water Splitting under UV Irradiation. J. Power Sources 2008, 183, 701–707. [Google Scholar] [CrossRef]
- Macaraig, L.; Chuangchote, S.; Sagawa, T. Electrospun SrTiO3 Nanofibers for Photocatalytic Hydrogen Generation. J. Mater. Res. 2014, 29, 123–130. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, G.; Wang, Y. Study on the Photocatalytic Property of La-Doped CoO/SrTiO3 for Water Decomposition to Hydrogen. Catal. Commun. 2007, 8, 926–930. [Google Scholar] [CrossRef]
- Sakata, Y.; Miyoshi, Y.; Maeda, T.; Ishikiriyama, K.; Yamazaki, Y.; Imamura, H.; Ham, Y.; Hisatomi, T.; Kubota, J.; Yamakata, A.; et al. Photocatalytic Property of Metal Ion Added SrTiO3 to Overall H2O Splitting. Appl. Catal. A Gen. 2016, 521, 227–232. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, C.; Chang, Y.; Feng, Y.; Yang, Z.; Yang, T.; Lou, L.-L.; Liu, S. Novel Three-Dimensionally Ordered Macroporous SrTiO3 Photocatalysts with Remarkably Enhanced Hydrogen Production Performance. Appl. Catal. B Environ. 2017, 200, 514–520. [Google Scholar] [CrossRef]
- Kumar, A.; Navakoteswara Rao, V.; Kumar, A.; Mushtaq, A.; Sharma, L.; Halder, A.; Pal, S.K.; Shankar, M.V.; Krishnan, V. Three-Dimensional Carbonaceous Aerogels Embedded with Rh-SrTiO3 for Enhanced Hydrogen Evolution Triggered by Efficient Charge Transfer and Light Absorption. ACS Appl. Energy Mater. 2020, 3, 12134–12147. [Google Scholar] [CrossRef]
- Lv, S.; Wang, Y.; Zhou, Y.; Liu, Q.; Song, C.; Wang, D. Oxygen Vacancy Stimulated Direct Z-Scheme of Mesoporous Cu2O/TiO2 for Enhanced Photocatalytic Hydrogen Production from Water and Seawater. J. Alloy. Compd. 2021, 868, 159144. [Google Scholar] [CrossRef]
- Lou, Y.; Zhang, Y.; Cheng, L.; Chen, J.; Zhao, Y. A Stable Plasmonic Cu@Cu2O/ZnO Heterojunction for Enhanced Photocatalytic Hydrogen Generation. ChemSusChem 2018, 11, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Kahng, S.; Hyeun Kim, J. Z-Scheme Assisted ZnO/Cu2O-CuO Photocatalysts to Increase Photoactive Electrons in Hydrogen Evolution by Water Splitting. Sol. Energy Mater. Sol. Cells 2020, 204, 110211. [Google Scholar] [CrossRef]
- Xiong, Z.; Lei, Z.; Kuang, C.-C.; Chen, X.; Gong, B.; Zhao, Y.; Zhang, J.; Zheng, C.; Wu, J.C.S. Selective Photocatalytic Reduction of CO2 into CH4 over Pt-Cu2O TiO2 Nanocrystals: The Interaction between Pt and Cu2O Cocatalysts. Appl. Catal. B Environ. 2017, 202, 695–703. [Google Scholar] [CrossRef]
- Chen, B.-R.; Nguyen, V.-H.; Wu, J.C.S.; Martin, R.; Kočí, K. Production of Renewable Fuels by the Photohydrogenation of CO2: Effect of the Cu Species Loaded onto TiO2 Photocatalysts. Phys. Chem. Chem. Phys. 2016, 18, 4942–4951. [Google Scholar] [CrossRef]
| Photo Catalyst | Synthesis Method | Cocatalyst (wt%) | Light Source | Reaction Solution | H2 Yield (umol g−1 h−1) | Ref. |
|---|---|---|---|---|---|---|
| 0.9%La: SrTiO3 | FSP | 1% Pt | Hg (250 W) | H2O + 20% CH3OH | 11,978 | This work |
| 0.25%La:SrTiO/0.5% CuO | FSP | 1% Pt | Hg (250 W) | H2O + 20% CH3OH | 5907 | This work |
| Ag-STO | Microwave-assisted hydrothermal | 2% Ag | UV-low pressure Hg | 50% H2O + 50% ethanol | 463 | [63] |
| Pt- SrTiO3 | Polymerized-Complex | 0.32% Pt | Hg (500 W) | Pure water + 40% methanol | 3200 | [64] |
| Nanofibers SrTiO3 | Electrospinning method | - | Hg (450 W) | Pure water + 40% methanol | 160 | [65] |
| La: CoO/SrTiO3 | Solid statereaction | - | Hg (400 W) | Pure water + Na2CO3 | 2800 | [66] |
| Na:SrTiO3 | polymerizable complex | 0.3% Rh | Hg (450 W) | Pure Water | 1600 | [67] |
| Macroporous SrTiO3 | emulsion polymerization method | 0.7% Pt | Xe (300 W) | Pure water + 25% methanol | 3599 | [68] |
| g-C3N4/Rh-SrTiO3/RGO | Multiple methods | 0.58% Rh | Xe (260 W) | Pure water + 10% TEOA | 3467 | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psathas, P.; Zindrou, A.; Papachristodoulou, C.; Boukos, N.; Deligiannakis, Y. In Tandem Control of La-Doping and CuO-Heterojunction on SrTiO3 Perovskite by Double-Nozzle Flame Spray Pyrolysis: Selective H2 vs. CH4 Photocatalytic Production from H2O/CH3OH. Nanomaterials 2023, 13, 482. https://doi.org/10.3390/nano13030482
Psathas P, Zindrou A, Papachristodoulou C, Boukos N, Deligiannakis Y. In Tandem Control of La-Doping and CuO-Heterojunction on SrTiO3 Perovskite by Double-Nozzle Flame Spray Pyrolysis: Selective H2 vs. CH4 Photocatalytic Production from H2O/CH3OH. Nanomaterials. 2023; 13(3):482. https://doi.org/10.3390/nano13030482
Chicago/Turabian StylePsathas, Pavlos, Areti Zindrou, Christina Papachristodoulou, Nikos Boukos, and Yiannis Deligiannakis. 2023. "In Tandem Control of La-Doping and CuO-Heterojunction on SrTiO3 Perovskite by Double-Nozzle Flame Spray Pyrolysis: Selective H2 vs. CH4 Photocatalytic Production from H2O/CH3OH" Nanomaterials 13, no. 3: 482. https://doi.org/10.3390/nano13030482
APA StylePsathas, P., Zindrou, A., Papachristodoulou, C., Boukos, N., & Deligiannakis, Y. (2023). In Tandem Control of La-Doping and CuO-Heterojunction on SrTiO3 Perovskite by Double-Nozzle Flame Spray Pyrolysis: Selective H2 vs. CH4 Photocatalytic Production from H2O/CH3OH. Nanomaterials, 13(3), 482. https://doi.org/10.3390/nano13030482









