Nano-Conversion of Ineffective Cephalosporins into Potent One against Resistant Clinical Uro-Pathogens via Gold Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AuNPs Synthesis
2.3. AuNPs Characterization
2.3.1. Spectrophotometry
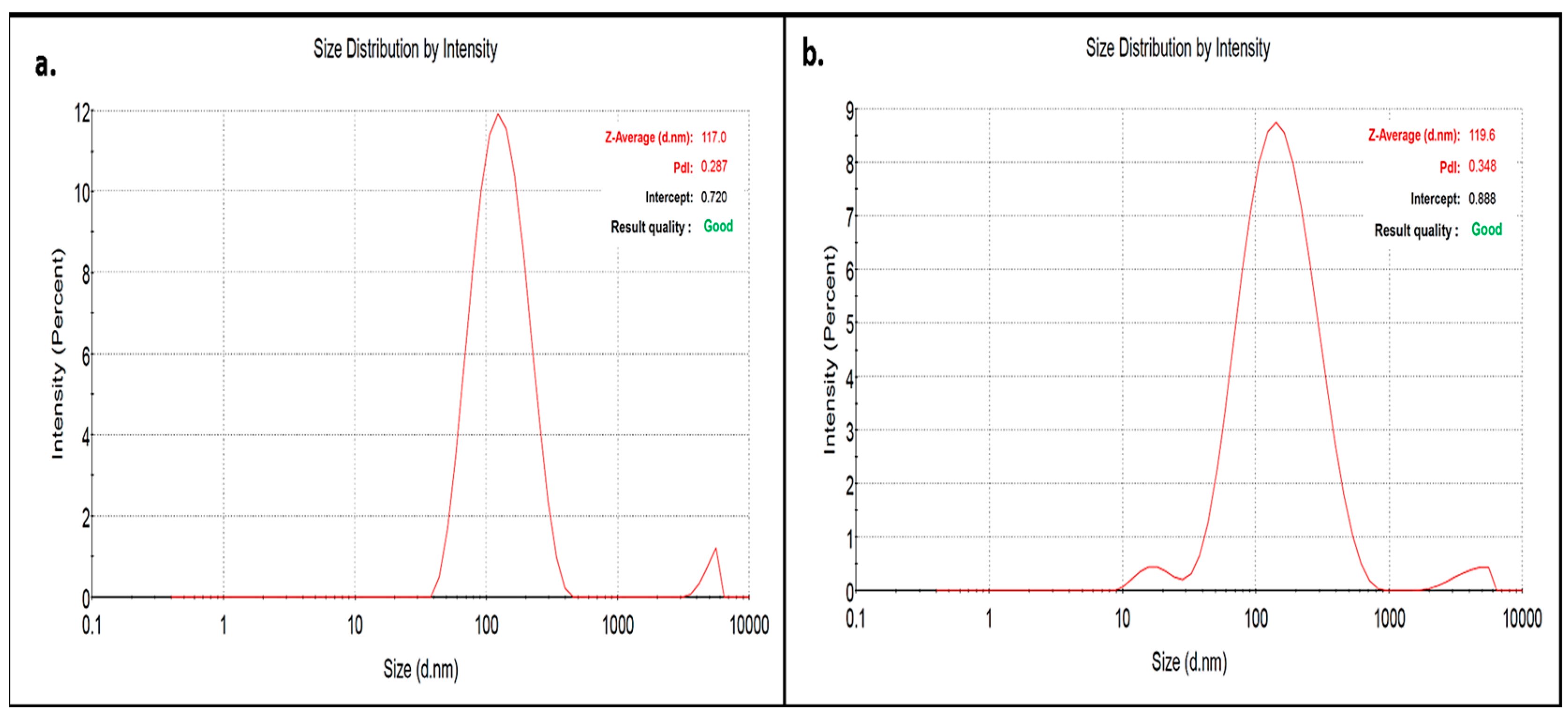
2.3.2. Zeta Sizing and Zeta Potential
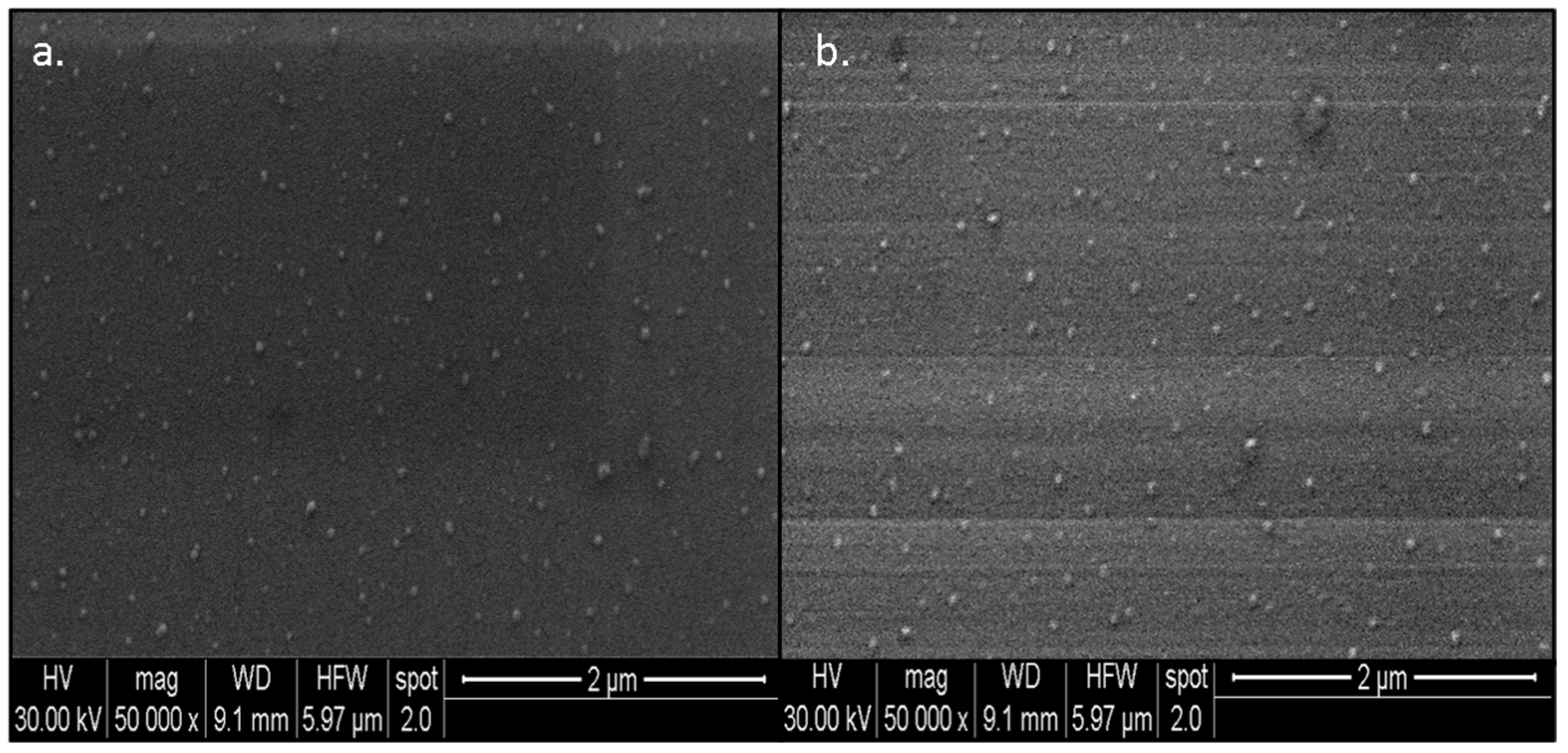
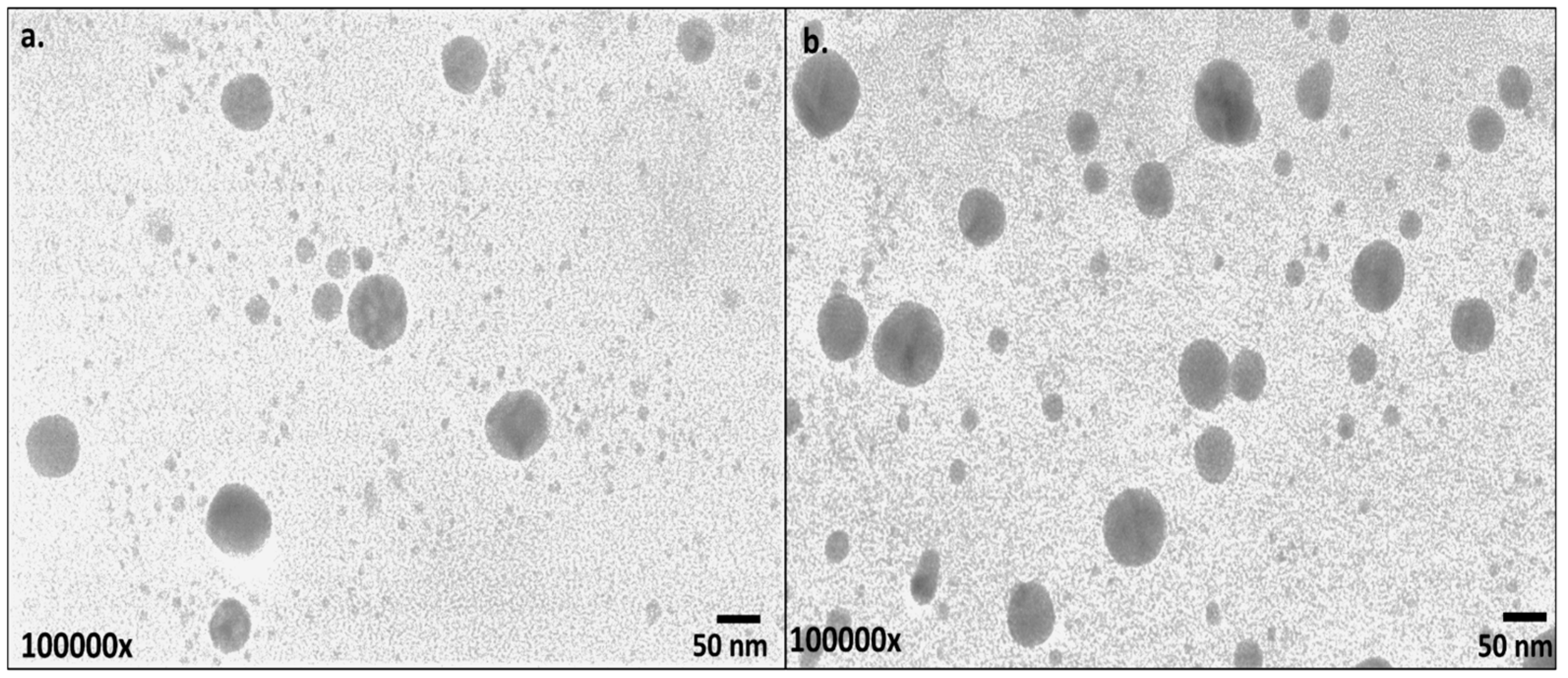
2.3.3. Electron Microscopy
2.4. Estimation of Loading Efficiency
2.5. Assessment of Antibacterial Potential of CT-AuNPs and CX-AuNPs
2.5.1. Resistant Uropathogenic Strains
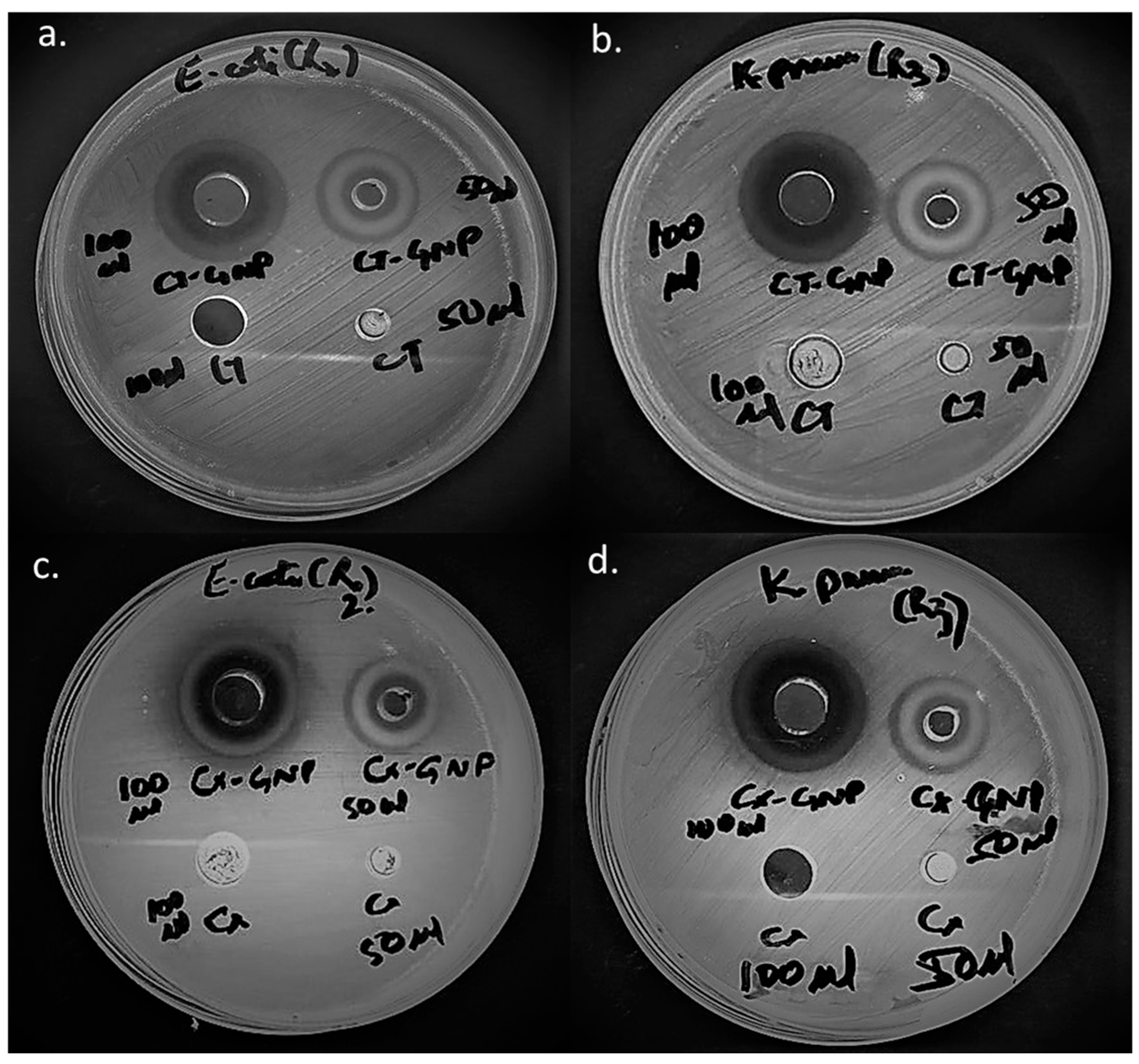
2.5.2. Antibacterial Assessment by Agar Well Diffusion
2.5.3. Antibacterial Assessment by Calculating Minimal Inhibitory Concentration
3. Results and Discussion
3.1. Synthesis of CT-AuNPs and CX-AuNPs
3.2. Characterization of CT-AuNPs and CX-AuNPs
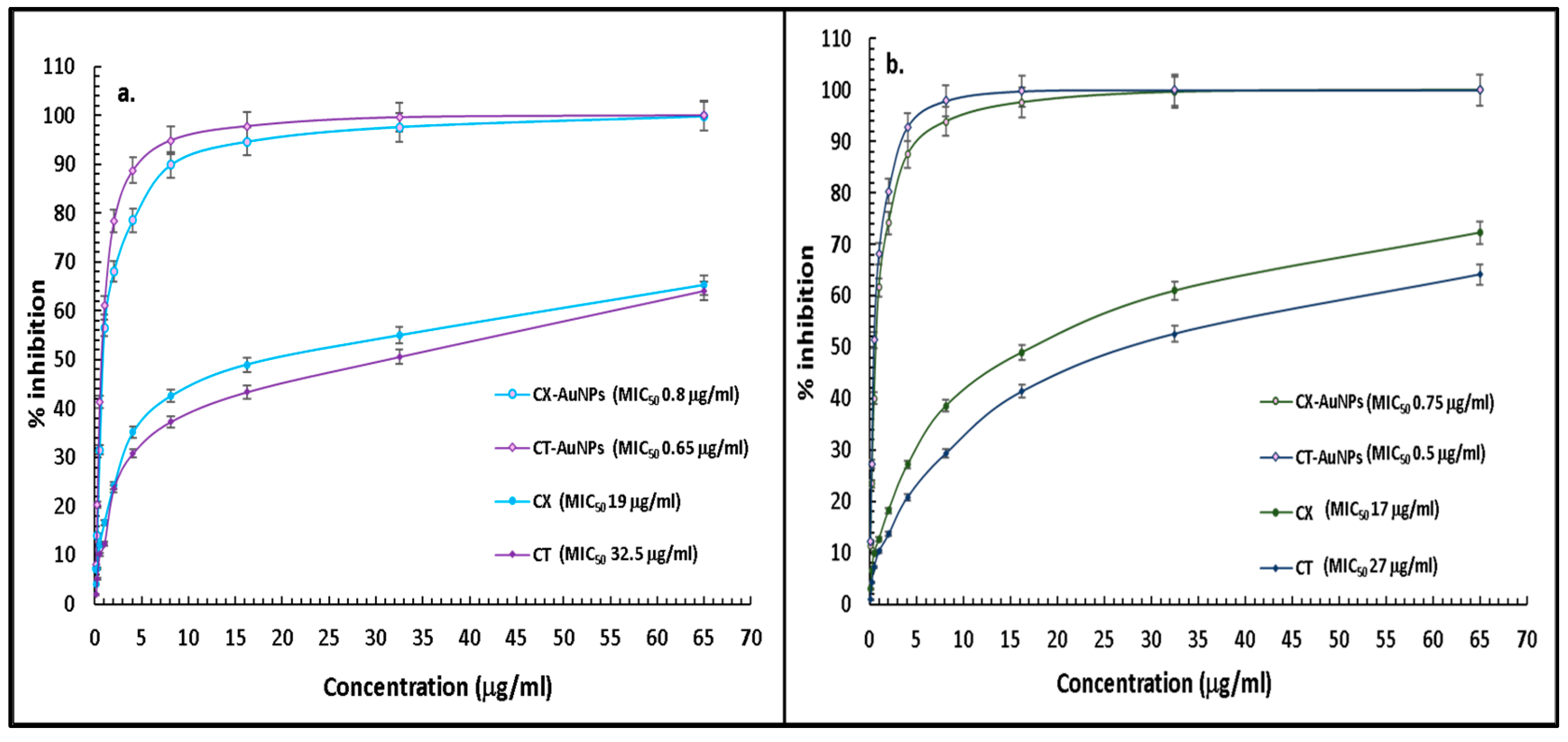
3.3. Comparative Antibacterial Assessment of CT-AuNPs and CX-AuNPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.R.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.C.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet (Lond. Engl.) 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Wang, T.; Xu, N.; Lu, T.; Hong, W.; Penuelas, J.; Gillings, M.; Wang, M.; Gao, W.; et al. Assessment of global health risk of antibiotic resistance genes. Nat. Commun. 2022, 13, 1553. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; Esiovwa, R.; Connolly, J.; Hursthouse, A.; Henriquez, F. Antimicrobial Resistance as a Global Health Threat: The Need to Learn Lessons from the COVID-19 Pandemic. Glob. Policy 2022, 13, 179–192. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 18 December 2022).
- Shaikh, S.; Fatima, J.; Shakil, S.; Rizvi, S.M.; Kamal, M.A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J. Biol. Sci. 2015, 22, 90–101. [Google Scholar] [CrossRef]
- Shaikh, S.; Fatima, J.; Shakil, S.; Danish Rizvi, S.M.; Kamal, M.A. Prevalence of multidrug resistant and extended spectrum beta-lactamase producing Pseudomonas aeruginosa in a tertiary care hospital. Saudi J. Biol. Sci. 2015, 22, 62–64. [Google Scholar] [CrossRef][Green Version]
- Shaikh, S.; Fatima, J.; Shakil, S.; Rizvi, S.M.; Kamal, M.A. Risk factors for acquisition of extended spectrum beta lactamase producing Escherichia coli and Klebsiella pneumoniae in North-Indian hospitals. Saudi J. Biol. Sci. 2015, 22, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Ahmad, A.; Pathak, N. Non-clonal Dissemination of Extended-Spectrum Beta-Lactamase-Producing Pseudomonas aeruginosa Strains of Clinical Origin. Iran. J. Sci. Technol. Trans. A Sci. 2017, 41, 1011–1015. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Alafnan, A.; Rizvi, S.M.; Alshammari, A.S.; Faiyaz, S.S.; Lila, A.S.; Katamesh, A.A.; Khafagy, E.-S.; Alotaibi, H.F.; Ahmed, A.B.F. Gold Nanoparticle-Based Resuscitation of Cefoxitin against Clinical Pathogens: A Nano-Antibiotic Strategy to Overcome Resistance. Nanomaterials 2022, 12, 3643. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Huwaimel, B.; Alobaida, A.; Hussain, T.; Rafi, Z.; Mehmood, K.; Abdallah, M.H.; Hagbani, T.A.; Rizvi, S.M.D.; Moin, A.; et al. Delafloxacin-Capped Gold Nanoparticles (DFX-AuNPs): An Effective Antibacterial Nano-Formulation of Fluoroquinolone Antibiotic. Materials 2022, 15, 5709. [Google Scholar] [CrossRef]
- Al Hagbani, T.; Rizvi, S.M.D.; Hussain, T.; Mehmood, K.; Rafi, Z.; Moin, A.; Abu Lila, A.S.; Alshammari, F.; Khafagy, E.S.; Rahamathulla, M.; et al. Cefotaxime Mediated Synthesis of Gold Nanoparticles: Characterization and Antibacterial Activity. Polymers 2022, 14, 771. [Google Scholar] [CrossRef]
- Hagbani, T.A.; Yadav, H.; Moin, A.; Lila, A.S.A.; Mehmood, K.; Alshammari, F.; Khan, S.; Khafagy, E.S.; Hussain, T.; Rizvi, S.M.D.; et al. Enhancement of Vancomycin Potential against Pathogenic Bacterial Strains via Gold Nano-Formulations: A Nano-Antibiotic Approach. Materials 2022, 15, 1108. [Google Scholar] [CrossRef]
- Alshammari, F.; Alshammari, B.; Moin, A.; Alamri, A.; Al Hagbani, T.; Alobaida, A.; Baker, A.; Khan, S.; Rizvi, S.M.D. Ceftriaxone Mediated Synthesized Gold Nanoparticles: A Nano-Therapeutic Tool to Target Bacterial Resistance. Pharmaceutics 2021, 13, 1896. [Google Scholar] [CrossRef]
- Soliman, W.E.; Khan, S.; Rizvi, S.M.D.; Moin, A.; Elsewedy, H.S.; Abulila, A.S.; Shehata, T.M. Therapeutic Applications of Biostable Silver Nanoparticles Synthesized Using Peel Extract of Benincasa hispida: Antibacterial and Anticancer Activities. Nanomaterials 2020, 10, 1954. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.M.D.; Hussain, T.; Ahmed, A.B.F.; Alshammari, T.M.; Moin, A.; Ahmed, M.Q.; Barreto, G.E.; Kamal, M.A.; Ashraf, G.M. Gold nanoparticles: A plausible tool to combat neurological bacterial infections in humans. Biomed. Pharmacother. 2018, 107, 7–18. [Google Scholar] [CrossRef]
- Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Hussain, T.; Alshammari, T.M.; Ahmad, W.; Tabrez, S.; Al-Qahtani, M.H.; Abuzenadah, A.M. Synthesis and Characterization of Cefotaxime Conjugated Gold Nanoparticles and Their Use to Target Drug-Resistant CTX-M-Producing Bacterial Pathogens. J. Cell Biochem. 2017, 118, 2802–2808. [Google Scholar] [CrossRef]
- Khandelwal, P.; Singh, D.K.; Poddar, P. Advances in the Experimental and Theoretical Understandings of Antibiotic Conjugated Gold Nanoparticles for Antibacterial Applications. ChemistrySelect 2019, 4, 6719–6738. [Google Scholar] [CrossRef]
- Allied Market Research. Available online: https://www.alliedmarketresearch.com/gold-nanoparticles-market-A08997 (accessed on 18 December 2022).
- Globenewswire. Available online: https://www.globenewswire.com/news-release/2022/10/13/2534150/0/en/Global-Gold-Nanoparticles-Market-to-Reach-7-6-Billion-by-2027.html (accessed on 18 December 2022).
- IMARCGROUP. Available online: https://www.imarcgroup.com/gold-nanoparticles-market (accessed on 18 December 2022).
- Rai, A.; Prabhune, A.; Perry, C.C. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J. Mater. Chem. 2010, 20, 6789–6798. [Google Scholar] [CrossRef]
- Khandelwal, P.; Singh, D.K.; Sadhu, S.; Poddar, P. Study of the nucleation and growth of antibiotic labeled Au NPs and blue luminescent Au8 quantum clusters for Hg2+ ion sensing, cellular imaging and antibacterial applications. Nanoscale 2015, 7, 19985–20002. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Maeda, T.; Okumura, K.; Uda, M.; Nakamura, M.; Kashiwagi, T.; Tsunoda, T. Process Development and Pilot-Scale Synthesis of Cefotetan. Org. Process Res. Dev. 2004, 8, 915–919. [Google Scholar] [CrossRef]
- Ramadan, A.A.; Mandil, H.; Dahhan, M. UV-VIS spectrophotometric study for determination of cefixime in pure form and in pharmaceuticals through complex-ation with Cu(II) using acetate-NaOH buffer in water:methanol. Int. J. Pharm. Phar. Sci. 2013, 51, 428–433. [Google Scholar]
- Gomes, M.J.; Martins, S.; Ferreira, D.; Segundo, M.A.; Reis, S. Lipid nanoparticles for topical and transdermal application for alopecia treatment: Development, physicochemical characterization, and in vitro release and penetration studies. Int. J. Nanomed. 2014, 9, 1231–1242. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Rusu, A.; Lungu, I.-A. The new fifth-generation cephalosporins–A balance between safety and efficacy. Ro. J. Pharm. Pract. 2020, 13, 121–126. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, X.; Zhang, L.; Xiong, Z.; Ma, Y.; Wei, Y.; Chen, Z.; Bai, J.; Liao, M.; Zhang, J. Fourth Generation Cephalosporin Resistance Among Salmonella enterica Serovar Enteritidis Isolates in Shanghai, China Conferred by bla (CTX-M-55) Harboring Plasmids. Front. Microbiol. 2020, 11, 910. [Google Scholar] [CrossRef] [PubMed]
- Lester, R.; Musicha, P.; Kawaza, K.; Langton, J.; Mango, J.; Mangochi, H.; Bakali, W.; Pearse, O.; Mallewa, J.; Denis, B.; et al. Effect of resistance to third-generation cephalosporins on morbidity and mortality from bloodstream infections in Blantyre, Malawi: A prospective cohort study. Lancet Microbe 2022, 3, e922–e930. [Google Scholar] [CrossRef]
- Chang, C.Y.; Huang, P.H.; Lu, P.L. The Resistance Mechanisms and Clinical Impact of Resistance to the Third Generation Cephalosporins in Species of Enterobacter cloacae Complex in Taiwan. Antibiotics 2022, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.R.; Papp-Wallace, K.M.; Drawz, S.M.; Bonomo, R.A. Novel β-lactamase inhibitors: A therapeutic hope against the scourge of multidrug resistance. Front. Microbiol. 2013, 4, 392. [Google Scholar] [CrossRef]
- Ripoll, A.; Galán, J.C.; Rodríguez, C.; Tormo, N.; Gimeno, C.; Baquero, F.; Martínez-Martínez, L.; Cantón, R. Detection of resistance to beta-lactamase inhibitors in strains with CTX-M beta-lactamases: A multicenter external proficiency study using a well-defined collection of Escherichia coli strains. J. Clin. Microbiol. 2014, 52, 122–129. [Google Scholar] [CrossRef]
- Helfand, M.S.; Bethel, C.R.; Hujer, A.M.; Hujer, K.M.; Anderson, V.E.; Bonomo, R.A. Understanding Resistance to β-Lactams and β-Lactamase Inhibitors in the SHV β-Lactamase: LESSONS FROM THE MUTAGENESIS OF SER-130. J. Biol. Chem. 2003, 278, 52724–52729. [Google Scholar] [CrossRef]
- Suchomel, P.; Kvitek, L.; Prucek, R.; Panacek, A.; Halder, A.; Vajda, S.; Zboril, R. Simple size-controlled synthesis of Au nanoparticles and their size-dependent catalytic activity. Sci. Rep. 2018, 8, 4589. [Google Scholar] [CrossRef]
- Piella, J.; Bastús, N.G.; Puntes, V. Size-Controlled Synthesis of Sub-10-nanometer Citrate-Stabilized Gold Nanoparticles and Related Optical Properties. Chem. Mater. 2016, 28, 1066–1075. [Google Scholar] [CrossRef]
- Elia, P.; Zach, R.; Hazan, S.; Kolusheva, S.; Porat, Z.e.; Zeiri, Y. Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int. J. Nanomed. 2014, 9, 4007–4021. [Google Scholar]
- Muddapur, U.M.; Alshehri, S.; Ghoneim, M.M.; Mahnashi, M.H.; Alshahrani, M.A.; Khan, A.A.; Iqubal, S.M.S.; Bahafi, A.; More, S.S.; Shaikh, I.A.; et al. Plant-Based Synthesis of Gold Nanoparticles and Theranostic Applications: A Review. Molecules 2022, 27, 1391. [Google Scholar] [CrossRef]
- Khan, S.; Danish Rizvi, S.M.; Avaish, M.; Arshad, M.; Bagga, P.; Khan, M.S. A novel process for size controlled biosynthesis of gold nanoparticles using bromelain. Mater. Lett. 2015, 159, 373–376. [Google Scholar] [CrossRef]
- Amina, S.J.; Guo, B. A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Int. J. Nanomed. 2020, 15, 9823. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.S.; Pandey, E.; Kadian, J.P.; Chauhan, B. Green Synthesis of Gold Nanoparticles: A Novel, Environment-Friendly, Economic, Safe Approach. Biomed. Pharmacol. J. 2021, 14, 2041–2406. [Google Scholar] [CrossRef]
- Sengani, M.; Grumezescu, A.M.; Rajeswari, V.D. Recent trends and methodologies in gold nanoparticle synthesis–A prospective review on drug delivery aspect. OpenNano 2017, 2, 37–46. [Google Scholar] [CrossRef]
- Khan, S.; Haseeb, M.; Baig, M.H.; Bagga, P.S.; Siddiqui, H.H.; Kamal, M.A.; Khan, M.S. Improved efficiency and stability of secnidazole–An ideal delivery system. Saudi J. Biol. Sci. 2015, 22, 42–49. [Google Scholar] [CrossRef]
- Khatoon, N.; Alam, H.; Khan, A.; Raza, K.; Sardar, M. Ampicillin Silver Nanoformulations against Multidrug resistant bacteria. Sci. Rep. 2019, 9, 6848. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska-Prończuk, J.; Morga, M.; Adamczyk, Z.; Oćwieja, M.; Zimowska, M. Homogeneous gold nanoparticle monolayers—QCM and electrokinetic characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2017, 514, 226–235. [Google Scholar] [CrossRef]
- Pourali, P.; Benada, O.; Pátek, M.; Neuhöferová, E.; Dzmitruk, V.; Benson, V. Response of Biological Gold Nanoparticles to Different pH Values: Is It Possible to Prepare Both Negatively and Positively Charged Nanoparticles? Appl. Sci. 2021, 11, 11559. [Google Scholar] [CrossRef]
- Kato, H.; Nakamura, A.; Takahashi, K.; Kinugasa, S. Accurate Size and Size-Distribution Determination of Polystyrene Latex Nanoparticles in Aqueous Medium Using Dynamic Light Scattering and Asymmetrical Flow Field Flow Fractionation with Multi-Angle Light Scattering. Nanomaterials 2012, 2, 15–30. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 2337. [Google Scholar] [CrossRef]
- Xu, R. Progress in nanoparticles characterization: Sizing and zeta potential measurement. Particuology 2008, 6, 112–115. [Google Scholar] [CrossRef]
- Zhou, W.; Apkarian, R.P.; Wang, Z.L.; Joy, D.C. Fundamentals of Scanning Electron Microscopy (SEM). In Scanning Microscopy for Nanotechnology; Zhou, W., Wang, Z.L., Eds.; Springer: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Wilson, B.K.; Prud′homme, R.K. Nanoparticle size distribution quantification from transmission electron microscopy (TEM) of ruthenium tetroxide stained polymeric nanoparticles. J. Colloid Interface Sci. 2021, 604, 208–220. [Google Scholar] [CrossRef]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef]
- Goldstein, E. Rise in the prevalence of resistance to extended-spectrum cephalosporins in the USA, nursing homes and antibiotic prescribing in outpatient and inpatient settings. J. Antimicrob. Chemother. 2021, 76, 2745–2747. [Google Scholar] [CrossRef]
- Zamudio, R.; Boerlin, P.; Beyrouthy, R.; Madec, J.-Y.; Schwarz, S.; Mulvey, M.R.; Zhanel, G.G.; Cormier, A.; Chalmers, G.; Bonnet, R.; et al. Dynamics of extended-spectrum cephalosporin resistance genes in Escherichia coli from Europe and North America. Nat. Commun. 2022, 13, 7490. [Google Scholar] [CrossRef]
- Meyer, E.; Schwab, F.; Schroeren-Boersch, B.; Gastmeier, P. Dramatic increase of third-generation cephalosporin-resistant E. coli in German intensive care units: Secular trends in antibiotic drug use and bacterial resistance, 2001 to 2008. Crit Care 2010, 14, R113. [Google Scholar] [CrossRef] [PubMed]
- van der Steen, M.; Leenstra, T.; Kluytmans, J.A.; van der Bij, A.K. Trends in Expanded-Spectrum Cephalosporin-Resistant Escherichia coli and Klebsiella pneumoniae among Dutch Clinical Isolates, from 2008 to 2012. PLoS ONE 2015, 10, e0138088. [Google Scholar] [CrossRef]
- Aracil-García, B.; Oteo-Iglesias, J.; Cuevas-Lobato, Ó.; Lara-Fuella, N.; Pérez-Grajera, I.; Fernández-Romero, S.; Pérez-Vázquez, M.; Campos, J. Rapid increase in resistance to third generation cephalosporins, imipenem and co-resistance in Klebsiella pneumoniae from isolated from 7140 blood-cultures (2010–2014) using EARS-Net data in Spain. Enferm. Infecc. Microbiol. Clin. 2017, 35, 480–486. [Google Scholar] [CrossRef]
- Maurya, N.; Jangra, M.; Tambat, R.; Nandanwar, H. Alliance of Efflux Pumps with β-Lactamases in Multidrug-Resistant Klebsiella pneumoniae Isolates. Microb. Drug Resist. 2019, 25, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Xavier, D.E.; Picão, R.C.; Girardello, R.; Fehlberg, L.C.; Gales, A.C. Efflux pumps expression and its association with porin down-regulation and beta-lactamase production among Pseudomonas aeruginosa causing bloodstream infections in Brazil. BMC Microbiol. 2010, 10, 217. [Google Scholar] [CrossRef]
- Martínez-Martínez, L. Extended-spectrum beta-lactamases and the permeability barrier. Clin. Microbiol. Infect. 2008, 14 (Suppl. S1), 82–89. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Khare, T.; Mahalunkar, S.; Shriram, V.; Gosavi, S.; Kumar, V. Embelin-loaded chitosan gold nanoparticles interact synergistically with ciprofloxacin by inhibiting efflux pumps in multidrug-resistant Pseudomonas aeruginosa and Escherichia coli. Environ. Res. 2021, 199, 111321. [Google Scholar] [CrossRef]


| E. coli | K. pneumoniae | |
|---|---|---|
| CX-GNPs (3.25 mg) | 17 mm | 18 mm |
| CX-GNPs (6.5 mg) | 22 mm | 23 mm |
| CT-GNPs (3.25 mg) | 18 mm | 19 mm |
| CT-GNPs (6.5 mg) | 23 mm | 24 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizvi, S.M.D.; Hussain, T.; Alshammari, F.; Sonbol, H.; Ahmad, N.; Faiyaz, S.S.M.; Kamal, M.A.; Khafagy, E.-S.; Moin, A.; Abu Lila, A.S. Nano-Conversion of Ineffective Cephalosporins into Potent One against Resistant Clinical Uro-Pathogens via Gold Nanoparticles. Nanomaterials 2023, 13, 475. https://doi.org/10.3390/nano13030475
Rizvi SMD, Hussain T, Alshammari F, Sonbol H, Ahmad N, Faiyaz SSM, Kamal MA, Khafagy E-S, Moin A, Abu Lila AS. Nano-Conversion of Ineffective Cephalosporins into Potent One against Resistant Clinical Uro-Pathogens via Gold Nanoparticles. Nanomaterials. 2023; 13(3):475. https://doi.org/10.3390/nano13030475
Chicago/Turabian StyleRizvi, Syed Mohd Danish, Talib Hussain, Farhan Alshammari, Hana Sonbol, Nabeel Ahmad, Syed Shah Mohammed Faiyaz, Mohammad Amjad Kamal, El-Sayed Khafagy, Afrasim Moin, and Amr Selim Abu Lila. 2023. "Nano-Conversion of Ineffective Cephalosporins into Potent One against Resistant Clinical Uro-Pathogens via Gold Nanoparticles" Nanomaterials 13, no. 3: 475. https://doi.org/10.3390/nano13030475
APA StyleRizvi, S. M. D., Hussain, T., Alshammari, F., Sonbol, H., Ahmad, N., Faiyaz, S. S. M., Kamal, M. A., Khafagy, E.-S., Moin, A., & Abu Lila, A. S. (2023). Nano-Conversion of Ineffective Cephalosporins into Potent One against Resistant Clinical Uro-Pathogens via Gold Nanoparticles. Nanomaterials, 13(3), 475. https://doi.org/10.3390/nano13030475








