Hydrothermal Synthesis and Properties of Nanostructured Silica Containing Lanthanide Type Ln–SiO2 (Ln = La, Ce, Pr, Nd, Eu, Gd, Dy, Yb, Lu)
Abstract
1. Introduction
2. Materials and Methods
2.1. Precursors and Reagents
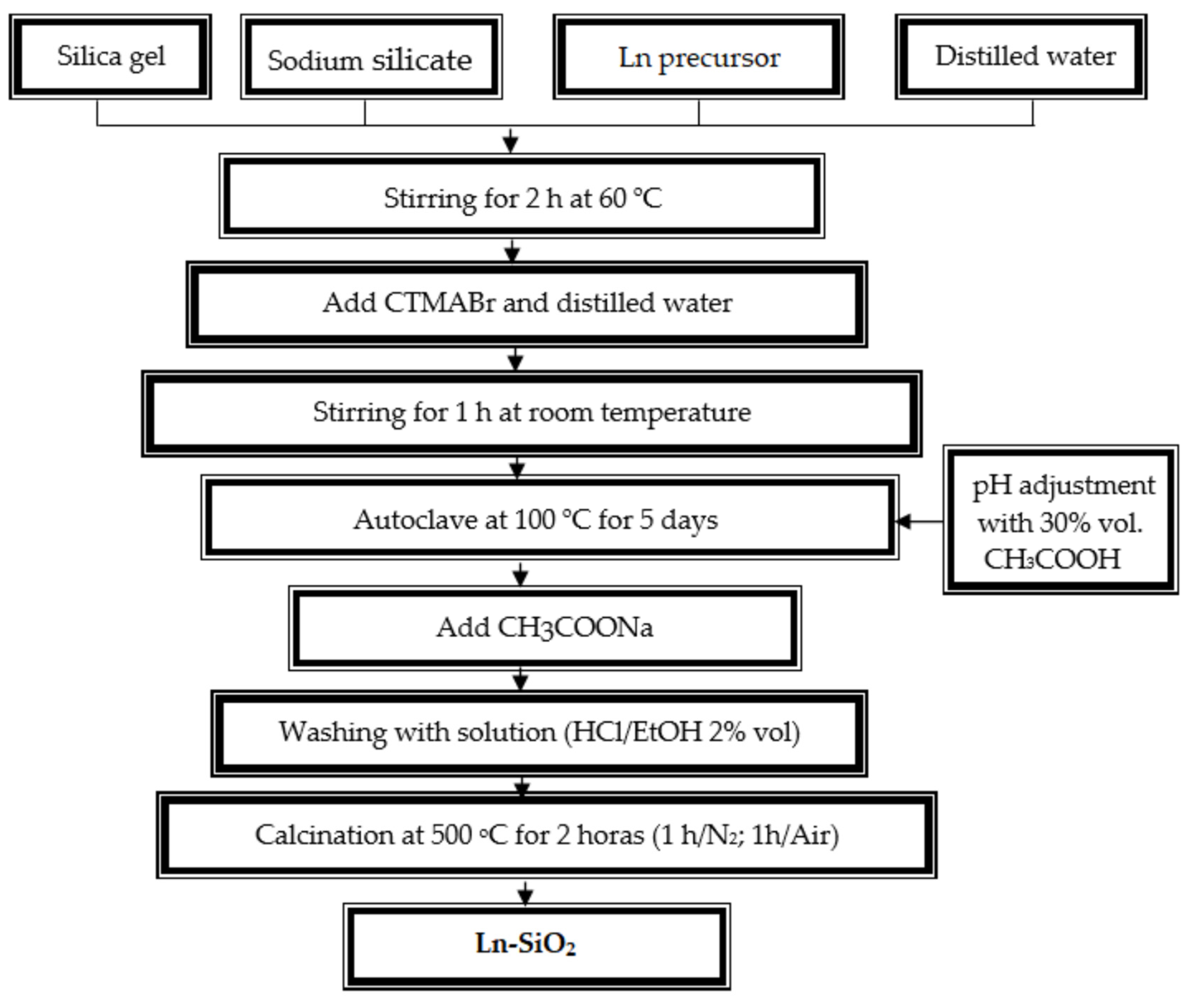
2.2. Hydrothermal Synthesis Procedures
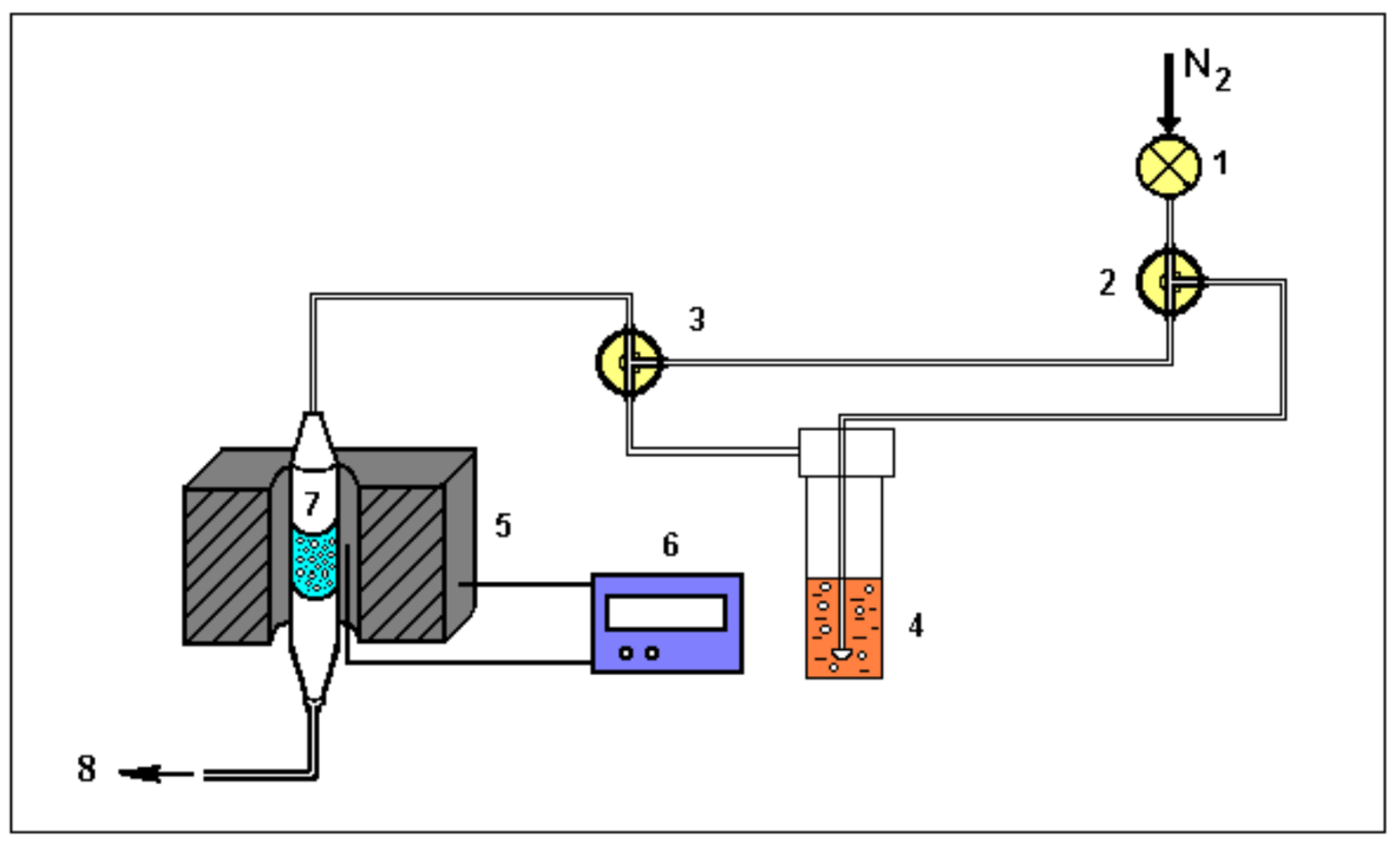
2.3. Characterization of the Materials
2.4. Surface Acidity Measurements
3. Results
3.1. Chemical Composition from XRF
3.2. Crystallographic Properties from XRD
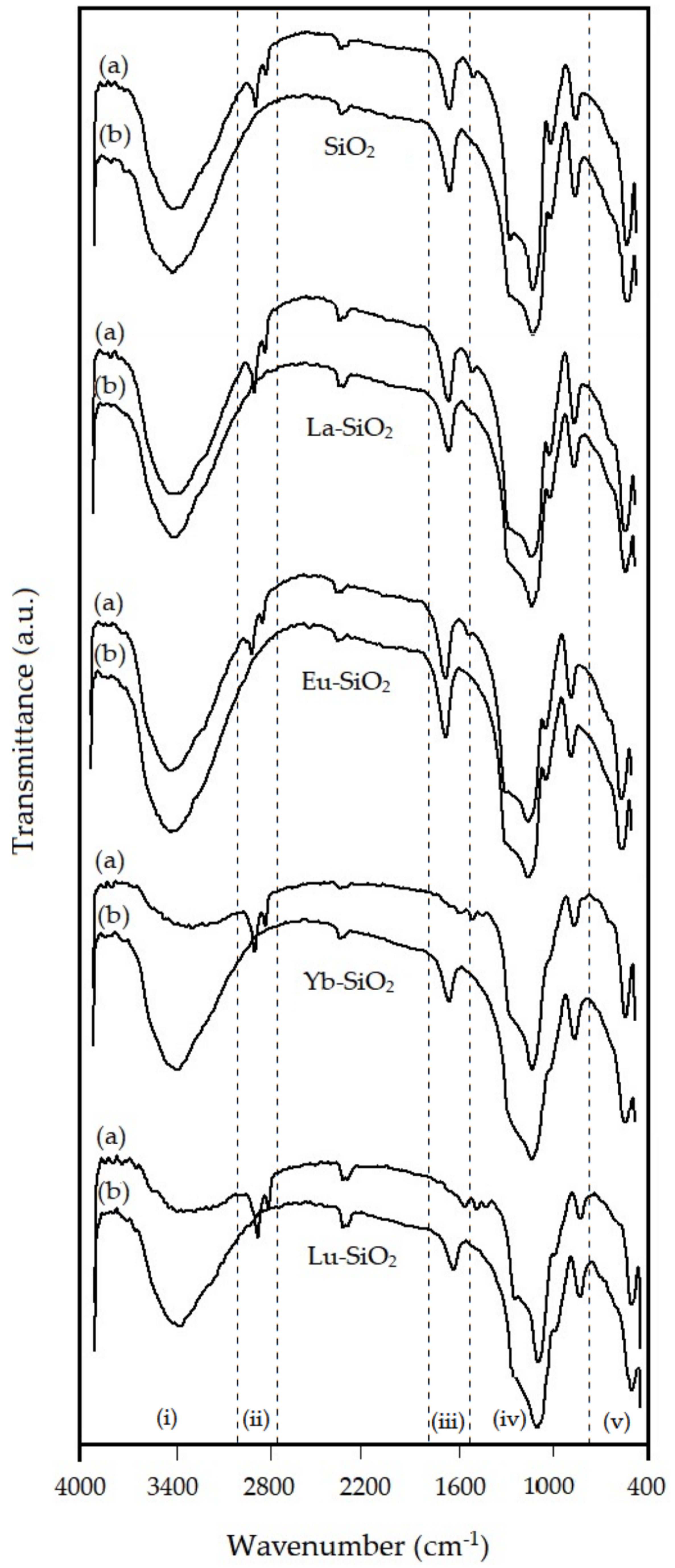
3.3. Structural Properties from FTIR
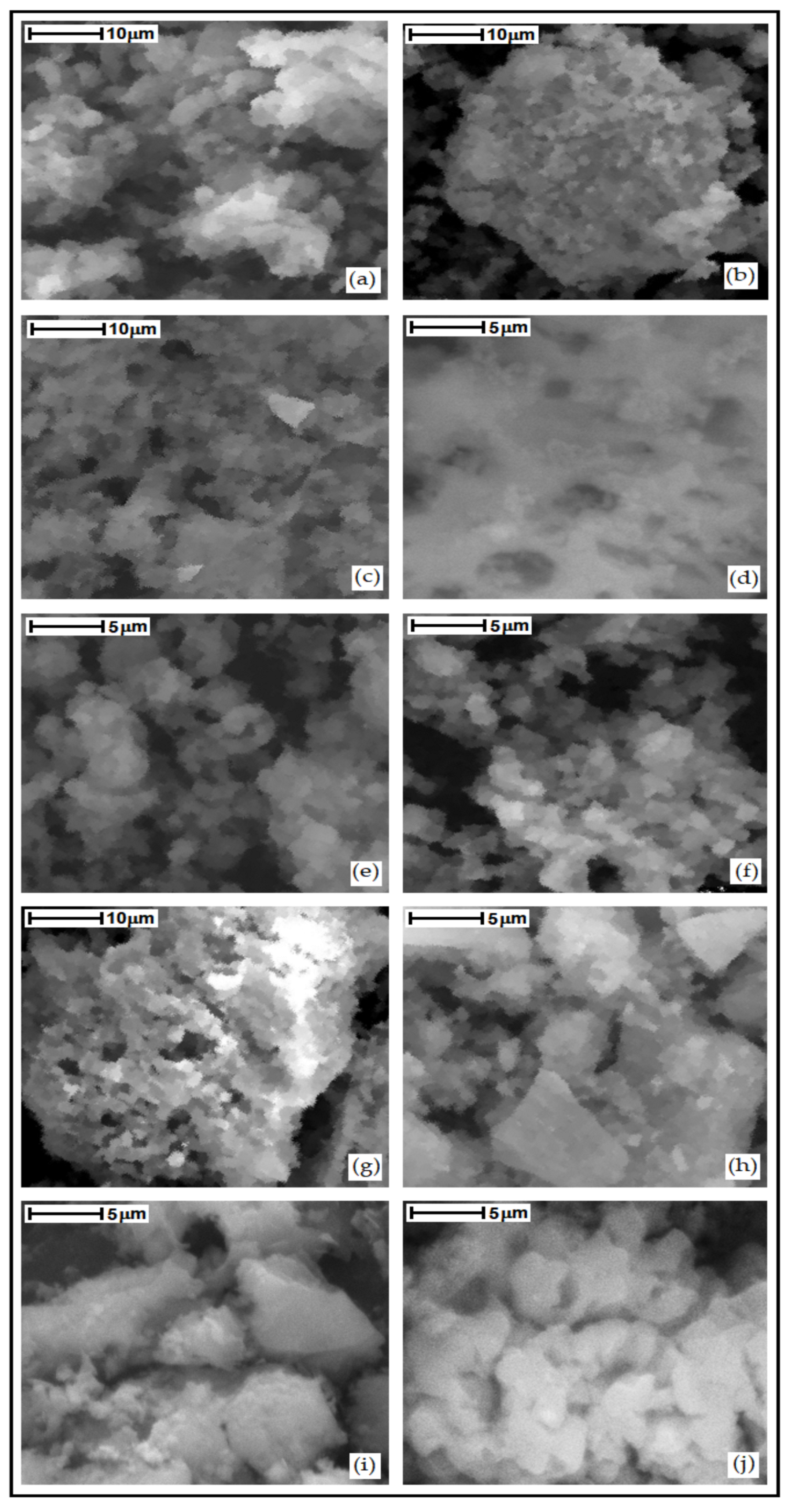
3.4. Scanning Electron Microscopy
3.5. Thermal Analysis
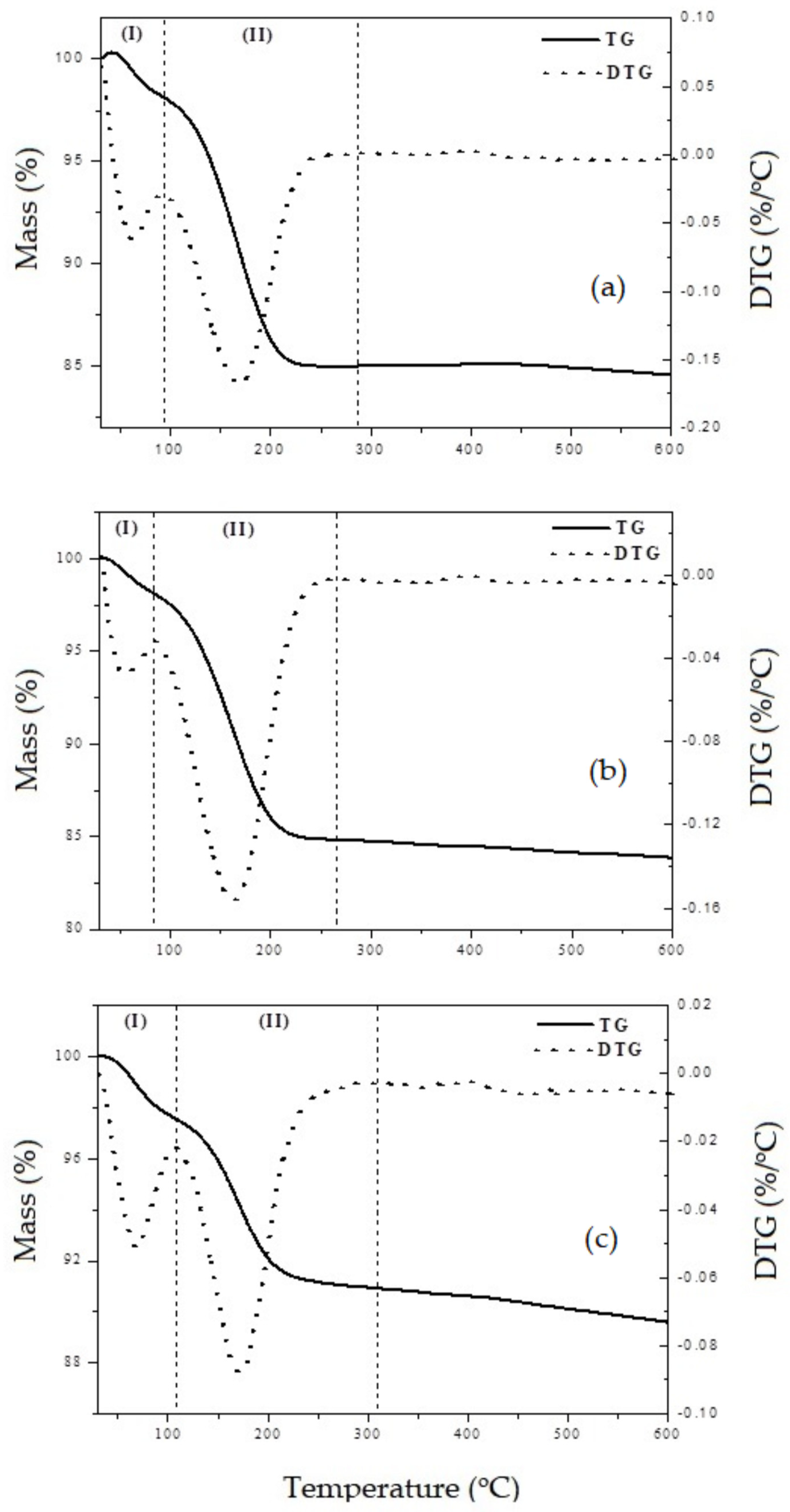
3.6. Surface Acidity Properties from TG
3.7. Surface Area from BET
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.; Olson, D.H.; Sheppard, E.W.; Mccullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Corma, A.; Martinez, A. Zeolites in refining and petrochemistry. Stud. Surf. Sci. Catal. 2005, 157, 337–361. [Google Scholar]
- Kim, J.B.; Inui, T. Synthesis of metal-incorporated mesoporous crystalline silicates for oligomerization of propene. Catal. Lett. 1996, 36, 255–261. [Google Scholar] [CrossRef]
- Mokaya, R.; Jones, W.; Luan, Z.; Alba, M.D.; Klinowski, J. Acidity and catalytic activity of the mesoporous aluminosilicate molecular sieve MCM-41. Catal. Lett. 1996, 37, 113–120. [Google Scholar] [CrossRef]
- Ren, Y.; Yue, B.; Gu, M.; He, H. Progress of the Application of Mesoporous Silica-Supported Heteropolyacids in Heterogeneous Catalysis and Preparation of Nanostructured Metal Oxides. Materials 2010, 3, 764–785. [Google Scholar]
- Popa, A.; Sasca, V.; Kiss, E.E.; Marinkovic-Neducin, R.; Holclajtner-Antunovic, I. Mesoporous silica directly modified by incorporation or impregnation of some heteropolyacids: Synthesis and structural characterization. Mater. Res. Bull. 2011, 46, 19–25. [Google Scholar]
- Saleheen, M.; Heyden, A. Liquid-Phase Modeling in Heterogeneous Catalysis. ACS Catal. 2018, 8, 2188–2194. [Google Scholar]
- Imamura, H.; Kumai, T.; Nishimura, K.; Nuruyu, T.; Sakata, Y. Partial Liquid-Phase Hydrogenation of Benzene to Cylohexene on SiO2 Immobilized Lanthanide (Eu and Yb) Catalysts. Catal. Lett. 2002, 82, 69–71. [Google Scholar] [CrossRef]
- Wolny, A.; Chrobok, A. Silica-Based Supported Ionic Liquid-like Phases as Heterogeneous Catalysts. Molecules 2022, 27, 5900. [Google Scholar]
- Cano, M.L.; Corma, A.; Fornés, V.; García, H.; Miranda, M.A.; Baerlocher, C.; Lengauer, C. Triarylmethylium Cations Encapsulated within Zeolite Supercages. J. Am. Chem. Soc. 1996, 118, 11006–11013. [Google Scholar] [CrossRef]
- Leon, R.; Margolese, D.; Stucky, G.D.; Petroff, P.M. Nanocrystalline Ge filaments in the pores of a mesosilicate. Phys. Rev. B 1995, 52, R2285. [Google Scholar] [CrossRef]
- Wu, C.G.; Bein, T. Conducting Polyaniline Filaments in a Mesoporous Channel Host. Science 1994, 264, 1757–1759. [Google Scholar] [CrossRef] [PubMed]
- Jarmolińska, S.; Feliczak-Guzik, A.; Nowak, I. Synthesis, Characterization and Use of Mesoporous Silicas of the Following Types SBA-1, SBA-2, HMM-1 and HMM-2. Materials 2020, 13, 4385. [Google Scholar] [CrossRef]
- Narayanan, V. Synthesis of Mesoporous Silica Microsphere from Dual Surfactant. Mater. Res. 2008, 11, 443–446. [Google Scholar] [CrossRef]
- Zdarta, J.; Jesionowski, T. Silica and Silica-Based Materials for Biotechnology, Polymer Composites, and Environmental Protection. Materials 2022, 15, 7703. [Google Scholar] [CrossRef]
- Stein, A.; Melde, B.J.; Schroden, R.C. Hybrid Inorganic ± Organic Mesoporous Silicates–Nanoscopic Reactors Coming of Age. Adv. Mater. 2000, 12, 1403–1419. [Google Scholar] [CrossRef]
- Corma, A.; Fornés, V.; Garcia, H.; Miranda, M.A.; Sabater, M.J. Photoinduced Electron Transfer within Zeolite Cavities: Cis-Stilbene Isomerization Photosensitized by 2,4,6-Triphenylpyrylium Cation Imprisoned inside Zeolite Y. J. Amer. Chem. Soc. 1994, 116, 2276–2280. [Google Scholar] [CrossRef]
- Wu, C.G.; Bein, T. Polyaniline wires in oxidant-containing mesoporous channel hosts. Chem. Mater. 1994, 6, 1109–1112. [Google Scholar] [CrossRef]
- Wu, C.G.; Bein, T. Conducting polymer wires in mesopore hosts. Stud. Surf. Sci. Catal. 1994, 84, 2269–2276. [Google Scholar]
- Lewellyn, P.L.; Ciesla, U.; Decher, H.; Stadler, R.; Schüth, F.; Unger, K.K. MCM-41 and related materials as media for controlled polymerization processes. Stud. Surf. Sci. Catal. 1994, 84, 2013–2020. [Google Scholar]
- Wu, C.G.; Bein, T. Conducting carbon wires in ordered, nanometer-sized channels. Science 1994, 266, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, X.; Deng, R.; Zhou, L.; Yu, Y.; Li, Y. Synthesis and Near Infrared Luminescence Properties of a Series of Lanthanide Complexes with POSS Modified Ligands. Molecules 2019, 24, 1253. [Google Scholar] [CrossRef] [PubMed]
- Čížková, M.; Mezricky, D.; Rucki, M.; Tóth, T.M.; Náhlík, V.; Lanta, V.; Bišová, K.; Zachleder, V.; Vítová, M. Bio-mining of Lanthanides from Red Mud by Green Microalgae. Molecules 2019, 24, 1356. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Kiss, M.P.; Valicsek, Z.; Horváth, O. Formation, Photophysics, and Photochemistry of Anionic Lanthanide(III) Mono- and Bisporphyrins. Molecules 2019, 24, 1309. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.S.; Kuehl, G.H.; Olson, D.H.; Schlenker, J.L.; Stucky, G.D.; Vartuli, J.C. Sensor device containing mesoporous crystalline materia. U.S. Patent 5,364,797, 15 November 1994. [Google Scholar]
- Kim, D. Recent Developments in Lanthanide-Doped Alkaline Earth Aluminate Phosphors with Enhanced and Long-Persistent Luminescence. Nanomaterials 2021, 11, 723. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Material. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Miki, T.; Ogawa, T.; Haneda, M.; Nakuda, N.; Ueno, A.; Tateishi, S.; Matsuura, S.; Sato, M. Enhanced oxygen storage capacity of cerium oxides in cerium dioxide/lanthanum sesquioxide/alumina containing precious metals. J. Chem. Phys. 1990, 94, 6464–6467. [Google Scholar] [CrossRef]
- Souza, M.J.B.; Lima, S.H.; Araujo, A.S.; Pedrosa, A.M.G.; Coutinho, A.C.S.L.S. Determination of the acidity of MCM-41 with different Si/Al ratios by the temperature programmed Desorption of Pyridine. Adsorpt. Sci. Technol. 2007, 25, 751–756. [Google Scholar] [CrossRef]
- Araujo, A.S.; Aquino, J.M.F.B.; Souza, M.J.B.; Silva, A.O.S. Synthesis, characterization and catalytic application of cerium-modified MCM-41. J. Solid State Chem. 2003, 171, 371–374. [Google Scholar] [CrossRef]
- Jiang, J.C.; Grahan, G.W.; Mccabe, R.W.; Schwank, J. Microstructure of a Pd/ceria–zirconia catalyst after high-temperature aging. Catal. Lett. 1998, 53, 37–42. [Google Scholar] [CrossRef]
- Trif, E.; Strugaru, D.; Ivan, I.; Russu, R.; Gheorghe, G.; Nicula, A. Thermal properties of Y-type zeolites. J. Therm. Anal. 1994, 41, 871–880. [Google Scholar] [CrossRef]
- Zhang, R.; Li, F.; Shi, Q.; Luo, L. The effects of rare earths on supported amorphous NiB/Al2O3 catalysts. Appl. Catal. A 2001, 205, 279–284. [Google Scholar] [CrossRef]
- Trigueiro, F.E.; Monteiro, D.F.J.; Zotin, F.M.Z.; Sousa-Aguiar, E.F. Thermal stability of Y zeolites containing different rare earth cations. J. Alloys Comp. 2002, 344, 337–341. [Google Scholar] [CrossRef]
- He, N.; Bao, S.L.; Xu, Q. Synthesis and characterization of FeSiMCM-41 and LaSiMCM-41. Stud. Surf. Sci. Catal. 1997, 105, 85–92. [Google Scholar] [CrossRef]
- He, N.; Lu, Z.; Yuan, C.; Hong, J.; Yang, C.; Bao, S.; Xu, Q. Effect of trivalent elements on the thermal and hydrothermal stability of MCM-41 mesoporous molecular materials. Supramol. Sci. 1998, 5, 533–558. [Google Scholar] [CrossRef]
- Zhang, W. Rare earth stabilization of mesoporous alumina molecular sieves assembled through an N°I° pathway. Chem. Commun. 1998, 1185–1186. [Google Scholar] [CrossRef]
- Araujo, A.S.; Jaroniec, M. Synthesis and properties of lanthanide incorporated mesoporous molecular sieves. J. Colloid Interface Sci. 1999, 218, 462–467. [Google Scholar] [CrossRef]
- Felsche, J. The crystal chemistry of the rare earth silicates. In Rare Earths; Institut Fur Kristallographie der ETH: Zurich, Switzerland, 1972. [Google Scholar]
- Belotti, A.; Liu, J.; Curcio, A.; Wang, J.; Wang, Z.; Quattrocchi, E.; Effat, M.B.; Ciucci, F. Introducing Ag in Ba0.9La0.1FeO3-δ: Combining cationic substitution with metal particle decoration. Mater. Rep. Energy 2021, 1, 100018. [Google Scholar] [CrossRef]
- Choppin, G.R. Covalency in f-element bonds. J. Alloys Compd. 2002, 344, 55–59. [Google Scholar] [CrossRef]
- Bagusa, P.S.; Nelin, C.J. Covalent interactions in oxides. J. Electron Spectrosc. Relat. Phenom. 2014, 194, 37–44. [Google Scholar] [CrossRef]
- Putz, A.-M.; Ivankov, O.I.; Kuklin, A.I.; Ryukhtin, V.; Ian, C.; Ciopec, M.; Negrea, A.; Trif, L.; Horváth, Z.E.; Almásy, L. Ordered Mesoporous Silica Prepared in Different Solvent Conditions: Application for Cu(II) and Pb(II) Adsorption. Gels 2022, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.S.; Jaroniec, M. Thermogravimetric monitoring of the MCM-41 synthesis. Thermochim. Acta 2000, 363, 175–180. [Google Scholar] [CrossRef]
- Majchrzak-Kucęba, I. Thermogravimetry applied to characterization of fly ash-based MCM-41 mesoporous materials. J. Therm. Anal. Calorim. 2012, 107, 911–921. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 1st ed.; Pergamon Press: New York, NY, USA, 1984. [Google Scholar]
- Farneth, W.E.; Gorte, R.J. Methods for Characterizing Zeolite Acidity. Chem. Rev. 1995, 95, 615–635. [Google Scholar] [CrossRef]
- Takahashi, M.; Iwasawa, Y.; Ogasawara, S. The nature of adsorbed sites on catalysts: II. Behavior of basic compounds on silica-alumina catalyst at elevated temperatures. J. Catal. 1976, 45, 15–24. [Google Scholar] [CrossRef]
- Antochshuk, V.; Araujo, A.S.; Jaroniec, M. Functionalized MCM-41 and CeMCM-41 materials synthesized via interfacial reactions. J. Phys. Chem. B 2000, 104, 9713–9719. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Berlini, C.; Ferraris, G.; Guidotti, M.; Moretti, G.; Psaro, R.; Ravasio, N. A comparison between [Ti]-MCM-41 and amorphous mesoporous silica–titania as catalysts for the epoxidation of bulky unsaturated alcohols. Micropor. Mesopor. Mater. 2001, 44, 595–602. [Google Scholar] [CrossRef]
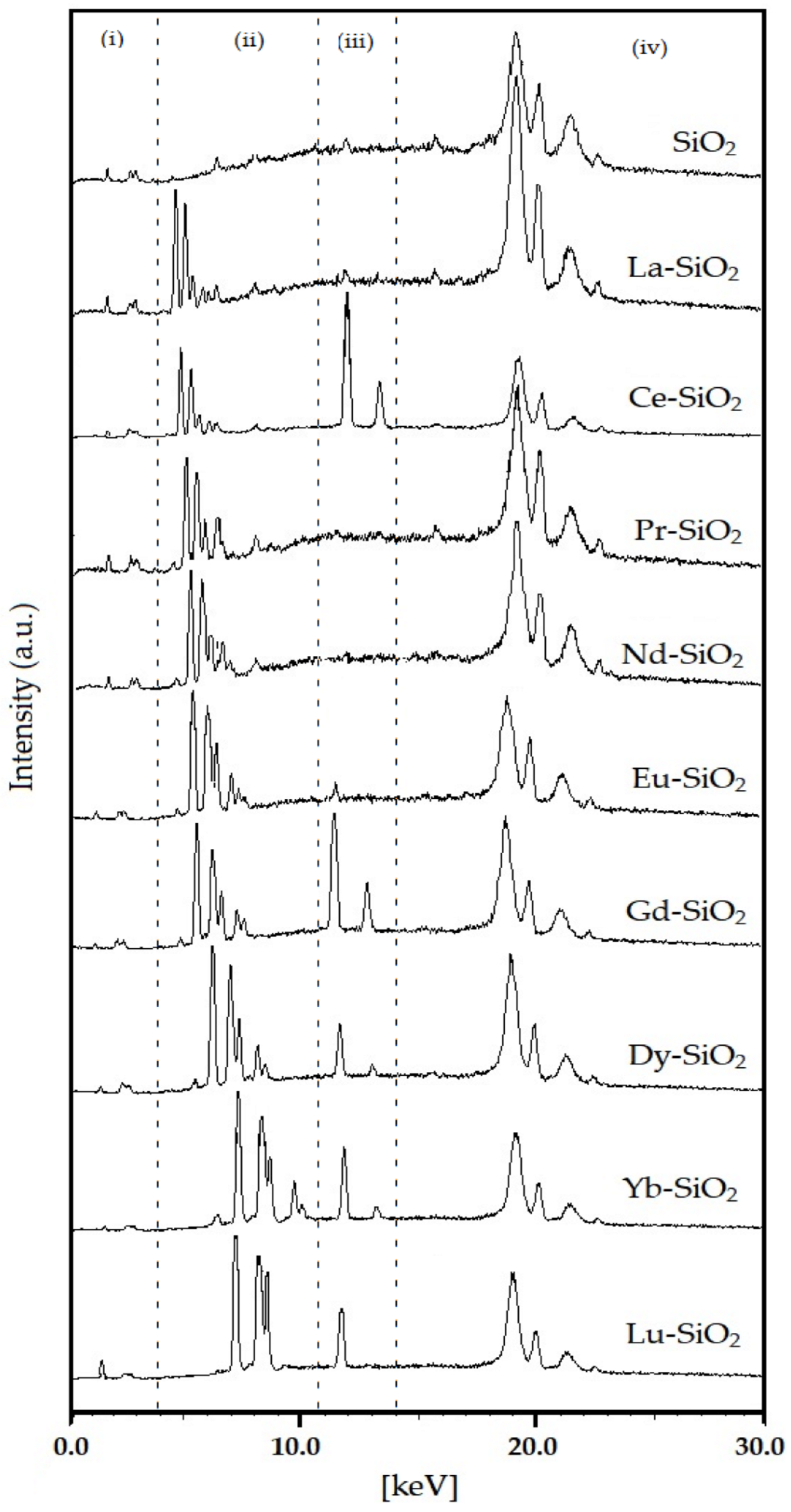
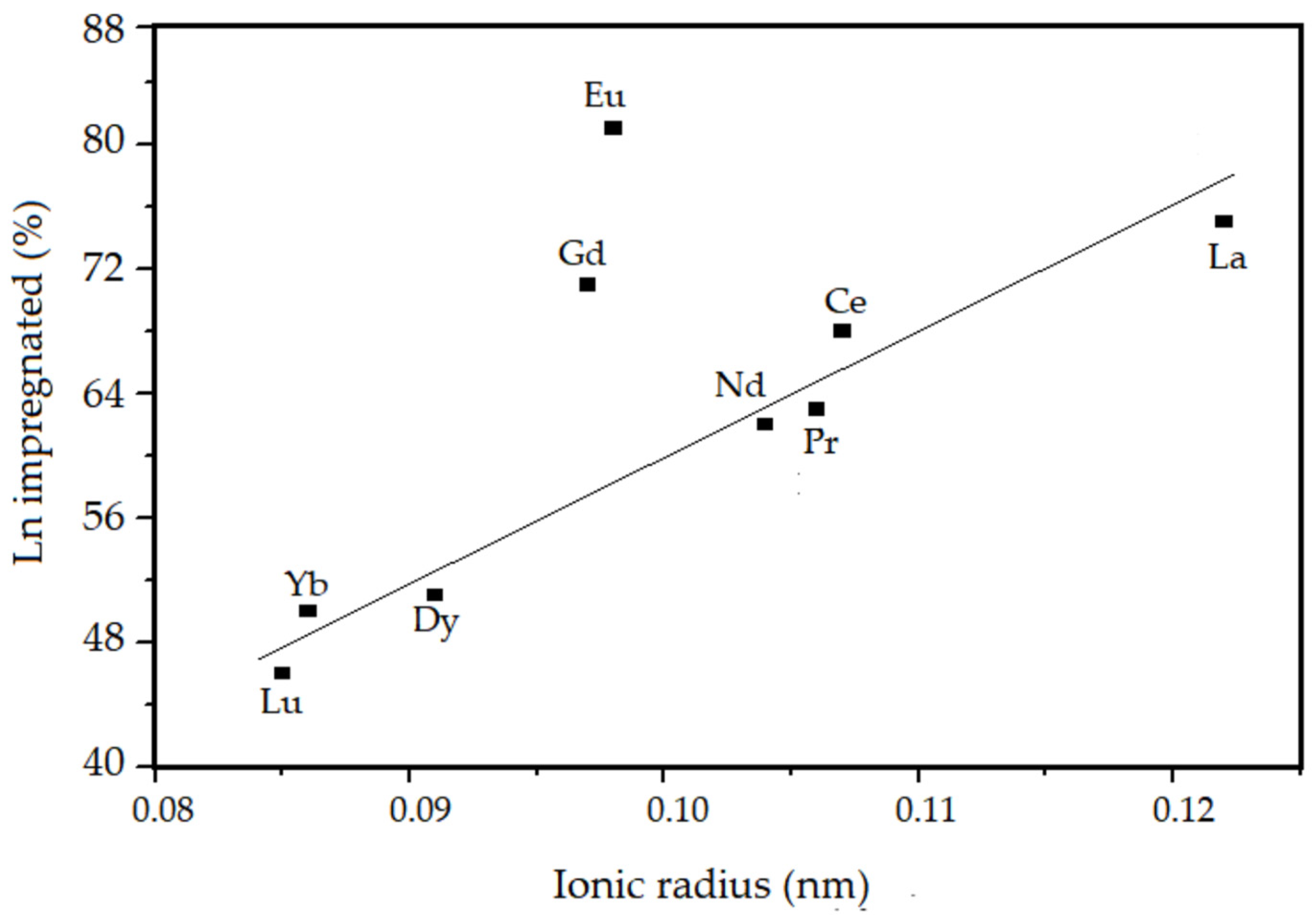
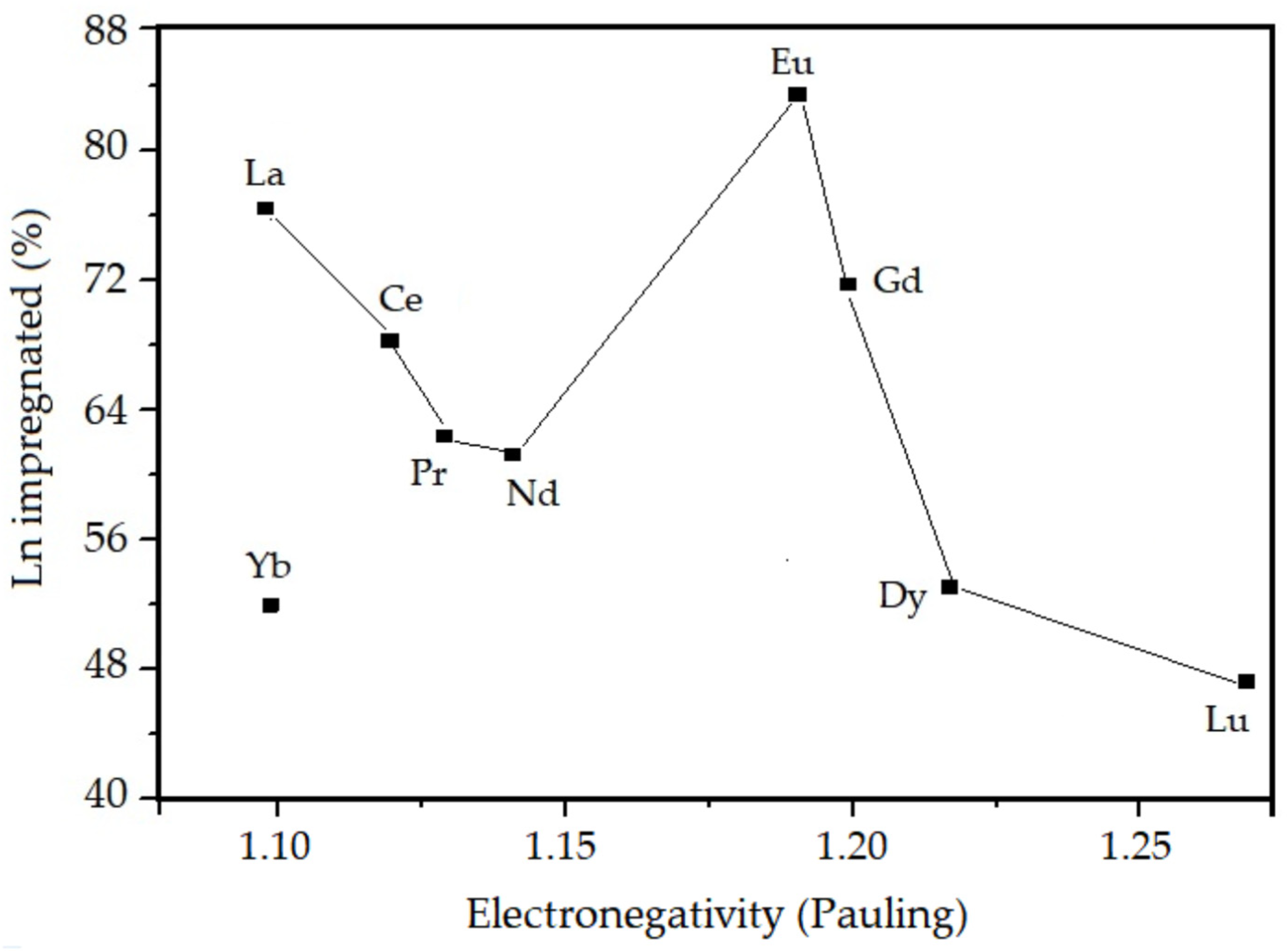
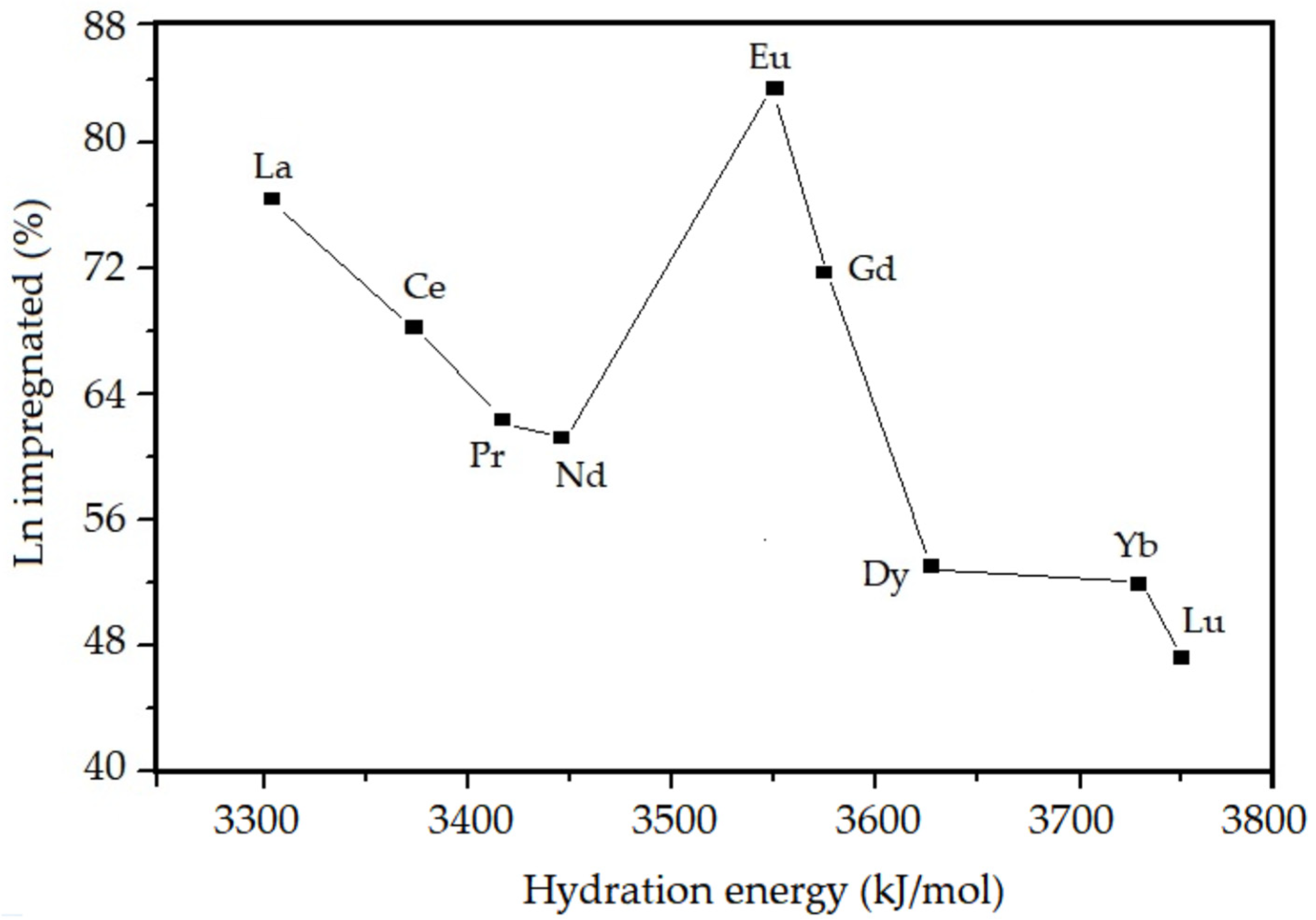



| Sample | Chemical Composition of the Oxides (%) | Thoerical Molar Ratio Si/Ln | Experimental Molar Ratio Si/Ln | Amount of Ln Incorporated in the SiO2 (%) | |
|---|---|---|---|---|---|
| SiO2 | Ln2O3 | ||||
| SiO2 | 100 | - | - | - | - |
| La–SiO2 | 95.52 | 4.48 | 50 | 68 | 75 |
| Ce–SiO2 | 96.58 | 3.62 | 50 | 73 | 68 |
| Pr–SiO2 | 96.58 | 3.42 | 50 | 80 | 63 |
| Nd–SiO2 | 96.64 | 3.36 | 50 | 81 | 62 |
| Eu–SiO2 | 95.47 | 4.53 | 50 | 62 | 81 |
| Gd–SiO2 | 95.85 | 4.15 | 50 | 70 | 71 |
| Dy–SiO2 | 96.94 | 3.06 | 50 | 98 | 51 |
| Yb–SiO2 | 96.86 | 3.14 | 50 | 101 | 50 |
| Lu–SiO2 | 97.04 | 2.96 | 50 | 109 | 46 |
| Sample | 2θ (Degree) | (khl) | d (nm) | a0 (nm) |
|---|---|---|---|---|
| SiO2 | 2.19 | (100) | 4.03 | 4.65 |
| La–SiO2 | 2.26 | (100) | 3.91 | 4.51 |
| Ce–SiO2 | 2.26 | (100) | 3.91 | 4.51 |
| Pr–SiO2 | 2.22 | (100) | 3.98 | 4.59 |
| Nd–SiO2 | 2.28 | (100) | 3.88 | 4.48 |
| Eu–SiO2 | 2.30 | (100) | 3.84 | 4.43 |
| Gd–SiO2 | 2.26 | (100) | 3.91 | 4.51 |
| Dy–SiO2 | 2.43 | (100) | 3.64 | 4.20 |
| Yb–SiO2 | 2.09 | (100) | 3.94 | 4.55 |
| Lu–SiO2 | 2.24 | (100) | 3.94 | 4.55 |
| Sample | Temperature Range (°C) | Mass Loss (%) | ||||
|---|---|---|---|---|---|---|
| (I) | (II) | (III) | (I) | (II) | (III) | |
| SiO2 | 31–137 | 137–382 | 382–531 | 9.26 | 21.58 | 3.50 |
| La–SiO2 | 31–136 | 146–353 | 353–464 | 7.36 | 9.23 | 1.79 |
| Ce–SiO2 | 31–139 | 129–365 | 365–553 | 2.77 | 31.79 | 6.28 |
| Pr–SiO2 | 31–137 | 137–357 | 357–448 | 2.11 | 15.69 | 3.53 |
| Nd–SiO2 | 30–139 | 139–356 | 356–454 | 8.31 | 8.46 | 3.37 |
| Eu–SiO2 | 30–137 | 137–345 | 345–494 | 7.51 | 5.98 | 1.99 |
| Gd–SiO2 | 31–130 | 130–365 | 365–563 | 4.07 | 32.07 | 6.08 |
| Dy–SiO2 | 31–141 | 148–352 | 352–492 | 4.25 | 8.96 | 4.84 |
| Yb–SiO2 | 31–142 | 142–360 | 360–467 | 2.09 | 23.40 | 4.76 |
| Lu–SiO2 | 30–142 | 142–368 | 368–540 | 6.72 | 24.67 | 4.94 |
| Sample | Temperature Range (°C) | Mass Loss (%) | Acidity (mmol/g) | ||
|---|---|---|---|---|---|
| I | II | I | II | ||
| SiO2 | 30–93 | 93–286 | 1.9 | 13.0 | 2.2 |
| La–SiO2 | 30–110 | 110–279 | 2.2 | 5.5 | 2.3 |
| Ce–SiO2 | 30–83 | 83–266 | 1.9 | 13.3 | 2.3 |
| Pr–SiO2 | 30–82 | 82–277 | 1.7 | 13.7 | 2.3 |
| Nd–SiO2 | 30–97 | 97–269 | 2.8 | 10.1 | 1.6 |
| Eu–SiO2 | 30–92 | 92–344 | 2.5 | 14.5 | 2.5 |
| Gd–SiO2 | 31–91 | 91–274 | 1.5 | 9.8 | 1.5 |
| Dy–SiO2 | 31–88 | 88–271 | 2.0 | 9.8 | 1.5 |
| Yb–SiO2 | 31–7 | 77–276 | 1.1 | 10.1 | 1.6 |
| Lu–SiO2 | 30–108 | 108–309 | 2.4 | 6.7 | 1.0 |
| Sample | BET Surface Area (m2/g) | Pore Size Diameter (nm) | Silica Wall Thickness (nm) |
|---|---|---|---|
| SiO2 | 668 | 3.1 | 1.58 |
| La–SiO2 | 732 | 3.2 | 1.31 |
| Ce–SiO2 | 734 | 3.4 | 1.09 |
| Pr–SiO2 | 699 | 3.4 | 1.23 |
| Nd–SiO2 | 651 | 2.7 | 1.76 |
| Eu–SiO2 | 721 | 2.6 | 1.83 |
| Gd–SiO2 | 782 | 3.5 | 1.01 |
| Dy–SiO2 | 376 | 3.7 | 0.52 |
| Yb–SiO2 | 411 | 3.4 | 1.13 |
| Lu–SiO2 | 412 | 3.7 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, J.M.F.; Fernandes, G.J.T.; Araujo, M.D.S.; Melo, D.M.A.; Gondim, A.D.; Fernandes, V.J., Jr.; Araujo, A.S. Hydrothermal Synthesis and Properties of Nanostructured Silica Containing Lanthanide Type Ln–SiO2 (Ln = La, Ce, Pr, Nd, Eu, Gd, Dy, Yb, Lu). Nanomaterials 2023, 13, 382. https://doi.org/10.3390/nano13030382
Barros JMF, Fernandes GJT, Araujo MDS, Melo DMA, Gondim AD, Fernandes VJ Jr., Araujo AS. Hydrothermal Synthesis and Properties of Nanostructured Silica Containing Lanthanide Type Ln–SiO2 (Ln = La, Ce, Pr, Nd, Eu, Gd, Dy, Yb, Lu). Nanomaterials. 2023; 13(3):382. https://doi.org/10.3390/nano13030382
Chicago/Turabian StyleBarros, Joana M. F., Glauber J. T. Fernandes, Marcio D. S. Araujo, Dulce M. A. Melo, Amanda D. Gondim, Valter J. Fernandes, Jr., and Antonio S. Araujo. 2023. "Hydrothermal Synthesis and Properties of Nanostructured Silica Containing Lanthanide Type Ln–SiO2 (Ln = La, Ce, Pr, Nd, Eu, Gd, Dy, Yb, Lu)" Nanomaterials 13, no. 3: 382. https://doi.org/10.3390/nano13030382
APA StyleBarros, J. M. F., Fernandes, G. J. T., Araujo, M. D. S., Melo, D. M. A., Gondim, A. D., Fernandes, V. J., Jr., & Araujo, A. S. (2023). Hydrothermal Synthesis and Properties of Nanostructured Silica Containing Lanthanide Type Ln–SiO2 (Ln = La, Ce, Pr, Nd, Eu, Gd, Dy, Yb, Lu). Nanomaterials, 13(3), 382. https://doi.org/10.3390/nano13030382






