Abstract
In the presented study, a novel approach for thermal atomic layer deposition (ALD) of Al2O3 thin films using plasma-activated water (PAW) as a co-reactant, replacing traditionally employed deionized (DI) water, is introduced. Utilizing ex situ PAW achieves up to a 16.4% increase in the growth per cycle (GPC) of Al2O3 films, consistent with results from plasma-enhanced atomic layer deposition (PEALD). Time-resolved mass spectrometry (TRMS) revealed disparities in CH4 partial pressures between TMA reactions with DI water and PAW, with PAW demonstrating enhanced reactivity. Reactive oxygen species (ROS), namely H2O2 and O3, are posited to activate Si(100) substrate sites, thereby improving GPC and film quality. Specifically, Al2O3 films grown with PAW pH = 3.1 displayed optimal stoichiometry, reduced carbon content, and an expanded bandgap. This study thus establishes “PAW-ALD” as a descriptor for this ALD variation and highlights the significance of comprehensive assessments of PAW in ALD processes.
1. Introduction
Plasma-activated water (PAW) has gained widespread attention in recent years due to its increased chemical reactivity, which is achieved through the transfer of energy from a gaseous plasma to water without the use of any additional chemicals. Its versatility extends to fields like agriculture, medicine, and dentistry, thanks to its notable biochemical properties. In agriculture, PAW boosts seed germination and plant growth [1]. In medicine and dentistry, it is harnessed for cancer therapy, wound healing, biofilm removal, disinfection, and teeth whitening [2,3,4]. Furthermore, PAW augments food safety and quality, showcasing its adaptability in various applications [5,6]. The pronounced biochemical efficacy of PAW stems from the reactive oxygen and nitrogen species (RONS) present, such as nitrous acid (HNO2), hydrogen peroxide (H2O2), nitrite (NO2−), nitrate (NO3−), and ozone (O3) [2]. While their concentrations are low, their prolonged existence magnifies their impact. Post plasma activation, the RONS concentration in PAW can spike to several hundred milligrams per liter, accounting for less than 1% of the total volume [2,6,7], underscoring the potent effects of these species.
The utilization of PAW in life sciences is well established, yet its application in nanotechnology remains nascent, with only a handful of studies exploring its potential. P. Galář et al. demonstrated the treatment of silicon nanoparticles with PAW, leading to a significant increase in the photoluminescence quantum yield of these particles, as well as a notable improvement in their water dispersibility [8]. In another study, N. Sharmin et al. synthesized silver nanoparticles averaging 22 nm in size [9]. They employed the reactive nitrogen and oxygen species in PAW as reducing agents within a sodium alginate (SA) solution, which simultaneously acted as a stabilizer for the nanoparticles. However, in the specific area of nanostructured thin film growth through chemical or physical deposition methods, no research has been reported to date.
To expand the scope of PAW applications, this study explores the use of PAW as a co-reactant in the thermal ALD of Al2O3 thin films using trimethylaluminum (TMA) reactant. We activated DI water using a non-thermal plasma jet, then we introduced PAW to the ALD reactor’s co-reactant line. Al2O3 thin films were grown on Si(100) using PAW and activated for 4, 30, and 60 min, resulting in pH values of 3.5, 3.1, and 2.7, respectively. The concentration of RONS in each PAW sample was measured using UV-Vis absorption spectroscopy. To understand the surface chemisorption dynamics of RONS in PAW interacting with TMA, an in situ analysis was performed with TRMS. Additionally, thin films of Al2O3, derived from both deionized (DI) water and PAW, were subjected to comprehensive characterization. This involved mechanical profilometry, Fourier-Transform Infrared Spectroscopy (FT-IR), and X-ray Photoelectron Spectroscopy (XPS) to assess their properties.
2. Materials and Methods
2.1. PAW Synthesis and Characterization
PAW was prepared using a gliding arc plasma jet (GAPJ) at a flow rate of 5 L/min and a discharge power of 7 W. Additional details about GAPJ are available in the supplementary material. DI water (40 mL), boasting a resistivity of 10 µS/cm and a pH of 6.7, was positioned 0.3 cm from the GAPJ reactor nozzle, followed by plasma activation for 4, 30, and 60 min. The ex situ PAW’s physicochemical parameters were assessed with a multi-parameter water meter (Metrohm 913 phmeter, São Paulo, Brazil), and the RONS (H2O2, NO2-, NO3-, and HNO2) concentrations were ascertained through UV-Vis spectrophotometry (Thermo Fisher Scientific Inc., Waltham, MA, USA). The absolute concentrations of the RONS were determined using the procedure described in [10]. To determine the O3 concentration, the multiparameter photometer Exact Micro 20 (Industrial Test Systems, Rock Hill, SC, USA) was used. See Table 1 for the results.

Table 1.
Physicochemical parameters (pH, oxidation reduction potential (ORP), conductivity (σ), and total dissolved solids (TDS)) and RONS concentrations of DI water and PAWs.
2.2. Al2O3 Thin Film Deposition and In Situ Process Monitoring
After generation, each PAW was transferred into the cross-flow-type thermal ALD reactor as a co-reactant. The ALD cycle had four steps: 0.15 s with TMA (97%), 4.00 s N2 purge, 0.30 s of PAW or DI water, followed by another 4.00 s N2 purge. The reactor’s base pressure was below 10−2 mbar, with the deposition pressure maintaining around 0.5 mbar using 100 sccm of N2. Al2O3 films were cultivated on p-type Si(100) wafers at 150 °C, with 100 to 1500 reaction cycles for each PAW and DI water.
In this study, the TRMS was employed to track the primary species produced in each ALD pulse: methane (CH4+, 16 amu), water (H2O+, 18 amu), and ethane (C2H6+, 30 amu). The partial pressure of the surface reaction by-product species within each pulse was analyzed using a mass spectrometer (RGA-200, Stanford Research Systems, Sunnyvale, CA, USA), configured to the reactor exhaust to detect residual species via a micro-orifice.
2.3. Material Characterization
The thickness of the Al2O3 films was gauged using a KLA Tencor P-7 profilometer, by creating a step in specific regions of the samples using Kapton tape. The samples’ chemical bonding was examined with infrared measurements via an ATR-FTIR PerkinElmer 400 IR spectrometer at a 2 cm−1 resolution. Additionally, the films underwent characterization by XPS with a K-Alpha Thermo Scientific spectrometer (X-ray Al-Kα, hν = 1486.6 eV) under pressures less than 10−7 mbar. The pass energy applied to obtain the spectra was 200 eV for survey and 50 eV for high-resolution scans.
3. Results and Discussion
Table 1 shows a comprehensive overview of the changes in key parameters of PAW during the activation process. Notably, there is a significant decrease in pH over time, indicating increasing acidity, a common characteristic of PAW. This drop in pH is paralleled by marked changes in other properties. The ORP increases, reflecting a more oxidizing environment within the water. Conductivity and TDS both show a significant uptick. The increase in conductivity suggests a greater ion concentration, which is corroborated by the rise in TDS, indicating more dissolved substances. These changes are indicative of the complex chemical transformations occurring in PAW.
Furthermore, the concentration of various RONS, namely H2O2, HNO2, NO3−, NO2−, and O3, also increased. H2O2 and O3, known for their strong oxidative properties, show a particularly notable rise. The increase in these RONS concentrations aligns with the enhanced oxidative potential of PAW, as indicated by the ORP readings. Each of these RONS plays a unique role in determining the chemical and physical properties of PAW, contributing to its potential applications, particularly in the growth of oxides through ALD.
As the XPS results did not reveal the incorporation of nitrogen into Al2O3 films (see Figure S4 in the Supplementary Material), the following discussions will primarily focus on reactive oxygen species (ROS), such as H2O2 and O3, in the ALD process using PAW as a co-reactant.
Figure 1a depicts a linear growth in the observed ALD processes with each reaction cycle, emphasizing the self-limiting nature of the ALD process when using PAW. Figure 1b demonstrates the GPC as a function of co-reactant pH. The data underscore PAW’s potential to boost the GPC of Al2O3 films. This trend likely results from the RONS produced during the plasma activation of water, leading to a GPC increase of up to 16.4% in this study.
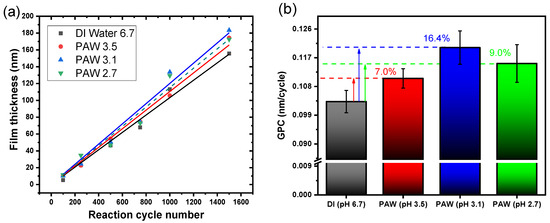
Figure 1.
(a) Al2O3 film thickness as a function of reaction cycle number for various co-reactants, and (b) GPC in relation to the pH of the co-reactant.
To elucidate the influence of RONS in PAW on the Al2O3 ALD chemisorption process, in situ TRMS was utilized. This method offered valuable insights into the gaseous by-products formed during the initial 20 ALD cycles on Si(100) substrates and on developing Al2O3 layers. The observed species mainly consisted of reaction by-products, specifically from interactions between TMA and PAW at a pH of 2.7 (illustrated in Figure 2a,b), as well as TMA in conjunction with DI water (shown in Figure 2c,d). Notably, the initial introduction of TMA resulted in the detection of methane (CH4+, m/e = 16) and ethane (C2H6+, m/e = 30) signals. While the methane signal was present during both TMA and H2O exposure phases, the ethane signal was uniquely observed during TMA exposure periods [11].
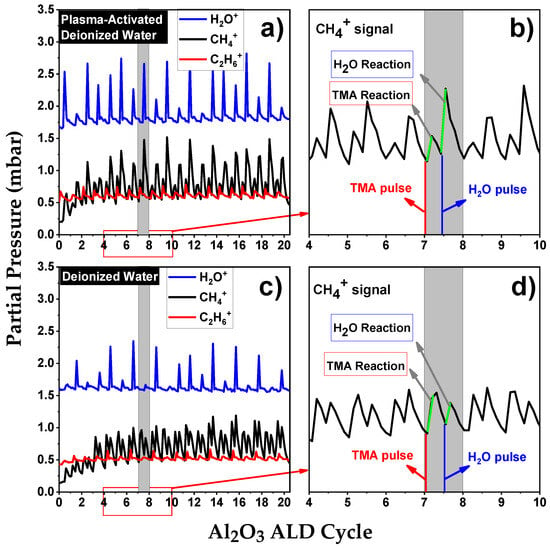
Figure 2.
TRMS of CH4+, H2O+, and C2H6+ during the first 20 cycles of Al2O3 thin film growth using (a,b) PAW with pH 2.7 and (c,d) DI water. The green segment on the TRMS graph signifies the changes in partial pressure during the pulsing intervals of TMA and PAW (or DI water), respectively.
It is important to note that during the initial ALD cycles on Si(100), characterized by a lack of substantial hydroxyl groups, relatively little CH4 is produced. The comparison between Figure 2a,c reveals that for PAW, from the second cycle onwards, the partial pressure of CH4+ exceeds that of C2H6+, while in the case of H2O, this phenomenon is only observed from the fourth cycle. As the ALD process progresses and multiple cycles are completed, Al2O3 forms a continuous film on the substrate. At this stage, TMA primarily interacts with the hydroxyl groups on the Al2O3 surface, resulting in an intensification of the CH4+ signal. Additionally, during the co-reactant pulse, the oxidant species oxidize the Al–CH3 surface, leading to the release of CH4. A comparison between Figure 2b,d reveals an increase in the partial pressure of CH4+ during the co-reactant pulse in the PAW-ALD process.
The oxidation process in PAW-ALD appears more intricate than in thermal ALD using H2O. The ROS in PAW, namely H2O2 and O3, could introduce alternative oxidation pathways for –CH3 surface groups. Seo et al. investigated the molecular reactivity of Al2O3 ALD at lower deposition temperatures using three different oxidants, proposing a reactivity sequence of H2O < H2O2 < O3 [12]. Indeed, Elliott et al. found that the thickness of Al2O3 films deposited using O3 is temperature-dependent [13]. Their observations indicated that at 150 °C, Al2O3 film thickness using O3 increased by up to 18.75% compared to films grown with H2O as a co-reactant. Nam et al. also explored the use of O3 as a co-reactant at low processing temperatures (50–125 °C). However, they observed that at 125 °C, the resulting Al2O3 film was thinner compared to the one produced using H2O as the co-reactant [14]. In the case of H2O2, literature evidence suggests that it can easily adsorb onto surfaces, thereby enhancing surface oxidation [12,14]. Al2O3 films deposited solely with H2O2 [14,15], or a combination of H2O2 and H2O [16], exhibited increased thickness compared to those grown with H2O at 150 °C.
These results are consistent with the pronounced CH4+ signal observed in Figure 2b. Moreover, an increase in the partial pressure of higher hydrocarbons, such as C2H6+, during the TMA exposure phase (as shown in Figure 2a) suggests the possibility of alternative reaction pathways, similar to those observed in PEALD [17]. The interaction between O3 and the –CH3 surface groups predominantly results in the production of CO2 and H2O (as indicated by the observed increase in H2O+ in Figure 2a when using PAW). However, this interaction can also generate CH4 [18,19], contributing to an elevated partial pressure of CH4+ during the PAW pulse. Similarly, the reaction of H2O2 with the surface yields CH4 [12], further augmenting the partial pressure of this by-product.
Figure 3 presents the FT-IR spectra of the alumina films deposited using different PAW samples. Each spectrum is characterized by a broad band ranging from 400 to 1000 cm−1, indicative of Al–O interactions, which are typical in both AlO4 and AlO6 structures [20]. Within this range, the peak observed around 516 cm−1 can be attributed to the Al–O vibrational mode in AlO6 [20]. Furthermore, the absorption peak near 668 cm−1 is largely influenced by O–Al–O bending vibrations [21].
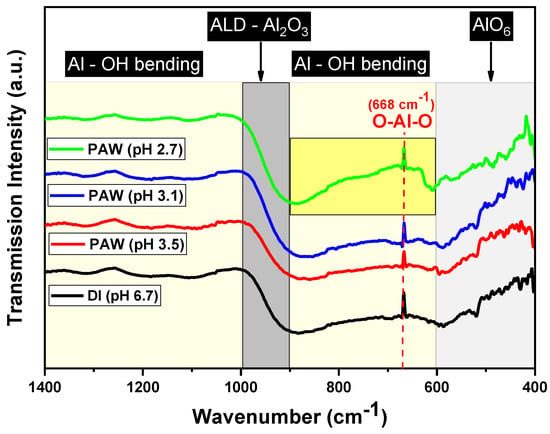
Figure 3.
FT-IR spectra of the Al2O3 thin films deposited with DI water and PAW at different pH values.
Significantly, the pH 2.7 PAW sample displays enhanced broad features in the 600–900 cm−1 region, commonly associated with Al–OH bending vibrations. The increased concentration of ROS in this sample may influence the chemisorption and subsequent growth of the alumina films. This influence could result in a greater incorporation of OH groups or induce a structural change in the alumina film. Such changes are reflected in the more pronounced Al–OH stretching vibration signals observed in the spectrum.
Additionally, the absence of a peak at 530 cm−1, as noted by Katamreddy et al., underscores the film’s amorphous nature [22]. The consistency observed in the 1000–1400 cm−1 region across all samples, typically associated with the bending vibrations of adsorbed water molecules or hydroxyl groups, suggests that the surface-bound water or hydroxyl groups are not significantly affected by the varying pH levels of the PAW used. Wang et al. successfully fabricated Al2O3 films possessing the aforementioned characteristics by employing O2 plasma and O3 as co-reactants [21].
Table 2 provides an analysis of the O/Al ratio, carbon content, and bandgap (Eg) of Al2O3 films, as determined by XPS. The film made with PAW at pH 3.1 shows an O/Al ratio close to the stoichiometric ratio and has lower carbon content than others. A consistent observation across PAW-treated films is their higher Eg values compared to those grown with DI water. The PAW 3.5 sample has the highest Eg (6.23 eV), followed by PAW 3.1 (6.09 eV) and PAW 2.7 (6.02 eV). In their recent study, Castillo-Saenz et al. determined the Eg of an Al2O3 thin film fabricated using O2 plasma, finding a value of 6.58 eV [23]. Elevated Eg values may suggest a uniform and dense film structure, advantageous for applications demanding enhanced electronic properties.

Table 2.
O/Al ratio, C concentration, and band gap (Eg) of the Al2O3 films.
4. Conclusions
The use of PAW as a co-reactant for thermal ALD (150 °C) of Al2O3 thin films results in a GPC enhancement of up to 16.4% (with PAW 3.1) compared to the conditions with DI water. This advancement holds industrial relevance, mirroring the gains observed in the PEALD process utilizing oxygen plasma. TRMS data indicates disparities in CH4+ partial pressures during TMA reactions with DI water versus PAW. Elevated CH4+ pressures are observed in PAW reactions, pointing to enhanced reactivity. Major influencing factors include H2O2 and O3, which potentially activate Si(100) and subsequent Al2O3 surface sites, thereby enhancing the GPC of Al2O3. Initial material analysis suggests that Al2O3 films grown using PAW demonstrate superior quality compared to those fabricated with DI water. Notably, films derived from PAW 3.1 presented a near stoichiometric ratio and reduced carbon concentration. For a comprehensive understanding of PAW’s role in the ALD process, in-depth investigations involving different temperatures to determine the optimal ALD window, exploration of varied metal precursors, and evaluation of diverse PAWs generated by alternate non-thermal plasmas are necessary. The term “PAW-ALD” is proposed to characterize this enhanced ALD variant.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nano13243110/s1, Figure S1. (a) Schematic illustration of the experimental setup, and (b) photograph of the FVFR gliding arc reactor used for activating deionized (DI) water. The value of d was fixed in 3 mm. Figure S2. (a) Voltage waveform for the GAPJ operating with an airflow of 5 L min−1, (b) Current waveform for the GAPJ operating with an airflow of 5 L min−1, and (c) Discharge power waveform of the GAPJ used to calculate the mean power using Equation (S1). Figure S3. Deep UV absorption spectra of PAW at different pH values: (a) 2.7, (b) 3.1, and (c) 3.5, with non-activated water used as the baseline. Gaussian deconvolution of the deep UV absorption spectrum of each investigated PAW was performed based on literature data. Figure S4. Survey XPS spectra for Al2O3 thin films deposited using: (a) DI water, (b) PAW at pH 3.5, (c) PAW at pH 3.1, and (d) PAW at pH 2.7. Figure S5. High-resolution XPS spectra of the Al2p peak and the corresponding deconvoluted peak for Al2O3 samples deposited using: (a) DI water, (b) PAW at pH 3.5, (c) PAW at pH 3.1, and (d) PAW at pH 2.7. Figure S6. High-resolution XPS spectra of the O1s peak and the corresponding deconvoluted peaks for Al2O3 samples deposited using: (a) DI water, (b) PAW at pH 3.5, (c) PAW at pH 3.1, and (d) PAW at pH 2.7. Figure S7. High-resolution XPS spectra of the C1s peaks and the corresponding deconvoluted peaks for Al2O3 samples deposited using: (a) DI water, (b) PAW at pH 3.5, (c) PAW at pH 3.1, and (d) PAW at pH 2.7. References [24,25,26,27,28,29,30,31,32,33] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, J.C. and R.P.; methodology, J.C., W.C. and R.P.; software, J.C., W.C. and J.K.; formal analysis, J.C., W.C. and R.P.; investigation, J.C., W.C., J.K., B.N., D.L., A.d.S.S. and R.P.; resources, R.P.; data curation, W.C. and R.P.; writing—original draft preparation, J.C., W.C. and R.P.; writing—review and editing, D.L., A.d.S.S. and R.P.; supervision, R.P.; project administration, R.P.; funding acquisition, R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPESP, grant numbers 2018/01265-1 and 2019/05856-7.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank Julio Cesar Sagas from the Universidade Estadual de Santa Catarina (UDESC) for the XPS measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma Activated Water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Milhan, N.V.M.; Chiappim, W.; Sampaio, A.d.G.; Vegian, M.R.d.C.; Pessoa, R.S.; Koga-Ito, C.Y. Applications of Plasma-Activated Water in Dentistry: A Review. Int. J. Mol. Sci. 2022, 23, 4131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; (Ken) Ostrikov, K.; Bazaka, K. Plasma-activated water: Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Julák, J.; Hujacová, A.; Scholtz, V.; Khun, J.; Holada, K. Contributions to the chemistry of plasma-activated water. Plasma Phys. Rep. 2018, 44, 125–136. [Google Scholar] [CrossRef]
- Gao, Y.; Francis, K.; Zhang, X. Review on formation of cold plasma activated water (PAW) and the applications in food and agriculture. Food Res. Int. 2022, 157, 111246. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Fan, L.; Li, Y.; Dong, S.; Li, K.; Bai, Y. A review on recent advances in plasma-activated water for food safety: Current applications and future trends. Crit. Rev. Food Sci. Nutr. 2022, 62, 2250–2268. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, Y.; Wu, R.A.; Liao, X.; Liu, D.; Cullen, P.J.; Zhou, R.-W.; Ding, T. Diagnostic analysis of reactive species in plasma-activated water (PAW): Current advances and outlooks. J. Phys. D Appl. Phys. 2022, 55, 023002. [Google Scholar] [CrossRef]
- Galář, P.; Khun, J.; Fučíková, A.; Dohnalová, K.; Popelář, T.; Matulková, I.; Valenta, J.; Scholtz, V.; Kůsová, K. Non-thermal pulsed plasma activated water: Environmentally friendly way for efficient surface modification of semiconductor nanoparticles. Green Chem. 2021, 23, 898–911. [Google Scholar] [CrossRef]
- Sharmin, N.; Pang, C.; Sone, I.; Walsh, J.L.; Fernández, C.G.; Sivertsvik, M.; Fernández, E.N. Synthesis of Sodium Alginate–Silver Nanocomposites Using Plasma Activated Water and Cold Atmospheric Plasma Treatment. Nanomaterials 2021, 11, 2306. [Google Scholar] [CrossRef]
- Sampaio, A.d.G.; Chiappim, W.; Milhan, N.V.M.; Botan Neto, B.; Pessoa, R.; Koga-Ito, C.Y. Effect of the pH on the Antibacterial Potential and Cytotoxicity of Different Plasma-Activated Liquids. Int. J. Mol. Sci. 2022, 23, 13893. [Google Scholar] [CrossRef]
- Chen, L.; Warburton, R.E.; Chen, K.-S.; Libera, J.A.; Johnson, C.; Yang, Z.; Hersam, M.C.; Greeley, J.P.; Elam, J.W. Mechanism for Al2O3 Atomic Layer Deposition on LiMn2O4 from in Situ Measurements and Ab Initio Calculations. Chem 2018, 4, 2418–2435. [Google Scholar] [CrossRef]
- Seo, S.; Nam, T.; Lee, H.-B.-R.; Kim, H.; Shong, B. Molecular oxidation of surface –CH3 during atomic layer deposition of Al2O3 with H2O, H2O2, and O3: A theoretical study. Appl. Surf. Sci. 2018, 457, 376–380. [Google Scholar] [CrossRef]
- Elliott, S.D.; Scarel, G.; Wiemer, C.; Fanciulli, M.; Pavia, G. Ozone-Based Atomic Layer Deposition of Alumina from TMA: Growth, Morphology, and Reaction Mechanism. Chem. Mater. 2006, 18, 3764–3773. [Google Scholar] [CrossRef]
- Nam, T.; Lee, H.; Seo, S.; Cho, S.M.; Shong, B.; Lee, H.-B.-R.; Kim, H. Moisture barrier properties of low-temperature atomic layer deposited Al2O3 using various oxidants. Ceram. Int. 2019, 45, 19105–19112. [Google Scholar] [CrossRef]
- Fan, J.-F.; Sugioka, K.; Toyoda, K. Low-Temperature Growth of Thin Films of Al2O3 by Sequential Surface Chemical Reaction of trimethylaluminum and H2O2. Jpn. J. Appl. Phys. 1991, 30, L1139. [Google Scholar] [CrossRef]
- Yasuhito, K.; Hayato, M.; Hiroshi, T.; Tadaki, M.; Takashi, K.; Nobuyasu, T.; Spiegelman, J. ALD Process Using Hydrogen Peroxide (H2O2mix) for High Aspect Ratio Structures. TAIYO NIPPON SANSO Technical Report No.4. 2022. Available online: https://www.tn-sanso.co.jp/Portals/0/resources/en/rd/giho/pdf/41/tnscgiho41_E03.pdf (accessed on 2 December 2023).
- Heil, S.B.S.; van Hemmen, J.L.; van de Sanden, M.C.M.; Kessels, W.M.M. Reaction mechanisms during plasma-assisted atomic layer deposition of metal oxides: A case study for Al2O3. J. Appl. Phys. 2008, 103, 103302. [Google Scholar] [CrossRef]
- Rose, M.; Niinistö, J.; Endler, I.; Bartha, J.W.; Kücher, P.; Ritala, M. In Situ Reaction Mechanism Studies on Ozone-Based Atomic Layer Deposition of Al2O3 and HfO2. ACS Appl. Mater. Interfaces 2010, 2, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Kayanuma, M.; Choe, Y.-K.; Hagiwara, T.; Kameda, N.; Shimoi, Y. Theoretical Study of the Mechanism for the Reaction of Trimethylaluminum with Ozone. ACS Omega 2021, 6, 26282–26292. [Google Scholar] [CrossRef]
- Gao, M.; Liu, B.; Zhao, P.; Yi, X.; Shen, X.; Xu, Y. Mechanical Strengths and Thermal Properties of Titania-Doped Alumina Aerogels and the Application as High-Temperature Thermal Insulator. J. Sol-Gel Sci. Technol. 2019, 91, 514–522. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Liu, H.; Chen, Z.; Xiong, P.; Xu, X.; Chen, F.; Li, K.; Duan, Y. Effect of Various Oxidants on Reaction Mechanisms, Self-Limiting Natures and Structural Characteristics of Al2O3 Films Grown by Atomic Layer Deposition. Adv. Mater. Interfaces 2018, 1701248. [Google Scholar] [CrossRef]
- Katamreddy, R.; Inman, R.; Jursich, G.; Soulet, A.; Takoudis, C. ALD and Characterization of Aluminum Oxide Deposited on Si(100) using Tris(diethylamino) Aluminum and Water Vapor. J. Electrochem. Soc. 2006, 153, C701–C706. [Google Scholar] [CrossRef]
- Castillo-Saenz, J.; Nedev, N.; Valdez-Salas, B.; Curiel-Alvarez, M.; Mendivil-Palma, M.I.; Hernandez-Como, N.; Martinez-Puente, M.; Mateos, D.; Perez-Landeros, O.; Martinez-Guerra, E. Properties of Al2O3 Thin Films Grown by PE-ALD at Low Temperature Using H2O and O2 Plasma Oxidants. Coatings 2021, 11, 1266. [Google Scholar] [CrossRef]
- Doria, A.C.O.C.; Figueira, F.R.; De Lima, J.S.B.; Figueira, J.A.N.; Castro, A.H.R.; Sismanoglu, B.N.; Petraconi, G.; Maciel, H.S.; Khouri, S.; Pessoa, R.S. Inactivation of Candida Albicans Biofilms by Atmospheric Gliding Arc Plasma Jet: Effect of Gas Chemistry/Flow and Plasma Pulsing. Plasma Res. Express 2019, 1. [Google Scholar] [CrossRef]
- Chiappim, W.; Sampaio, A.; da, G.; Miranda, F.; Fraga, M.; Petraconi, G.; da Silva Sobrinho, A.; Kostov, K.; Koga-Ito, C.; Pessoa, R. Antimicrobial Effect of Plasma-Activated Tap Water on Staphylococcus Aureus, Escherichia Coli, and Candida Albicans. Water 2021, 13, 1480. [Google Scholar] [CrossRef]
- Oh, J.S.; Szili, E.J.; Ogawa, K.; Short, R.D.; Ito, M.; Furuta, H.; Hatta, A. UV-Vis Spectroscopy Study of Plasma-Activated Water: Dependence of the Chemical Composition on Plasma Exposure Time and Treatment Distance. Jpn. J. Appl. Phys. 2018, 57. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, C.; Liu, D.; He, T.; Guo, L.; Xu, D.; Kong, M.G. Quantifying the Concentration and Penetration Depth of Long-Lived RONS in Plasma-Activated Water by UV Absorption Spectroscopy. AIP Adv. 2019, 9. [Google Scholar] [CrossRef]
- Tachibana, K.; Nakamura, T. Examination of UV-Absorption Spectroscopy for Analysis of O3, NO2−, and HNO2 Compositions and Kinetics in Plasma-Activated Water. Jpn. J. Appl. Phys. 2020, 59. [Google Scholar] [CrossRef]
- Szili, E.J.; Oh, J.S.; Hong, S.H.; Hatta, A.; Short, R.D. Probing the Transport of Plasma-Generated RONS in an Agarose Target as Surrogate for Real Tissue: Dependency on Time, Distance and Material Composition. J. Phys. D Appl. Phys. 2015, 48. [Google Scholar] [CrossRef]
- Hu, B.; Yao, M.; Xiao, R.; Chen, J.; Yao, X. Optical properties of amorphous Al2O3 thin films prepared by a sol–gel process. Ceram. Int. 2014, 40, 14133–14139. [Google Scholar] [CrossRef]
- Beladiya, V.; Faraz, T.; Kessels, W.M.M.; Tünnermann, A.; Szeghalmi, A. Controlling mechanical, structural, and optical properties of Al2O3 thin films deposited by plasma-enhanced atomic layer deposition with substrate biasing. Proc. SPIE 2018, 10691, 106910. [Google Scholar] [CrossRef]
- Alshehri, A.H.; Mistry, K.; Nguyen, V.H.; Ibrahim, K.H.; Muñoz-Rojas, D.; Yavuz, M.; Musselman, K.P. Quantum-Tunneling Metal-Insulator-Metal Diodes Made by Rapid Atmospheric Pressure Chemical Vapor Deposition. Adv. Funct. Mater. 2019, 29, 1805533. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Kempiński, M.; Jancelewicz, M.; Załęski, K.; Jurga, S.; Smyntyna, V. Structural and XPS Characterization of ALD Al2O3 Coated Porous Silicon. Vacuum 2015, 113, 52–58. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).