Stepwise−Process−Controlled Ligand Management Strategy for Efficient and Stable Perovskite Quantum Dot Solar Cells
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Synthesis and Post−Treatment of CsPbI3 Quantum Dots
2.3. Device Fabrication
2.4. Characterization
3. Results and Discussion
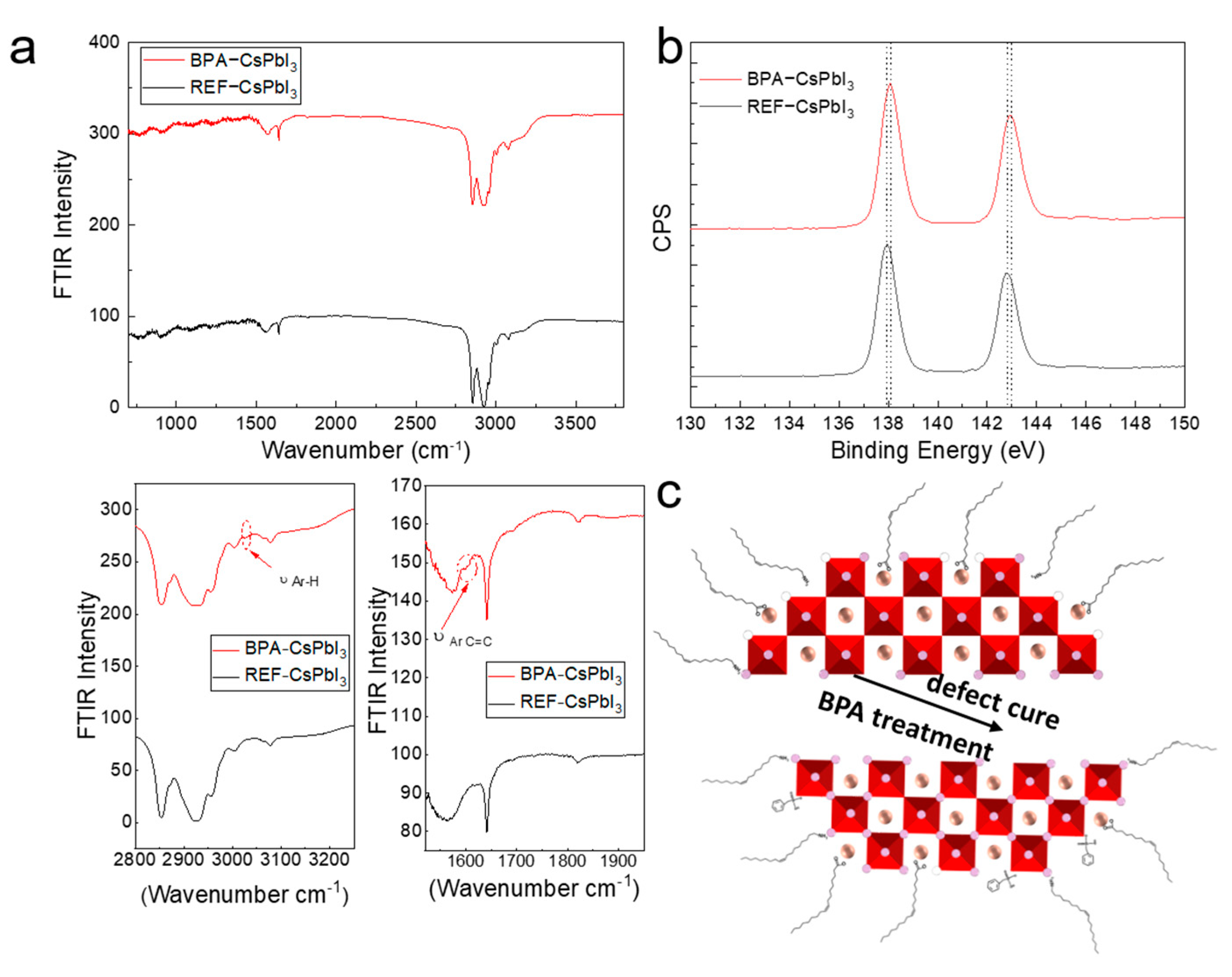
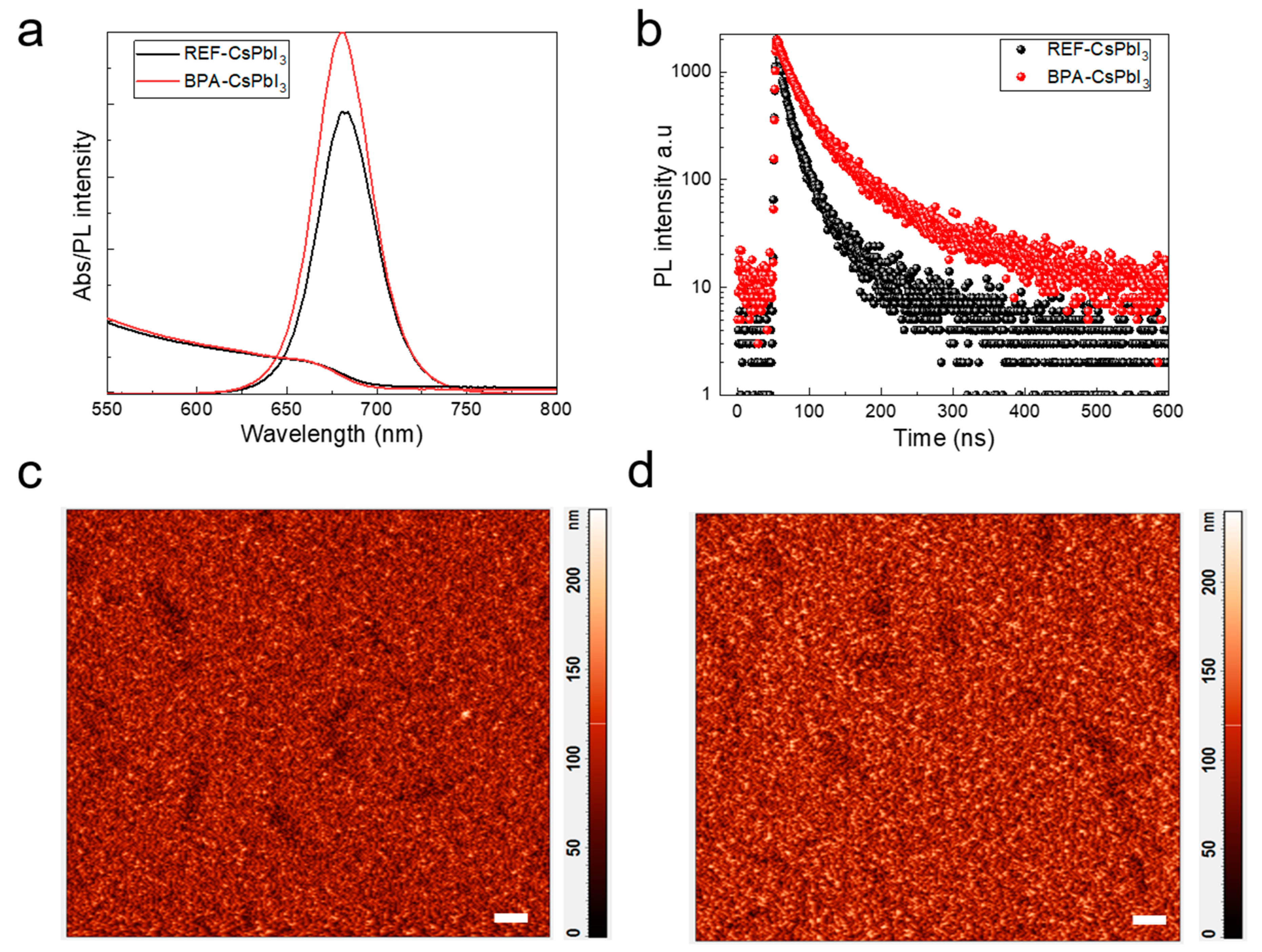
3.1. Characterization of CsPbI3 QDs
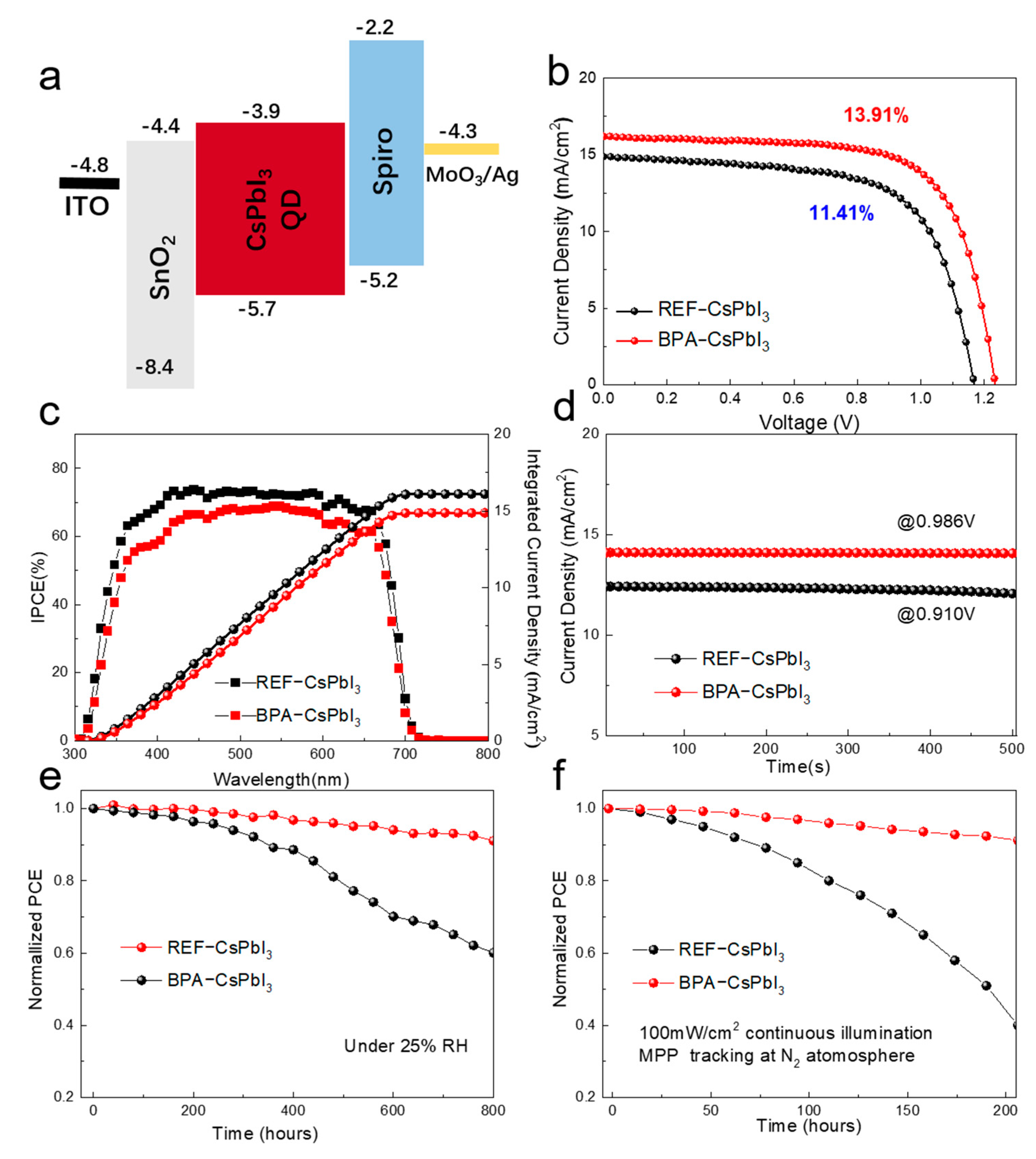
3.2. Device Performance and Stability Analysis
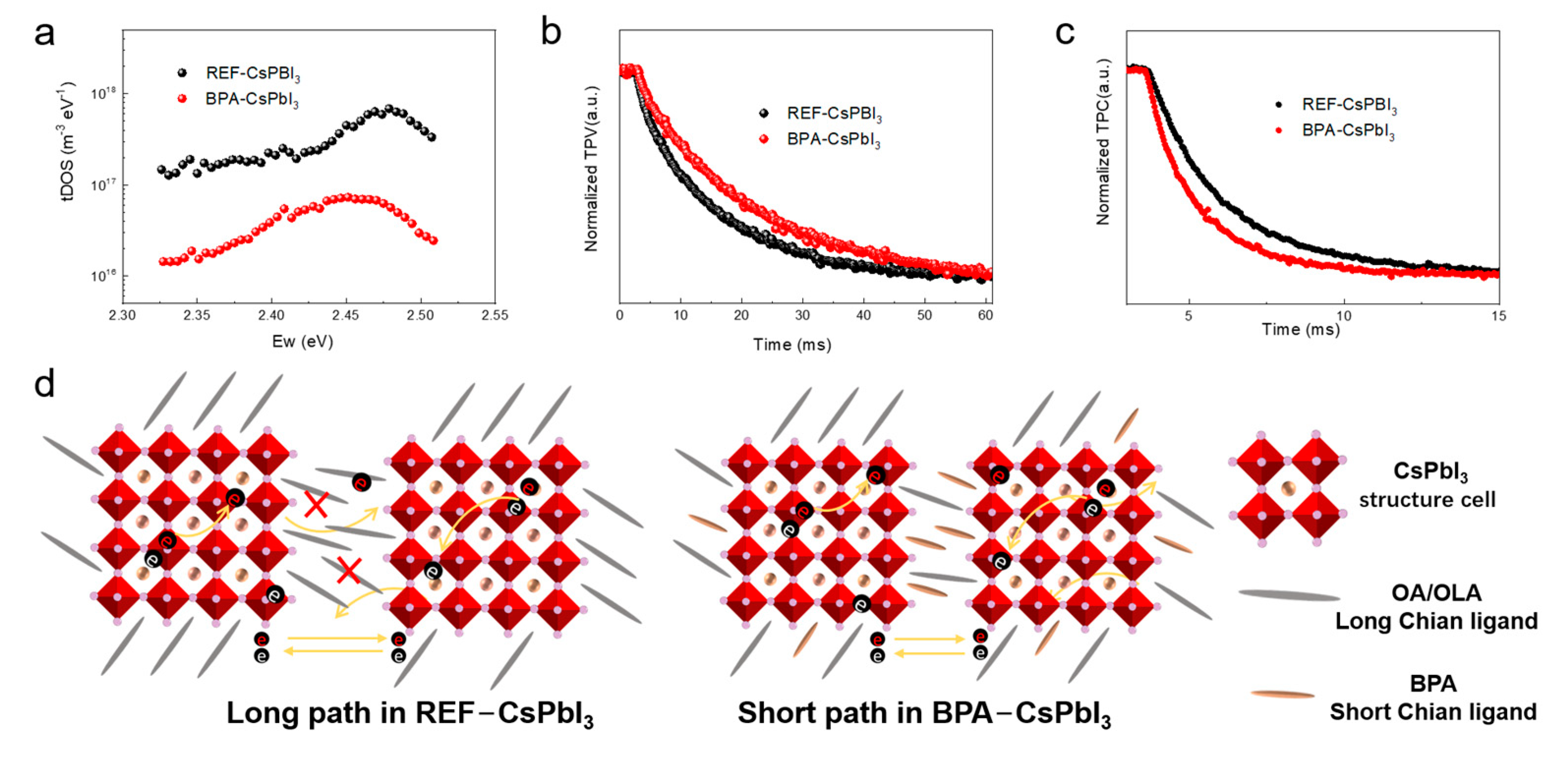
3.3. Mechanism Analysis of Defect Passivation and Charge Transport
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Renewable Energy Laboratory. Best Research−Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell−efficiency.html (accessed on 1 November 2023.).
- Park, J.; Kim, J.; Yun, H.S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature 2023, 616, 724–730. [Google Scholar] [CrossRef]
- Eperon, G.E.; Paternò, G.M.; Sutton, R.J.; Zampetti, A.; Haghighirad, A.A.; Cacialli, F.; Snaith, H.J. Inorganic caesium lead iodide perovskite solar cells. J. Mater. Chem. A 2015, 3, 19688–19695. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Frazer, L.; Clark, D.J.; Kim, Y.S.; Rhim, S.H.; Freeman, A.J.; Ketterson, J.B.; Jang, J.I.; Kanatzidis, M.G. Hybrid Germanium Iodide Perovskite Semiconductors: Active Lone Pairs, Structural Distortions, Direct and Indirect Energy Gaps, and Strong Nonlinear Optical Properties. J. Am. Chem. Soc. 2015, 137, 6804–6819. [Google Scholar] [CrossRef] [PubMed]
- Mubiayi, K.P.; Moloto, N.; Moloto, M.J. Effect of diphenylphosphinic acid on cesium lead iodide perovskite stability. Crystengcomm 2018, 20, 5275–5280. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Mei, X.Y.; Jia, D.L.; Chen, J.X.; Zheng, S.Y.; Zhang, X.L. Approaching high−performance light−emitting devices upon perovskite quantum dots: Advances and prospects. Nano Today 2022, 43, 101449. [Google Scholar] [CrossRef]
- Jia, D.L.; Chen, J.X.; Yu, M.; Liu, J.H.; Johansson, E.M.J.; Hagfeldt, A.; Zhang, X.L. Dual Passivation of CsPbI Perovskite Nanocrystals with Amino Acid Ligands for Efficient Quantum Dot Solar Cells. Small 2020, 16, 2001772. [Google Scholar] [CrossRef]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot−induced phase stabilization of α−CsPbI perovskite for high−efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef]
- Sanehira, E.M.; Marshall, A.R.; Christians, J.A.; Harvey, S.P.; Ciesielski, P.N.; Wheeler, L.M.; Schulz, P.; Lin, L.Y.; Beard, M.C.; Luther, J.M. Enhanced mobility CsPbI quantum dot arrays for record−efficiency, high−voltage photovoltaic cells. Sci. Adv. 2017, 3, eaao4204. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, J.Y.; Zhang, X.L.; Ling, X.F.; Larson, B.W.; Zhao, Q.; Yang, Y.G.; Shi, Y.; Luther, J.M.; Ma, W.L. Surface Ligand Management Aided by a Secondary Amine Enables Increased Synthesis Yield of CsPbI Perovskite Quantum Dots and High Photovoltaic Performance. Adv. Mater. 2020, 32, 2000449. [Google Scholar] [CrossRef]
- Yuan, J.B.; Zhang, X.L.; Sun, J.G.; Patterson, R.; Yao, H.F.; Xue, D.; Wang, Y.; Ji, K.; Hu, L.; Huang, S.J.; et al. Hybrid Perovskite Quantum Dot/Non−Fullerene Molecule Solar Cells with Efficiency Over 15%. Adv. Funct. Mater. 2021, 31, 2101272. [Google Scholar] [CrossRef]
- Shi, J.W.; Li, F.C.; Jin, Y.; Liu, C.; Cohen−Kleinstein, B.; Yuan, S.; Li, Y.Y.; Wang, Z.K.; Yuan, J.Y.; Ma, W.L. In Situ Ligand Bonding Management of CsPbI Perovskite Quantum Dots Enables High−Performance Photovoltaics and Red Light−Emitting Diodes. Angew. Chem. Int. Edit 2020, 59, 22230–22237. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Siegler, T.D.; Thomas, C.J.; Abney, M.K.; Shah, T.; De Gorostiza, A.; Greene, R.M.; Korgel, B.A. A “Tips and Tricks” Practical Guide to the Synthesis of Metal Halide Perovskite Nanocrystals. Chem. Mater. 2020, 32, 5410–5423. [Google Scholar] [CrossRef]
- Ling, X.F.; Yuan, J.Y.; Zhang, X.L.; Qian, Y.L.; Zakeeruddin, S.M.; Larson, B.W.; Zhao, Q.; Shi, J.W.; Yang, J.C.; Ji, K.; et al. Guanidinium−Assisted Surface Matrix Engineering for Highly Efficient Perovskite Quantum Dot Photovoltaics. Adv. Mater. 2020, 32, 2001906. [Google Scholar] [CrossRef]
- Wheeler, L.M.; Sanehira, E.M.; Marshall, A.R.; Schulz, P.; Suri, M.; Anderson, N.C.; Christians, J.A.; Nordlund, D.; Sokaras, D.; Kroll, T.; et al. Targeted Ligand−Exchange Chemistry on Cesium Lead Halide Perovskite Quantum Dots for High−Efficiency Photovoltaics. J. Am. Chem. Soc. 2018, 140, 10504–10513. [Google Scholar] [CrossRef]
- Kim, J.; Cho, S.; Dinic, F.; Choi, J.; Choi, C.; Jeong, S.M.; Lee, J.S.; Voznyy, O.; Ko, M.J.; Kim, Y. Hydrophobic stabilizer−anchored fully inorganic perovskite quantum dots enhance moisture resistance and photovoltaic performance. Nano Energy 2020, 75, 104985. [Google Scholar] [CrossRef]
- Zhang, X.L.; Huang, H.H.; Maung, Y.M.; Yuan, J.Y.; Ma, W.L. Aromatic amine−assisted pseudo−solution−phase ligand exchange in CsPbI perovskite quantum dot solar cells. Chem. Commun. 2021, 57, 7906–7909. [Google Scholar] [CrossRef]
- Ding, S.S.; Steele, J.A.; Chen, P.; Lin, T.E.; He, D.X.; Zhang, C.X.; Fan, X.Q.; Solano, E.; Whittaker, A.K.; Hao, M.M.; et al. Ligand−Mediated Homojunction Structure for High−Efficiency FAPbI Quantum Dot Solar Cells. Adv. Energy Mater. 2023, 2301817. [Google Scholar] [CrossRef]
- Jia, D.L.; Chen, J.X.; Qiu, J.M.; Ma, H.L.; Yu, M.; Liu, J.H.; Zhang, X.L. Tailoring solvent−mediated ligand exchange for CsPbI perovskite quantum dot solar cells with efficiency exceeding 16.5%. Joule 2022, 6, 1632–1653. [Google Scholar] [CrossRef]
- Jia, D.L.; Chen, J.X.; Mei, X.Y.; Fan, W.T.; Luo, S.; Yu, M.; Liu, J.H.; Zhang, X.L. Surface matrix curing of inorganic CsPbI perovskite quantum dots for solar cells with efficiency over 16%. Energy Environ. Sci. 2021, 14, 4599–4609. [Google Scholar] [CrossRef]
- Koole, R.; Liljeroth, P.; Donegá, C.D.; Vanmaekelbergh, D.; Meijerink, A. Electronic coupling and exciton energy transfer in CdTe quantum−dot molecules. J. Am. Chem. Soc. 2006, 128, 10436–10441. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Zhang, X.L.; Yuan, J.Y.; Wang, Y.; Shi, G.Z.; Patterson, R.; Shi, J.W.; Ling, X.F.; Hu, L.; Wu, T.; et al. Tuning the Surface−Passivating Ligand Anchoring Position Enables Phase Robustness in CsPbI Perovskite Quantum Dot Solar Cells. ACS Energy Lett. 2020, 5, 3322–3329. [Google Scholar] [CrossRef]
- Vickers, E.T.; Graham, T.A.; Chowdhury, A.H.; Bahrami, B.; Dreskin, B.W.; Lindley, S.; Naghadeh, S.B.; Qiao, Q.Q.; Zhang, J.Z. Improving Charge Carrier Delocalization in Perovskite Quantum Dots by Surface Passivation with Conductive Aromatic Ligands. ACS Energy Lett. 2018, 3, 2931–2939. [Google Scholar] [CrossRef]
- Yao, J.S.; Ge, J.; Wang, K.H.; Zhang, G.; Zhu, B.S.; Chen, C.; Zhang, Q.; Luo, Y.; Shu, H.; Yao, H.B. Few−nanometer−sized α−CspbI3 quantum dots enabled by strontium substitution and iodide passivation for efficient red−light emitting diodes. J. Am. Chem. Soc. 2019, 141, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.B.; Yin, C.Y.; Yang, F.; Yao, Y.; Yuan, F.L.; Chen, H.T.; Wang, R.W.; Bai, S.; Tu, G.L.; Hou, L.T. Highly Luminescent and Stable CsPbI Perovskite Nanocrystals with Sodium Dodecyl Sulfate Ligand Passivation for Red−Light−Emitting Diodes. J. Phys. Chem. Lett. 2021, 12, 2437–2443. [Google Scholar] [CrossRef]
- Shen, W.; Dai, Y.J.; Cai, B.; Chen, S.; Yang, H.; Ma, Y.Z.; Chen, Y.F.; Su, Z.; Zhang, J.B.; Qiu, Y.; et al. Ligand−Assisted Breaking Crystal Symmetry to Achieve Stable γ−CsPbI Nanorods with Strong Polarization Response. ACS Energy Lett. 2023, 8, 2561–2569. [Google Scholar] [CrossRef]
- Luo, D.Y.; Su, R.; Zhang, W.; Gong, Q.H.; Zhu, R. Minimizing non−radiative recombination losses in perovskite solar cells. Nat. Rev. Mater. 2020, 5, 44–60. [Google Scholar] [CrossRef]
- Li, B.; Chang, B.H.; Pan, L.; Li, Z.H.; Fu, L.; He, Z.B.; Yin, L.W. Tin−Based Defects and Passivation Strategies in Tin−Related Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 3752–3772. [Google Scholar] [CrossRef]
- Zhang, F.; Bi, D.Q.; Pellet, N.; Xiao, C.X.; Li, Z.; Berry, J.J.; Zakeeruddin, S.M.; Zhu, K.; Grätzel, M. Suppressing defects through the synergistic effect of a Lewis base and a Lewis acid for highly efficient and stable perovskite solar cells. Energy Environ. Sci. 2018, 11, 3480–3490. [Google Scholar] [CrossRef]
- Shao, Y.H.; Xiao, Z.G.; Bi, C.; Yuan, Y.B.; Huang, J.S. Origin and elimination of photocurrent hysteresis by fullerene passivation in CHNHPbI3 planar heterojunction solar cells. Nat. Commun. 2014, 5, 5784. [Google Scholar] [CrossRef]
- Bi, C.; Wang, Q.; Shao, Y.C.; Yuan, Y.B.; Xiao, Z.G.; Huang, J.S. Non−wetting surface−driven high−aspect−ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 2015, 6, 7747. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.G.; Li, B.; Hu, L.; Guo, J.J.; Ling, X.F.; Zhang, X.L.; Zhang, C.; Wu, X.X.; Huang, H.H.; Han, C.X.; et al. Hybrid Block Copolymer/Perovskite Heterointerfaces for Efficient Solar Cells. Adv. Mater. 2023, 35, 2206047. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ling, X.; Yang, D.; Li, F.; Zhou, S.; Shi, J.; Qian, Y.; Hu, J.; Sun, Y.; Yang, Y.; et al. Band−Aligned Polymeric Hole Transport Materials for Extremely Low Energy Loss α−CsPbI3 Perovskite Nanocrystal Solar Cells. Joule 2018, 2, 2450. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Yang, Z.; Li, Q.; Wei, W.; Hu, B.; Chen, W. Cu12Sb4S13 Quantum Dots with Ligand Exchange as Hole Transport Materials in All−Inorganic Perovskite CsPbI3 Quantum Dot Solar Cells. ACS Appl. Energy Mater. 2020, 3, 3521. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J.; Park, J.Y.; Min, J.; Yun, S.; Park, T.; Kim, Y.; Choi, J. Suppressed Degradation and Enhanced Performance of CsPbI3 Perovskite Quantum Dot Solar Cells via Engineering of Electron Transport Layers. ACS Appl. Mater. Interfaces 2021, 13, 6119–6129. [Google Scholar] [CrossRef]
- Shivarudraiah, S.B.; Ng, M.; Li, C.H.A.; Halpert, J.E. All−Inorganic, Solution−Processed, Inverted Cspbi3 Quantum Dot Solar Cells with a Pce of 13.1% Achieved Via a Layer−by−Layer Fai Treatment. ACS Appl. Energy Mater. 2020, 3, 5620–5627. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Guo, W.; Xu, J.; Xu, R.; Xi, J.; Dong, H.; Wu, Z. Stepwise−Process−Controlled Ligand Management Strategy for Efficient and Stable Perovskite Quantum Dot Solar Cells. Nanomaterials 2023, 13, 3032. https://doi.org/10.3390/nano13233032
Dai J, Guo W, Xu J, Xu R, Xi J, Dong H, Wu Z. Stepwise−Process−Controlled Ligand Management Strategy for Efficient and Stable Perovskite Quantum Dot Solar Cells. Nanomaterials. 2023; 13(23):3032. https://doi.org/10.3390/nano13233032
Chicago/Turabian StyleDai, Jinfei, Wei Guo, Jie Xu, Ruoyao Xu, Jun Xi, Hua Dong, and Zhaoxin Wu. 2023. "Stepwise−Process−Controlled Ligand Management Strategy for Efficient and Stable Perovskite Quantum Dot Solar Cells" Nanomaterials 13, no. 23: 3032. https://doi.org/10.3390/nano13233032
APA StyleDai, J., Guo, W., Xu, J., Xu, R., Xi, J., Dong, H., & Wu, Z. (2023). Stepwise−Process−Controlled Ligand Management Strategy for Efficient and Stable Perovskite Quantum Dot Solar Cells. Nanomaterials, 13(23), 3032. https://doi.org/10.3390/nano13233032







