First-Principles Dynamics Investigation of Germanium as an Anode Material in Multivalent-Ion Batteries
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deng, W.; Liu, W.; Zhu, H.; Chen, L.; Liao, H.; Chen, H. Click-chemistry and ionic cross-linking induced double cross-linking ionogel electrolyte for flexible lithium-ion batteries. J. Energy Storage 2023, 72, 108509. [Google Scholar]
- Li, L.; Nam, J.S.; Kim, M.S.; Wang, Y.; Jiang, S.; Hou, H.; Kim, I.-D. Sulfur–Carbon Electrode with PEO-LiFSI-PVDF Composite Coating for High-Rate and Long-Life Lithium–Sulfur Batteries. Adv. Energy Mater. 2023, 13, 2302139. [Google Scholar] [CrossRef]
- Deng, W.-N.; Li, Y.-H.; Xu, D.-F.; Zhou, W.; Xiang, K.-X.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium–selenium batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+ diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Deng, W.; Xu, Y.; Zhang, X.; Li, C.; Liu, Y.; Xiang, K.; Chen, H. (NH4)2Co2V10O28·16H2O/(NH4)2V10O25·8H2O heterostructure as cathode for high-performance aqueous Zn-ion batteries . J. Alloys Compd. 2022, 903, 163824. [Google Scholar] [CrossRef]
- Guo, Z.; Han, X.; Zhang, C.; He, S.; Liu, K.; Hu, J.; Yang, W.; Jian, S.; Jiang, S.; Duan, G. Activation of biomass-derived porous carbon for supercapacitors: A review. Chin. Chem. Lett. 2023, in press. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and its derived composite electrodes toward supercapacitors: Fabrication, properties, and challenges. J. Bioresour. Bioprod. 2022, 7, 245–269. [Google Scholar]
- Wei, C.; Tan, L.; Zhang, Y.; Wang, Z.; Feng, J.; Qian, Y. Towards better Mg metal anodes in rechargeable Mg batteries: Challenges, strategies, and perspectives. Energy Storage Mater. 2022, 52, 299–319. [Google Scholar]
- Huang, W.; Zhang, K.; Yuan, B.; Yang, L.; Zhu, M. Predominant intercalation of H+ enables ultrahigh rate capability of oxygen deficient MoO3 for aqueous Al-ion batteries. Energy Storage Mater. 2022, 50, 152–160. [Google Scholar] [CrossRef]
- Yaghoobnejad Asl, H.; Manthiram, A. Mass Transfer of Divalent Ions in an Oxide Host: Comparison of Mg2+ and Zn2+ Diffusion in Hexagonal KxW3O9 Bronze. Chem. Mater. 2019, 31, 2296–2307. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, K.; Gao, Y.; Wang, C. Challenges and Perspectives of Organic Multivalent Metal-Ion Batteries. Adv. Mater. 2022, 34, 2200662. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Wang, J.; Ji, R.; Yuan, H.; Wang, Y.; Wang, H. Recent progress of flexible aqueous multivalent ion batteries. Carbon Energy 2022, 4, 411–445. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, Y.; Chen, R.; Xu, Z.; Li, J.; Zong, W.; Li, H.; Li, Z.; Zhang, Z.; Zhu, J.; et al. Highly Reversible Zinc Metal Anode in a Dilute Aqueous Electrolyte Enabled by a pH Buffer Additive. Angew. Chem. Int. Ed. 2022, 62, e202212695. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H.; Ren, B.; Jiang, J.; Qu, G.; Yang, J.; Chen, G.; Li, H.; Zhi, C.; Liu, Z. Unlocking High-Performance Ammonium-Ion Batteries: Activation of In-Layer Channels for Enhanced Ion Storage and Migration. Adv. Mater. 2023, 35, 2304209. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, C.; Chai, L.; Zhang, W.; Li, Z. Bimetallic Rechargeable Al/Zn Hybrid Aqueous Batteries Based on Al–Zn Alloys with Composite Electrolytes. Adv. Mater. 2022, 34, 2206099. [Google Scholar] [CrossRef]
- Li, J.; Han, C.; Ou, X.; Tang, Y. Concentrated Electrolyte for High-Performance Ca-Ion Battery Based on Organic Anode and Graphite Cathode. Angew. Chem. Int. Ed. 2022, 134, e202116668. [Google Scholar] [CrossRef]
- Xiao, X.; Zheng, Z.; Zhong, X.; Gao, R.; Piao, Z.; Jiao, M.; Zhou, G. Rational Design of Flexible Zn-Based Batteries for Wearable Electronic Devices. ACS Nano 2023, 17, 1764–1802. [Google Scholar] [CrossRef]
- Zhao, S.; Li, C.; Zhang, X.; Li, N.; Wang, T.; Li, X.; Wang, C.; Qu, G.; Xu, X. An advanced Ca/Zn hybrid battery enabled by the dendrite-free zinc anode and a reversible calcification/decalcification NASICON cathode. Sci. Bull. 2023, 68, 56–64. [Google Scholar] [CrossRef]
- Shi, F.; Chen, C.; Xu, Z.-L. Recent Advances on Electrospun Nanofiber Materials for Post-lithium Ion Batteries. Adv. Fiber Mater. 2021, 3, 275–301. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Z.; Wu, J.; Liu, B.; Zhang, Y.; Huang, S. Effect of Oxygen Vacancies and F-Doping on TiO2(B) as Anode for Mg-Ion Batteries. J. Phys. Chem. C 2023, 127, 14086–14097. [Google Scholar] [CrossRef]
- Li, W.; Ma, Y.; Shi, H.; Jiang, K.; Wang, D. Cu7Te4 as an Anode Material and Zn Dendrite Inhibitor for Aqueous Zn-Ion Battery. Adv. Funct. Mater. 2022, 32, 2205602. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, Y.; Shu, X.; Xu, T.; Jing, Y. Computational insights into the rational design of organic electrode materials for metal ion batteries. Comput. Mol. Sci. 2023, 13, e1660. [Google Scholar] [CrossRef]
- Xie, Y.; Duan, C.; Yang, Z.; Feng, Y.; Yao, J. Post-treatment of ZIF-67/Ni3S4 Nanoparticle Composites by Metal Ions and Solvent-Assisted Etching as Advanced Electrodes for Supercapacitors. ACS Appl. Nano Mater. 2023, 6, 12105–12113. [Google Scholar] [CrossRef]
- Liu, H.; Wu, T.; Zhang, L.; Wang, X.; Li, H.; Liu, S.; Zhang, Q.; Zhang, X.; Yu, H. Germanium Nanowires via Molten-Salt Electrolysis for Lithium Battery Anode. ACS Nano 2022, 16, 14402–14411. [Google Scholar] [CrossRef]
- Jo, C.; Wen, B.; Jeong, H.; Park, S.K.; Son, Y.; Volder, M.D. Spinodal Decomposition Method for Structuring Germanium–Carbon Li-Ion Battery Anodes. ACS Nano 2023, 17, 8403–8410. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Q.; Guo, X.; Huang, J.; Tan, H.; Du, X.; Zhu, Y.; Zhang, B. Critical roles of microstructure and interphase on the stability of microsized germanium anode. J. Power Sources 2021, 481, 228916. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, S.; Chen, M.; Wu, L. Uniformly Confined Germanium Quantum Dots in 3D Ordered Porous Carbon Framework for High-Performance Li-ion Battery. Adv. Funct. Mater. 2020, 30, 2000373. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, Z.; Yang, Z.; Xiao, D.; Hu, P.; Xu, H.; Duan, Y.; Pang, S.; Gu, L.; Cui, G. Hierarchically Designed Germanium Microcubes with High Initial Coulombic Efficiency toward Highly Reversible Lithium Storage. Chem. Mater. 2015, 27, 2189–2194. [Google Scholar] [CrossRef]
- Liu, Q.; Hou, J.; Xu, C.; Chen, Z.; Qin, R.; Liu, H. TiO2 particles wrapped onto macroporous germanium skeleton as high performance anode for lithium-ion batteries. Chem. Eng. J. 2020, 381, 122649. [Google Scholar] [CrossRef]
- Mo, R.; Rooney, D.; Sun, K. Hierarchical graphene-scaffolded mesoporous germanium dioxide nanostructure for high-performance flexible lithium-ion batteries. Energy Storage Mater. 2020, 29, 198–206. [Google Scholar] [CrossRef]
- Ngo, D.T.; Le, H.T.T.; Kim, C.; Lee, J.-Y.; Fisher, J.G.; Kim, I.-D.; Park, C.-J. Mass-scalable synthesis of 3D porous germanium–carbon composite particles as an ultra-high rate anode for lithium ion batteries. Energy Environ. Sci. 2015, 8, 3577–3588. [Google Scholar] [CrossRef]
- Li, P.; Zhang, H.; Meng, L.; Ding, Y.; Wang, J.; Lou, X.; Bai, H. In Situ Synthesis of Germanium Particles Decorated in Conjugated N-doped Carbon Matrix: Boosting the Performance of the Lithium-Ion Battery. ACS Appl. Energy Mater. 2022, 6, 362–370. [Google Scholar] [CrossRef]
- Metcalf, T.H.; Liu, X.; Jernigan, G.; Culbertson, J.C.; Abernathy, M.; Molina-Ruiz, M.; Hellman, F. Internal friction measurements of low energy excitations in amorphous germanium thin films. J. Alloys Compd. 2021, 856, 157616. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.-Y.; Chang, B.; Wang, K.-X. Recent progress on germanium-based anodes for lithium ion batteries: Efficient lithiation strategies and mechanisms. Energy Storage Mater. 2020, 30, 146–169. [Google Scholar] [CrossRef]
- Chou, C.-Y.; Hwang, G.S. On the origin of the significant difference in lithiation behavior between silicon and germanium. J. Power Sources 2014, 263, 252–258. [Google Scholar] [CrossRef]
- Kohandehghan, A.; Cui, K.; Kupsta, M.; Ding, J.; Memarzadeh Lotfabad, E.; Kalisvaart, W.P.; Mitlin, D. Activation with Li Enables Facile Sodium Storage in Germanium. Nano Lett. 2014, 14, 5873–5882. [Google Scholar] [CrossRef]
- Jung, S.C.; Jung, D.S.; Choi, J.W.; Han, Y.-K. Atom-Level Understanding of the Sodiation Process in Silicon Anode Material. J. Phys. Chem. Lett. 2014, 5, 1283–1288. [Google Scholar] [CrossRef]
- Jung, S.C.; Kim, H.-J.; Kang, Y.-J.; Han, Y.-K. Advantages of Ge anode for Na-ion batteries: Ge vs. Si and Sn. J. Alloys Compd. 2016, 688, 158–163. [Google Scholar] [CrossRef]
- Lu, X.; Adkins, E.R.; He, Y.; Zhong, L.; Luo, L.; Mao, S.X.; Wang, C.-M.; Korgel, B.A. Germanium as a Sodium Ion Battery Material: In Situ TEM Reveals Fast Sodiation Kinetics with High Capacity. Chem. Mater. 2016, 28, 1236–1242. [Google Scholar] [CrossRef]
- Liu, J.; Muhammad, S.; Wei, Z.; Zhu, J.; Duan, X. Hierarchical N-doping germanium/carbon nanofibers as anode for high-performance lithium-ion and sodium-ion batteries. Nanotech. 2019, 31, 015402. [Google Scholar] [CrossRef]
- Wu, F.; Yang, H.; Bai, Y.; Wu, C. Paving the path toward reliable cathode materials for aluminum-ion batteries. Adv. Mater. 2019, 31, 1806510. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, L.; Cao, X.; Zhou, J.; Pan, A.; Fang, G.; Wang, S.; Liang, S. Ion migration and defect effect of electrode materials in multivalent-ion batteries. Prog. Mater. Sci. 2022, 125, 100911. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, Y.; Meng, C.; Liu, X.; Li, B.; Xia, S. Amorphous Germanium Nanomaterials as High-Performance Anode for Lithium and Sodium-Ion Batteries. Adv. Mater. Technol. 2023, 8, 2201817. [Google Scholar] [CrossRef]
- Hüger, E.; Strauß, F.; Stahn, J.; Deubener, J.; Bruns, M.; Schmidt, H. In-situ Measurement of Self-Atom Diffusion in Solids Using Amorphous Germanium as a Model System. Sci. Rep. 2018, 8, 17607. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.C.; Choi, J.W.; Han, Y.-K. Anisotropic volume expansion of crystalline silicon during electrochemical lithium insertion: An atomic level rationale. Nano Lett. 2012, 12, 5342–5347. [Google Scholar] [CrossRef]
- Lee, S.; Jung, S.C.; Han, Y.-K. First-principles molecular dynamics study on ultrafast potassium ion transport in silicon anode. J. Power Sources 2019, 415, 119–125. [Google Scholar] [CrossRef]
- Lee, S.; Ko, M.; Jung, S.C.; Han, Y.-K. Silicon as the Anode Material for Multivalent-Ion Batteries: A First-Principles Dynamics Study. ACS Appl. Mater. Interfaces 2020, 12, 55746–55755. [Google Scholar] [CrossRef]
- Jung, S.C.; Han, Y.-K. How Do Li Atoms Pass through the Al2O3 Coating Layer during Lithiation in Li-ion Batteries? J. Phys. Chem. Lett. 2013, 4, 2681–2685. [Google Scholar] [CrossRef]
- Jung, S.C.; Kim, H.-J.; Choi, J.W.; Han, Y.-K. Sodium Ion Diffusion in Al2O3: A Distinct Perspective Compared with Lithium Ion Diffusion. Nano Lett. 2014, 14, 6559–6563. [Google Scholar] [CrossRef]
- Lee, S.W.; McDowell, M.T.; Choi, J.W.; Cui, Y. Anomalous Shape Changes of Silicon Nanopillars by Electrochemical Lithiation. Nano Lett. 2011, 11, 3034–3039. [Google Scholar] [CrossRef]
- Aaltonen, T.; Nilsen, O.; Magrasó, A.; Fjellvåg, H. Atomic Layer Deposition of Li2O–Al2O3 Thin Films. Chem. Mater. 2011, 23, 4669–4675. [Google Scholar] [CrossRef]
- Han, X.; Liu, Y.; Jia, Z.; Chen, Y.-C.; Wan, J.; Weadock, N.; Gaskell, K.J.; Li, T.; Hu, L. Atomic-Layer-Deposition Oxide Nanoglue for Sodium Ion Batteries. Nano Lett. 2014, 14, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Jarolimek, K.; Hazrati, E.; de Groot, R.A.; de Wijs, G.A. Band Offsets at the Interface between Crystalline and Amorphous Silicon from First Principles. Phys. Rev. Appl. 2017, 8, 014026. [Google Scholar] [CrossRef]
- Jarolimek, K.; de Groot, R.A.; de Wijs, G.A.; Zeman, M. First-principles study of hydrogenated amorphous silicon. Phys. Rev. B 2009, 79, 155206. [Google Scholar] [CrossRef]
- Pulay, P. Convergence acceleration of iterative sequences. The case of SCF iteration. Chem. Phys. Lett. 1980, 73, 393–398. [Google Scholar] [CrossRef]
- Lewis, L.J. Fifty years of amorphous silicon models: The end of the story? J. Non-Cryst. Solids 2022, 580, 121383. [Google Scholar] [CrossRef]
- Dalba, G.; Fornasini, P.; Grazioli, M.; Rocca, F. Local disorder in crystalline and amorphous germanium. Phys. Rev. B 1995, 52, 11034. [Google Scholar] [CrossRef]
- Qian, L.; Chen, J.F.; Li, Y.H.; Wu, L.; Wang, H.F.; Chen, A.P.; Hu, P.; Zheng, L.R.; Yang, H.G. Orange Zinc Germanate with Metallic Ge–Ge Bonds as a Chromophore-Like Center for Visible-Light-Driven Water Splitting. Angew. Chem. 2015, 127, 11629–11633. [Google Scholar] [CrossRef]
- Rao, Y.K.; Belton, G.R. Thermodynamic properties of Mg−Ge alloys. Metall. Trans. 1971, 2, 2215–2219. [Google Scholar] [CrossRef]
- Palenzona, A.; Manfrinetti, P.; Fornasini, M.L. The phase diagram of the Ca–Ge system. J. Alloys Compd. 2002, 345, 144–147. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Y.; Zhang, Y.; Qin, C.; Yu, H.; Bakenov, Z.; Wang, Z. Sn modified nanoporous Ge for improved lithium storage performance. J. Colloid Interface Sci. 2021, 602, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zou, Y.; Shen, X.; Qiu, J.; Lian, J.; Pu, J.; Li, S.; Du, F.-H.; Li, S.-Q.; Ji, Z.; et al. Ge nanoparticles uniformly immobilized on 3D interconnected porous graphene frameworks as anodes for high-performance lithium-ion batteries. Energy Chem. 2022, 69, 161–173. [Google Scholar] [CrossRef]
- Fang, S.; Tong, Z.; Zhang, X. 3D nitrogen-doped carbon foam supported Ge@C composite as anode for high performance lithium-ion battery. Chem. Eng. J. 2017, 322, 188–195. [Google Scholar] [CrossRef]
- Kennedy, T.; Brandon, M.; Ryan, K.M. Advances in the application of silicon and germanium nanowires for high-performance lithium-ion batteries. Adv. Mater. 2016, 28, 5696–5704. [Google Scholar] [CrossRef]
- Chen, Z.; Soltani, A.; Chen, Y.; Zhang, Q.; Davoodi, A.; Hosseinpour, S.; Peukert, W.; Liu, W. Emerging Organic Surface Chemistry for Si Anodes in Lithium-Ion Batteries: Advances, Prospects, and Beyond. Adv. Energy Mater. 2022, 12, 2200924. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Q.; Zhang, H.; Cheng, J.; Li, Y.; Zeng, Z.; Zhang, S.; Xu, X.; Ji, F.; Li, D.; et al. The application road of silicon-based anode in lithium-ion batteries: From liquid electrolyte to solid-state electrolyte. Energy Storage Mater. 2023, 55, 244–263. [Google Scholar] [CrossRef]
- Jung, S.C.; Choi, J.-H.; Han, Y.-K. The origin of excellent rate and cycle performance of Sn4P3 binary electrodes for sodium-ion batteries. J. Mater. Chem. A 2018, 6, 1772–1779. [Google Scholar] [CrossRef]
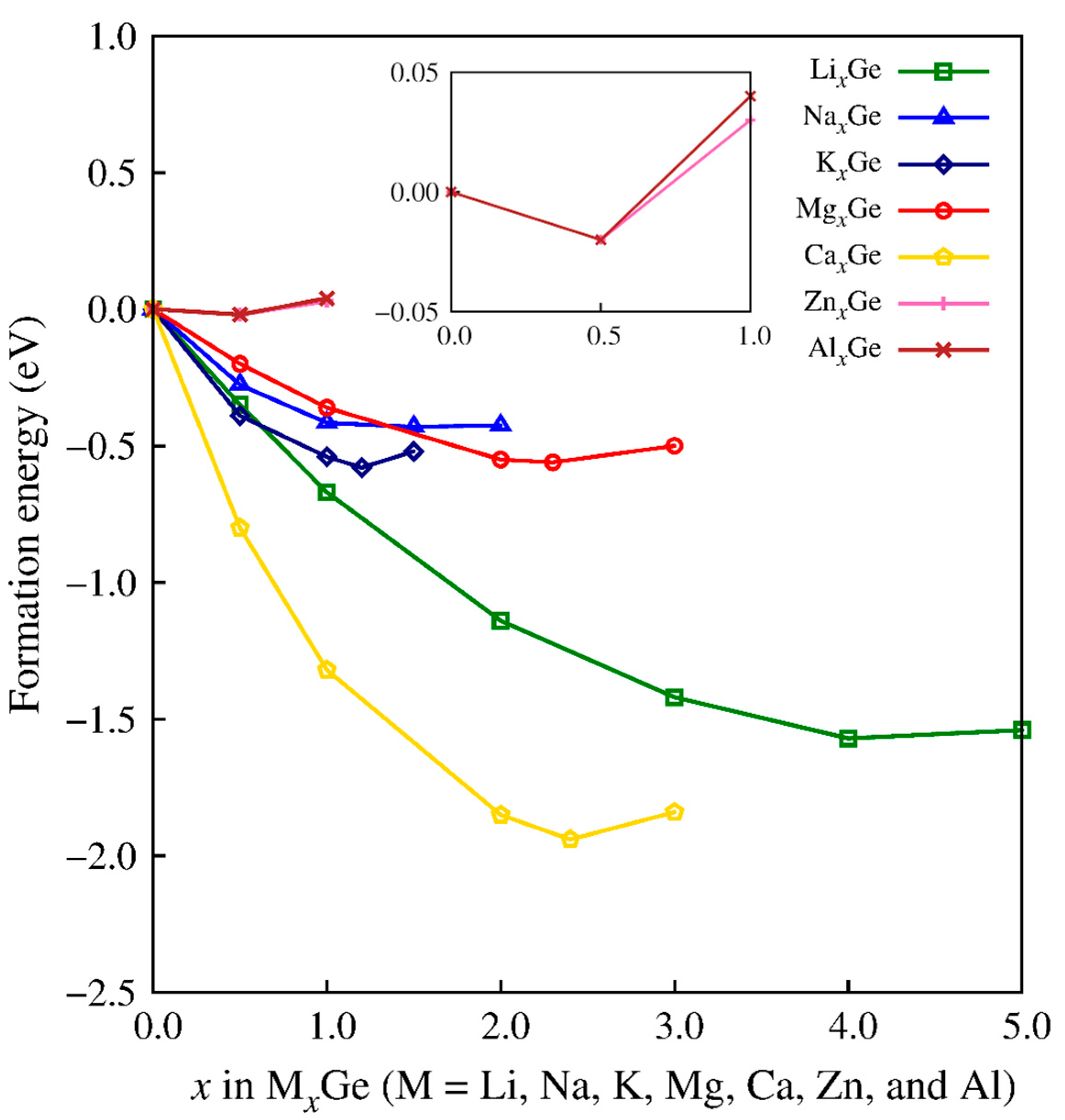
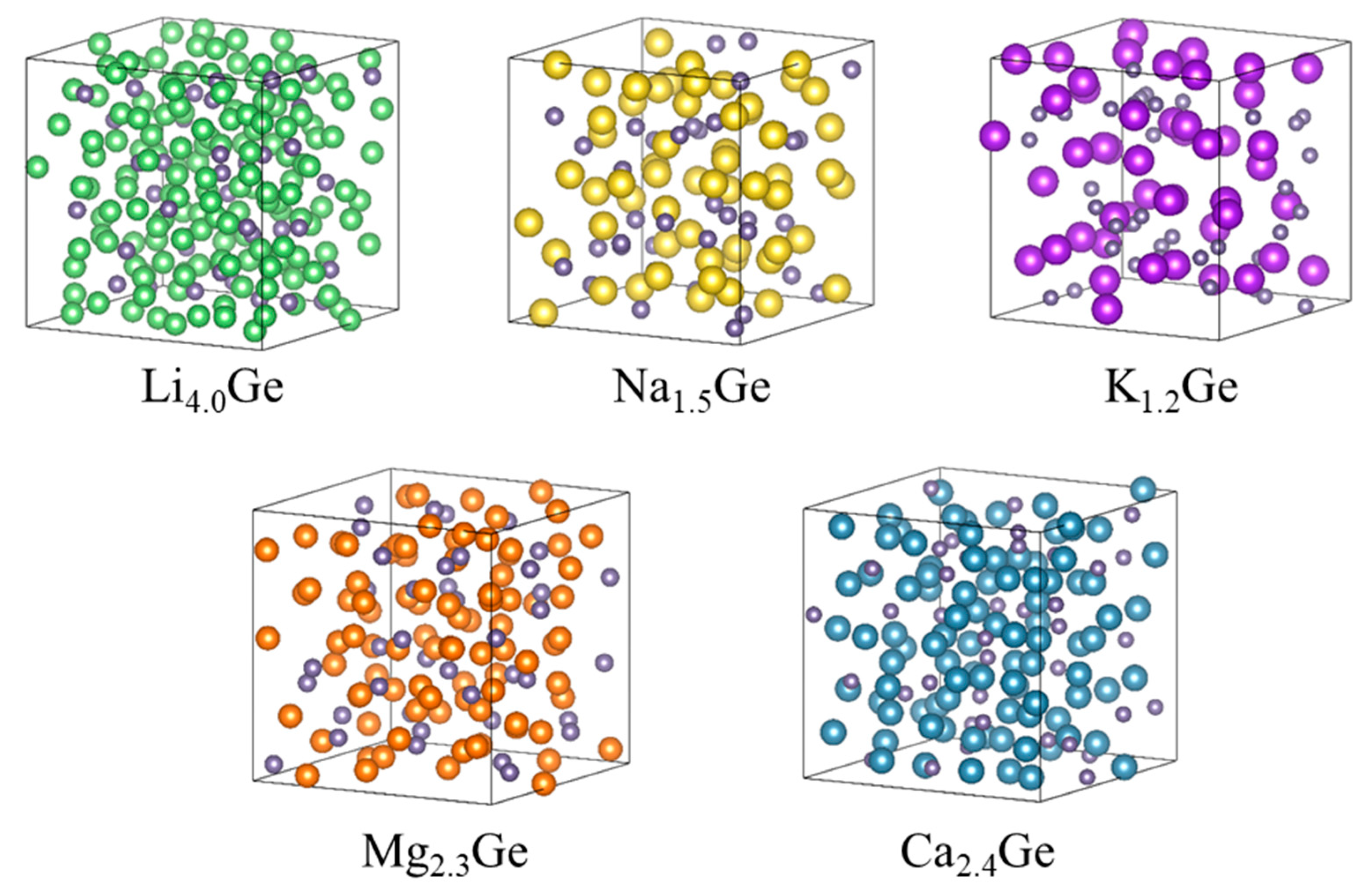
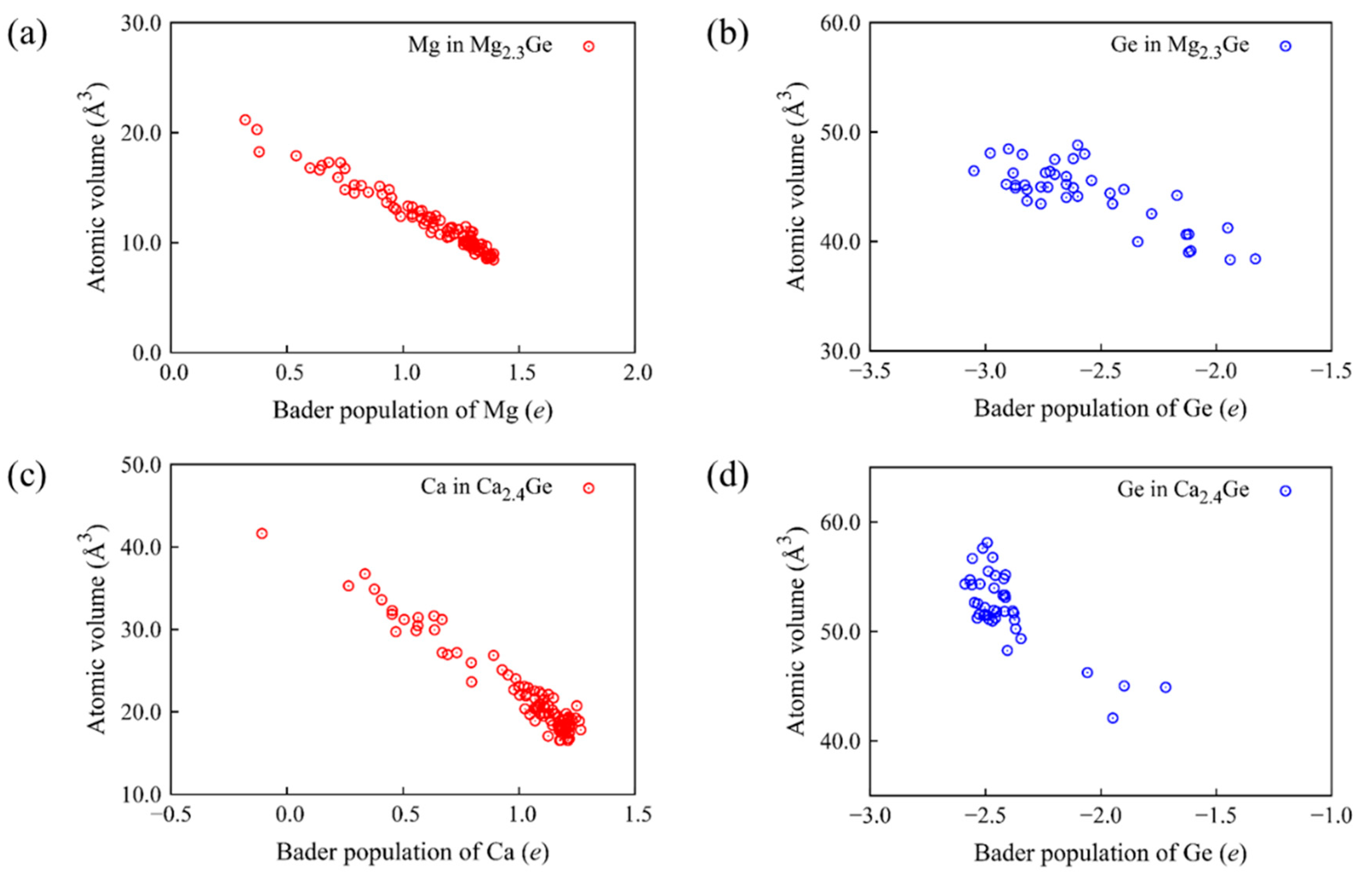
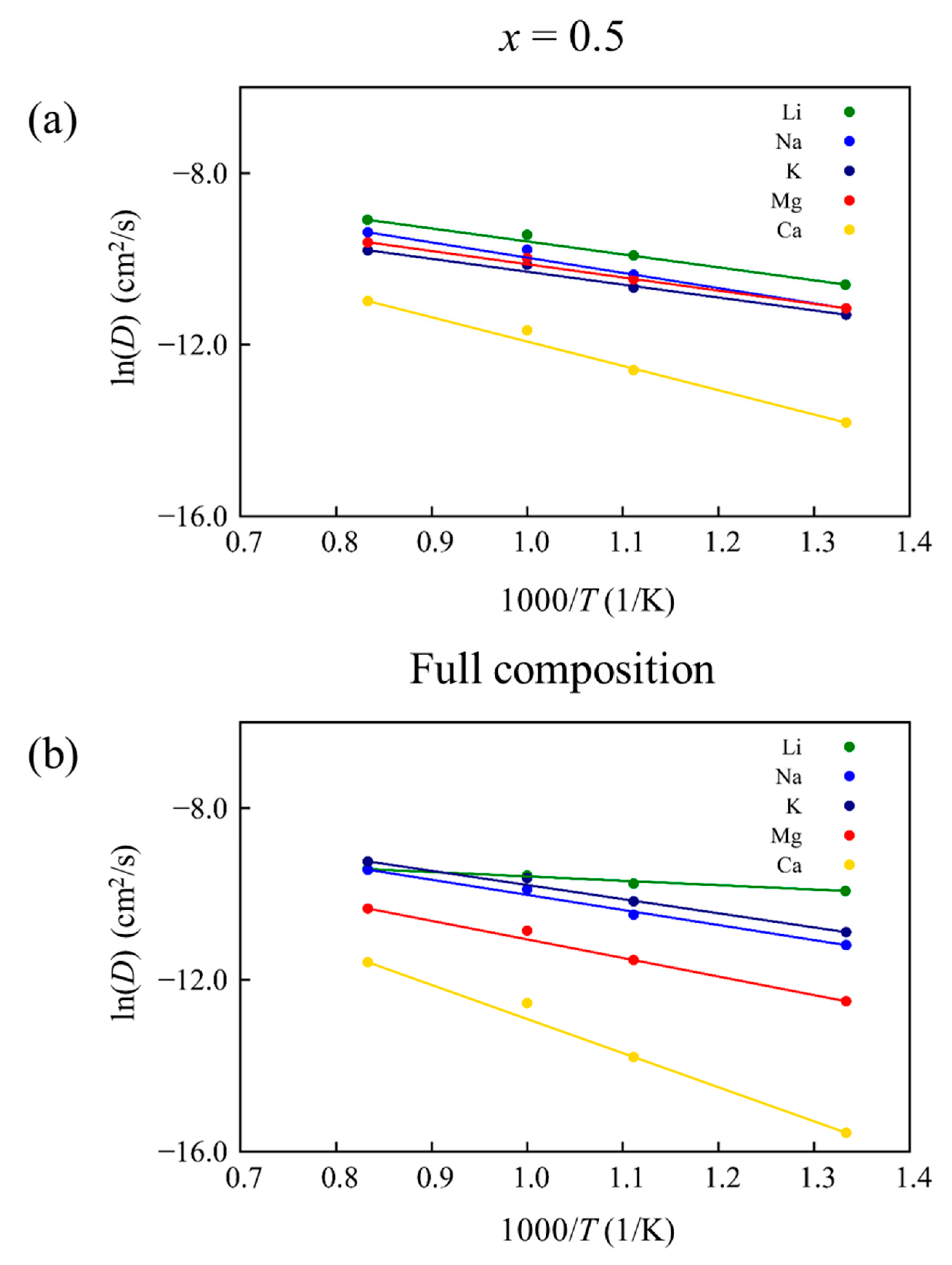
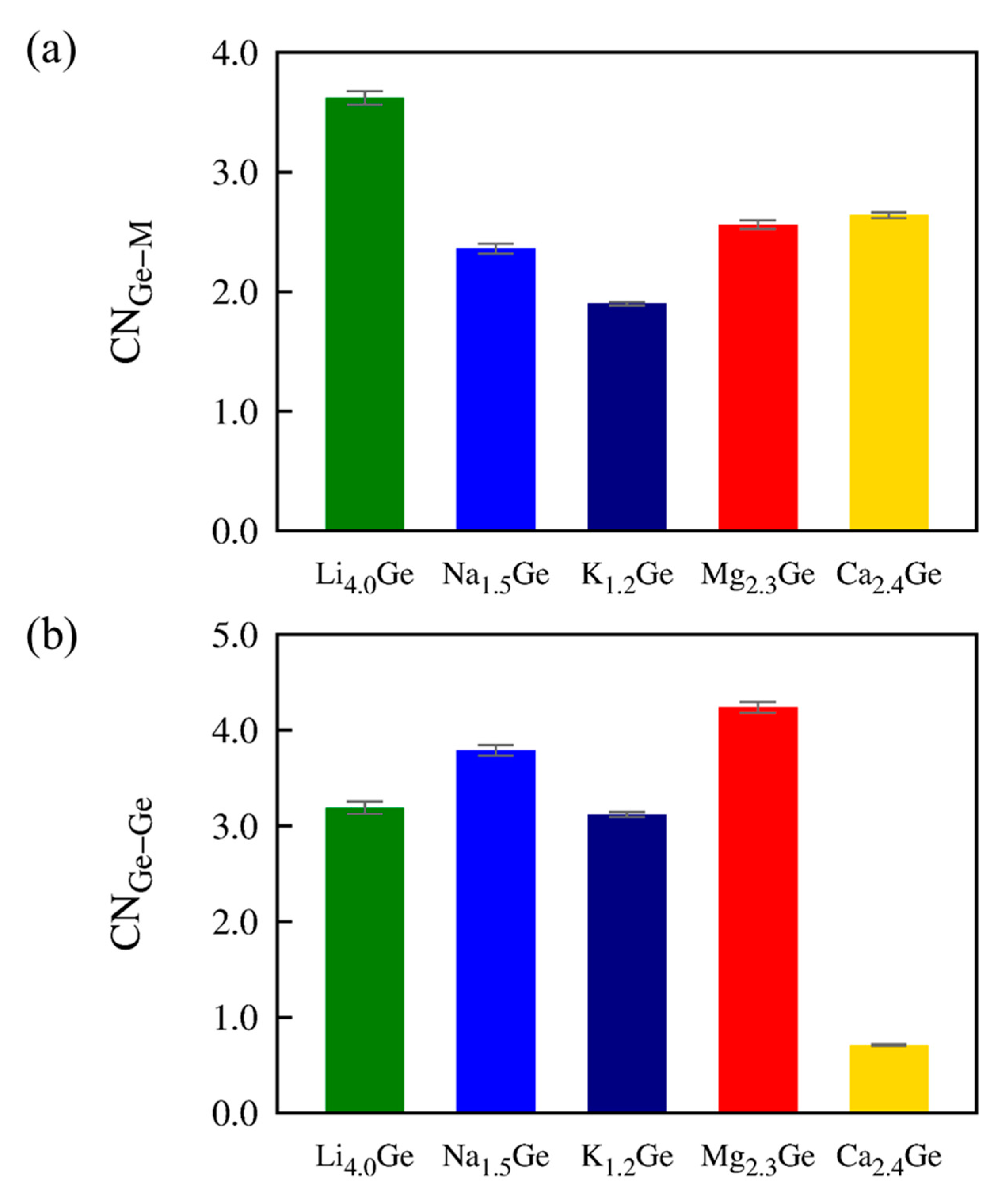
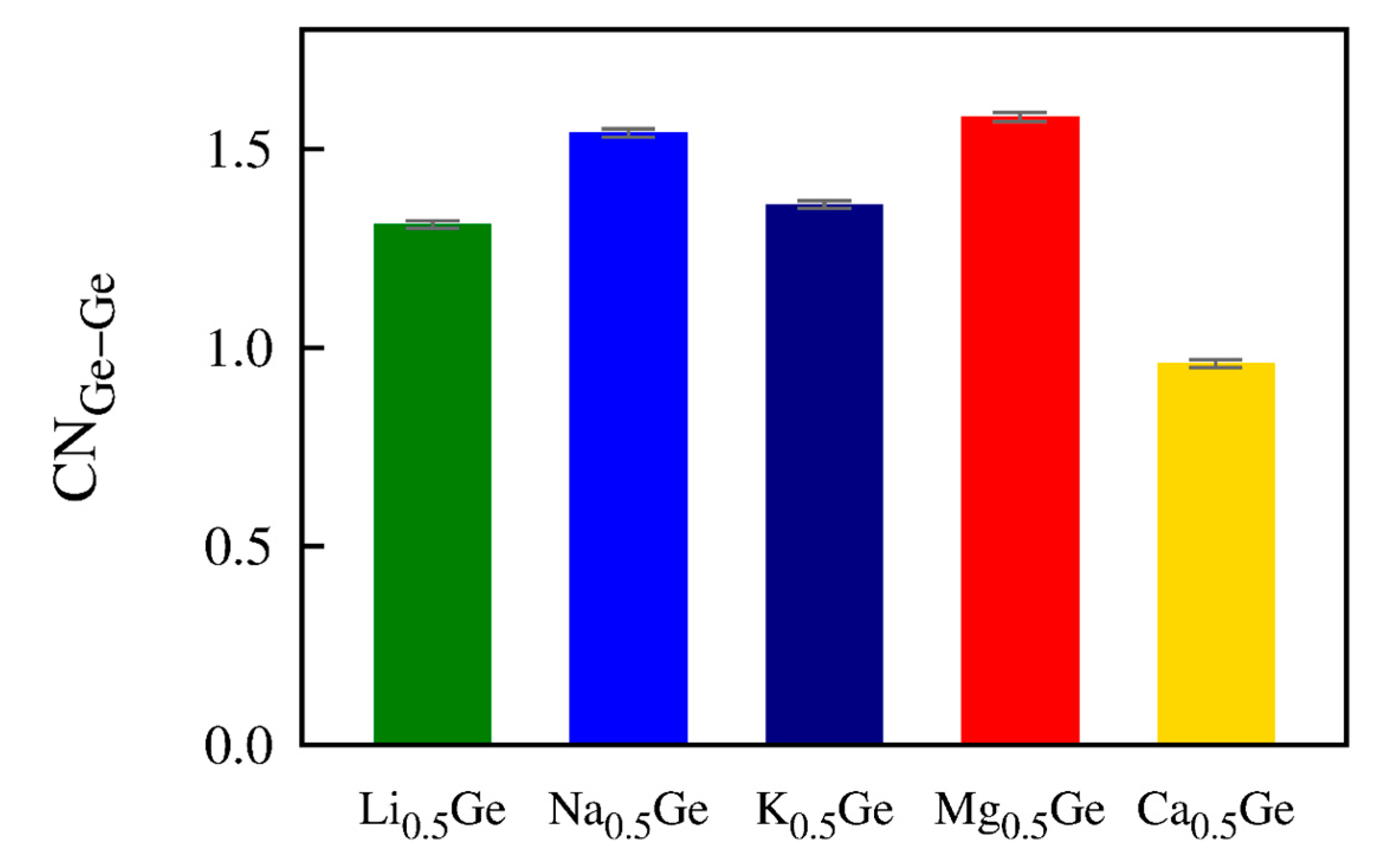
| Carrier Ion | ED | D0 | D |
|---|---|---|---|
| Li | 0.26 | 1.4 × 10−3 | 5.9 × 10−8 |
| Na | 0.31 | 1.7 × 10−3 | 1.2 × 10−8 |
| K | 0.26 | 7.0 × 10−4 | 2.8 × 10−9 |
| Mg | 0.27 | 8.7 × 10−4 | 3.0 × 10−8 |
| Ca | 0.49 | 1.9 × 10−3 | 1.1 × 10−11 |
| Carrier Ion | Capacity | Expansion Ratio | Diffusivity |
|---|---|---|---|
| Li | 1476 | 253 | 5.9 × 10−8 |
| Na | 553 | 207 | 1.2 × 10−8 |
| K | 443 | 351 | 2.8 × 10−8 |
| Mg | 1697 | 231 | 3.0 × 10−8 |
| Ca | 1771 | 389 | 1.1 × 10−11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.; Hwang, U.; Lee, S.; Han, Y.-K. First-Principles Dynamics Investigation of Germanium as an Anode Material in Multivalent-Ion Batteries. Nanomaterials 2023, 13, 2868. https://doi.org/10.3390/nano13212868
Kim C, Hwang U, Lee S, Han Y-K. First-Principles Dynamics Investigation of Germanium as an Anode Material in Multivalent-Ion Batteries. Nanomaterials. 2023; 13(21):2868. https://doi.org/10.3390/nano13212868
Chicago/Turabian StyleKim, Chaewon, Useul Hwang, Sangjin Lee, and Young-Kyu Han. 2023. "First-Principles Dynamics Investigation of Germanium as an Anode Material in Multivalent-Ion Batteries" Nanomaterials 13, no. 21: 2868. https://doi.org/10.3390/nano13212868
APA StyleKim, C., Hwang, U., Lee, S., & Han, Y.-K. (2023). First-Principles Dynamics Investigation of Germanium as an Anode Material in Multivalent-Ion Batteries. Nanomaterials, 13(21), 2868. https://doi.org/10.3390/nano13212868






