Metal Oxide Nanostructures Enhanced Microfluidic Platform for Efficient and Sensitive Immunofluorescence Detection of Dengue Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dengue Virus and Monoclonal Antibodies Preparation
2.2. Captured ELISA
2.3. Synthesis of ZnO Nanorods via Hydrothermal Growth Method
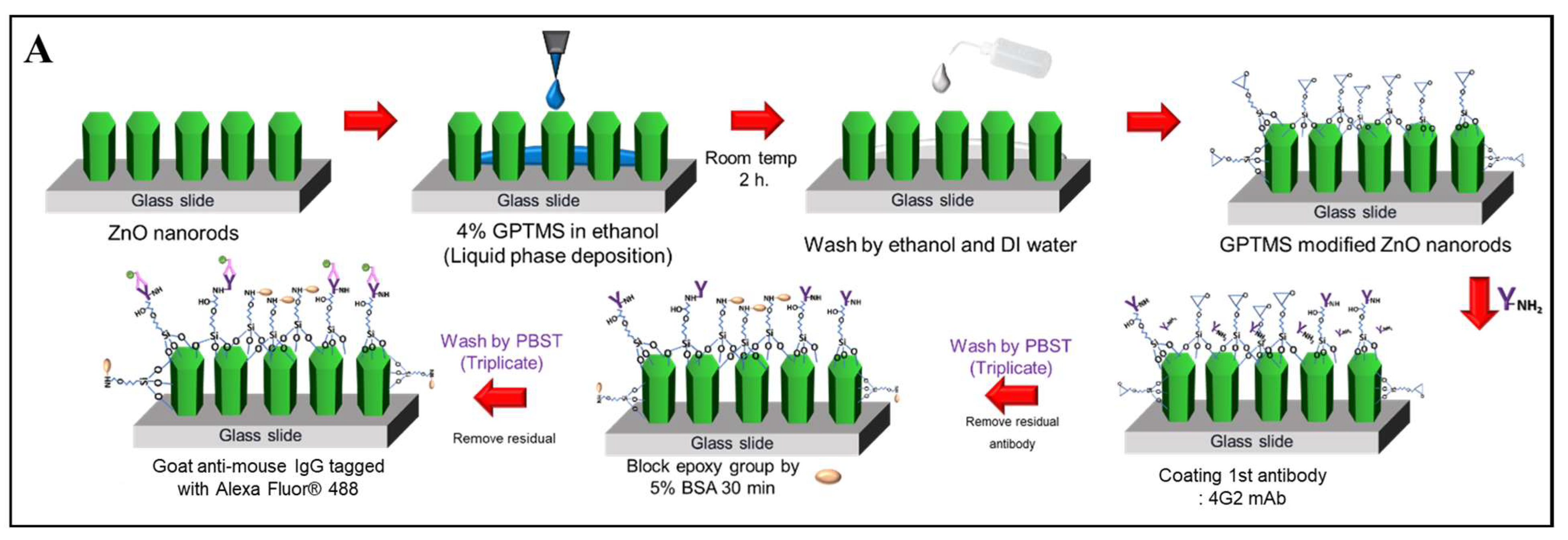
2.4. Conjugation of Monoclonal Antibodies (mAbs) onto ZnO Nanorods
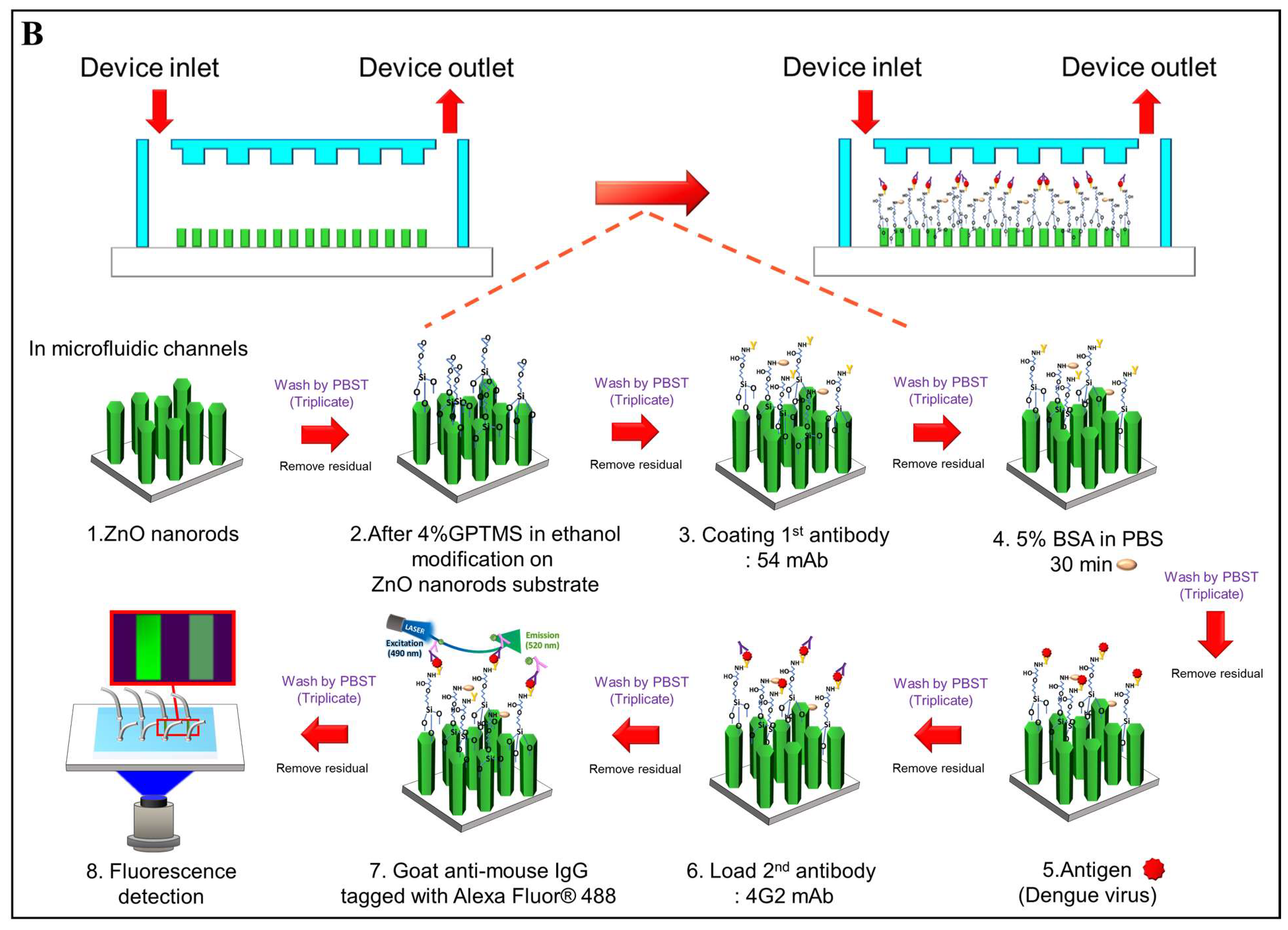
2.5. Microfluidic Chip Design and Fabrication
2.6. Sensitivity of the DENV-3 Quantitative Assay Using Immunofluorescence on ZnO-Nanorod-Integrated Microfluidic Platform
2.7. XRD Analysis
2.8. SEM Imaging
2.9. FT-IR Analysis
3. Results and Discussion
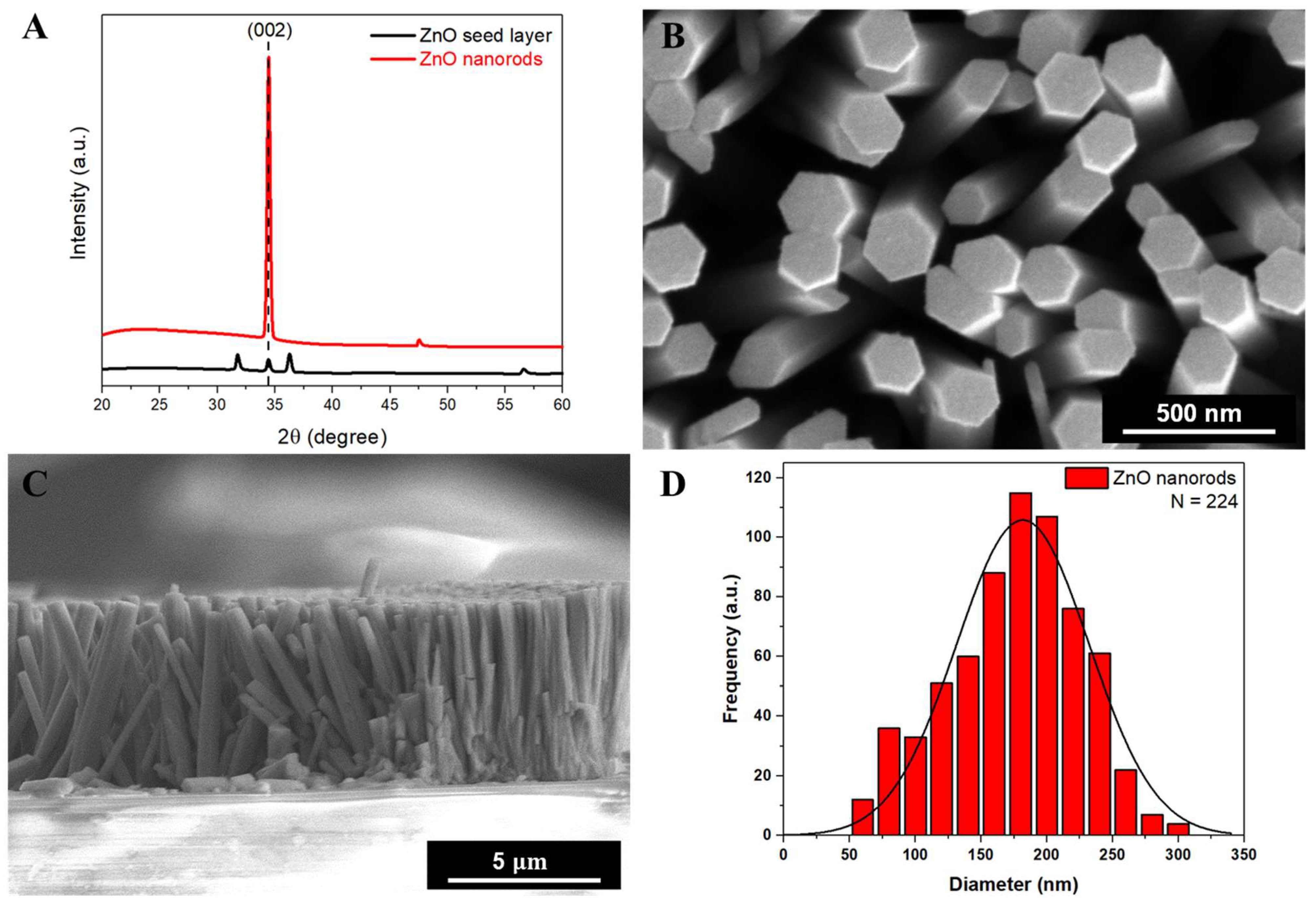
3.1. The Seed-Assisted Hydrothermal Synthesis Method for ZnO Nanorods and Their Characterization
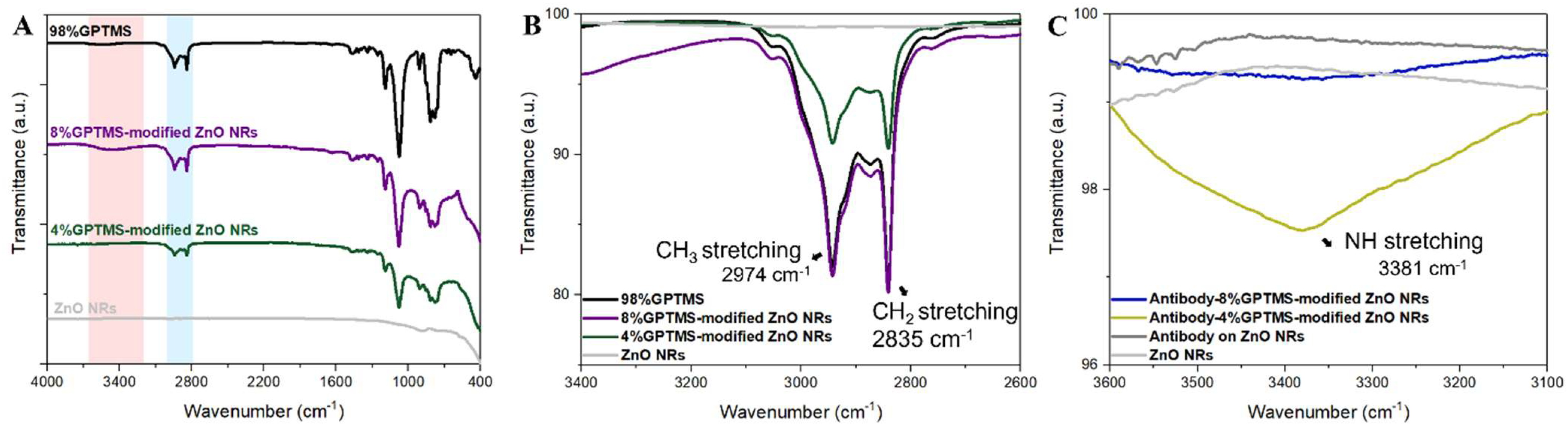
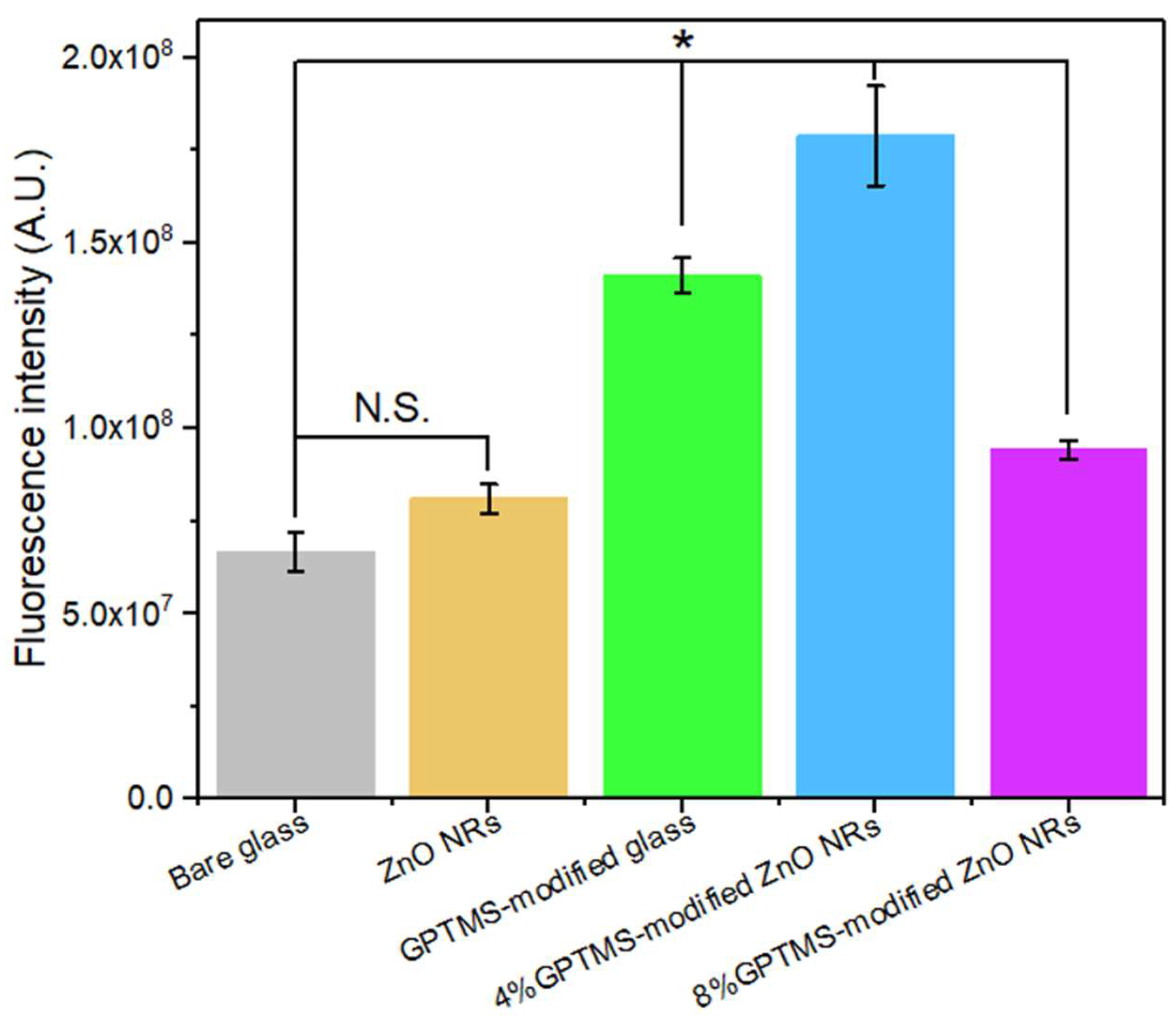
3.2. Comparative Analysis of Functionalization Efficiency on ZnO Nanorod Substrate and Bare Glass Substrate
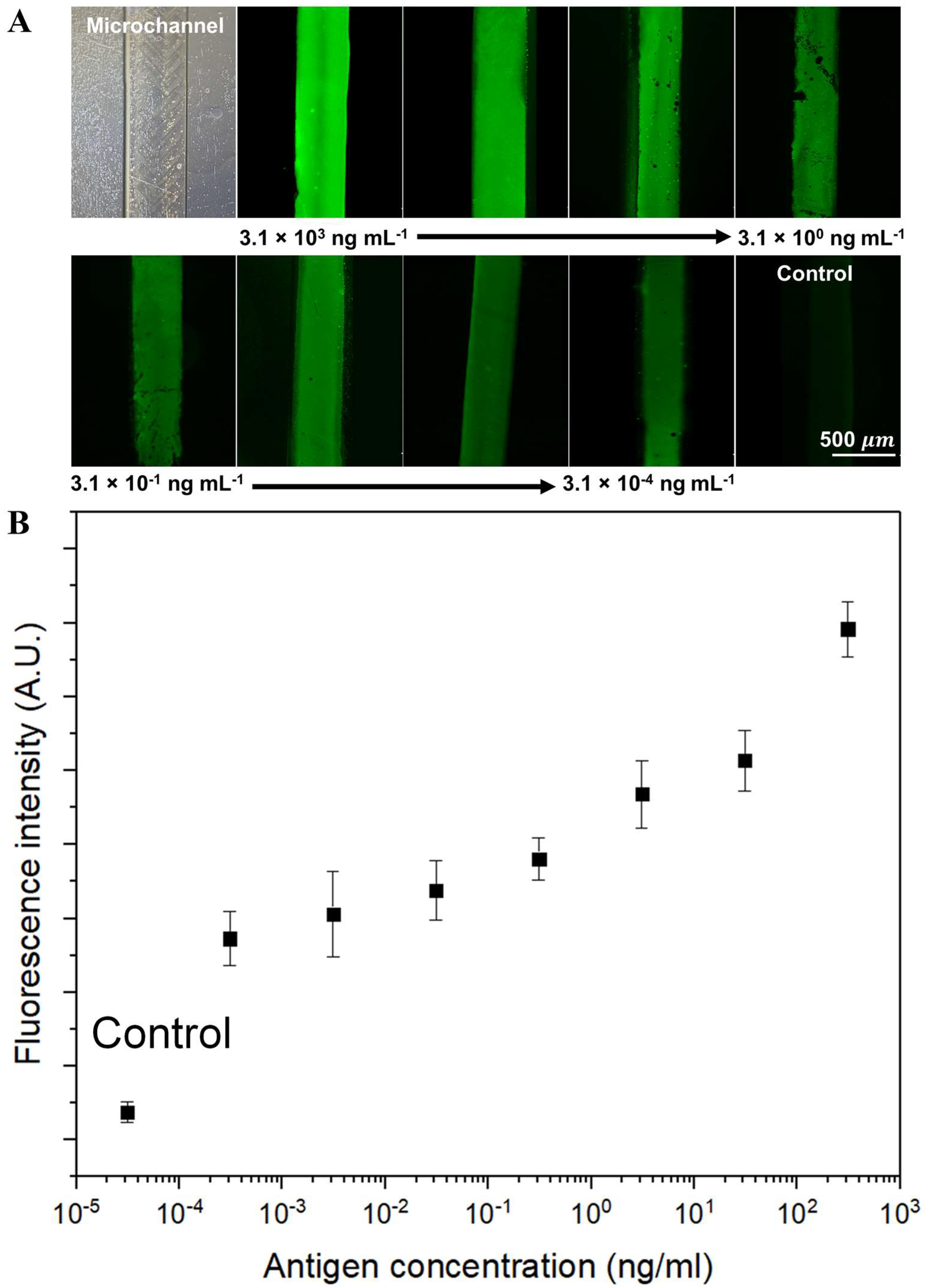
3.3. Optimization and Validation of a ZnO-NR-Surface-Integrated Microfluidic Platform for the Immunofluorescence Detection of DENV-3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faye, O.; Freire, C.C.; Iamarino, A.; Faye, O.; de Oliveira, J.V.; Diallo, M.; Zanotto, P.M.; Sall, A.A. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl. Trop. Dis. 2014, 8, e2636. [Google Scholar] [CrossRef]
- Hui, D.S.; Wong, P.C.; Wang, C. SARS: Clinical features and diagnosis. Respirology 2003, 8, S20–S24. [Google Scholar] [CrossRef]
- De Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef]
- Tarim, E.A.; Anil Inevi, M.; Ozkan, I.; Kecili, S.; Bilgi, E.; Baslar, M.S.; Ozcivici, E.; Oksel Karakus, C.; Tekin, H.C. Microfluidic-based technologies for diagnosis, prevention, and treatment of COVID-19: Recent advances and future directions. Biomed. Microdevices 2023, 25, 10. [Google Scholar] [CrossRef]
- Zhai, P.; Ding, Y.; Wu, X.; Long, J.; Zhong, Y.; Li, Y. The epidemiology, diagnosis and treatment of COVID-19. Int. J. Antimicrob. Agents 2020, 55, 105955. [Google Scholar] [CrossRef]
- Rai, P.; Kumar, B.K.; Deekshit, V.K.; Karunasagar, I.; Karunasagar, I. Detection technologies and recent developments in the diagnosis of COVID-19 infection. Appl. Microbiol. Biotechnol. 2021, 105, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Louten, J. Chapter 7—Detection and Diagnosis of Viral Infections. In Essential Human Virology; Louten, J., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 111–132. [Google Scholar]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue infection. Nat. Rev. Dis. Primers 2016, 2, 16055. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martinez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Parkash, O.; Shueb, R.H. Diagnosis of Dengue Infection Using Conventional and Biosensor Based Techniques. Viruses 2015, 7, 5410–5427. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Biosensors and Microfluidic Biosensors: From Fabrication to Application. Biosensors 2022, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.T.; Peh, A.E.; Chee, C.Y.; Fink, K.; Chow, V.T.; Ng, M.M.; Toh, C.S. Electrochemical impedance spectroscopy characterization of nanoporous alumina dengue virus biosensor. Bioelectrochemistry 2012, 88, 15–21. [Google Scholar] [CrossRef]
- Loureiro, F.C.C.L.; Neff, H.; Melcher, E.U.K.; Roque, R.A.; de Figueiredo, R.M.P.; Thirstrup, C.; Borre, M.B.; Lima, A.M.N. Simplified immunoassay for rapid Dengue serotype diagnosis, revealing insensitivity to non-specific binding interference. Sens. Bio-Sens. Res. 2017, 13, 96–103. [Google Scholar] [CrossRef]
- Siew, Q.Y.; Tan, S.H.; Pang, E.L.; Loh, H.S.; Tan, M.T.T. A graphene-based dengue immunosensor using plant-derived envelope glycoprotein domain III (EDIII) as the novel probe antigen. Analyst 2021, 146, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Park, M.; Alfson, K.J.; Tamhankar, M.; Carrion, R.; Patterson, J.L.; Griffiths, A.; He, Q.; Yildiz, A.; Mathies, R.; et al. Rapid and Fully Microfluidic Ebola Virus Detection with CRISPR-Cas13a. ACS Sens. 2019, 4, 1048–1054. [Google Scholar] [CrossRef]
- Laleh, S.; Ibarlucea, B.; Stadtmuller, M.; Cuniberti, G.; Medina-Sanchez, M. Portable microfluidic impedance biosensor for SARS-CoV-2 detection. Biosens. Bioelectron. 2023, 236, 115362. [Google Scholar] [CrossRef] [PubMed]
- Della Ventura, B.; Gelzo, M.; Battista, E.; Alabastri, A.; Schirato, A.; Castaldo, G.; Corso, G.; Gentile, F.; Velotta, R. Biosensor for Point-of-Care Analysis of Immunoglobulins in Urine by Metal Enhanced Fluorescence from Gold Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 3753–3762. [Google Scholar] [CrossRef]
- Deliorman, M.; Janahi, F.K.; Sukumar, P.; Glia, A.; Alnemari, R.; Fadl, S.; Chen, W.; Qasaimeh, M.A. AFM-compatible microfluidic platform for affinity-based capture and nanomechanical characterization of circulating tumor cells. Microsyst. Nanoeng. 2020, 6, 20. [Google Scholar] [CrossRef]
- Iswardy, E.; Tsai, T.C.; Cheng, I.F.; Ho, T.C.; Perng, G.C.; Chang, H.C. A bead-based immunofluorescence-assay on a microfluidic dielectrophoresis platform for rapid dengue virus detection. Biosens. Bioelectron. 2017, 95, 174–180. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Hong, S.; Lee, D.; Cui, T.; Goyal, S.M. Carbon nanotube based sensors for the detection of viruses. Sens. Actuators B Chem. 2011, 155, 67–74. [Google Scholar] [CrossRef]
- Gajos, K.; Szafraniec, K.; Petrou, P.; Budkowski, A. Surface density dependent orientation and immunological recognition of antibody on silicon: TOF-SIMS and surface analysis of two covalent immobilization methods. Appl. Surf. Sci. 2020, 518, 146269. [Google Scholar] [CrossRef]
- Darwish, N.T.; Sekaran, S.D.; Alias, Y.; Khor, S.M. Immunofluorescence-based biosensor for the determination of dengue virus NS1 in clinical samples. J. Pharm. Biomed. Anal. 2018, 149, 591–602. [Google Scholar] [CrossRef]
- Tiwari, S.; Vinchurkar, M.; Rao, V.R.; Garnier, G. Zinc oxide nanorods functionalized paper for protein preconcentration in biodiagnostics. Sci. Rep. 2017, 7, 43905. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-S.; Moon, D.-S.; Lee, J.-K. Quantitative Analysis and Efficient Surface Modification of Silica Nanoparticles. J. Nanomater. 2012, 2012, 48. [Google Scholar] [CrossRef]
- Yu, X.; Xia, Y.; Tang, Y.; Zhang, W.L.; Yeh, Y.T.; Lu, H.; Zheng, S.Y. A Nanostructured Microfluidic Immunoassay Platform for Highly Sensitive Infectious Pathogen Detection. Small 2017, 13, 1700425. [Google Scholar] [CrossRef]
- Viter, R.; Khranovskyy, V.; Starodub, N.; Ogorodniichuk, Y.; Gevelyuk, S.; Gertnere, Z.; Poletaev, N.; Yakimova, R.; Erts, D.; Smyntyna, V.; et al. Application of Room Temperature Photoluminescence From ZnO Nanorods for Salmonella Detection. IEEE Sens. J. 2014, 14, 2028–2034. [Google Scholar] [CrossRef]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications. Biosens. Bioelectron. 2019, 141, 111417. [Google Scholar] [CrossRef] [PubMed]
- Kasamechonchung, P.; Horprathum, M.; Boonpavanitchakul, K.; Supaka, N.; Prompinit, P.; Kangwansupamonkon, W.; Somboonkaew, A.; Wetcharungsri, J.; Pratontep, S.; Porntheeraphat, S.; et al. Morphology-controlled seed-assisted hydrothermal ZnO nanowires via critical concentration for nucleation and their photoluminescence properties. Phys. Status Solidi 2015, 212, 394–400. [Google Scholar] [CrossRef]
- Phetban, P.; Khemasiri, N.; Kalasung, S.; Jessadaluk, S.; Horprathum, M.; Eiamchai, P.; Pornthreeraphat, S.; Phromyothin, D. Fabrication of Zinc Oxide Nanorods for Photoelectrochemical Water Splitting Application. Key Eng. Mater. 2016, 675–676, 117–120. [Google Scholar] [CrossRef]
- Guo, L.; Shi, Y.; Liu, X.; Han, Z.; Zhao, Z.; Chen, Y.; Xie, W.; Li, X. Enhanced fluorescence detection of proteins using ZnO nanowires integrated inside microfluidic chips. Biosens. Bioelectron. 2018, 99, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Zhang, M.; Zhang, J.X.J. Microfluidics for ZnO micro-/nanomaterials development: Rational design, controllable synthesis, and on-chip bioapplications. Biomater. Sci. 2020, 8, 1783–1801. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, D.; Hou, C.; Liu, L.; Chen, J.; Huang, H.; Zhang, Q.; Duan, Y.; Li, Y.; Wang, H. Enhanced immunofluorescence detection of a protein marker using a PAA modified ZnO nanorod array-based microfluidic device. Nanoscale 2018, 10, 17663–17670. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, Y.; Tang, Y.; Cheng, G.; Yu, X.; He, H.; Cao, G.; Lu, H.; Liu, Z.; Zheng, S.Y. Smartphone-Based Point-of-Care Microfluidic Platform Fabricated with a ZnO Nanorod Template for Colorimetric Virus Detection. ACS Sens. 2019, 4, 3298–3307. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.F.; Binduga-Gajewska, I.; Kauffman, E.B.; Kramer, L.D. Quantitation of flaviviruses by fluorescent focus assay. J. Virol. Methods 2006, 134, 183–189. [Google Scholar] [CrossRef]
- Galula, J.U.; Salem, G.M.; Destura, R.V.; Remenyi, R.; Chao, D.Y. Comparable Accuracies of Nonstructural Protein 1- and Envelope Protein-Based Enzyme-Linked Immunosorbent Assays in Detecting Anti-Dengue Immunoglobulin G Antibodies. Diagnostics 2021, 11, 741. [Google Scholar] [CrossRef]
- Chao, D.-Y.; Yi Jung, L.; Shen, W.-F.; Tu, W.-C.; Galula, J.; Wu, H.-C. Comparison of E and NS1 antigens capture ELISA to detect dengue viral antigens from mosquitoes. J. Vector Borne Dis. 2015, 52, 134–141. [Google Scholar]
- Lardeux, F.; Torrico, G.; Aliaga, C. Calculation of the ELISA’s cut-off based on the change-point analysis method for detection of Trypanosoma cruzi infection in Bolivian dogs in the absence of controls. Memórias Do Inst. Oswaldo Cruz 2016, 111, 501–504. [Google Scholar] [CrossRef]
- He, Y.; Yanagida, T.; Nagashima, K.; Zhuge, F.; Meng, G.; Xu, B.; Klamchuen, A.; Rahong, S.; Kanai, M.; Li, X.; et al. Crystal-Plane Dependence of Critical Concentration for Nucleation on Hydrothermal ZnO Nanowires. J. Phys. Chem. C 2013, 117, 1197–1203. [Google Scholar] [CrossRef]
- Lawson, R.A.; Robinson, A.P.G. Overview of materials and processes for lithography. In Materials and Processes for Next Generation Lithography; Frontiers of Nanoscience; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–90. [Google Scholar]
- Nadine, S.; Chung, A.; Diltemiz, S.E.; Yasuda, B.; Lee, C.; Hosseini, V.; Karamikamkar, S.; de Barros, N.R.; Mandal, K.; Advani, S.; et al. Advances in microfabrication technologies in tissue engineering and regenerative medicine. Artif. Organs 2022, 46, E211–E243. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef]
- Satyanarayana, S.; Karnik, R.N.; Majumdar, A. Stamp-and-stick room-temperature bonding technique for microdevices. J. Microelectromechanical Syst. 2005, 14, 392–399. [Google Scholar] [CrossRef]
- Mousavi, M.; Fini, E. Silanization Mechanism of Silica Nanoparticles in Bitumen Using 3-Aminopropyl Triethoxysilane (APTES) and 3-Glycidyloxypropyl Trimethoxysilane (GPTMS). ACS Sustain. Chem. Eng. 2020, 8, 3231–3240. [Google Scholar] [CrossRef]
- Ma, S.; Liu, W.; Wei, Z.; Li, H. Mechanical and Thermal Properties and Morphology of Epoxy Resins Modified by a Silicon Compound. J. Macromol. Sci. Part A 2010, 47, 1084–1090. [Google Scholar] [CrossRef]
- Monteiro, D.A.; Gozzi, G.; Chinaglia, D.L.; Oliveira, O.N.; de Vicente, F.S. Proton conduction mechanisms in GPTMS/TEOS-derived organic/silica hybrid films prepared by sol-gel process. Synth. Met. 2020, 267, 116448. [Google Scholar] [CrossRef]
- Luzinov, I.; Julthongpiput, D.; Liebmann-Vinson, A.; Cregger, T.; Foster, M.D.; Tsukruk, V.V. Epoxy-Terminated Self-Assembled Monolayers: Molecular Glues for Polymer Layers. Langmuir 2000, 16, 504–516. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, R.; Guo, L.-H. One-step and high-density protein immobilization on epoxysilane-modified silica nanoparticles. Chin. Sci. Bull. 2009, 54, 2620–2626. [Google Scholar] [CrossRef]
- Lien, K.Y.; Lin, J.L.; Liu, C.Y.; Lei, H.Y.; Lee, G.B. Purification and enrichment of virus samples utilizing magnetic beads on a microfluidic system. Lab Chip 2007, 7, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Rashid, J.I.A.; Yusof, N.A.; Abdullah, J.; Hashim, U.; Hajian, R. A Novel Disposable Biosensor Based on SiNWs/AuNPs Modified-Screen Printed Electrode for Dengue Virus DNA Oligomer Detection. IEEE Sens. J. 2015, 15, 4420–4427. [Google Scholar] [CrossRef]
- Nawaz, M.H.; Hayat, A.; Catanante, G.; Latif, U.; Marty, J.L. Development of a portable and disposable NS1 based electrochemical immunosensor for early diagnosis of dengue virus. Anal. Chim. Acta 2018, 1026, 1–7. [Google Scholar] [CrossRef]
- Prabowo, M.H.; Chatchen, S.; Rijiravanich, P.; Limkittikul, K.; Surareungchai, W. Dengue NS1 detection in pediatric serum using microfluidic paper-based analytical devices. Anal. Bioanal. Chem. 2020, 412, 2915–2925. [Google Scholar] [CrossRef] [PubMed]
| Detection Method | Analyte | Matrix | Detection Method | Detection Range | Detection Limit | Detection Time | Ref. |
|---|---|---|---|---|---|---|---|
| Electrochemical impedance spectroscopy for dengue virus biosensor | Dengue serotype type 2 | - | IgG antibody | 1–900 PFU mL−1 | - | - | [13] |
| Magnetic beads on a microfluidic system | Dengue serotype type 2 | serum | Antibody | 101–106 PFU mL−1 | 102 PFU mL−1 | - | [50] |
| A microfluidic dielectrophoresis platform | Dengue serotype type 2 | - | Anti-DENV envelope protein antibody | 0–106 PFU mL−1 | 104 PFU mL−1 | 5 min (pre-fabricated immunoassay is available) | [20] |
| SPR biosensor | Dengue serotype 2 and 3 | serum | Monoclonal anti-flavivirus antibodies | - | 2 × 104 particles. mL−1 | 30 min (pre-fabricated immunoassay is available) | [14] |
| Biosensor based on SiNW/AuNP-modified screen-printed electrode | Dengue serotype type 1,2,3 and 4 | - | DNA | 1.0 × 10−11–1.0 × 10−7 M | 1.63 × 10−12 M | 10 min 35 s | [51] |
| SPCE-portable NS1-based electrochemical immunosensor | Dengue virus NS1 | serum | Anti-NS1 monoclonal antibody | 1–200 ng mL−1 | 0.30 ng mL−1 | Assay time is not specified | [52] |
| SERS-based lateral flow biosensor | Dengue nonstructural protein 1 | - | Polyclonal primary anti NS1antibody | 15–500 ng mL−1 | 15 ng mL−1 | Assay time is not specified | [23] |
| Graphene-SPCE | Dengue virus antibodies | serum | Envelope glycoprotein domain III (EDIII) antigen | 125–2000 ng mL−1 | 22.50 ng mL−1 | Assay time is not specified | [15] |
| Microfluidic paper-based analytical devices (μPADs) | Dengue NS1 | serum | Anti-NS1 monoclonal antibody | 0–1 μg mL−1 | 74.8 ng mL−1 | 20–30 min | [53] |
| Nanomaterial-enhanced microfluidic platforms | Dengue serotype type 3 | Culture supernatants | Anti-DENV envelope protein monoclonal antibody | 3.1 × 10−4–3.1 × 103 ng mL−1 | 3.1 × 10−4 ng mL−1 | 15 min | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pormrungruang, P.; Phanthanawiboon, S.; Jessadaluk, S.; Larpthavee, P.; Thaosing, J.; Rangkasikorn, A.; Kayunkid, N.; Waiwijit, U.; Horprathum, M.; Klamchuen, A.; et al. Metal Oxide Nanostructures Enhanced Microfluidic Platform for Efficient and Sensitive Immunofluorescence Detection of Dengue Virus. Nanomaterials 2023, 13, 2846. https://doi.org/10.3390/nano13212846
Pormrungruang P, Phanthanawiboon S, Jessadaluk S, Larpthavee P, Thaosing J, Rangkasikorn A, Kayunkid N, Waiwijit U, Horprathum M, Klamchuen A, et al. Metal Oxide Nanostructures Enhanced Microfluidic Platform for Efficient and Sensitive Immunofluorescence Detection of Dengue Virus. Nanomaterials. 2023; 13(21):2846. https://doi.org/10.3390/nano13212846
Chicago/Turabian StylePormrungruang, Pareesa, Supranee Phanthanawiboon, Sukittaya Jessadaluk, Preeda Larpthavee, Jiraphon Thaosing, Adirek Rangkasikorn, Navaphun Kayunkid, Uraiwan Waiwijit, Mati Horprathum, Annop Klamchuen, and et al. 2023. "Metal Oxide Nanostructures Enhanced Microfluidic Platform for Efficient and Sensitive Immunofluorescence Detection of Dengue Virus" Nanomaterials 13, no. 21: 2846. https://doi.org/10.3390/nano13212846
APA StylePormrungruang, P., Phanthanawiboon, S., Jessadaluk, S., Larpthavee, P., Thaosing, J., Rangkasikorn, A., Kayunkid, N., Waiwijit, U., Horprathum, M., Klamchuen, A., Pruksamas, T., Puttikhunt, C., Yasui, T., Djamal, M., Rahong, S., & Nukeaw, J. (2023). Metal Oxide Nanostructures Enhanced Microfluidic Platform for Efficient and Sensitive Immunofluorescence Detection of Dengue Virus. Nanomaterials, 13(21), 2846. https://doi.org/10.3390/nano13212846








