A “Green” Stirring Plasma Functionalization Strategy for Controllable Oxygen-Containing Functional Groups on Octa-Methyl POSS Microstructure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
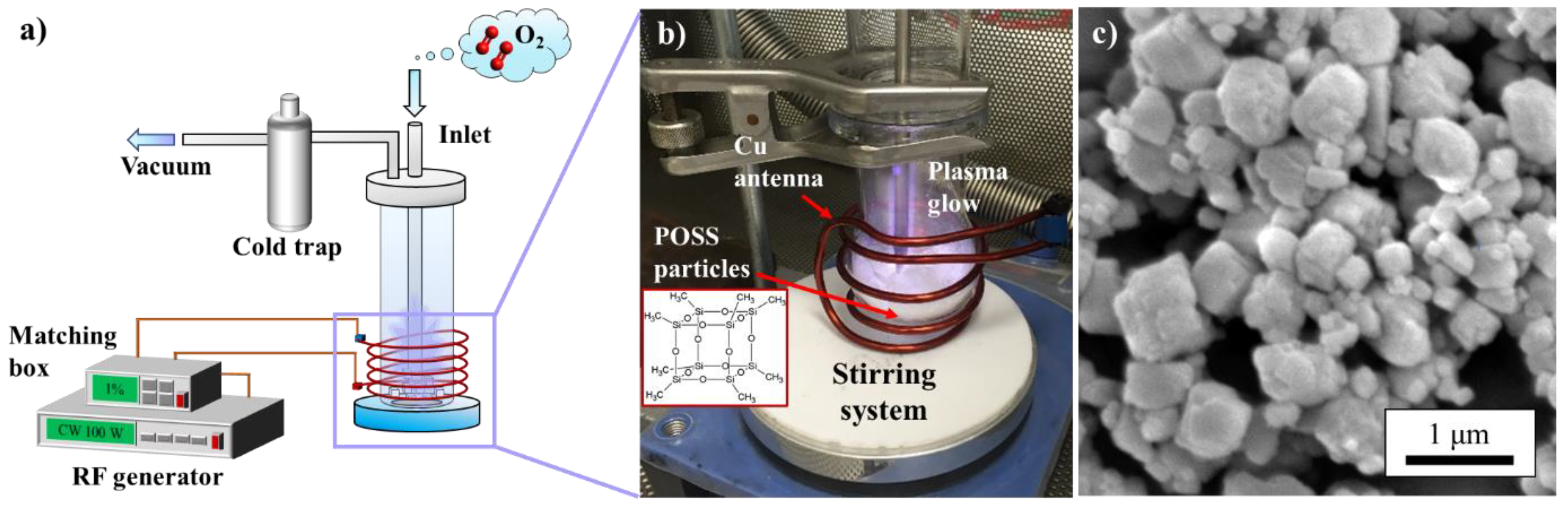
2.3. Plasma Functionalization by Stirring Plasma Treatment
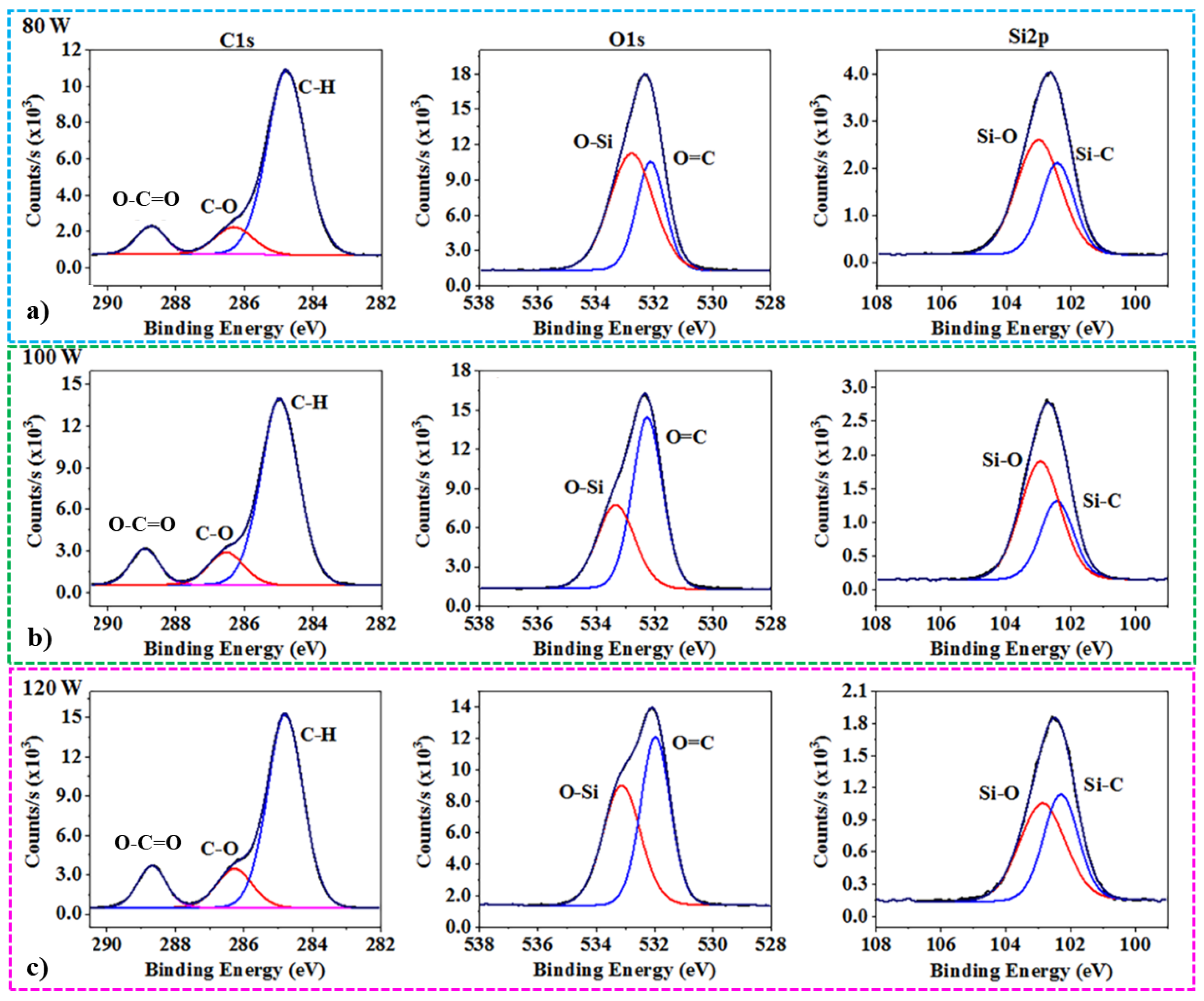
2.4. Characterizations
3. Results
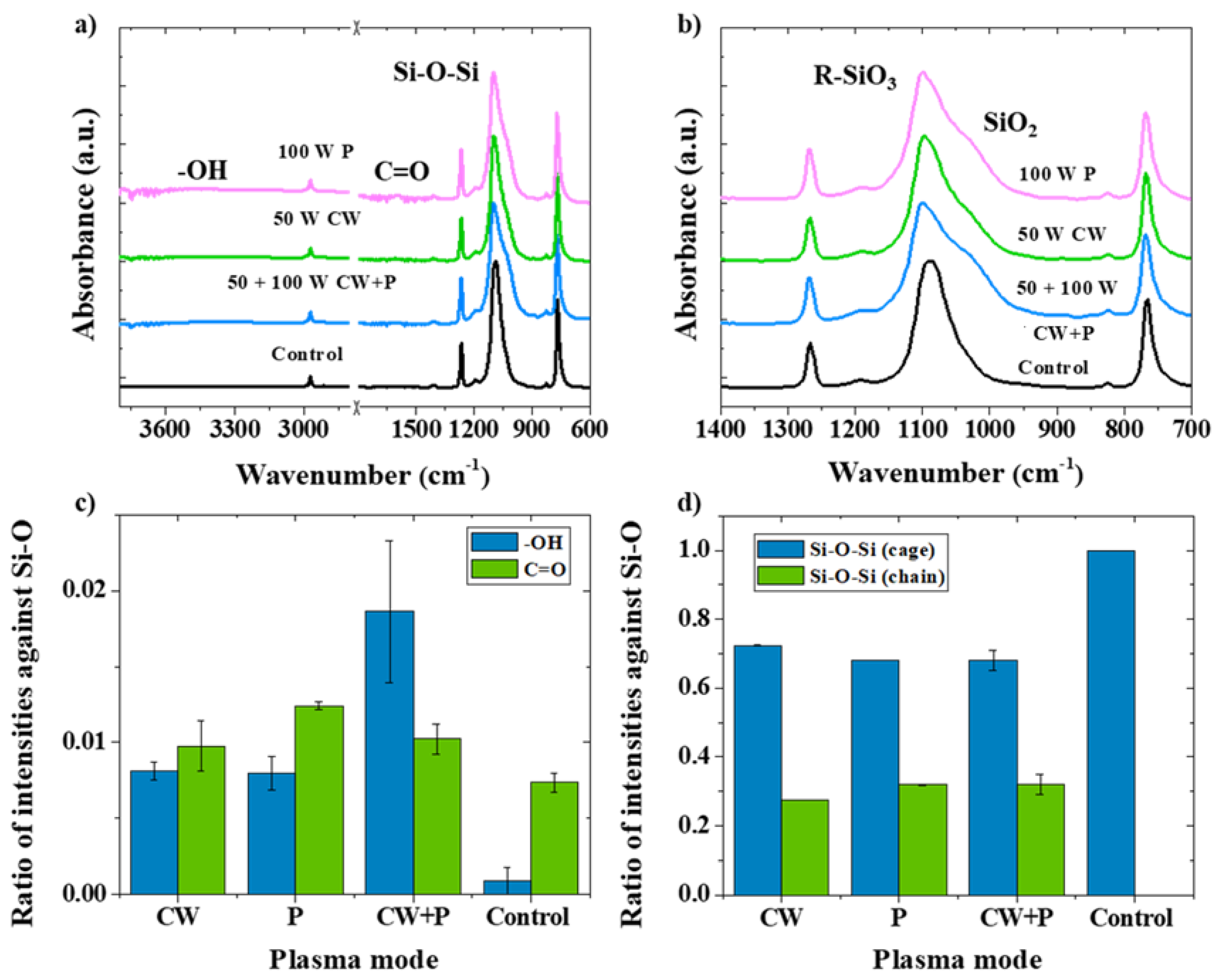
3.1. Plasma Strategies for “Green” Functionalization on Octa-Methyl POSS
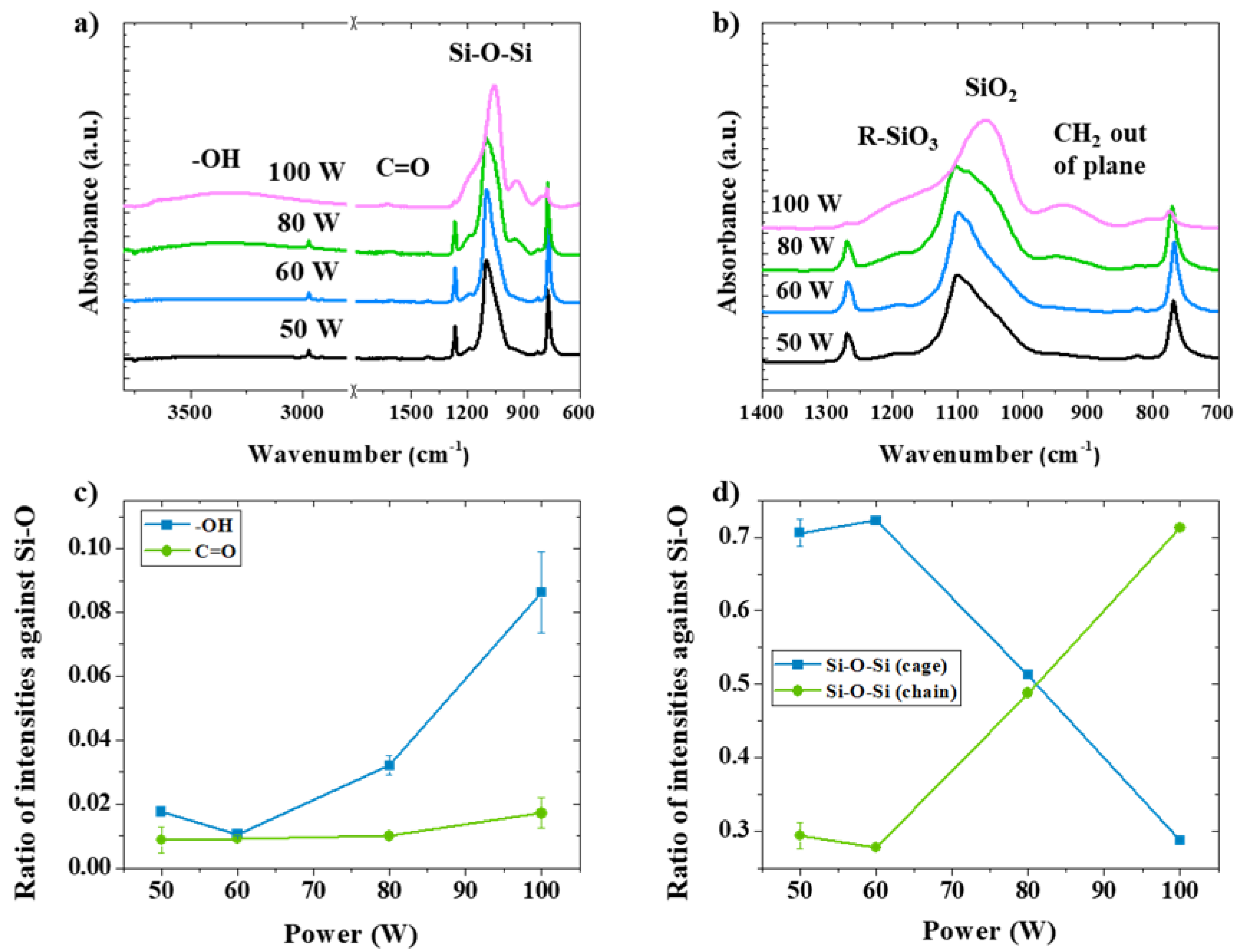
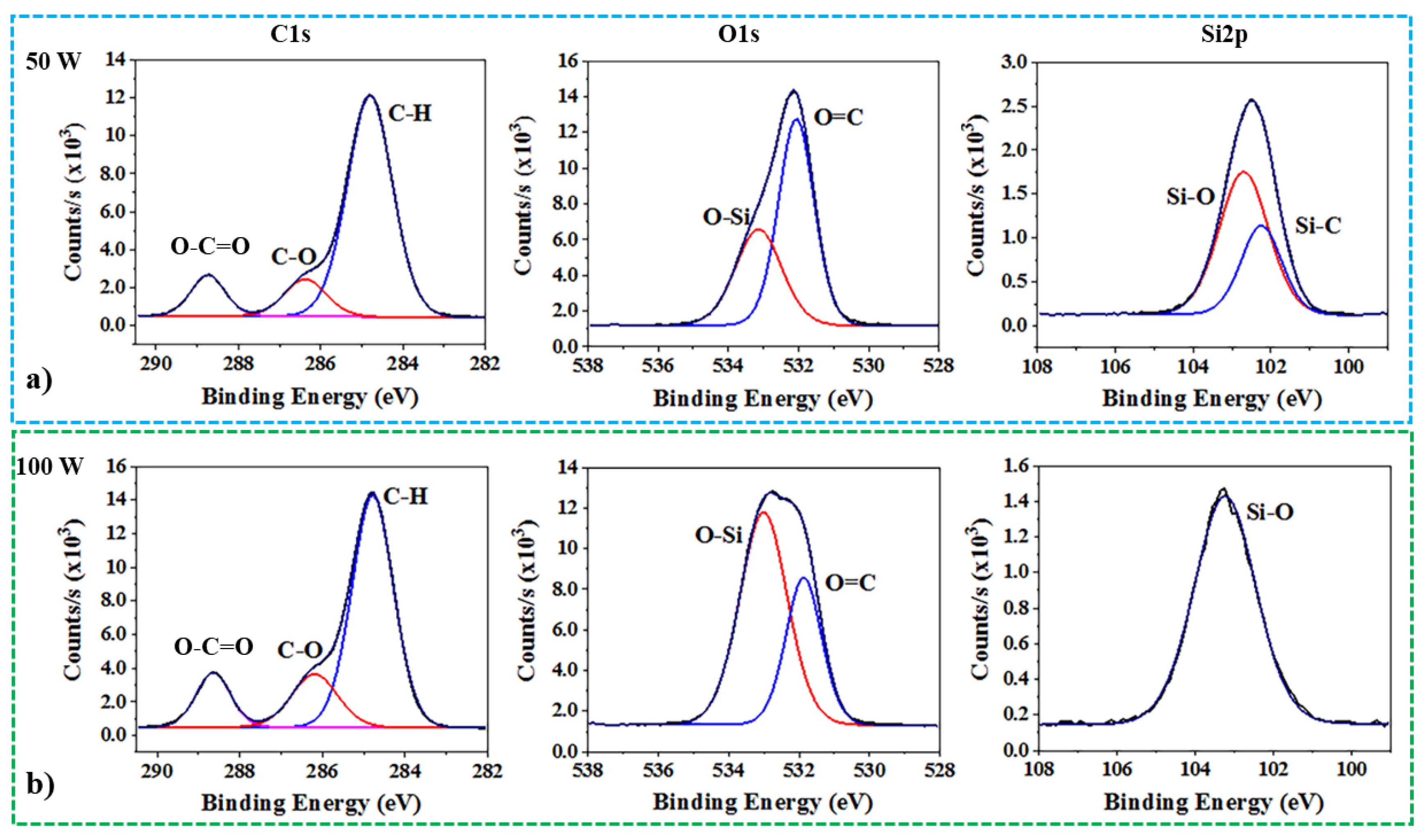
3.2. The Introduction of Hydroxyl Groups (OH) in CW Plasma
3.3. The Introduction of Carboxyl Groups (COOH) in Combined Mode Plasma
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, N.; Xu, Z.; Zheng, S.; Dai, H.; Wang, L.; Tian, Y.; Dong, Z.; Jiang, L. Superamphiphilic TiO2 composite surface for protein antifouling. Adv. Mater. 2021, 33, 2003559. [Google Scholar] [CrossRef]
- Zhang, B.; Wong, P.W.; Guo, J.; Zhou, Y.; Wang, Y.; Sun, J.; Jiang, M.; Wang, Z.; An, A.K. Transforming Ti3C2Tx MXene’s intrinsic hydrophilicity into superhydrophobicity for efficient photothermal membrane desalination. Nat. Commun. 2022, 13, 3315. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, S.; Liang, T.; Zhang, Q.; Fan, Z.; Yin, H.; Huang, K.-W.; Zhang, X.; Lai, Z.; Sheng, P. High-flux water desalination with interfacial salt sieving effect in nanoporous carbon composite membranes. Nat. Nanotechnol. 2018, 13, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.; Li, X.; Gao, W.; Zhang, L.; Liu, J.; Zheng, Y.; Chen, H.; Shi, J. Injectable 2D MoS2-integrated drug delivering implant for highly efficient NIR-triggered synergistic tumor hyperthermia. Adv. Mater. 2015, 27, 7117–7122. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhu, C.; Zeng, Z.; Pan, F.; Wan, F.; Lei, L.; Nyström, G.; Fu, Z. Bioinspired cellulose-integrated MXene-based hydrogels for multifunctional sensing and electromagnetic interference shielding. Interdiscip. Mater. 2022, 1, 495–506. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Dong, W.-X.; Deng, Y.; Chen, L.-F.; Zhao, C.-F.; Zhang, C.-L.; Zhou, J.; Qu, Y.-F.; Li, Y.-S.; Li, D.-J.; et al. Biomass-based biomimetic-oriented Janus nanoarchitecture for efficient heavy-metal enrichment and interfacial solar water sanitation. Interdiscip. Mater. 2022, 1, 537–547. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, L.; Lao, J.; Luo, K.; Liu, X.; Sui, K.; Gao, J.; Jiang, L. Reliable and low temperature actuation of water and oil slugs in Janus photothermal slippery tube. ACS Appl. Mater. Interfaces 2022, 14, 17968–17974. [Google Scholar] [CrossRef]
- Zhang, L.; Kan, X.; Huang, T.; Lao, J.; Luo, K.; Gao, J.; Liu, X.; Sui, K.; Jiang, L. Electric field modulated water permeation through laminar Ti3C2Tx MXene membrane. Water Res. 2022, 219, 118598. [Google Scholar] [CrossRef]
- Moghaddam, S.; Pengwang, E.; Jiang, Y.-B.; Garcia, A.R.; Burnett, D.J.; Brinker, C.J.; Masel, R.I.; Shannon, M.A. An inorganic–organic proton exchange membrane for fuel cells with a controlled nanoscale pore structure. Nat. Nanotechnol. 2010, 5, 230–236. [Google Scholar] [CrossRef]
- Kang, J.; Ko, Y.; Kim, J.P.; Kim, J.Y.; Kim, J.; Kwon, O.; Kim, K.C.; Kim, D.W. Microwave-assisted design of nanoporous graphene membrane for ultrafast and switchable organic solvent nanofiltration. Nat. Commun. 2023, 14, 901. [Google Scholar] [CrossRef]
- Li, P.; Gu, Z.; Bai, C.; Xu, Y.; Tang, J.; Ren, J.; Xu, L.; Yu, S. Effects of MOFs size and functional group on the pore structure and performance of thin-film nanocomposite membranes. J. Membr. Sci. 2023, 686, 121979. [Google Scholar] [CrossRef]
- Devaraju, S.; Alagar, M. Chapter 1—POSS nanoparticles: Synthesis, characterization, and properties. In Polyhedral Oligomeric Silsesquioxane (POSS) Polymer Nanocomposites; Thomas, S., Somasekharan, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–25. [Google Scholar]
- Li, G.; Wang, L.; Ni, H.; Pittman, C.U. Polyhedral oligomeric silsesquioxane (POSS) polymers and copolymers: A review. J. Inorg. Organomet. Polym. Mater. 2001, 11, 123–154. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Katha, A.R.; Pandian, S.; Kolake, S.M.; Han, S. Polyamide–POSS hybrid membranes for seawater desalination: Effect of POSS inclusion on membrane properties. J. Membr. Sci. 2014, 461, 89–95. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Fan, B.C.; Liu, Q.L.; Zhu, A.M.; Shi, F.F. A novel poly(dimethyl siloxane)/poly(oligosilsesquioxanes) composite membrane for pervaporation desulfurization. J. Membr. Sci. 2011, 366, 335–341. [Google Scholar] [CrossRef]
- Zhang, D.; Kanezashi, M.; Tsuru, T.; Yamamoto, K.; Gunji, T.; Adachi, Y.; Ohshita, J. Preparation of thermally stable 3-glycidyloxypropyl-POSS-derived polysilsesquioxane RO membranes for water desalination. J. Membr. Sci. 2023, 668, 121213. [Google Scholar] [CrossRef]
- Rodriguez Herrero, Y.; Ullah, A. Hydrophobic polyhedral oligomeric silsesquioxane support enhanced methanol production from CO2 hydrogenation. ACS Appl. Mater. Interfaces 2023, 15, 14399–14414. [Google Scholar] [CrossRef]
- Chen, X.; Porto, C.L.; Chen, Z.; Merenda, A.; Allioux, F.-M.; d’Agostino, R.; Magniez, K.; Dai, X.J.; Palumbo, F.; Dumée, L.F. Single step synthesis of Janus nano-composite membranes by atmospheric aerosol plasma polymerization for solvents separation. Sci. Total Environ. 2018, 645, 22–33. [Google Scholar] [CrossRef]
- Duan, J.; Litwiller, E.; Pinnau, I. Preparation and water desalination properties of POSS-polyamide nanocomposite reverse osmosis membranes. J. Membr. Sci. 2015, 473, 157–164. [Google Scholar] [CrossRef]
- Wang, J.; Du, W.; Zhang, Z.; Gao, W.; Li, Z. Biomass/polyhedral oligomeric silsesquioxane nanocomposites: Advances in preparation strategies and performances. J. Appl. Polym. Sci. 2021, 138, 49641. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Carniato, F.; Frache, A.; Boccaleri, E.; Camino, G. Polyhedral oligomeric silsesquioxanes (POSS) thermal degradation. Thermochim. Acta 2006, 440, 36–42. [Google Scholar] [CrossRef]
- Wang, M.; Chi, H.; Joshy, K.S.; Wang, F. Progress in the synthesis of bifunctionalized polyhedral oligomeric silsesquioxane. Polymers 2019, 11, 2098. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Z.; Dumée, L.F.; O’Dell, L.A.; du Plessis, J.; d’Agostino, R.; Dai, X.J.; Magniez, K. Grafting of N-moieties onto octa-methyl polyhedral oligomeric silsesquioxane microstructures by sequential continuous wave and pulsed plasma. Plasma Process. Polym. 2017, 14, 1600244. [Google Scholar] [CrossRef]
- Kan, C.-W.; Lam, C.-F.; Chan, C.-K.; Ng, S.-P. Using atmospheric pressure plasma treatment for treating grey cotton fabric. Carbohyd. Polym. 2014, 102, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.K.; Shancita, I.; Bhattacharia, S.K.; Pantoya, M.L. Surface modifications of plasma treated aluminum particles and direct evidence for altered reactivity. Mater. Des. 2021, 210, 110119. [Google Scholar] [CrossRef]
- Kondratowicz, I.; Nadolska, M.; Şahin, S.; Łapiński, M.; Prześniak-Welenc, M.; Sawczak, M.; Yu, E.H.; Sadowski, W.; Żelechowska, K. Tailoring properties of reduced graphene oxide by oxygen plasma treatment. Appl. Surf. Sci. 2018, 440, 651–659. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Mozetič, M.; Primc, G. Surface modification of PS polymer by oxygen-atom treatment from remote plasma: Initial kinetics of functional groups formation. Appl. Surf. Sci. 2021, 561, 150058. [Google Scholar] [CrossRef]
- Longo, R.C.; Ranjan, A.; Ventzek, P.L.G. Density functional theory study of oxygen adsorption on polymer surfaces for atomic-layer etching: Implications for semiconductor device fabrication. ACS Appl. Nano Mater. 2020, 3, 5189–5202. [Google Scholar] [CrossRef]
- Felten, A.; Bittencourt, C.; Pireaux, J.J.; Van Lier, G.; Charlier, J.C. Radio-frequency plasma functionalization of carbon nanotubes surface O2, NH3, and CF4 treatments. J. Appl. Phys. 2005, 98, 074308. [Google Scholar] [CrossRef]
- Sahoo, G.; Polaki, S.R.; Ghosh, S.; Krishna, N.G.; Kamruddin, M.; Ostrikov, K. Plasma-tuneable oxygen functionalization of vertical graphenes enhance electrochemical capacitor performance. Energy Stor. Mater. 2018, 14, 297–305. [Google Scholar] [CrossRef]
- Kim, G.W.; Ha, J.W. Single-particle study: Effects of oxygen plasma treatment on structural and spectral changes of anisotropic gold nanorods. Phys. Chem. Chem. Phys. 2020, 22, 11767–11770. [Google Scholar] [CrossRef] [PubMed]
- Duch, J.; Mazur, M.; Golda-Cepa, M.; Podobiński, J.; Piskorz, W.; Kotarba, A. Insight into the modification of electrodonor properties of multiwalled carbon nanotubes via oxygen plasma: Surface functionalization versus amorphization. Carbon 2018, 137, 425–432. [Google Scholar] [CrossRef]
- Narushima, K.; Tsutsui, Y.; Kasukabe, K.; Inagaki, N.; Isono, Y.; Islam, M.R. Surface modification of polymer films by pulsed oxygen plasma. Jpn. J. Appl. Phys. 2007, 46, 4246. [Google Scholar] [CrossRef]
- Li, L.; Dai, X.J.; Xu, H.S.; Zhao, J.H.; Yang, P.; Maurdev, G.; du Plessis, J.; Lamb, P.R.; Fox, B.L.; Michalski, W.P. Combined continuous wave and pulsed plasma modes: For more stable interfaces with higher functionality on metal and semiconductor surfaces. Plasma Process. Polym. 2009, 6, 615–619. [Google Scholar] [CrossRef]
- Dai, X.J.; Plessis, J.d.; Kyratzis, I.L.; Maurdev, G.; Huson, M.G.; Coombs, C. Controlled amine functionalization and hydrophilicity of a poly(lactic acid) fabric. Plasma Process. Polym. 2009, 6, 490–497. [Google Scholar] [CrossRef]
- Chen, Z.; Dai, X.J.; Magniez, K.; Lamb, P.R.; Fox, B.L.; Wang, X. Improving the mechanical properties of multiwalled carbon nanotube/epoxy nanocomposites using polymerization in a stirring plasma system. Compos. Part A Appl. Sci. Manuf. 2014, 56, 172–180. [Google Scholar] [CrossRef]
- Alam, A.; Wan, C.; McNally, T. Surface amination of carbon nanoparticles for modification of epoxy resins: Plasma-treatment vs. Wet-chemistry approach. Eur. Polym. J. 2017, 87, 422–448. [Google Scholar] [CrossRef]
- Shi, D.; Lian, J.; He, P.; Wang, L.M.; van Ooij, W.J.; Schulz, M.; Liu, Y.; Mast, D.B. Plasma deposition of ultrathin polymer films on carbon nanotubes. Appl. Phys. Lett. 2002, 81, 5216–5218. [Google Scholar] [CrossRef]
- Jauberteau, J.L.; Jauberteau, I. Comparison of hexamethyldisiloxane dissociation processes in plasma. J. Phys. Chem. A 2012, 116, 8840–8850. [Google Scholar] [CrossRef]
- Cicala, G.; Creatore, M.; Favia, P.; Lamendola, R.; d’Agostino, R. Modulated rf discharges as an effective tool for selecting excited species. Appl. Phys. Lett. 1999, 75, 37–39. [Google Scholar] [CrossRef]
- Favia, P.; Stendardo, M.V.; d’Agostino, R. Selective grafting of amine groups on polyethylene by means of NH3−H2 RF glow discharges. Plasma. Polym. 1996, 1, 91–112. [Google Scholar] [CrossRef]
- Francescangeli, A.; Palumbo, F.; d’Agostino, R.; Defranoux, C. Pulsed plasma deposition from vinyltrimethylsilane/oxygen mixtures. Plasma Process. Polym. 2009, 6, 132–138. [Google Scholar] [CrossRef]
- Su, C.-H.; Chiu, Y.-P.; Teng, C.-C.; Chiang, C.-L. Preparation, characterization and thermal properties of organic–inorganic composites involving epoxy and polyhedral oligomeric silsesquioxane (POSS). J. Polym. Res. 2010, 17, 673–681. [Google Scholar] [CrossRef]
- Liu, Y.R.; Huang, Y.D.; Liu, L. Thermal stability of POSS/methylsilicone nanocomposites. Compos. Sci. Technol. 2007, 67, 2864–2876. [Google Scholar] [CrossRef]
- Augustine, B.H.; Hughes, W.C.; Zimmermann, K.J.; Figueiredo, A.J.; Guo, X.; Chusuei, C.C.; Maidment, J.S. Plasma surface modification and characterization of POSS-based nanocomposite polymeric thin films. Langmuir 2007, 23, 4346–4350. [Google Scholar] [CrossRef]
- Duch, J.; Gołda-Cępa, M.; Piskorz, W.; Rysz, J.; Kotarba, A. Stability of oxygen-functionalized graphenic surfaces: Theoretical and experimental insights into electronic properties and wettability. Appl. Surf. Sci. 2021, 539, 148190. [Google Scholar] [CrossRef]


| Plasma Treatments | CW Conditions | P Conditions | ||
|---|---|---|---|---|
| RF Power (W) | Duration (min) | RF Power (W) | Duration (min) | |
| Various plasma modes | 50 | 11 | - | |
| - | 100 | 55 | ||
| 50 | 5 | 100 | 30 | |
| Various RF powers for CW plasma | 50 | 5 | - | |
| 60 | 5 | - | ||
| 80 | 5 | - | ||
| 100 | 5 | - | ||
| Various RF powers for P plasma in CW + P mode | 50 | 5 | 50 | 30 |
| 50 | 5 | 80 | 30 | |
| 50 | 5 | 100 | 30 | |
| 50 | 5 | 120 | 30 | |
| 50 | 5 | 150 | 30 | |
| Assignment | Band Range (cm−1) |
|---|---|
| O-H (hydroxyl) | 3200–3500 |
| C-H | 2700–2990 |
| C=O (carbonyl or carboxyl) | 1580–1650 |
| C-H | 1250–1280 |
| Si-O | 1075–1135 |
| Si-C | 720–860 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Magniez, K.; Zhang, P.; Kujawski, W.; Chen, Z.; Dumée, L.F. A “Green” Stirring Plasma Functionalization Strategy for Controllable Oxygen-Containing Functional Groups on Octa-Methyl POSS Microstructure. Nanomaterials 2023, 13, 2770. https://doi.org/10.3390/nano13202770
Chen X, Magniez K, Zhang P, Kujawski W, Chen Z, Dumée LF. A “Green” Stirring Plasma Functionalization Strategy for Controllable Oxygen-Containing Functional Groups on Octa-Methyl POSS Microstructure. Nanomaterials. 2023; 13(20):2770. https://doi.org/10.3390/nano13202770
Chicago/Turabian StyleChen, Xiao, Kevin Magniez, Pengchao Zhang, Wojciech Kujawski, Zhiqiang Chen, and Ludovic F. Dumée. 2023. "A “Green” Stirring Plasma Functionalization Strategy for Controllable Oxygen-Containing Functional Groups on Octa-Methyl POSS Microstructure" Nanomaterials 13, no. 20: 2770. https://doi.org/10.3390/nano13202770
APA StyleChen, X., Magniez, K., Zhang, P., Kujawski, W., Chen, Z., & Dumée, L. F. (2023). A “Green” Stirring Plasma Functionalization Strategy for Controllable Oxygen-Containing Functional Groups on Octa-Methyl POSS Microstructure. Nanomaterials, 13(20), 2770. https://doi.org/10.3390/nano13202770










