Bio-Based Pyrrole Compounds Containing Sulfur Atoms as Coupling Agents of Carbon Black with Unsaturated Elastomers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pyrrole Compounds, Adducts and Elastomer Composites
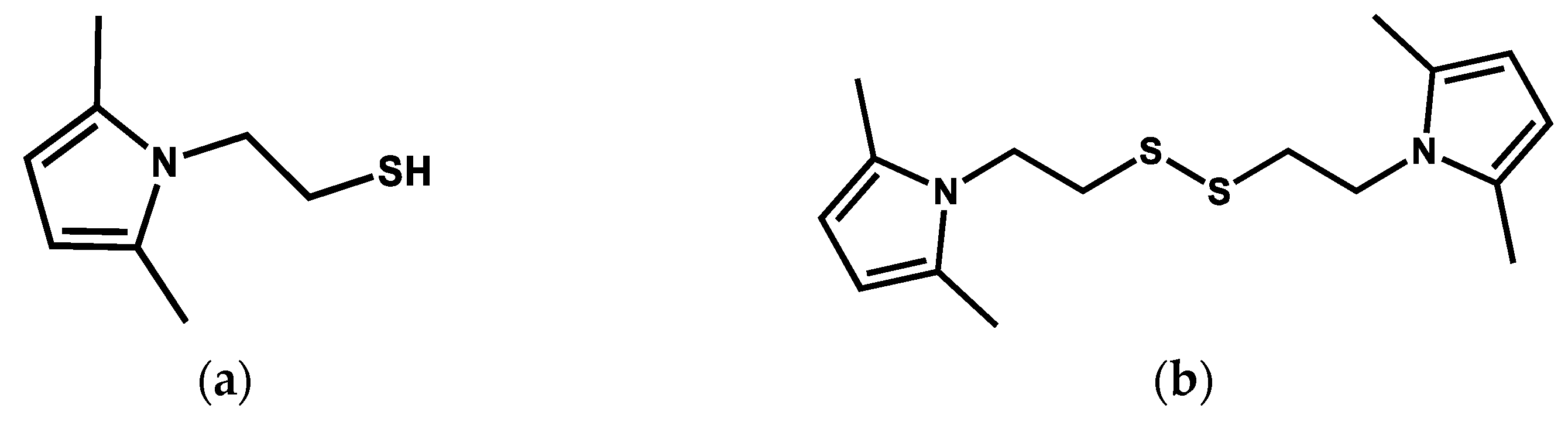
2.2.1. Synthesis of 2-(2,5-Dimethyl-1H-pyrrol-1-yl)ethane-1-thiol (Thiol Pyrrole, SHP)
2.2.2. Synthesis of 1,2-Bis(2-(2,5-dimethyl-1H-pyrrol-1-yl)ethyl)disulfide (Disulfide Pyrrole, SSP)
2.2.3. Preparation of Adducts of CB with Thiol Pyrrole (SHP) and Disulfide Pyrrole (SSP)
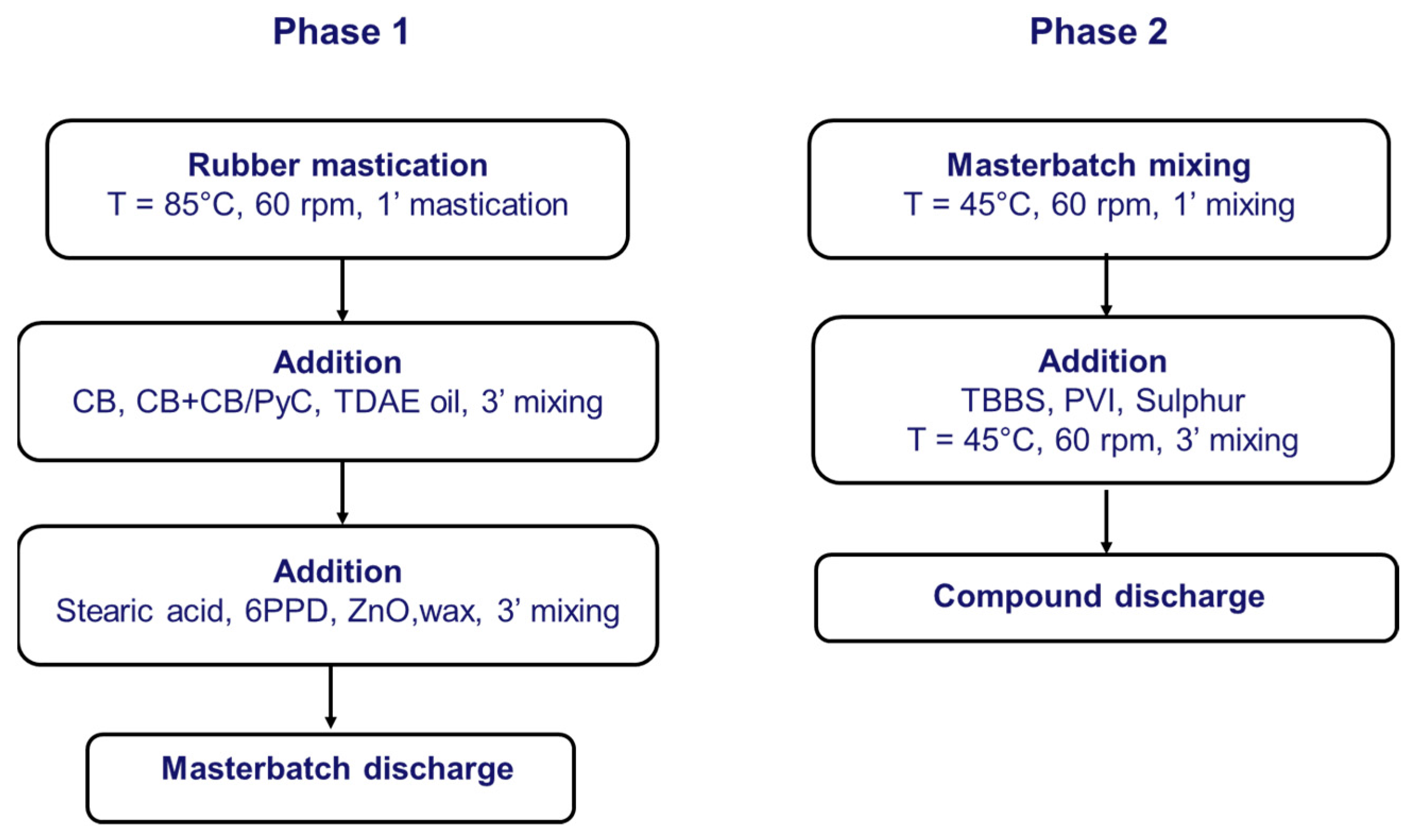
2.2.4. Preparation of Elastomer Composites
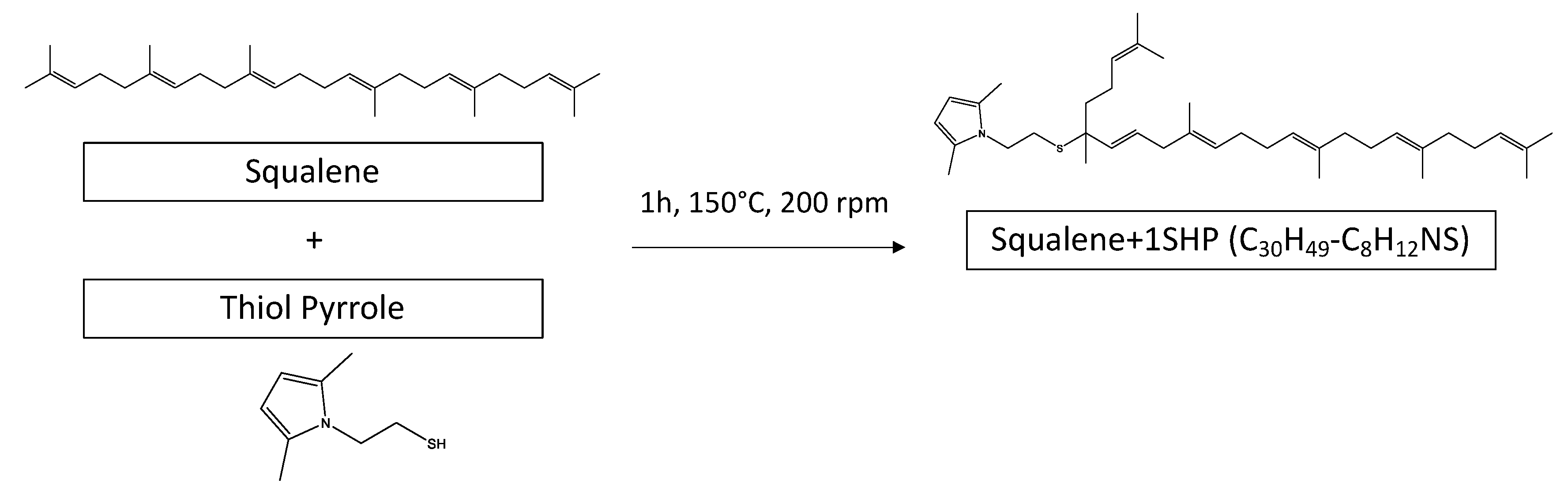
2.3. Reaction of SHP and SSP with Squalene
2.3.1. Reaction of SHP with Squalene
2.3.2. Reactivity of SHP with Squalene in the Presence of Vulcanizing Agents
2.4. Characterization of Low-Molar-Mass Chemicals: Pyrrole Compounds
2.4.1. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.4.2. Gas Chromatography–Mass Spectrometry (GC–MS)
2.4.3. HPLC–UV–MS Direct Injection Analyses
2.5. Characterization of CB/SHP and CB/SSP Adducts
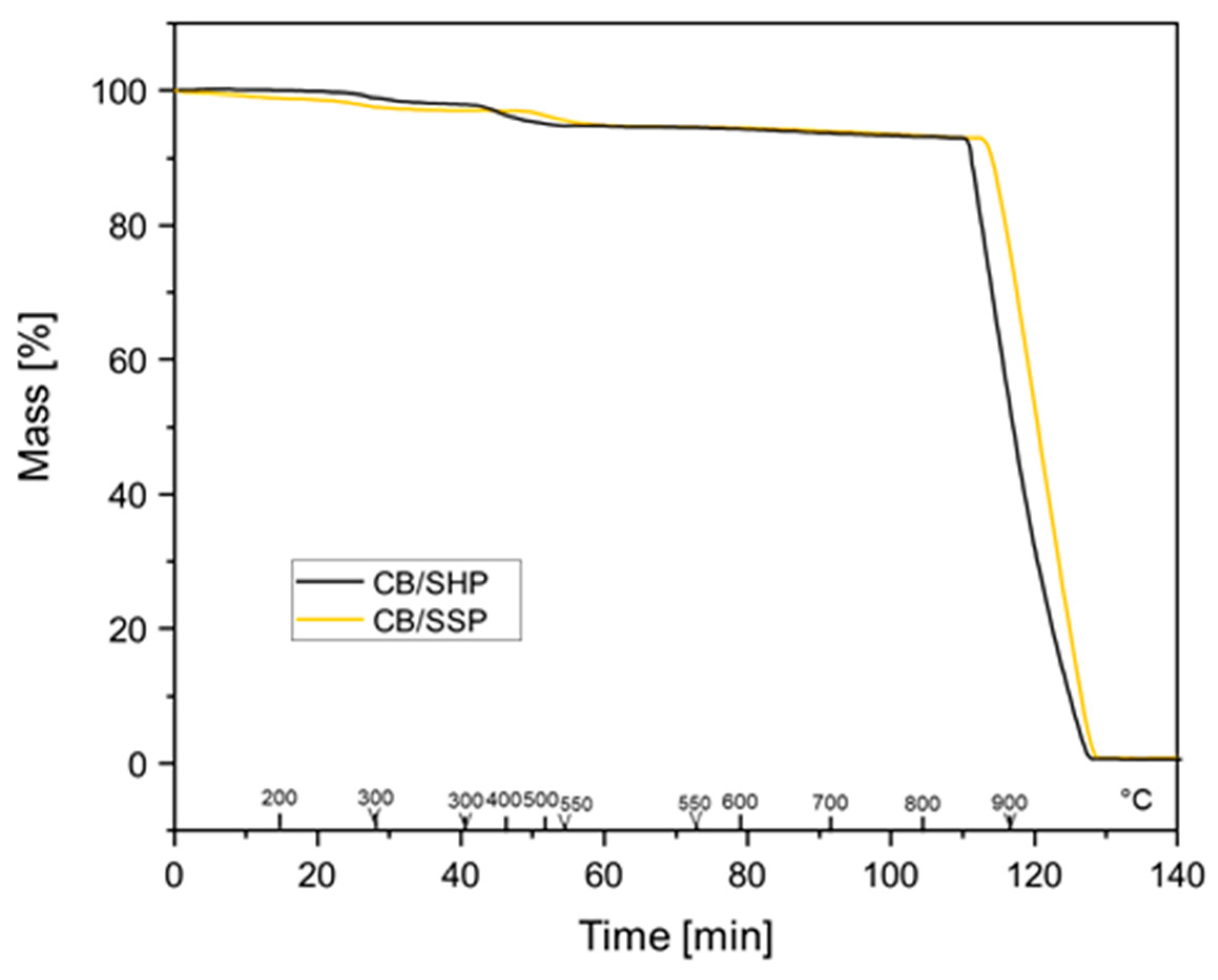
2.5.1. Thermogravimetric Analysis (TGA)
2.5.2. Amount of PyC in the CB/PyC Adduct (in Phc)
2.5.3. Functionalization Yield
2.6. Characterization of Elastomer Composites
2.6.1. Crosslinking
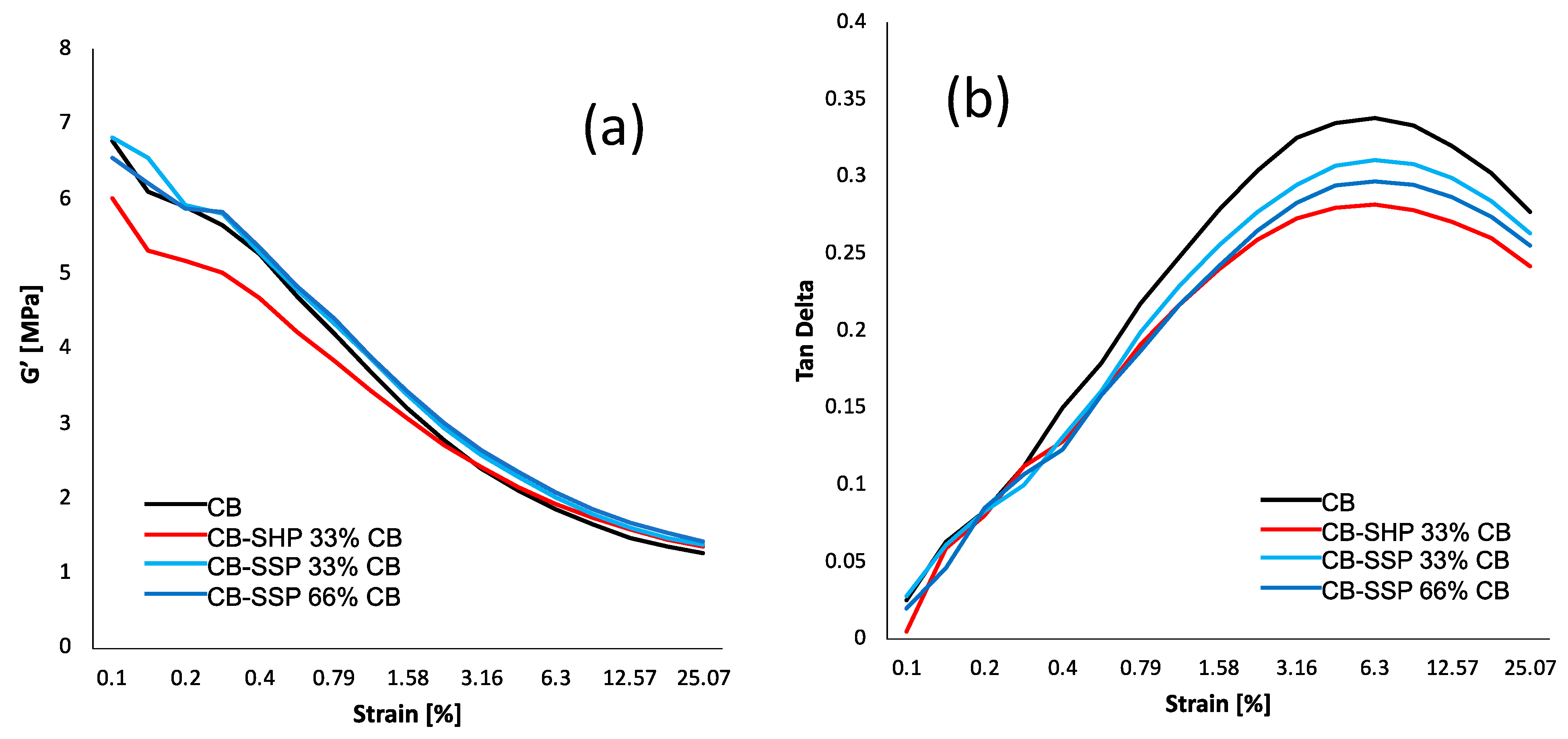
2.6.2. Dynamic-Mechanical Analyses in Shear Strain-Sweep Tests
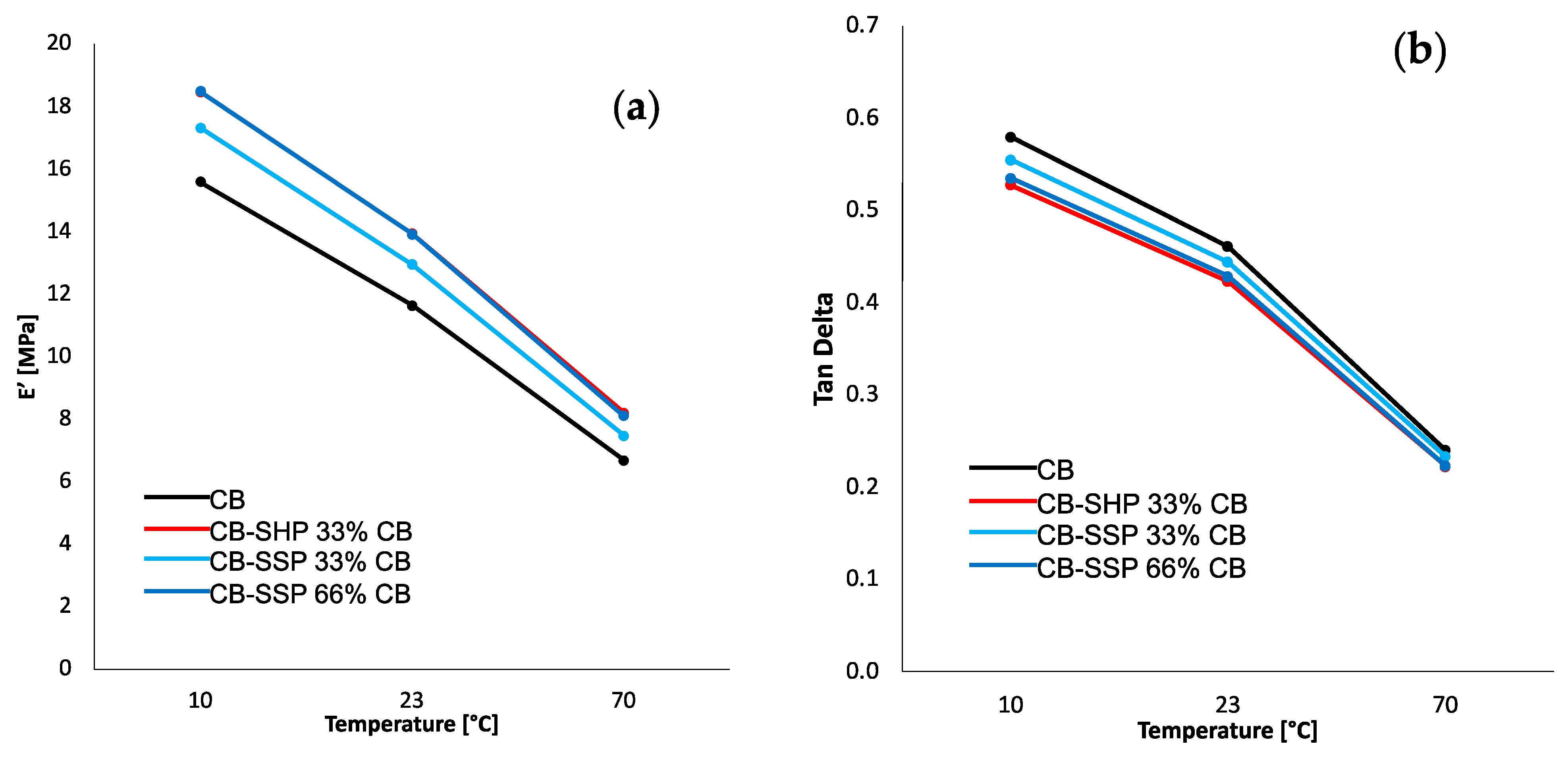
2.6.3. Dynamic-Mechanical Analyses in Compression
2.6.4. Quasi-Static Tensile Tests
2.6.5. Digital Filler Dispersion Analysis
3. Results and Discussion
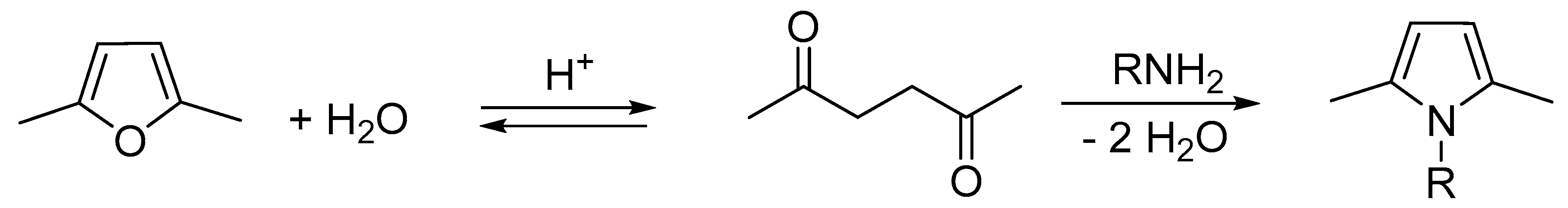
3.1. Synthesis of SHP and SSP
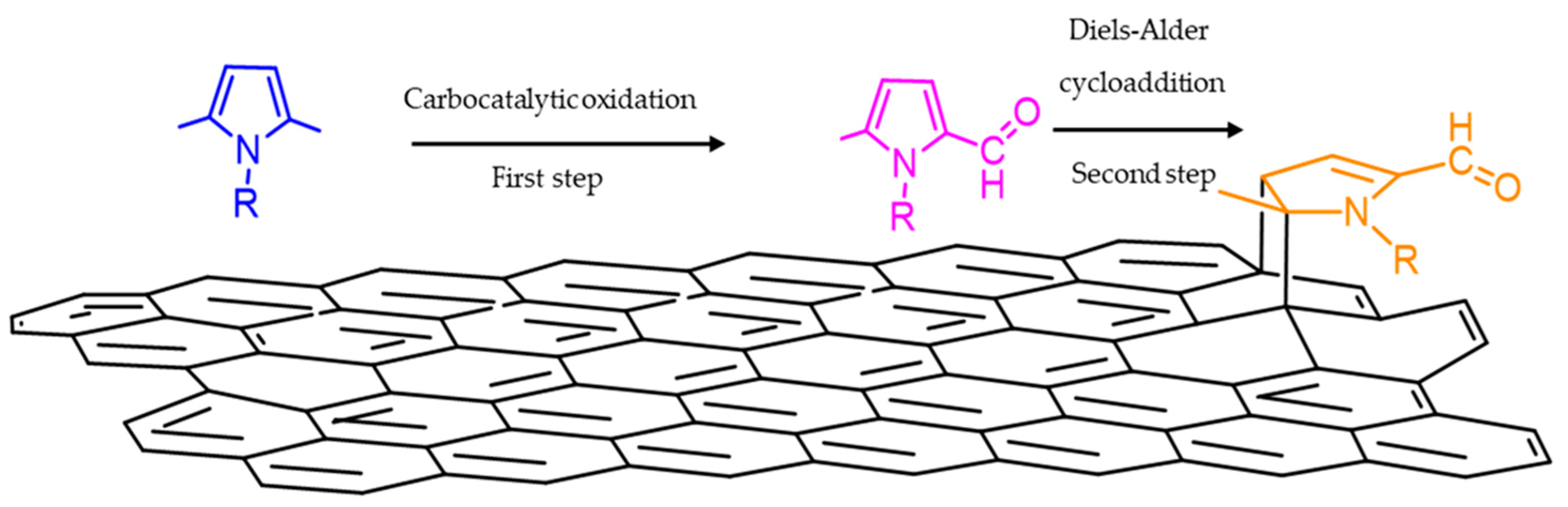
3.2. Functionalization of Carbon Black with SHP or SSP
3.3. Preparation of the Elastomer Composites
3.4. Characterization of Elastomeric Composites
3.4.1. Curing
3.4.2. Dynamic-Mechanical Properties in the Shear Mode
3.4.3. Dynamic-Mechanical Properties from Axial Compression Tests
3.4.4. Tensile Properties
3.4.5. Filler Dispersion Analysis
3.5. Study of the Reactivity of SHP and SSP with Squalene
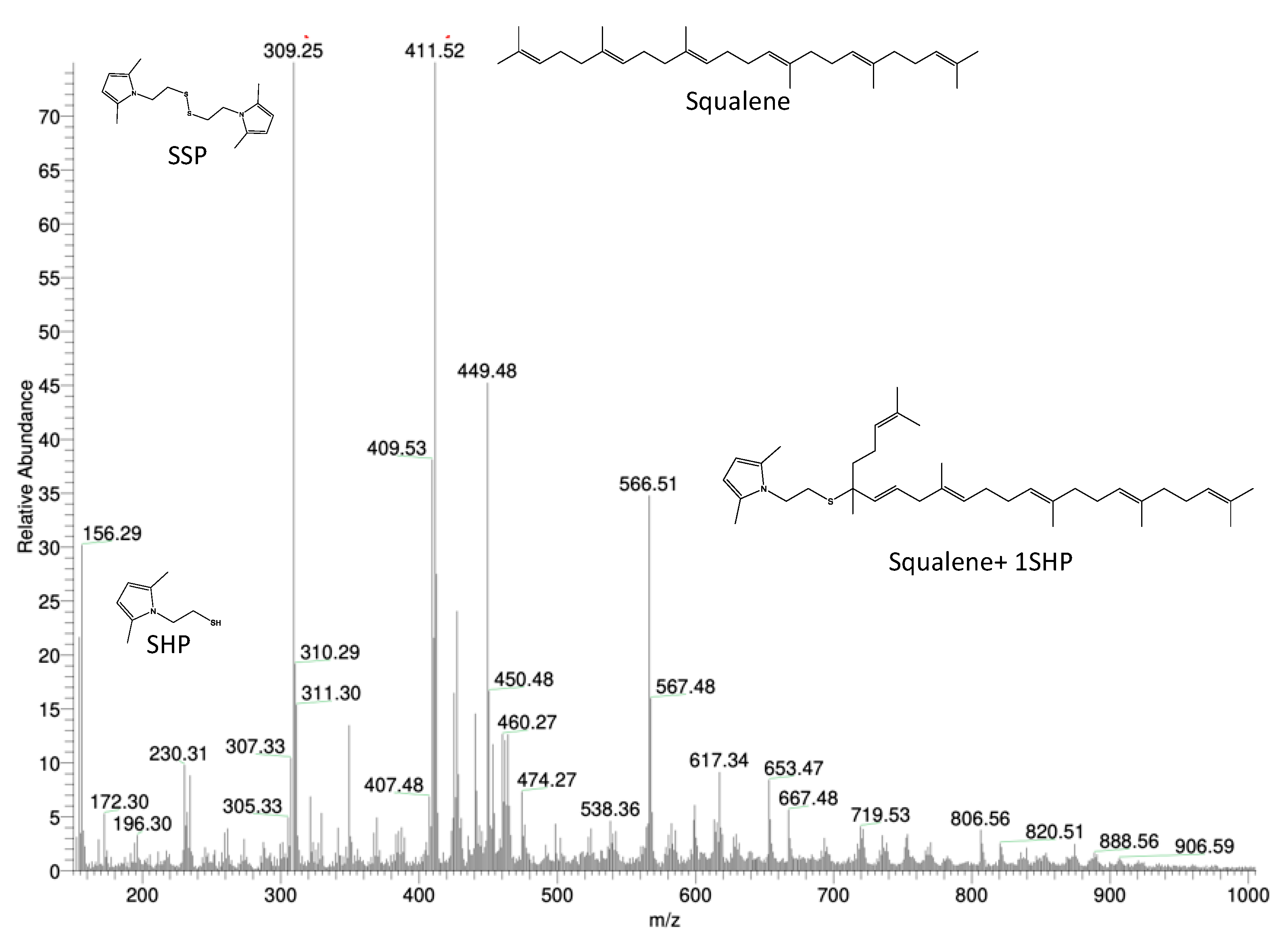
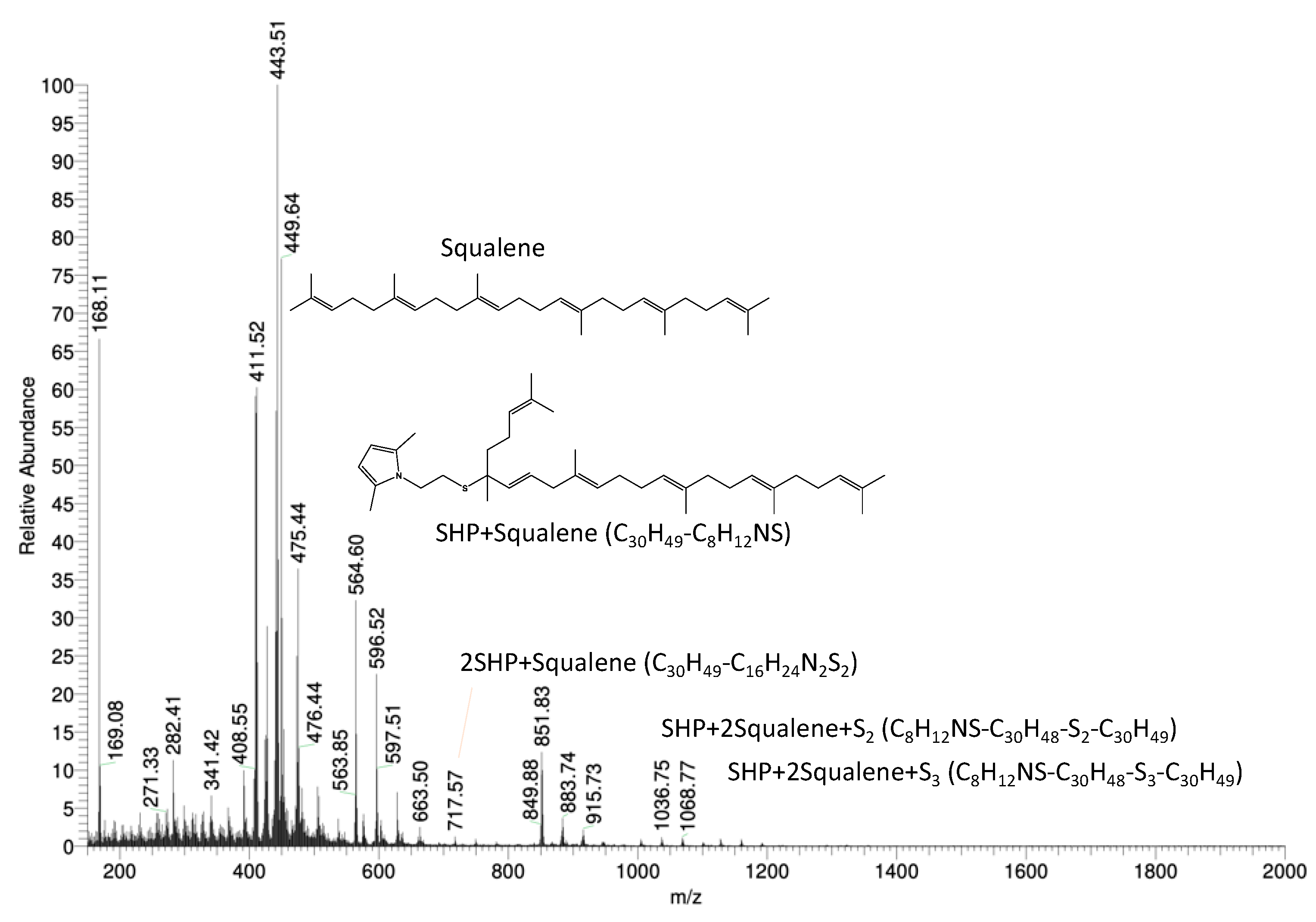
3.5.1. Reactivity of SHP with Squalene
3.5.2. Reactivity of SSP with Squalene
3.5.3. Reactivity of SHP with Squalene in the Presence of the Vulcanization System
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sustainable Development Goals Knowledge Platform. Available online: https://sustainabledevelopment.un.org/topics/sustainabletransport (accessed on 13 August 2023).
- Global Mobility Report. 2017. Available online: https://sdgs.un.org/sites/default/files/publications/2643Global_Mobility_Report_2017.pdf (accessed on 13 August 2023).
- Hall, D.E.; Moreland, J.C. Fundamentals of rolling resistance. Rubber Chem. Technol. 2001, 74, 525–539. [Google Scholar] [CrossRef]
- Rubber World 134 Years. Available online: https://rubberworld.com (accessed on 13 August 2023).
- Donnet, J.B.; Custodero, E.; Mark, J.E.; Erman, B.; Eirich, F.R. The Science and Technology of Rubber, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 8, pp. 367–400. [Google Scholar]
- Voet, A.; Morawski, J.C.; Donnet, J.B. Reinforcement of elastomers by silica. Rubber Chem. Technol. 1977, 50, 342. [Google Scholar] [CrossRef]
- Donnet, J.B. Carbon Black: Science and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Lolage, M.; Parida, P.; Chaskar, M.; Gupta, A.; Rautaray, D. Green Silica: Industrially scalable & sustainable approach towards achieving improved “nano filler–Elastomer” interaction and reinforcement in tire tread compounds. Sustain. Mater. Technol. 2020, 26, 00232. [Google Scholar]
- Shoul, B.; Marfavi, Y.; Sadeghi, B.; Kowsari, E.; Sadeghi, P.; Ramakrishna, S. Investigating the potential of sustainable use of green silica in the green tire industry: A review. Environ. Sci. Pollut. Res. 2022, 29, 51298–51317. [Google Scholar] [CrossRef] [PubMed]
- Kinney, C.R.; Friedman, L.D. Ozonization studies of coal constitution. J. Am. Chem. Soc. 1952, 74, 57–61. [Google Scholar] [CrossRef]
- Deitz, V.R.; Bitner, J.L. Interaction of ozone with adsorbent charcoals. Carbon 1973, 11, 393–401. [Google Scholar] [CrossRef]
- Cataldo, F.; Ursini, O. The role of carbon nanostructures in the ozonization of different carbon black grades, together with graphite and rubber crumb in an IR gas cell. Fuller. Nanotub. Carbon Nonstructures 2007, 15, 1–20. [Google Scholar] [CrossRef]
- Stenger, F.; Bergemann, K.; Nagel, M. Process for Aftertreating Carbon Black. U.S. Patent 8,574,527, 5 November 2013. [Google Scholar]
- Herd, C.; Edwards, C.; Curtis, J.; Crossley, S.; Schomberg, K.C.; Gross, T.; Steinhauser, N.; Kloppenberg, H.; Hardy, D.; Lucassen, A. Use of Surface-Treated Carbon Blacks in an Elastomer to Reduce Compound Hysteresis and Tire Rolling Resistance and Improve Wet Traction. U.S. Patent Application 13/391,085, 21 February 2013. [Google Scholar]
- Donnet, J.B.; Heuber, F.; Reitzer, C.; Odoux, J.; Riess, G. Etude de l’action chimique des oxydants sur le noir de carbone. Bull. Soc. Chim. Fr. 1962, 1927. [Google Scholar]
- Puri, B.R.; Kalra, K.C. The decomposition of hydrogen peroxide in the presence of carbon blacks. Carbon 1971, 9, 313–320. [Google Scholar] [CrossRef]
- Curtis, J.C.; Taylor, R.L.; Joyce, G.A. Hydrogen Peroxide Oxidation of Carbon Black. U.S. Patent 6,120,594, 19 September 2000. [Google Scholar]
- Pietrzak, R.; Wachowska, H. The influence of oxidation with HNO3 on the surface composition of high-sulphur coals: XPS study. Fuel Process. Technol. 2006, 87, 1021–1029. [Google Scholar] [CrossRef]
- Takada, T.; Nakahara, M.; Kumagai, H.; Sanada, Y. Surface modification and characterization of carbon black with oxygen plasma. Carbon 1996, 34, 1087–1091. [Google Scholar] [CrossRef]
- Ávila-Orta, C.A.; Cruz-Delgado, V.J.; Neira-Velázquez, M.G.; Hernández-Hernández, E.; Méndez-Padilla, M.G.; Medellín-Rodríguez, F.J. Surface modification of carbon nanotubes with ethylene glycol plasma. Carbon 2009, 47, 1916–1921. [Google Scholar] [CrossRef]
- Fulcheri, L.; Probst, N.; Flamant, G.; Fabry, F.; Grivei, E.; Bourrat, X. Plasma processing: A step towards the production of new grades of carbon black. Carbon 2002, 40, 169–176. [Google Scholar] [CrossRef]
- Fabry, F.; Flamant, G.; Fulcheri, L. Carbon black processing by thermal plasma. Analysis of the particle formation mechanism. Chem. Eng. Sci. 2001, 56, 2123–2132. [Google Scholar] [CrossRef]
- Park, S.J.; Cho, K.S.; Ryu, S.K. Filler–elastomer interactions: Influence of oxygen plasma treatment on surface and mechanical properties of carbon black/rubber composites. Carbon 2003, 41, 1437–1442. [Google Scholar] [CrossRef]
- Studebaker, M.L.; Huffman, E.W.D.; Wolfe, A.C.; Nabors, L.G. Oxygen-containing groups on the surface of carbon black. Ind. Eng. Chem. 1956, 48, 162–166. [Google Scholar] [CrossRef]
- Emmett, P.H. Adsorption and Pore-Size Measurements on Charcoals and Whetlerites. Chem. Rev. 1948, 43, 69–148. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, W.J.; van der Plas, T.h. Rev. Gen. Caoutchouc. 1964, 41, 453.
- Wang, X.; Lee, J.S.; Zhu, Q.; Liu, J.; Wang, Y.; Dai, S. Ammonia-treated ordered mesoporous carbons as catalytic materials for oxygen reduction reaction. Chem. Mater. 2010, 22, 2178–2180. [Google Scholar] [CrossRef]
- Belmont, J.A.; Tirumala, V.R.; Zhang, P. Elastomeric Composites Containing Modified Fillers and Functionalized Elastomers. U.S. Patent 10,640,630, 5 May 2020. [Google Scholar]
- Hoffmann, U.; Ohlrich, G. Oberflächenchemie des Kohlenstoffs. Angew. Chem. 1950, 62, 16. [Google Scholar] [CrossRef]
- Puri, B.R.; Hazra, R.S. Carbon-sulphur surface complexes on charcoal. Carbon 1971, 9, 123–134. [Google Scholar] [CrossRef]
- Studebaker, M.L.; Nabors, L.G. Sulfur group analyses in natural rubber vulcanizates. Rubber Chem. Technol. 1959, 32, 941–961. [Google Scholar] [CrossRef]
- Bonnetain, L. Désorption des oxydes de surface de graphites artificiels. J. De Chim. Phys. 1961, 58, 34–46. [Google Scholar] [CrossRef]
- Lang, F.M.; Magnier, P. Degazage du graphite nucleaire avant et apres purification—Influence de l’oxydation a l’air a 620 °C. Carbon 1964, 2, 7–14. [Google Scholar] [CrossRef]
- Barton, S.S.; Gillespie, D.; Harrison, B.H. Surface studies of carbon: Acidic oxides on spheron 6. Carbon 1973, 11, 649–654. [Google Scholar] [CrossRef]
- Adib, F.; Bagreev, A.; Bandosz, T.J. Adsorption/oxidation of hydrogen sulfide on nitrogen-containing activated carbons. Langmuir 2000, 16, 1980–1986. [Google Scholar] [CrossRef]
- Stacy, W.O.; Vastola, F.J.; Walker, P.L., Jr. Interaction of sulfur dioxide with active carbon. Carbon 1968, 6, 917–923. [Google Scholar] [CrossRef]
- Blayden, H.E.; Patrick, J.W. Solid complexes of carbon and sulphur—I. Sulphurised polymer car-bons. Carbon 1967, 5, 533–544. [Google Scholar] [CrossRef]
- Sykes, K.W.; White, P. The reactions of carbon with sulphur compounds. Part 4. Adsorption of gaseous sulphur and carbon disulphide by charcoal. Trans. Faraday Soc. 1956, 52, 660–671. [Google Scholar] [CrossRef]
- Wampler, W.; Jacobsson, B.M.; Nikiel, L.; Cameron, P.D.; Neilsen, J. Polysulfide Treatment of Carbon Black Filler and Elastomeric Compositions with Polysulfide Treated Carbon Black. U.S. Patent 9,005,359, 14 April 2015. [Google Scholar]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Metrics of green chemistry and sustainability: Past, present, and future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, M.S.; Barbera, V.; Sebastiano, R.; Valerio, A.M.; Leonardi, G.; Citterio, A. Adducts between Carbon Allotropes and Serinol Derivatives. U.S. Patent 10,160,652,B2, 25 December 2018. [Google Scholar]
- Galimberti, M.; Barbera, V. Adducts of Pyrrole Derivatives to Carbon Allotropes. WO Patent EP3538481B1, 17 June 2019. [Google Scholar]
- Magaletti, F.; Margani, F.; Monti, A.; Dezyani, R.; Prioglio, G.; Giese, U.; Barbera, V.; Galimberti, M.S. Adducts of Carbon Black with a Biosourced Janus Molecule for Elastomeric Composites with Lower Dissipation of Energy. Polymers 2023, 15, 3120. [Google Scholar] [CrossRef] [PubMed]
- Barbera, V.; Porta, A.; Brambilla, L.; Guerra, S.; Serafini, A.; Vareio, A.M.; Vitale, A.; Galimberti, M. Polyhydroxylated fewlayer graphene for the preparation of flexible conductive carbonpaper. RSC Adv. 2016, 6, 87767. [Google Scholar] [CrossRef]
- Locatelli, D.; Barbera, V.; Brambilla, L.; Castiglioni, C.; Sironi, A.; Galimberti, M. Tuning the solubility parameters of carbon nanotubes by means of their adducts with Janus pyrrole compounds. Nanomaterials 2020, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Barbera, V.; Brambilla, L.; Milani, A.; Palazzolo, A.; Castiglioni, C.; Vitale, A.; Bongiovanni, R.; Galimberti, M. Domino reaction for the sustainable functionalization of few-layer graphene. Nanomaterials 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.E.; Bowman, C.N. Thiol–ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, H.; Jiang, J.; Wang, Q.; Luo, Y.; Feng, P.; Zhang, C. A cysteine derivative-enabled ultrafast thiol–ene reaction for scalable synthesis of a fully bio-based internal emulsifier for high-toughness waterborne polyurethanes. Green Chem. 2020, 22, 5722–5729. [Google Scholar] [CrossRef]
- Sato, M.; Mihara, S.; Amino, N.; Dierkes, W.K.; Blume, A. Reactivity study of mercapto–silane and sulfide–silane with polymer. Rubber Chem. Technol. 2020, 93, 319–345. [Google Scholar] [CrossRef]
- Barbera, V.; Galimberti, M.; Giannini, L.; Naddeo, S. Process for the Preparation of Diketones and Pyrrole Derivatives. Patent Application n. PCT/IB2022/062453, 19 December 2022. [Google Scholar]
- Galimberti, M.S.; Barbera, V.; Prioglio, G.; Giannini, L. Adducts between Carbon Allotropes and Pyrrole Derivatives, Elastomer Mixtures Comprising Them and Tyres Comprising Such Mixtures. U.S. Patent Application 17/594,787, 7 July 2022. [Google Scholar]
- ISO 9924-1; Rubber and Rubber Products—Determination of the Composition of Vulcanizates and Uncured Compounds by Thermogravimetry—Part 1: Butadiene, Ethylene-Propylene Copolymer and Terpolymer, Isobutene-Isoprene, Isoprene and Styrene-Butadiene Rubbers. ISO: Geneva, Switzerland, 2016.
- ISO 37; Determination of Traction Properties of Vulcanized Rubber. ISO: Geneva, Switzerland, 2017.
- McCabe, W.L.; Julian, C.S.; Harriot, P. Unit Operations of Chemical Engineering; McGraw-Hill: New York, NY, USA, 1993; Volume 5. [Google Scholar]
- Dillon, J.H.; Prettyman, I.B.; Hall, G.L. Hysteretic and elastic properties of rubberlike materials under dynamic shear stresses. J. Appl. Phys. 1944, 15, 309. [Google Scholar] [CrossRef]
- Fletcher, W.P.; Gent, A.N. Nonlinearity in the dynamic properties of vulcanized rubber compounds. Trans. Inst. Rubber Ind. 1953, 29, 266. [Google Scholar] [CrossRef]
- Payne, A.R. The dynamic properties of carbon black-loaded natural rubber vulcanizates. Part I. J. Appl. Polym. Sci. 1962, 6, 57. [Google Scholar] [CrossRef]
- Warasitthinon, N.; Genix, A.C.; Sztucki, M.; Oberdisse, J.; Robertson, C.G. The Payne effect: Primarily polymer-related or filler-related phenomenon? Rubber Chem. Technol. 2019, 92, 599–611. [Google Scholar] [CrossRef]
- Wang, D.; Tang, Z.; Fang, S.; Wu, S.; Zeng, H.; Wang, A.; Guo, B. The use of inverse vulcanised polysulfide as an intelligent interfacial modifier in rubber/carbon black composites. Carbon 2021, 184, 409–417. [Google Scholar] [CrossRef]
- Wu, X.; Smith, J.A.; Petcher, S.; Zhang, B.; Parker, D.J.; Griffin, J.M.; Hasell, T. Catalytic inverse vulcanization. Nat. Commun. 2019, 10, 647. [Google Scholar] [CrossRef] [PubMed]
- Dondi, D.; Buttafava, A.; Zeffiro, A.; Palamini, C.; Lostritto, A.; Giannini, L.; Faucitano, A. The mechanisms of the sulphur-only and catalytic vulcanization of polybutadiene: An EPR and DFT study. Eur. Polym. J. 2015, 62, 222–235. [Google Scholar] [CrossRef]
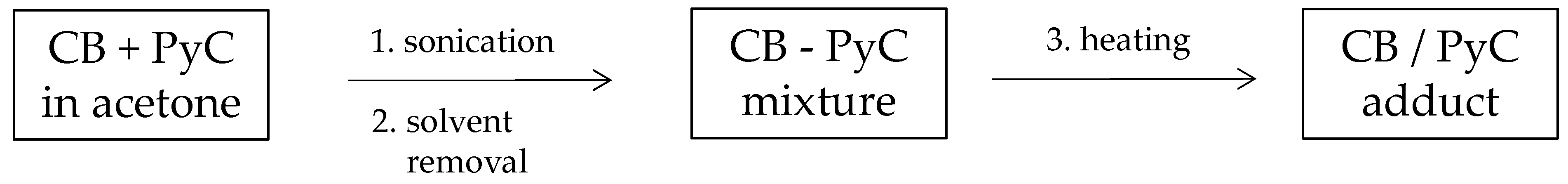
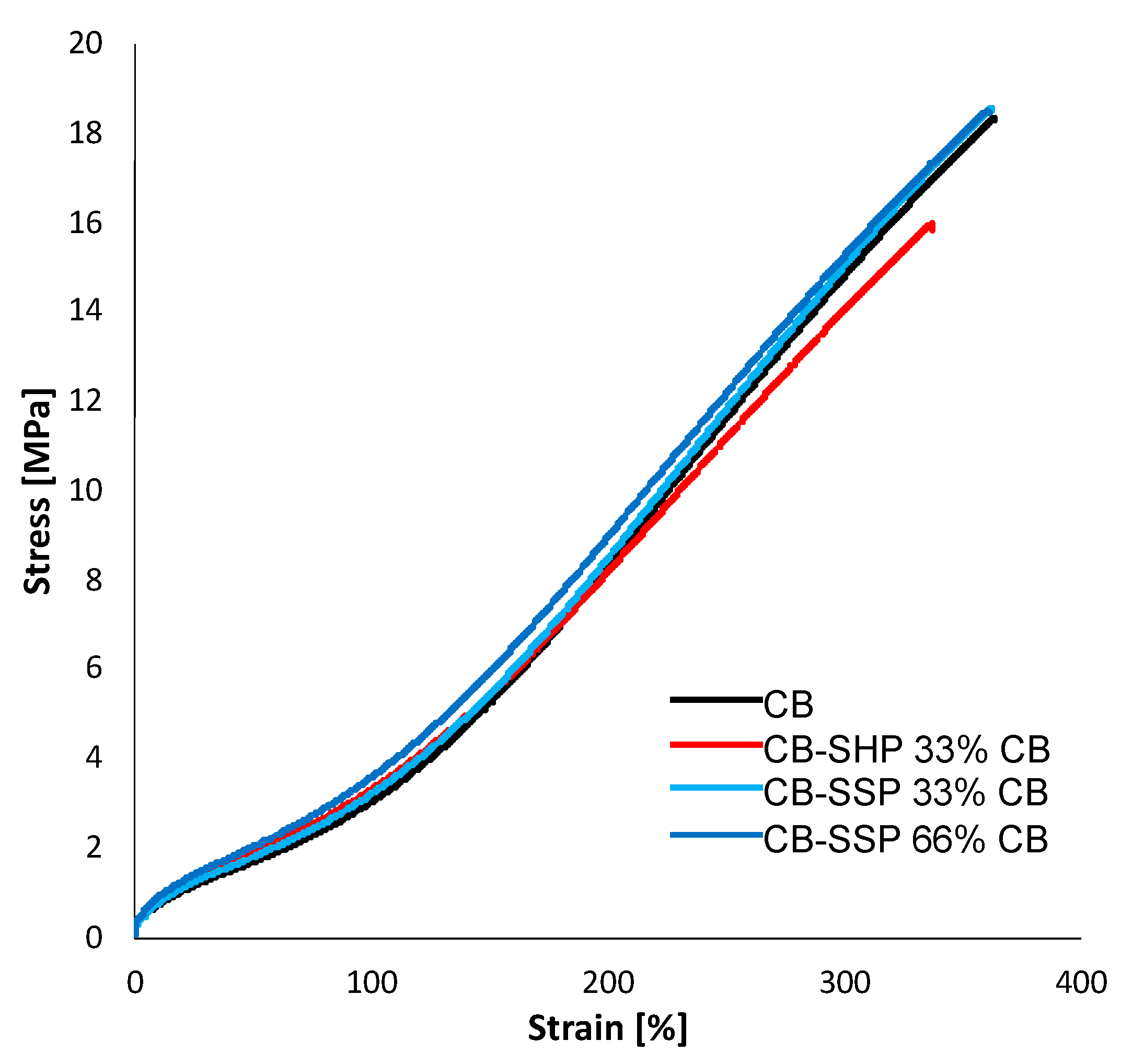
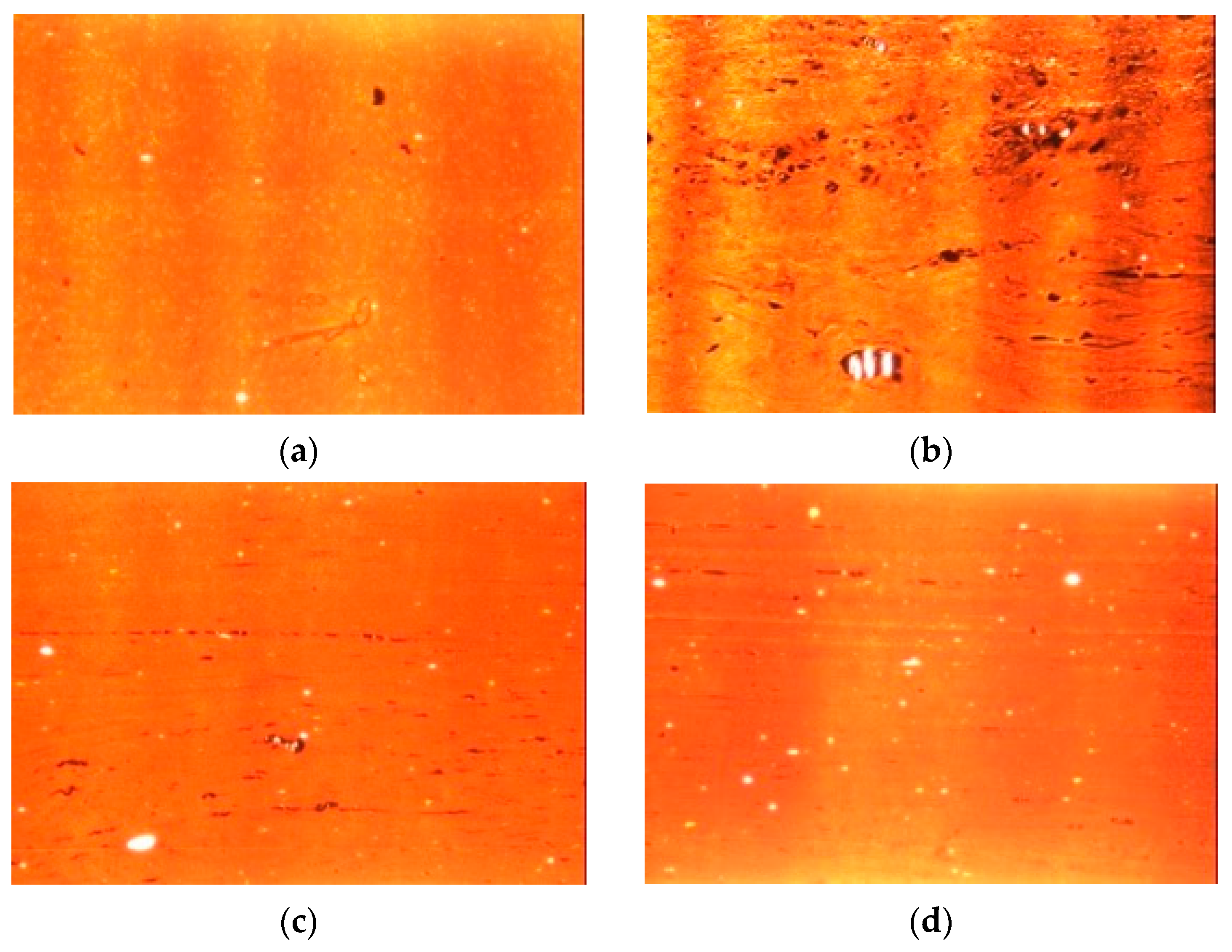
| Ingredient | CB | CB + SHP | CB/SHP 33% CB | CB/SSP 33% CB | CB/SSP 66% CB |
|---|---|---|---|---|---|
| S-SBR 4630 | 70 | 70 | 70 | 70 | 70 |
| NR | 30 | 30 | 30 | 30 | 30 |
| CB | 65 | 65 | 43.55 | 43.55 | 22.10 |
| SHP | 1.2 | ||||
| CB/SHP | 0 | 0 | 22.95 | 0 | 0 |
| CB | 21.45 | ||||
| SHP | 1.5 | ||||
| CB/SSP | 0 | 0 | 0 | 22.75 | 45.5 |
| CB | 21.45 | 42.9 | |||
| SSP | 1.3 | 2.6 |
| Sample | Mass Loss (%) | ||||
|---|---|---|---|---|---|
| T < 150 °C | 150 °C < T < 550 °C | 550 °C < T < 900 °C | T > 900 °C (d) | ||
| CB | (a) | 0.4 | 0.7 | 1.3 | 97.6 |
| (c) | 0.3 | 0.1 | 0.1 | 99.5 | |
| CB/SHP | (b) | 0.2 | 5.6 | 2.1 | 92.7 |
| (c) | 0.03 | 5.2 | 1.9 | 93 | |
| CB/SSP | (b) | 0.9 | 4.4 | 1.6 | 93 |
| (c) | 0.6 | 4.2 | 1.6 | 95.4 | |
| Property | CB | CB + SHP | CB/SHP 33% CB | CB/SSP 33% CB | CB/SSP 66% CB |
|---|---|---|---|---|---|
| ML (dNm) | 3.6 | 3.6 | 4.0 | 3.9 | 4.2 |
| MH (dNm) | 20.2 | 19.7 | 20.8 | 21.3 | 22.2 |
| MH − ML (dNm) | 16.6 | 16.1 | 16.8 | 17.5 | 18 |
| tS1 (min) | 3.3 | 2.4 | 3.1 | 3.1 | 3 |
| t90 (min) | 8.8 | 6.2 | 7.2 | 7.8 | 7.4 |
| (MH − ML)/(t90 − ts1) (dNm/min) a | 3.0 | 4.3 | 4.1 | 3.7 | 4.2 |
| Property | Temperature (°C) | CB | CB + SHP | CB-SHP 33% CB | CB-SSP 33% CB | CB-SSP 66% CB |
|---|---|---|---|---|---|---|
| E′ (MPa) | 10 | 15.60 | 14.92 | 18.48 | 17.33 | 18.49 |
| 23 | 11.64 | 11.21 | 13.94 | 12.95 | 13.92 | |
| 70 | 6.69 | 6.42 | 8.21 | 7.47 | 8.11 | |
| E″ (MPa) | 10 | 9.06 | 8.88 | 9.77 | 9.63 | 9.90 |
| 23 | 5.37 | 5.44 | 5.91 | 5.76 | 5.97 | |
| 70 | 1.61 | 1.71 | 1.83 | 1.75 | 1.82 | |
| Tan Delta | 10 | 0.58 | 0.60 | 0.53 | 0.56 | 0.54 |
| 23 | 0.46 | 0.49 | 0.42 | 0.44 | 0.43 | |
| 70 | 0.24 | 0.27 | 0.22 | 0.23 | 0.22 | |
| ΔE′ (E′@10 °C–E′@70 °C) (MPa) | / | 8.91 | 8.49 | 10.27 | 10.16 | 9.86 |
| Property | CB | CB + SHP | CB/SHP 33% CB | CB/SSP 33% CB | CB/SSP 66% CB |
|---|---|---|---|---|---|
| σ100 (MPa) | 0.14 | 3.08 ± 0.03 | 0.06 | 0.16 | 0.18 |
| σ200 (MPa) | 0.46 | 7.90 ± 0.15 | 0.01 | 0.36 | 0.35 |
| σ300 (MPa) | 0.64 | 14.50 ± 0.19 | 0.09 | 0.33 | 0.48 |
| σB (MPa) | 1.30 | 18.42 ± 0.53 | 0.68 | 0.12 | 0.28 |
| εB (%) | 28.24 | 366.54 ± 6.06 | 9.43 | 5.05 | 6.43 |
| Energy at break (MJ/m3) | 4.60 | 29.22 ± 1.45 | 1.65 | 0.57 | 0.30 |
| Property | CB | CB/SHP 33% CB | CB/SSP 33% CB | CB/SSP 66% CB |
|---|---|---|---|---|
| Filler (%) | 35.79 | 35.79 | 35.79 | 35.79 |
| N° of aggregates | 12 | 132 | 20 | 12 |
| m) | 13 | 9 | 11 | 11 |
| m) | 29 | 52 | 34 | 18 |
| Average | 20 | 18 | 20 | 15 |
| Standard deviation | 5 | 7 | 7 | 2 |
| Undispersed filler (%) | 0.2 | 1.5 | 0.3 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prioglio, G.; Naddeo, S.; Giese, U.; Barbera, V.; Galimberti, M. Bio-Based Pyrrole Compounds Containing Sulfur Atoms as Coupling Agents of Carbon Black with Unsaturated Elastomers. Nanomaterials 2023, 13, 2761. https://doi.org/10.3390/nano13202761
Prioglio G, Naddeo S, Giese U, Barbera V, Galimberti M. Bio-Based Pyrrole Compounds Containing Sulfur Atoms as Coupling Agents of Carbon Black with Unsaturated Elastomers. Nanomaterials. 2023; 13(20):2761. https://doi.org/10.3390/nano13202761
Chicago/Turabian StylePrioglio, Gea, Simone Naddeo, Ulrich Giese, Vincenzina Barbera, and Maurizio Galimberti. 2023. "Bio-Based Pyrrole Compounds Containing Sulfur Atoms as Coupling Agents of Carbon Black with Unsaturated Elastomers" Nanomaterials 13, no. 20: 2761. https://doi.org/10.3390/nano13202761
APA StylePrioglio, G., Naddeo, S., Giese, U., Barbera, V., & Galimberti, M. (2023). Bio-Based Pyrrole Compounds Containing Sulfur Atoms as Coupling Agents of Carbon Black with Unsaturated Elastomers. Nanomaterials, 13(20), 2761. https://doi.org/10.3390/nano13202761







