Fabrication of β-Ga2O3 Nanotubes via Sacrificial GaSb-Nanowire Templates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of β-Ga2O3 Nanotubes
2.2. Characterizations and In Situ TEM Heating Experiments
2.3. Photoelectric Tests of the β-Ga2O3 Nanotubes
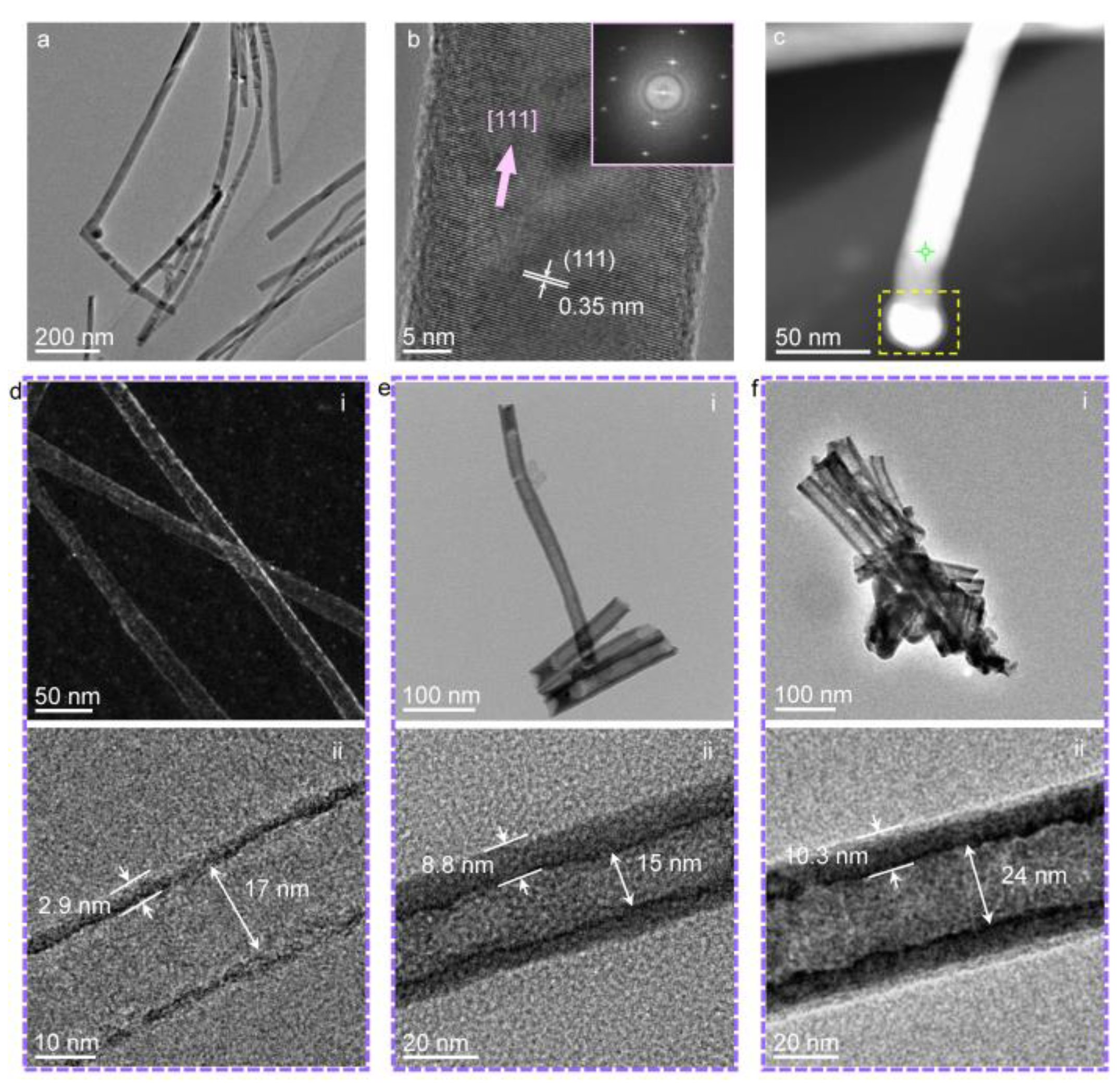
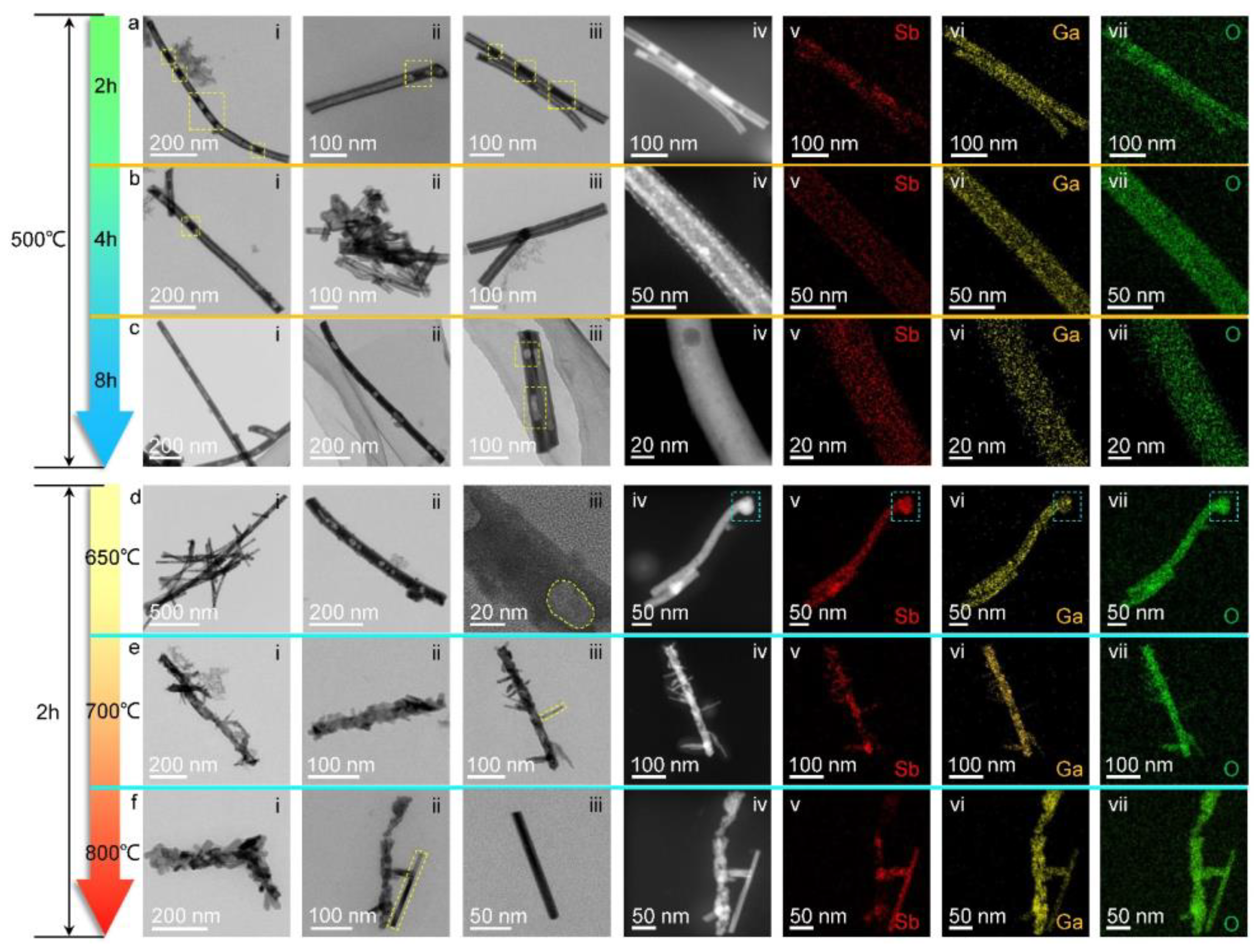
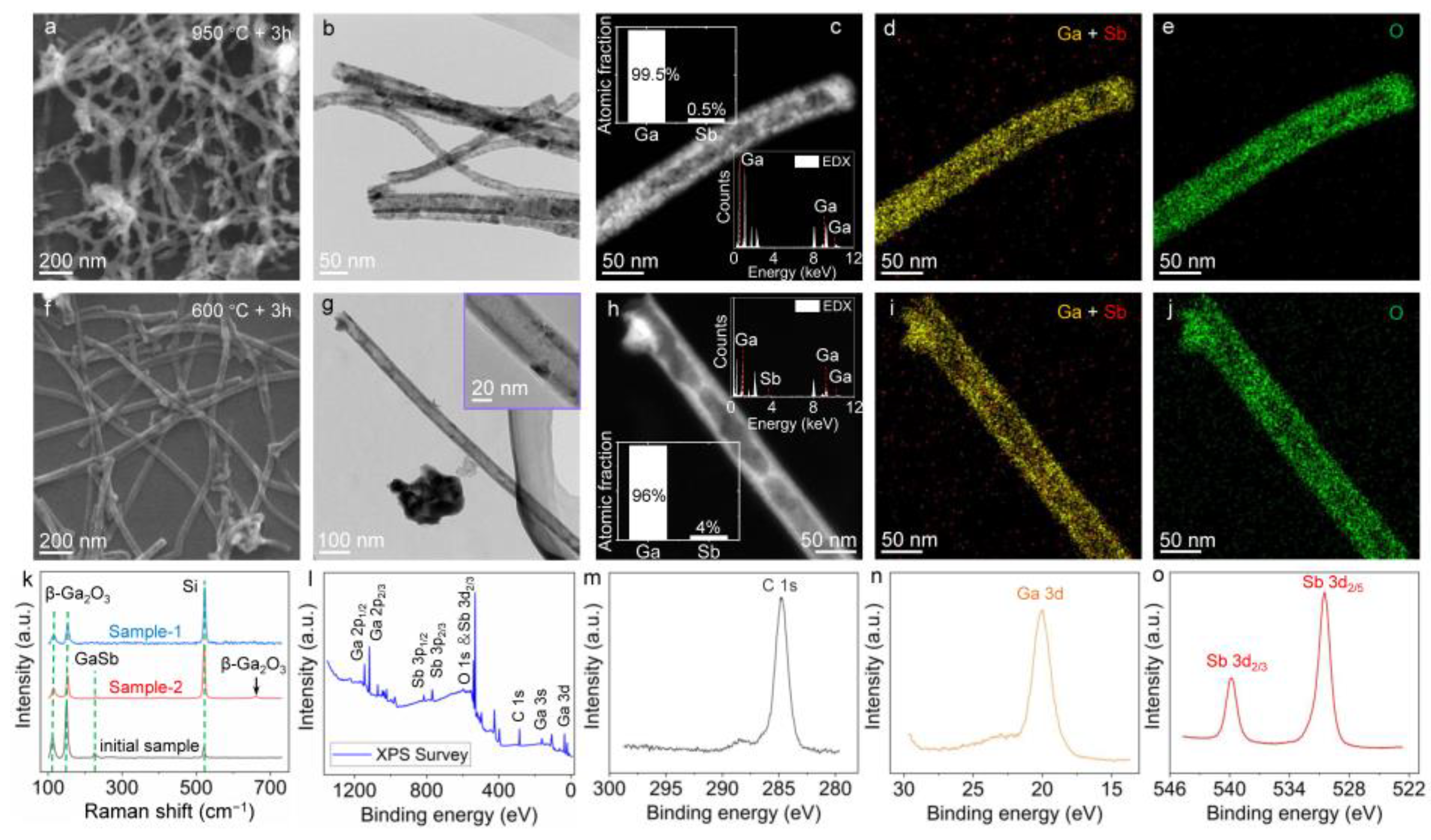
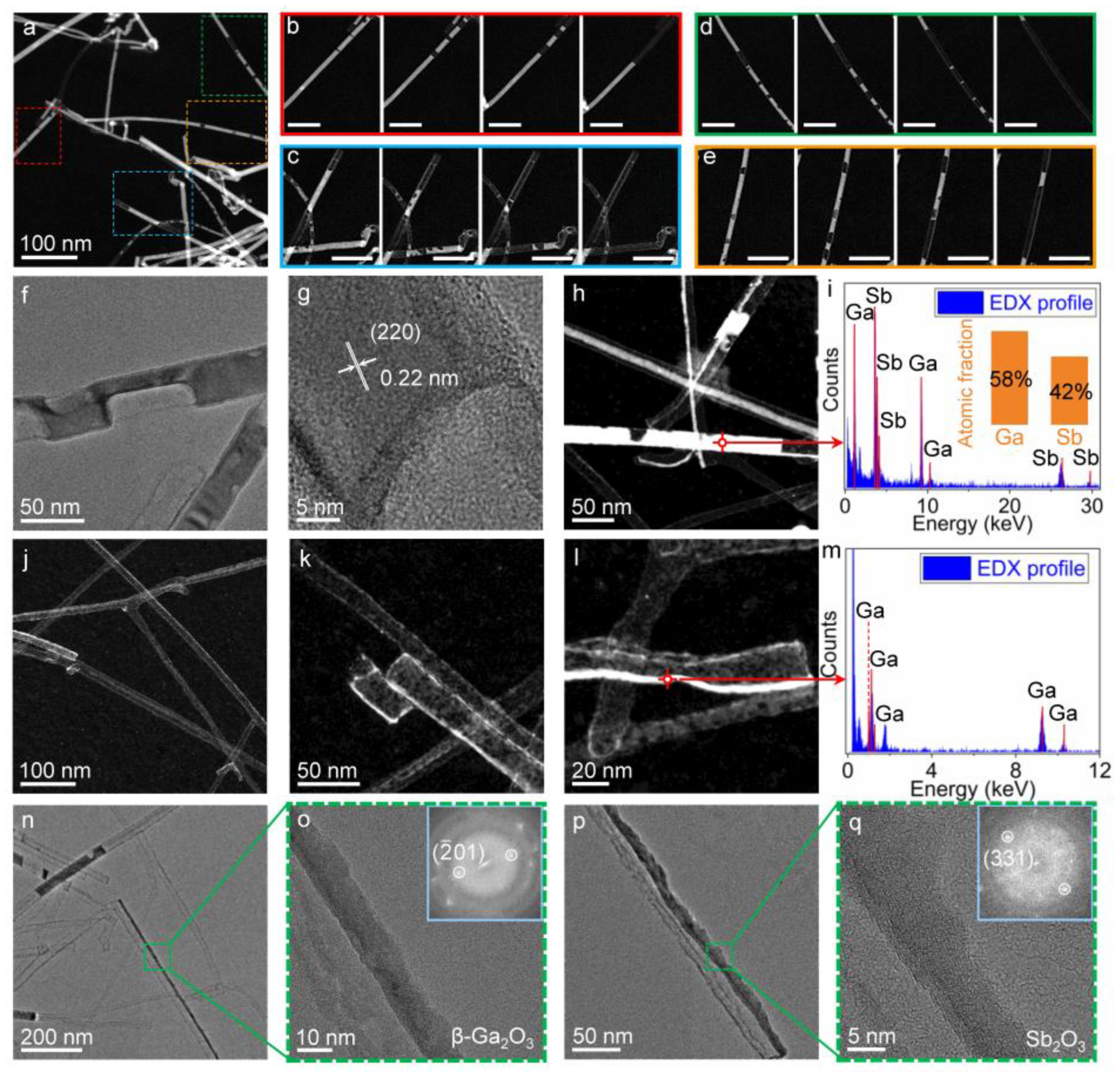
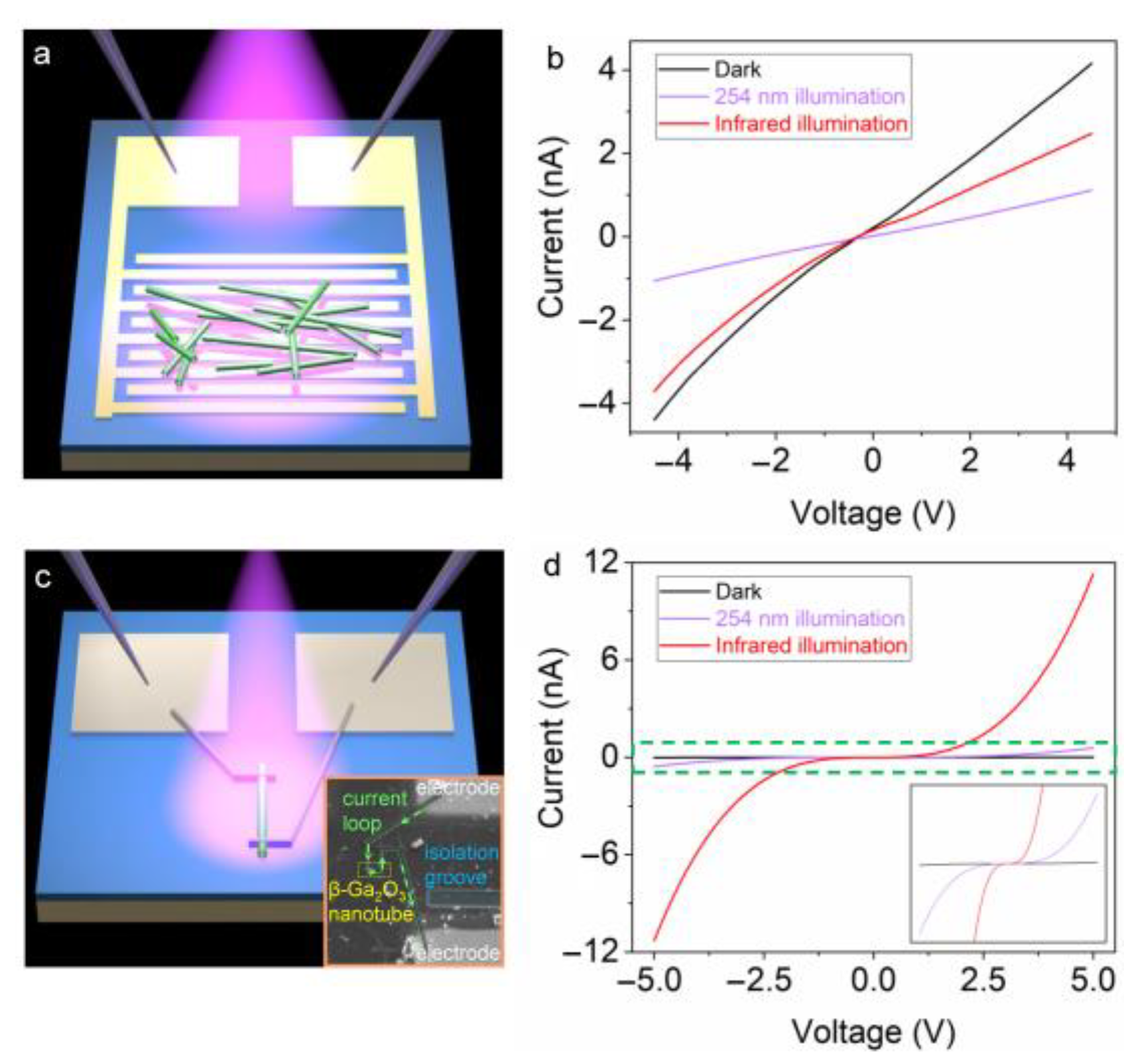
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro-Fernández, P.; Mance, D.; Liu, C.; Abdala, P.M.; Willinger, E.; Rossinelli, A.A.; Serykh, A.I.; Pidko, E.A.; Copéret, C.; Fedorov, A.; et al. Bulk and Surface Transformations of Ga2O3 Nanoparticle Catalysts for Propane Dehydrogenation Induced by a H2 Treatment. J. Catal. 2022, 408, 155–164. [Google Scholar] [CrossRef]
- Gong, N.W.; Lu, M.Y.; Wang, C.Y.; Chen, Y.; Chen, L.J. Au(Si)-Filled β-Ga2O3 Nanotubes as Wide Range High Temperature Nanothermometers. Appl. Phys. Lett. 2008, 92, 073101. [Google Scholar] [CrossRef]
- Graham, U.M.; Sharma, S.; Sunkara, M.K.; Davis, B.H. Nanoweb Formation: 2D Self-Assembly of Semiconductor Gallium Oxide Nanowires/Nanotubes. Adv. Funct. Mater. 2003, 13, 576–581. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, X.; Wang, J.; Ma, H.; Wang, Y.; Liu, W.; Wang, G.; Xiao, L.; Lu, J.; Zhuang, L. Two-Dimensional Ga2O3/C Nanosheets as Durable and High-Rate Anode Material for Lithium Ion Batteries. Langmuir 2019, 35, 13607–13613. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tokizono, T.; Liao, M.; Zhong, M.; Koide, Y.; Yamada, I.; Delaunay, J.-J. Efficient Assembly of Bridged β-Ga2O3 Nanowires for Solar-Blind Photodetection. Adv. Funct. Mater. 2010, 20, 3972–3978. [Google Scholar] [CrossRef]
- Lin, H.J.; Baltrus, J.P.; Gao, H.; Ding, Y.; Nam, C.Y.; Ohodnicki, P.; Gao, P.X. Perovskite Nanoparticle-Sensitized Ga2O3 Nanorod Arrays for CO Detection at High Temperature. ACS Appl. Mater. Interfaces 2016, 8, 8880–8887. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Jiang, Z.; Song, P.; Tian, P.; Hu, L.; Liu, R.; Fang, Z.; Kang, J.; Zhang, T.Y. Nanowire-Seeded Growth of Single-Crystalline (010) beta-Ga2O3 Nanosheets with High Field-Effect Electron Mobility and On/Off Current Ratio. Small 2019, 15, e1900580. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Li, S.; Sun, Z.; Chen, J.; Chen, L.; Pangmai, M.; Li, G.; Lan, S. Efficient White Light Emission from Ga/Ga2O3 Hybrid Nanoparticles. Adv. Opt. Mater. 2021, 9, 2100675. [Google Scholar] [CrossRef]
- Yang, G.; Jang, S.; Ren, F.; Pearton, S.J.; Kim, J. Influence of High-Energy Proton Irradiation on beta-Ga2O3 Nanobelt Field-Effect Transistors. ACS Appl. Mater. Interfaces 2017, 9, 40471–40476. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Zhang, Y.; Liu, D.; Tong, N.; Lin, J.; Chen, L.; Zhang, Z.; Wang, X. Phase Transition of Two-Dimensional beta-Ga2O3 Nanosheets from Ultrathin gamma-Ga2O3 Nanosheets and Their Photocatalytic Hydrogen Evolution Activities. ACS Omega 2018, 3, 14469–14476. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Huang, H.; Wang, Y.; Tong, N.; Lin, J.; Liu, D.; Wang, X. Oxygen Vacancy Modulation of Two-Dimensional gamma-Ga2O3 Nanosheets as Efficient Catalysts for Photocatalytic Hydrogen Evolution. Nanoscale 2018, 10, 21509–21517. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Li, X.X.; Ma, H.P.; Huang, W.; Ji, Z.G.; Xia, C.; Lu, H.L.; Zhang, D.W. Investigation of the Mechanism for Ohmic Contact Formation in Ti/Al/Ni/Au Contacts to beta-Ga2O3 Nanobelt Field-Effect Transistors. ACS Appl. Mater. Interfaces 2019, 11, 32127–32134. [Google Scholar] [CrossRef]
- Chen, J.X.; Li, X.X.; Tao, J.J.; Cui, H.Y.; Huang, W.; Ji, Z.G.; Sai, Q.L.; Xia, C.T.; Lu, H.L.; Zhang, D.W. Fabrication of a Nb-Doped beta-Ga2O3 Nanobelt Field-Effect Transistor and Its Low-Temperature Behavior. ACS Appl. Mater. Interfaces 2020, 12, 8437–8445. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; An, L.; Lu, C.G.; Liu, J. Conversion between hexagonal GaN and beta-Ga2O3 Nanowires and Their Electrical Transport Properties. Nano Lett. 2006, 6, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-J.; Gao, H.; Gao, P.-X. UV-Enhanced CO Sensing Using Ga2O3-Based Nanorod Arrays at Elevated Temperature. Appl. Phys. Lett. 2017, 110, 043101. [Google Scholar] [CrossRef]
- Xie, C.; Lu, X.T.; Ma, M.R.; Tong, X.W.; Zhang, Z.X.; Wang, L.; Wu, C.Y.; Yang, W.H.; Luo, L.B. Catalyst-Free Vapor–Solid Deposition Growth of β-Ga2O3 Nanowires for DUV Photodetector and Image Sensor Application. Adv. Opt. Mater. 2019, 7, 1901257. [Google Scholar] [CrossRef]
- Weng, T.-F.; Ho, M.-S.; Sivakumar, C.; Balraj, B.; Chung, P.-F. VLS Growth of Pure and Au Decorated β-Ga2O3 Nanowires for Room Temperature CO Gas Sensor and Resistive Memory Applications. Appl. Surf. Sci. 2020, 533, 147476. [Google Scholar] [CrossRef]
- Chen, X.; Liu, K.; Zhang, Z.; Wang, C.; Li, B.; Zhao, H.; Zhao, D.; Shen, D. Self-Powered Solar-Blind Photodetector with Fast Response Based on Au/beta-Ga2O3 Nanowires Array Film Schottky Junction. ACS Appl. Mater. Interfaces 2016, 8, 4185–4191. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, H.; Feng, C.; Zhang, Z.; Yu, H.; Zhang, C.; Lin, S.; Xu, C.; Bai, H.; Guo, F. Self-Powered Solar-Blind Photodetectors Based on α-Ga2O3 Nanorod Arrays. ACS Appl. Nano Mater. 2022, 5, 11956–11963. [Google Scholar] [CrossRef]
- Zou, R.; Zhang, Z.; Liu, Q.; Hu, J.; Sang, L.; Liao, M.; Zhang, W. High Detectivity Solar-Blind High-Temperature Deep-Ultraviolet Photodetector Based on Multi-Layered (l00) Facet-Oriented beta-Ga2O3 Nanobelts. Small 2014, 10, 1848–1856. [Google Scholar] [CrossRef]
- Kumar, V.B.; Mishra, R.K.; Pulidindi, I.N.; Porat, Z.E.; Luong, J.H.T.; Gedanken, A. Preparation and Catalytic Activity of Thermosensitive Ga2O3 Nanorods. Energy Fuels 2016, 30, 7419–7427. [Google Scholar] [CrossRef]
- Mohamed, S.H.; El-Hagary, M.; Althoyaib, S. Growth of β-Ga2O3 Nanowires and Their Photocatalytic and Optical Properties Using Pt as a Catalyst. J. Alloys Compd. 2012, 537, 291–296. [Google Scholar] [CrossRef]
- Chang, K.W.; Wu, J.J. Low-Temperature Growth of Well-Aligned β-Ga2O3 Nanowires from a Single-Source Organometallic Precursor. Adv. Mater. 2004, 16, 545–549. [Google Scholar] [CrossRef]
- Gao, Y.H.; Bando, Y.; Sato, T.; Zhang, Y.F.; Gao, X.Q. Synthesis, Raman Scattering and Defects of β-Ga2O3 Nanorods. Appl. Phys. Lett. 2002, 81, 2267–2269. [Google Scholar] [CrossRef]
- Geng, B.; Zhang, L.; Meng, G.; Xie, T.; Peng, X.; Lin, Y. Large-Scale Synthesis and Photoluminescence of Single-Crystalline β-Ga2O3 Nanobelts. J. Cryst. Growth 2003, 259, 291–295. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, N.H. Growth of β-Ga2O3 Nanobelts on Ir-Coated Substrates. Appl. Phys. A Mater. Sci. Process. 2005, 80, 537–540. [Google Scholar] [CrossRef]
- Li, J.Y.; Qiao, Z.Y.; Chen, X.L.; Chen, L.; Cao, Y.G.; He, M.; Li, H.; Cao, Z.M.; Zhang, Z. Synthesis of beta-Ga2O3 Nanorods. J. Alloys Compd. 2000, 306, 300–302. [Google Scholar] [CrossRef]
- Vanithakumari, S.C.; Nanda, K.K. A One-Step Method for the Growth of Ga2O3-Nanorod-Based White-Light-Emitting Phosphors. Adv. Mater. 2009, 21, 3581–3584. [Google Scholar] [CrossRef]
- Choi, Y.C.; Kim, W.S.; Park, Y.S.; Lee, S.M.; Bae, D.J.; Lee, Y.H.; Park, G.S.; Choi, W.B.; Lee, N.S.; Kim, J.M. Catalytic Growth of beta-Ga2O3 Nanowires by Arc Discharge. Adv. Mater. 2000, 12, 746–750. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Lin, C.; Lin, J. A Simple Method to Synthesize β-Ga2O3 Nanorods and Their Photoluminescence Properties. J. Cryst. Growth 2005, 280, 99–106. [Google Scholar] [CrossRef]
- Hu, J.Q.; Li, Q.; Meng, X.M.; Lee, C.S.; Lee, S.T. Synthesis of beta-Ga2O3 Nanowires by Laser Ablation. J. Phys. Chem. B 2002, 106, 9536–9539. [Google Scholar] [CrossRef]
- Cheng, B.; Samulski, E.T. Fabrication and Characterization of Nanotubular Semiconductor Oxides In2O3 and Ga2O3. J. Mater. Chem. 2001, 11, 2901–2902. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, L.; Li, Y.; Xiu, X.; Xie, Z.; Tao, T.; Liu, B.; Chen, P.; Zhang, R.; Zheng, Y. A Selective Etching Route for Large-Scale Fabrication of beta-Ga2O3 Micro-/Nanotube Arrays. Nanomaterials 2021, 11, 3327. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.X.; Han, N.; Fang, M.; Lin, H.; Cheung, H.Y.; Yip, S.; Wang, E.J.; Hung, T.; Wong, C.Y.; Ho, J.C. Surfactant-Assisted Chemical Vapour Deposition of High-Performance Small-Diameter GaSb Nanowires. Nat. Commun. 2014, 5, 5249. [Google Scholar] [CrossRef] [PubMed]
- Mel, A.A.; Tessier, P.Y.; Buffiere, M.; Gautron, E.; Ding, J.; Du, K.; Choi, C.H.; Konstantinidis, S.; Snyders, R.; Bittencourt, C.; et al. Controlling the Formation of Nanocavities in Kirkendall Nanoobjects through Sequential Thermal Ex Situ Oxidation and In Situ Reduction Reactions. Small 2016, 12, 2885–2892. [Google Scholar] [CrossRef]
- Niu, K.Y.; Park, J.; Zheng, H.; Alivisatos, A.P. Revealing Bismuth Oxide Hollow Nanoparticle Formation by the Kirkendall Effect. Nano Lett. 2013, 13, 5715–5719. [Google Scholar] [CrossRef]
- Yin, Y.D.; Rioux, R.M.; Erdonmez, C.K.; Hughes, S.; Somorjai, G.A.; Alivisatos, A.P. Formation of Hollow Nanocrystals Through the Nanoscale Kirkendall Effect. Science 2004, 304, 711–714. [Google Scholar] [CrossRef]
- Mizokawa, Y.; Komoda, O.; Iwasaki, H.; Nakamura, S. Xps Study on Oxide Gasb Interfacial Chemical-Reaction at Room-Temperature. Jpn. J. Appl. Phys. 1984, 23, L257–L259. [Google Scholar] [CrossRef]
- Mestl, G.; Ruiz, P.; Delmon, B.; Knozinger, H. Sb2O3/Sb2O4 in Reducing/Oxidizing Environments—An in-Situ Raman-Spectroscopy Study. J. Phys. Chem. 1994, 98, 11276–11282. [Google Scholar] [CrossRef]
- Yi, Z.; Han, Q.; Li, X.; Wu, Y.; Cheng, Y.; Wang, L. Two-Step Oxidation of Bulk Sb to One-Dimensional Sb2O4 Submicron-Tubes as Advanced Anode Materials for Lithium-Ion and Sodium-Ion Batteries. Chem. Eng. J. 2017, 315, 101–107. [Google Scholar] [CrossRef]
- Mishra, P.; Lin, C.-Y.; Cheng, C.-C.; Lee, M.-C.M. Facile Surface Treatment of Precursors before Rapid Melt Growth of GaSb on Silicon. Thin Solid Films 2021, 732, 138797. [Google Scholar] [CrossRef]
- Machon, D.; McMillan, P.F.; Xu, B.; Dong, J. High-Pressure Study of the β-to-α Transition in Ga2O3. Phys. Rev. B 2006, 73, 094125. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Zhang, A.; Liu, Q.; Shen, C.; Wu, F.; Xu, C.; Chen, M.; Fu, H.; Zhou, C. Quasi-Two-Dimensional β-Ga2O3 Field Effect Transistors with Large Drain Current Density and Low Contact Resistance via Controlled Formation of Interfacial Oxygen Vacancies. Nano Res. 2018, 12, 143–148. [Google Scholar] [CrossRef]
- Bourque, J.L.; Biesinger, M.C.; Baines, K.M. Chemical State Determination of Molecular Gallium Compounds Using XPS. Dalton Trans. 2016, 45, 7678–7696. [Google Scholar] [CrossRef] [PubMed]
- Garbassi, F. XPS and AES Study of Antimony Oxides. Surf. Interface Anal. 1980, 2, 165–169. [Google Scholar] [CrossRef]
- Yaws, C.L. The Yaws Handbook of Vapor Pressure: Antoine Coefficients; Gulf Professional Publishing: Houston, TX, USA, 2015. [Google Scholar]
- Wan, Q.; Zhang, N.L.; Liu, W.L.; Lin, C.L.; Wang, T.H. Memory and Negative Photoconductivity Effects of Ge Nanocrystals Embedded in ZrO2/Al2O3 Gate Dielectrics. Appl. Phys. Lett. 2003, 83, 138–140. [Google Scholar] [CrossRef]
- Choi, S.-H.; Elliman, R.G. Negative Photoconductivity in SiO2 Films Containing Si Nanocrystals. Appl. Phys. Lett. 1999, 74, 3987–3989. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shangguan, L.; He, L.-B.; Dong, S.-P.; Gao, Y.-T.; Sun, Q.; Zhu, J.-H.; Hong, H.; Zhu, C.; Yang, Z.-X.; Sun, L.-T. Fabrication of β-Ga2O3 Nanotubes via Sacrificial GaSb-Nanowire Templates. Nanomaterials 2023, 13, 2756. https://doi.org/10.3390/nano13202756
Shangguan L, He L-B, Dong S-P, Gao Y-T, Sun Q, Zhu J-H, Hong H, Zhu C, Yang Z-X, Sun L-T. Fabrication of β-Ga2O3 Nanotubes via Sacrificial GaSb-Nanowire Templates. Nanomaterials. 2023; 13(20):2756. https://doi.org/10.3390/nano13202756
Chicago/Turabian StyleShangguan, Lei, Long-Bing He, Sheng-Pan Dong, Yu-Tian Gao, Qian Sun, Jiong-Hao Zhu, Hua Hong, Chao Zhu, Zai-Xing Yang, and Li-Tao Sun. 2023. "Fabrication of β-Ga2O3 Nanotubes via Sacrificial GaSb-Nanowire Templates" Nanomaterials 13, no. 20: 2756. https://doi.org/10.3390/nano13202756
APA StyleShangguan, L., He, L.-B., Dong, S.-P., Gao, Y.-T., Sun, Q., Zhu, J.-H., Hong, H., Zhu, C., Yang, Z.-X., & Sun, L.-T. (2023). Fabrication of β-Ga2O3 Nanotubes via Sacrificial GaSb-Nanowire Templates. Nanomaterials, 13(20), 2756. https://doi.org/10.3390/nano13202756





