Thermal Transport and Rheological Properties of Hybrid Nanofluids Based on Vegetable Lubricants
Abstract
:1. Introduction
2. Materials and Methods
3. Results
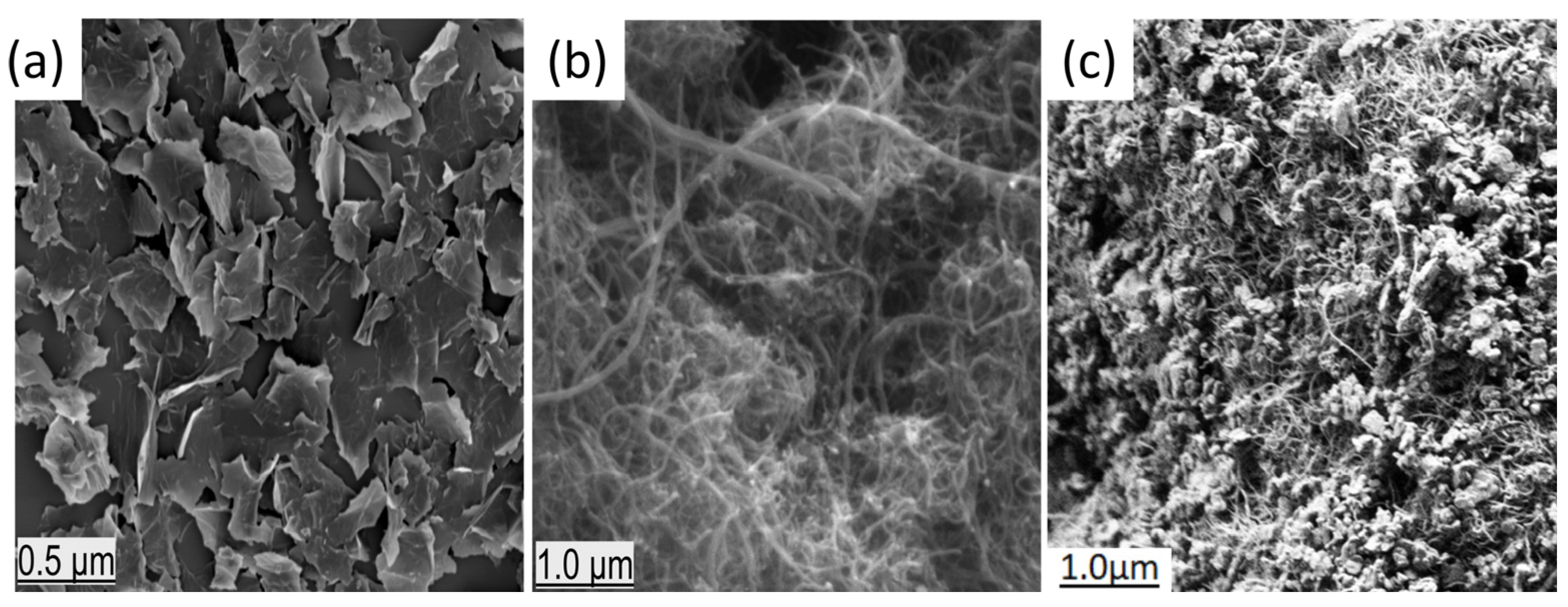
3.1. SEM Images
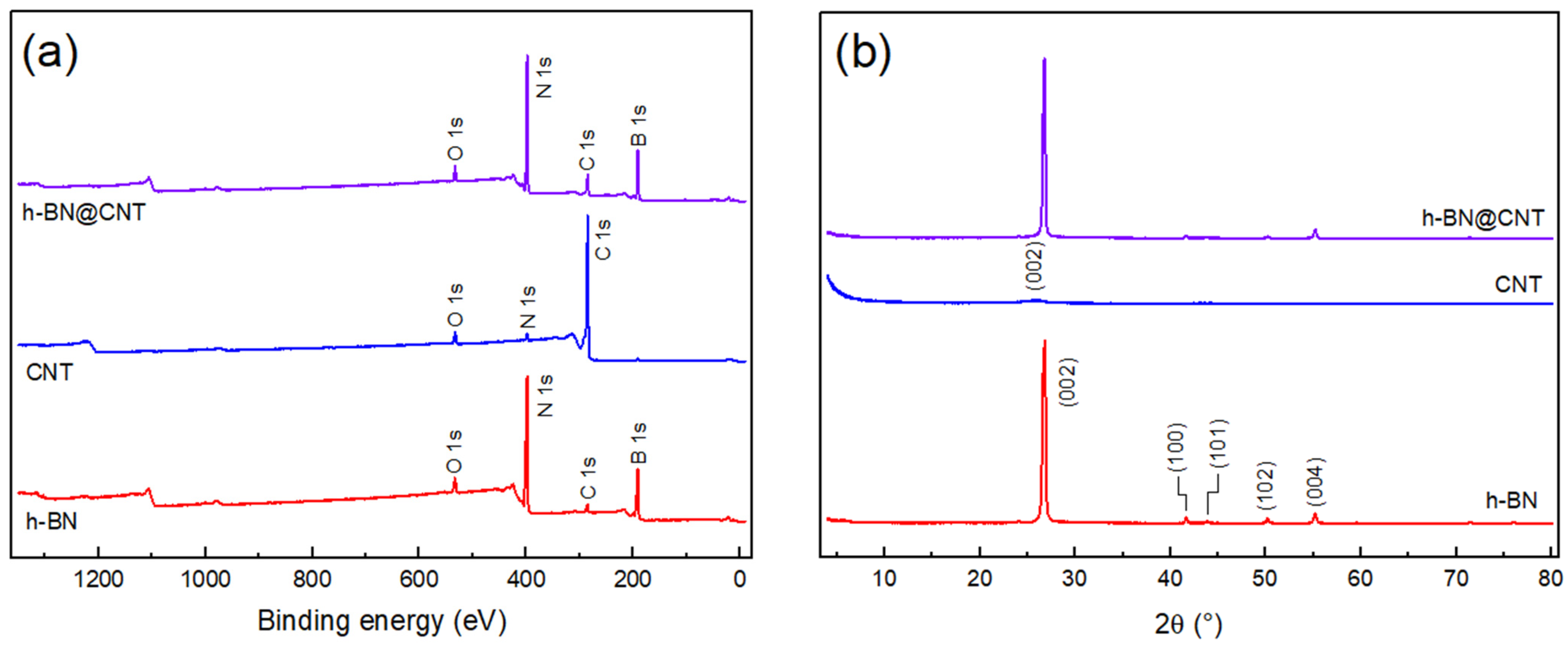
3.2. XRD and XPS Analysis
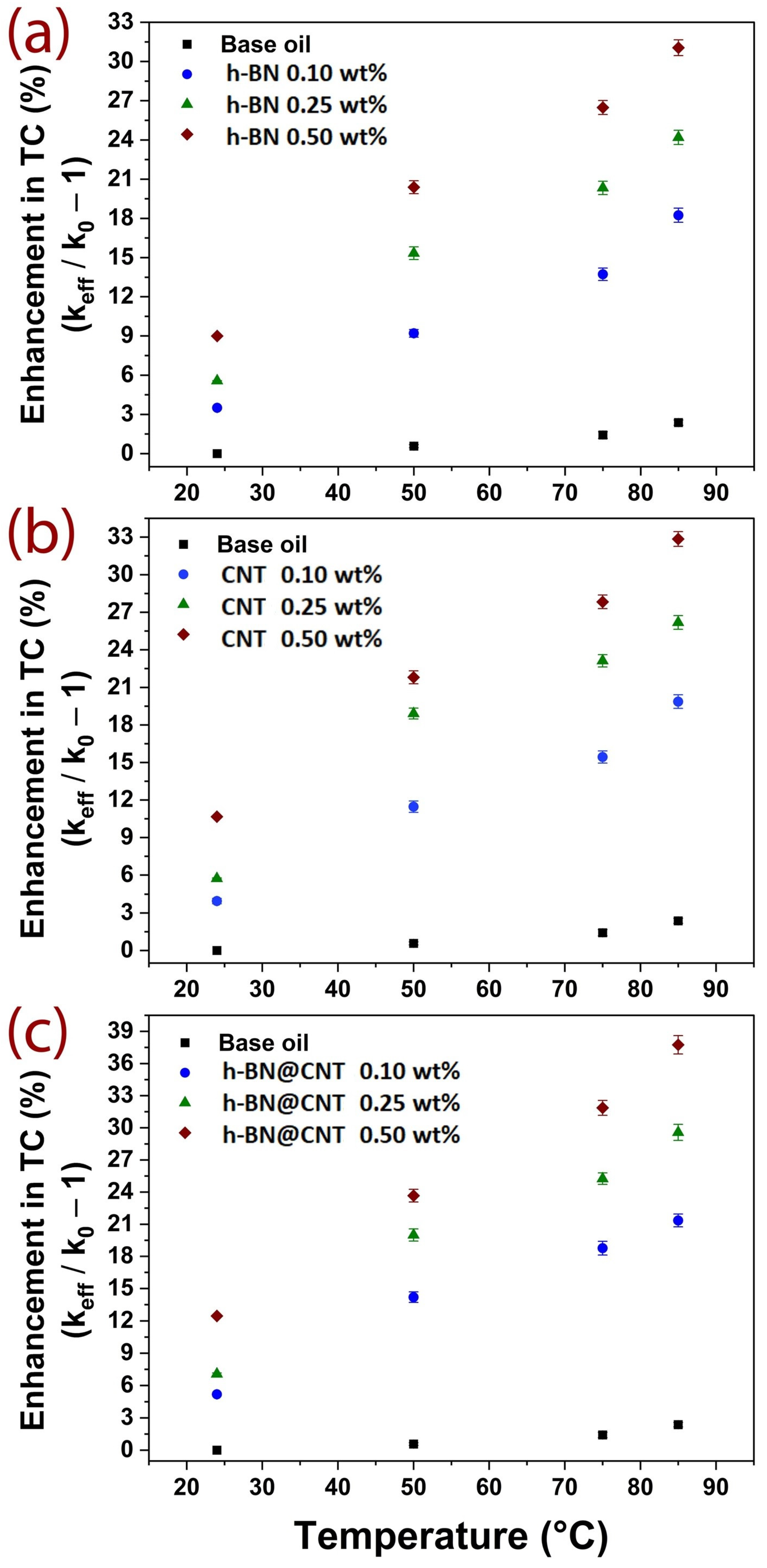
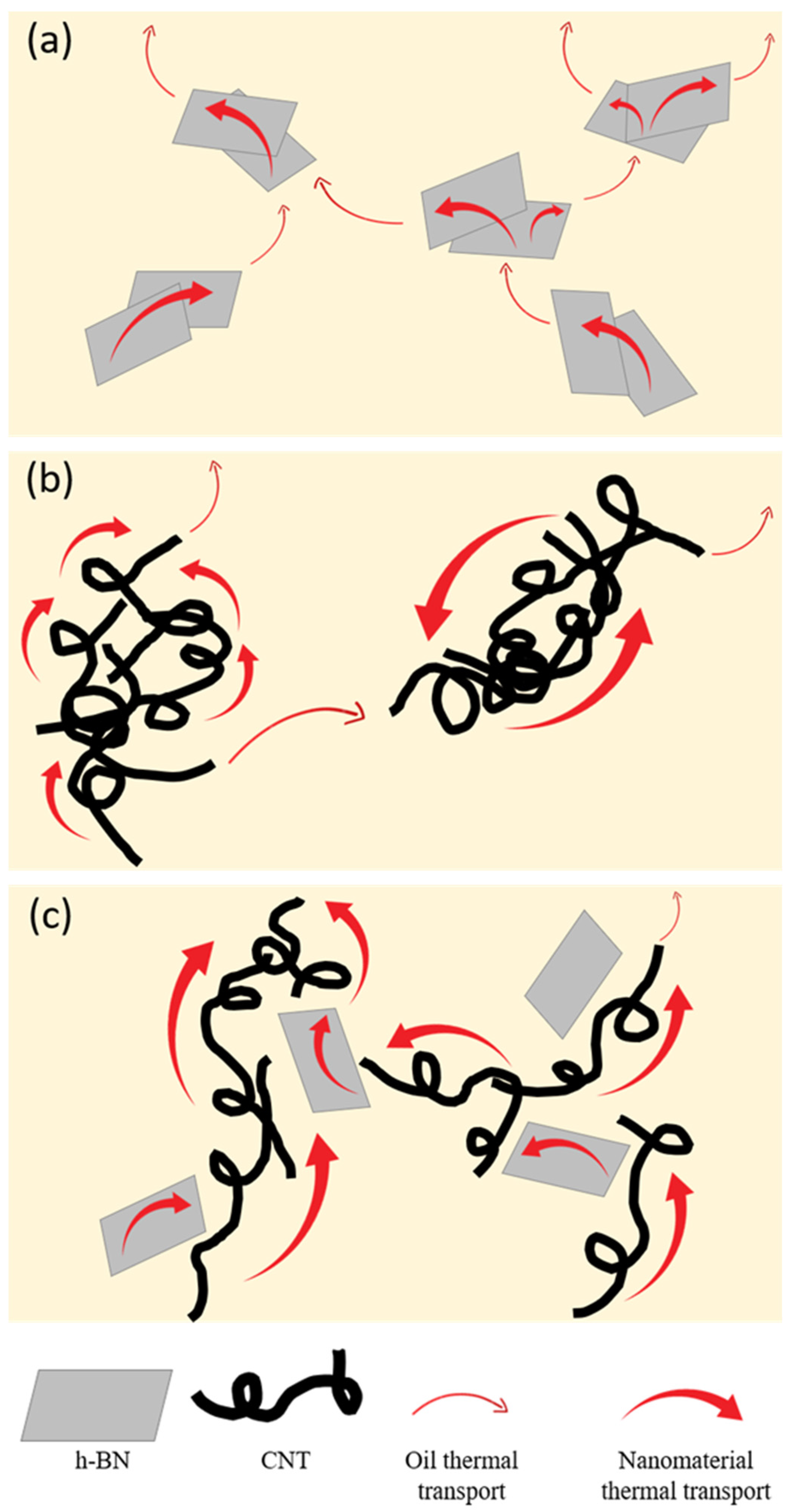
3.3. Thermal Transport Performance
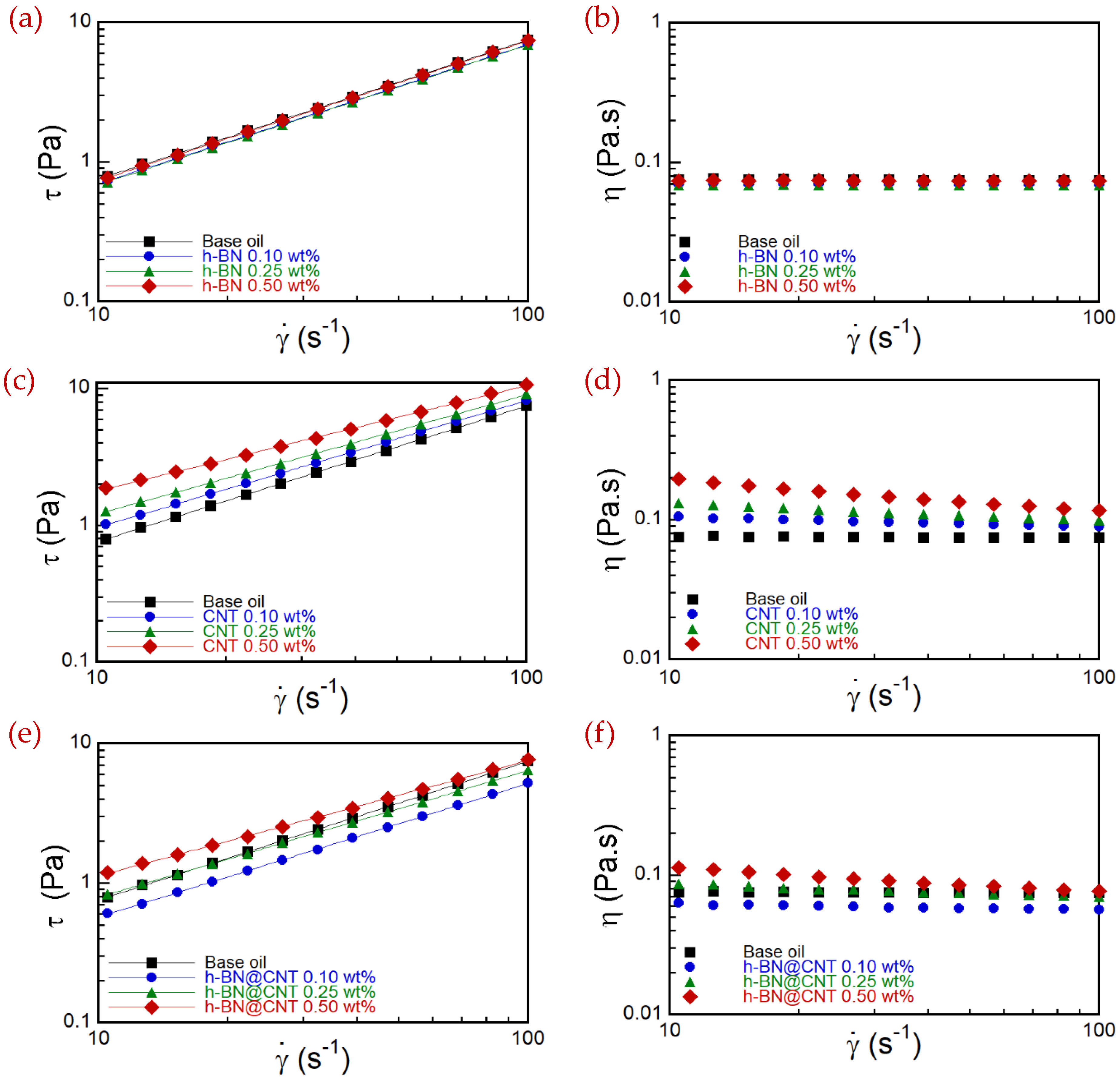
3.4. Rheology Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taha-Tijerina, J.; Ribeiro, H.; Aviña, K.; Martínez, J.M.; Godoy, A.P.; Cremonezzi, J.M.d.O.; Luciano, M.A.; Gimenes Benega, M.A.; Andrade, R.J.E.; Fechine, G.J.M.; et al. Thermal Conductivity Performance of 2D h-BN/MoS2/-Hybrid Nanostructures Used on Natural and Synthetic Esters. Nanomaterials 2020, 10, 1160. [Google Scholar] [CrossRef] [PubMed]
- Salah, N.; Abdel-wahab, M.S.; Alshahrie, A.; Alharbi, N.D.; Khan, Z.H. Carbon nanotubes of oil fly ash as lubricant additives for different base oils and their tribology performance. RSC Adv. 2017, 7, 40295–40302. [Google Scholar] [CrossRef]
- Taha-Tijerina, J.; Narayanan, T.N.; Gao, G.; Rohde, M.; Tsentalovich, D.A.; Pasquali, M.; Ajayan, P.M. Electrically Insulating Thermal Nano-Oils Using 2D Fillers. ACS Nano 2012, 6, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Taha-Tijerina, J.J.; Aviña, K.; Ulloa-Castillo, N.A.; Melo-Maximo, D.V. Thermal Transport and Physical Characteristics of Silver-Reinforced Biodegradable Nanolubricant. Sustainability 2023, 15, 8795. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Silva, S.S.; Reis, R.L. Challenges and opportunities on vegetable oils derived systems for biomedical applications. Biomater. Adv. 2022, 134, 112720. [Google Scholar] [CrossRef]
- Ramteke, S.M.; Chelladurai, H. Effects of hexagonal boron nitride based nanofluid on the tribological and performance, emission characteristics of a diesel engine: An experimental study. Eng. Rep. 2020, 2, e12216. [Google Scholar] [CrossRef]
- Roy, S.; Zhang, X.; Puthirath, A.B.; Meiyazhagan, A.; Bhattacharyya, S.; Rahman, M.M.; Babu, G.; Susarla, S.; Saju, S.K.; Tran, M.K.; et al. Structure, Properties and Applications of Two-Dimensional Hexagonal Boron Nitride. Adv. Mater. 2021, 33, 2101589. [Google Scholar] [CrossRef]
- Thakur, A.; Ganjoo, R.; Kumar, A. Surface Modified Carbon Nanotubes in Corrosion Protection. In Surface Modified Carbon Nanotubes Volume 1: Fundamentals, Synthesis and Recent Trends; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2022; Volume 1424, pp. 235–255. [Google Scholar]
- Pinto, G.M.; Cremonezzi, J.M.O.; Ribeiro, H.; Andrade, R.J.E.; Demarquette, N.R.; Fechine, G.J.M. From two-dimensional materials to polymer nanocomposites with emerging multifunctional applications: A critical review. Polym. Compos. 2023, 44, 1438–1470. [Google Scholar] [CrossRef]
- Ribeiro, H.; Trigueiro, J.P.C.; Lopes, M.C.; Pedrotti, J.J.; Woellner, C.F.; Silva, W.M.; Silva, G.G.; Ajayan, P.M. Enhanced thermal conductivity and mechanical properties of hybrid MoS2/h-BN polyurethane nanocomposites. J. Appl. Polym. Sci. 2018, 135, 46560. [Google Scholar] [CrossRef]
- Thomas, S.; Sobhan, C.B.; Taha-Tijerina, J.; Narayanan, T.N.; Ajayan, P.M. Investigations on Transient Natural Convection in Boron Nitride-Mineral Oil Nanofluid Systems. In Proceedings of the ASME 2012 International Mechanical Engineering Congress and Exposition, Houston, TX, USA, 9–15 November 2012; pp. 671–678. [Google Scholar]
- Taha-Tijerina, J.; Kochandra, R.; Autreto, P.; Narayanan, T.; Ajayan, P.; Sobhan, C. Thermal Transport Effects of h-BN 2D-Nanosheet Reinforced Fluids: A Molecular Dynamics and Experimental Analysis. J. Eng. Technol. 2019, 8, 356–370. [Google Scholar]
- Yu, B.; Fan, J.; He, J.; Liu, Y.; Wang, R.; Qi, K.; Han, P.; Luo, Z. Boron nitride nanosheets: Large-scale exfoliation in NaOH-LiCl solution and their highly thermoconductive insulating nanocomposite paper with PI via electrospinning-electrospraying. J. Alloys Compd. 2023, 930, 167303. [Google Scholar] [CrossRef]
- Yang, Y.; Grulke, E.A.; Zhang, Z.G.; Wu, G. Thermal and rheological properties of carbon nanotube-in-oil dispersions. J. Appl. Phys. 2006, 99, 114307. [Google Scholar] [CrossRef]
- Ahmad, S.; Ali, K.; Ashraf, M.; Khalifa, H.A.E.-W.; ElSeabee, F.A.A.; Din, E.S.M.T.E. Analysis of pure nanofluid (GO/engine oil) and hybrid nanofluid (GO–Fe3O4/engine oil): Novel thermal and magnetic features. Nanotechnol. Rev. 2022, 11, 2903–2915. [Google Scholar] [CrossRef]
- Wanatasanappan, V.V.; Rezman, M.; Abdullah, M.Z. Thermophysical Properties of Vegetable Oil-Based Hybrid Nanofluids Containing Al2O3-TiO2 Nanoparticles as Insulation Oil for Power Transformers. Nanomaterials 2022, 12, 3621. [Google Scholar] [CrossRef] [PubMed]
- Nabil, M.F.; Azmi, W.H.; Hamid, K.A.; Zawawi, N.N.M.; Priyandoko, G.; Mamat, R. Thermo-physical properties of hybrid nanofluids and hybrid nanolubricants: A comprehensive review on performance. Int. Commun. Heat Mass Transf. 2017, 83, 30–39. [Google Scholar] [CrossRef]
- Afrand, M. Experimental study on thermal conductivity of ethylene glycol containing hybrid nano-additives and development of a new correlation. Appl. Therm. Eng. 2017, 110, 1111–1119. [Google Scholar] [CrossRef]
- Lee, D.-K.; Yoo, J.; Kim, H.; Kang, B.-H.; Park, S.-H. Electrical and Thermal Properties of Carbon Nanotube Polymer Composites with Various Aspect Ratios. Materials 2022, 15, 1356. [Google Scholar] [CrossRef]
- Cai, Q.; Scullion, D.; Gan, W.; Falin, A.; Zhang, S.; Watanabe, K.; Taniguchi, T.; Chen, Y.; Santos, E.J.G.; Li, L.H. High thermal conductivity of high-quality monolayer boron nitride and its thermal expansion. Sci. Adv. 2019, 5, eaav0129. [Google Scholar] [CrossRef]
- Huang, Z.; He, G.; Li, J.; Wang, F.; Zhang, R.; Yao, D. Exponentially reduced carrier mobility of natural ester via blocking effect of 2D hexagonal boron nitride nanosheets. High Volt. 2021, 6, 219–229. [Google Scholar] [CrossRef]
- Küster, K.; Hooshmand, Z.; Rosenblatt, D.P.; Koslowski, S.; Le, D.; Starke, U.; Rahman, T.S.; Kern, K.; Schlickum, U. Growth of Graphene Nanoflakes/h-BN Heterostructures. Adv. Mater. Interfaces 2021, 8, 2100766. [Google Scholar] [CrossRef]
- Varga, M.; Izak, T.; Vretenar, V.; Kozak, H.; Holovsky, J.; Artemenko, A.; Hulman, M.; Skakalova, V.; Lee, D.S.; Kromka, A. Diamond/carbon nanotube composites: Raman, FTIR and XPS spectroscopic studies. Carbon 2017, 111, 54–61. [Google Scholar] [CrossRef]
- Parmee, R.J.; Collins, C.M.; Milne, W.I.; Cole, M.T. X-ray generation using carbon nanotubes. Nano Converg. 2015, 2, 1. [Google Scholar] [CrossRef]
- Shafi, W.K.; Charoo, M.S. An overall review on the tribological, thermal and rheological properties of nanolubricants. Tribol.-Mater. Surf. Interfaces 2021, 15, 20–54. [Google Scholar] [CrossRef]
- Yasmin, H.; Giwa, S.O.; Noor, S.; Aybar, H.Ş. Reproduction of Nanofluid Synthesis, Thermal Properties and Experiments in Engineering: A Research Paradigm Shift. Energies 2023, 16, 1145. [Google Scholar] [CrossRef]
- Apmann, K.; Fulmer, R.; Scherer, B.; Good, S.; Wohld, J.; Vafaei, S. Nanofluid Heat Transfer: Enhancement of the Heat Transfer Coefficient inside Microchannels. Nanomaterials 2022, 12, 615. [Google Scholar] [CrossRef]
- Rabinowitsch, B. Über die Viskosität und Elastizität von Solen. Z. Phys. Chem. 1929, 145A, 1–26. [Google Scholar] [CrossRef]
- Haroon, K.; John, T.; Fonte, C.P.; Mendoza, Ć.; Baker, M.; Martin, P. Investigating the Design and Implementation of an In-Line Near-Infrared Probe Using Computational Fluid Dynamics for Measurement of Non-Newtonian Fluids. Appl. Spectrosc. 2022, 76, 331–339. [Google Scholar] [CrossRef]
- E, S.; Zhu, Z.; Xie, L.; Li, Z.; Geng, R.; Li, T.; Li, C.; Lu, W.; Yao, Y. An integrated strategy towards the high-yield fabrication of soluble boron nitride nanosheets. Chem. Eng. J. 2019, 360, 1407–1415. [Google Scholar] [CrossRef]
- Trujillo de Santiago, G.; de Gante, C.; Garca-Lara, S.; Ballesc-Estrada, A.; Alvarez, M. Studying Mixing in Non-Newtonian Blue Maize Flour Suspension Using Color Analysis. PLoS ONE 2014, 9, e112954. [Google Scholar] [CrossRef]
- Lin-Gibson, S.; Pathak, J.A.; Grulke, E.A.; Wang, H.; Hobbie, E.K. Elastic Flow Instability in Nanotube Suspensions. Phys. Rev. Lett. 2004, 92, 048302. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Bazilevsky, A.V.; Yarin, A.L.; Megaridis, C.M. Elongational and shear rheology of carbon nanotube suspensions. Rheol. Acta 2009, 48, 597–609. [Google Scholar] [CrossRef]
- Soares, Y.C.F.; Yokoyama, D.D.; Costa, L.C.; de Oliveira Cremonezzi, J.M.; Ribeiro, H.; Naccache, M.F.; Andrade, R.J.E. Multifunctional hexagonal boron nitride dispersions based in xanthan gum for use in drilling fluids. Geoenergy Sci. Eng. 2023, 221, 111311. [Google Scholar] [CrossRef]
- Sajid, M.U.; Ali, H.M. Thermal conductivity of hybrid nanofluids: A critical review. Int. J. Heat Mass Transf. 2018, 126, 211–234. [Google Scholar] [CrossRef]
| General Properties | Standard | Units | |
|---|---|---|---|
| Density at 20 °C | ISO 3675 | <0.96 | g/cm3 |
| Viscosity at 40 °C | D445 | <50 | mm2/s |
| Dissipation Factor 25 °C | D924 | <0.20 | - |
| Acute Toxicity | OECD 202 | Non-Toxic | - |
| General Properties | h-BN | MWCNT | Units |
|---|---|---|---|
| Purity | 98 | >95 | % |
| Density | 2.29 | 1.7–2.1 | g/cm3 |
| Diameter | - | 8–15 | nm |
| Particle Size/Length | ~1 | 10–50 | µm |
| Thermal conductivity | 300–500 | 3000–2000 | W/m K |
| Suspension | Nanomaterial Concentration (wt.%) | K (Pa.sn) | n | Model |
|---|---|---|---|---|
| Base Oil | 0 | 0.075 | 1 | Newtonian |
| h-BN | 0.10 | 0.070 | 1 | Newtonian |
| 0.25 | 0.069 | 1 | Newtonian | |
| 0.50 | 0.074 | 1 | Newtonian | |
| CNT | 0.10 | 0.113 | 0.93 | Ostwald-de Waele |
| 0.25 | 0.160 | 0.87 | Ostwald-de Waele | |
| 0.50 | 0.299 | 0.77 | Ostwald-de Waele | |
| h-BN@CNT | 0.10 | 0.063 | 0.96 | Ostwald-de Waele |
| 0.25 | 0.099 | 0.91 | Ostwald-de Waele | |
| 0.50 | 0.169 | 0.82 | Ostwald-de Waele |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, H.; Taha-Tijerina, J.J.; Gomez, O.; Acosta, E.; Pinto, G.M.; Moraes, L.R.C.; Fechine, G.J.M.; Andrade, R.J.E.; Reinoza, J.; Padilla, V.; et al. Thermal Transport and Rheological Properties of Hybrid Nanofluids Based on Vegetable Lubricants. Nanomaterials 2023, 13, 2739. https://doi.org/10.3390/nano13202739
Ribeiro H, Taha-Tijerina JJ, Gomez O, Acosta E, Pinto GM, Moraes LRC, Fechine GJM, Andrade RJE, Reinoza J, Padilla V, et al. Thermal Transport and Rheological Properties of Hybrid Nanofluids Based on Vegetable Lubricants. Nanomaterials. 2023; 13(20):2739. https://doi.org/10.3390/nano13202739
Chicago/Turabian StyleRibeiro, Hélio, Jose Jaime Taha-Tijerina, Ofelia Gomez, Ever Acosta, Gabriel M. Pinto, Lorena R. C. Moraes, Guilhermino J. M. Fechine, Ricardo J. E. Andrade, Jefferson Reinoza, Victoria Padilla, and et al. 2023. "Thermal Transport and Rheological Properties of Hybrid Nanofluids Based on Vegetable Lubricants" Nanomaterials 13, no. 20: 2739. https://doi.org/10.3390/nano13202739
APA StyleRibeiro, H., Taha-Tijerina, J. J., Gomez, O., Acosta, E., Pinto, G. M., Moraes, L. R. C., Fechine, G. J. M., Andrade, R. J. E., Reinoza, J., Padilla, V., & Lozano, K. (2023). Thermal Transport and Rheological Properties of Hybrid Nanofluids Based on Vegetable Lubricants. Nanomaterials, 13(20), 2739. https://doi.org/10.3390/nano13202739










