Photocatalytic Hydrogen Production and Tetracycline Degradation Using ZnIn2S4 Quantum Dots Modified g-C3N4 Composites
Abstract
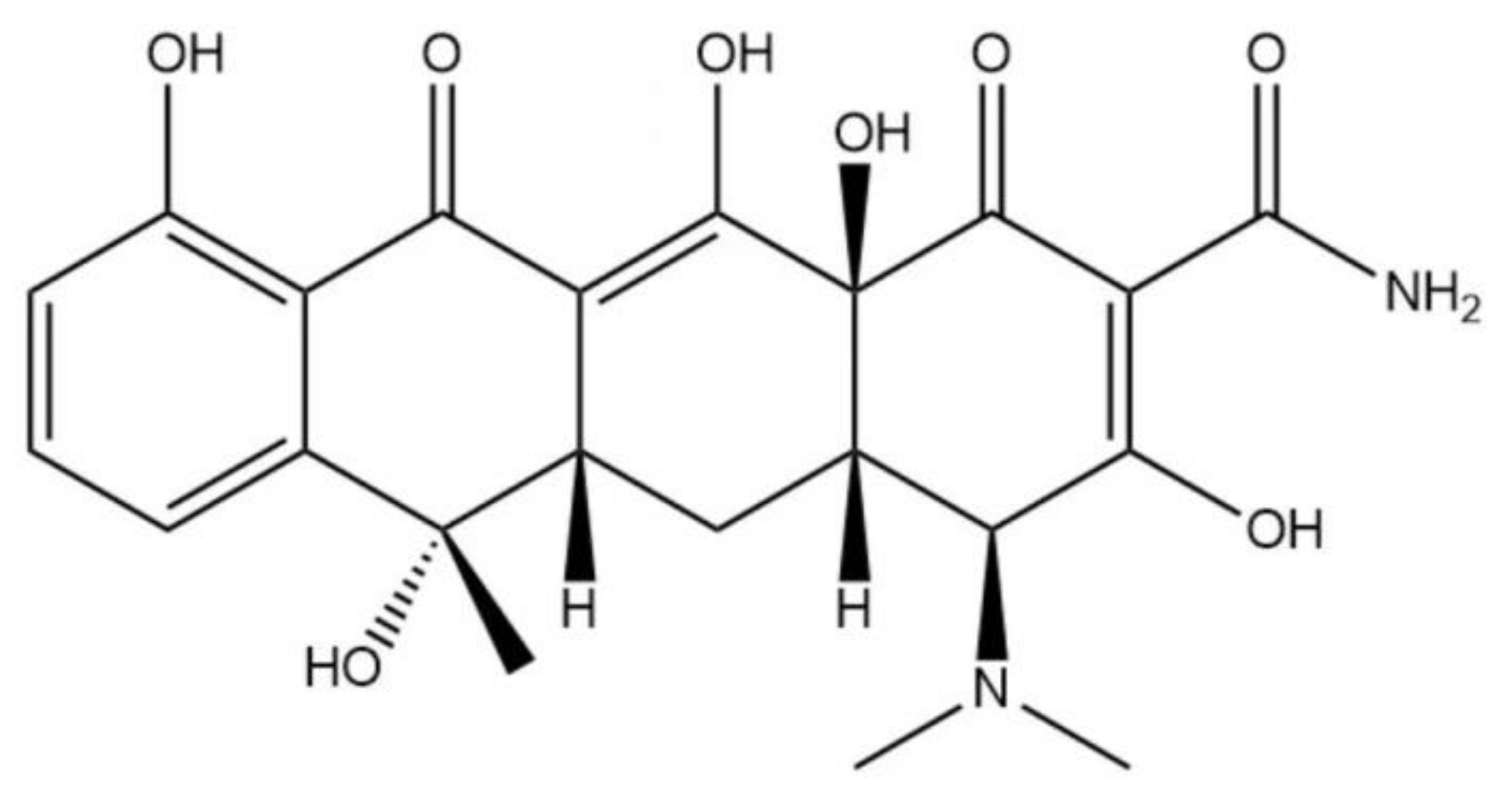
1. Introduction
2. Experimental Section
2.1. Material Preparation
2.2. Characterization
2.3. Photocatalytic Activity
2.4. Radical/Charge Capturing Experiment
2.5. Photoelectrochemical Test
3. Results
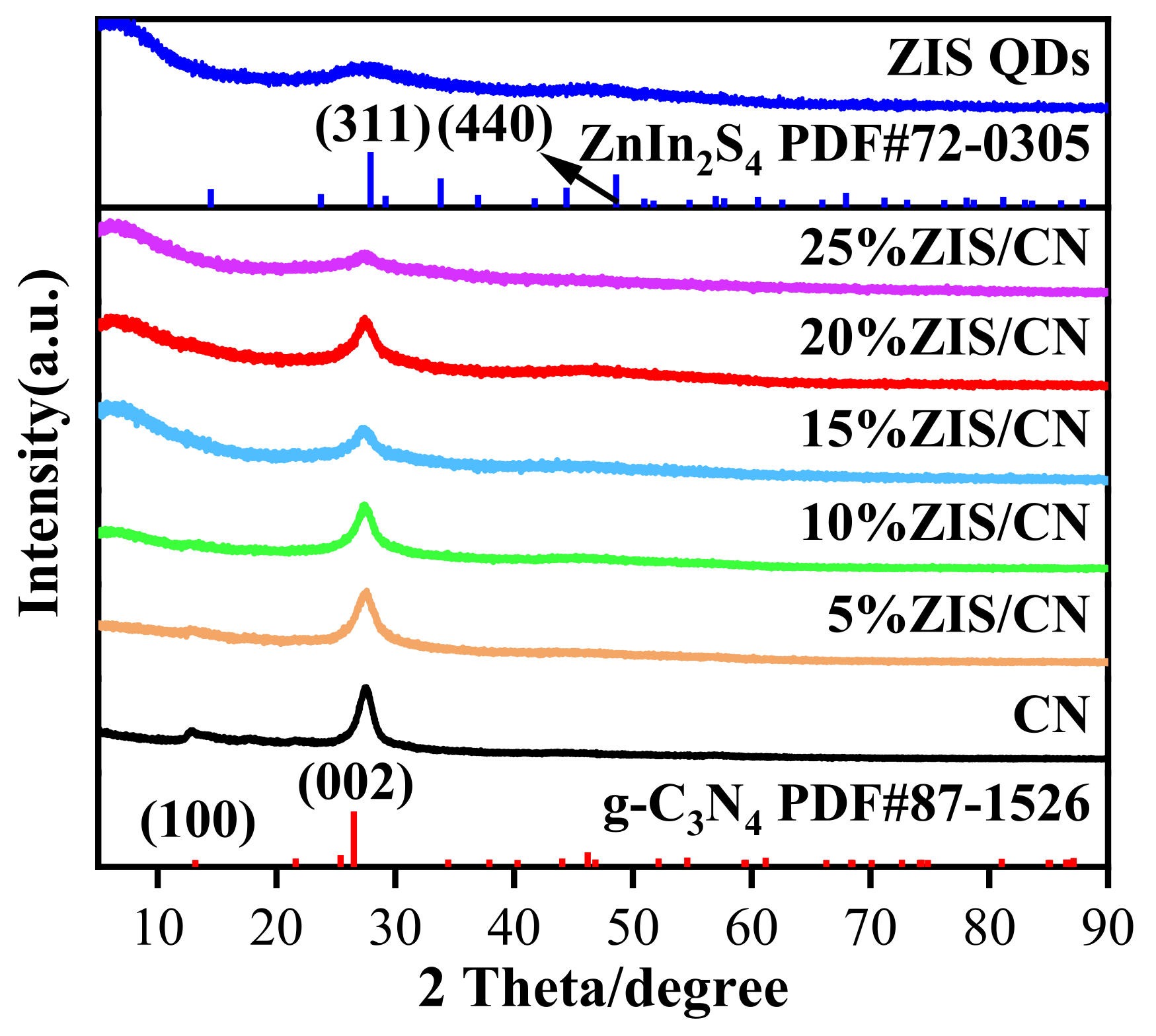
3.1. XRD
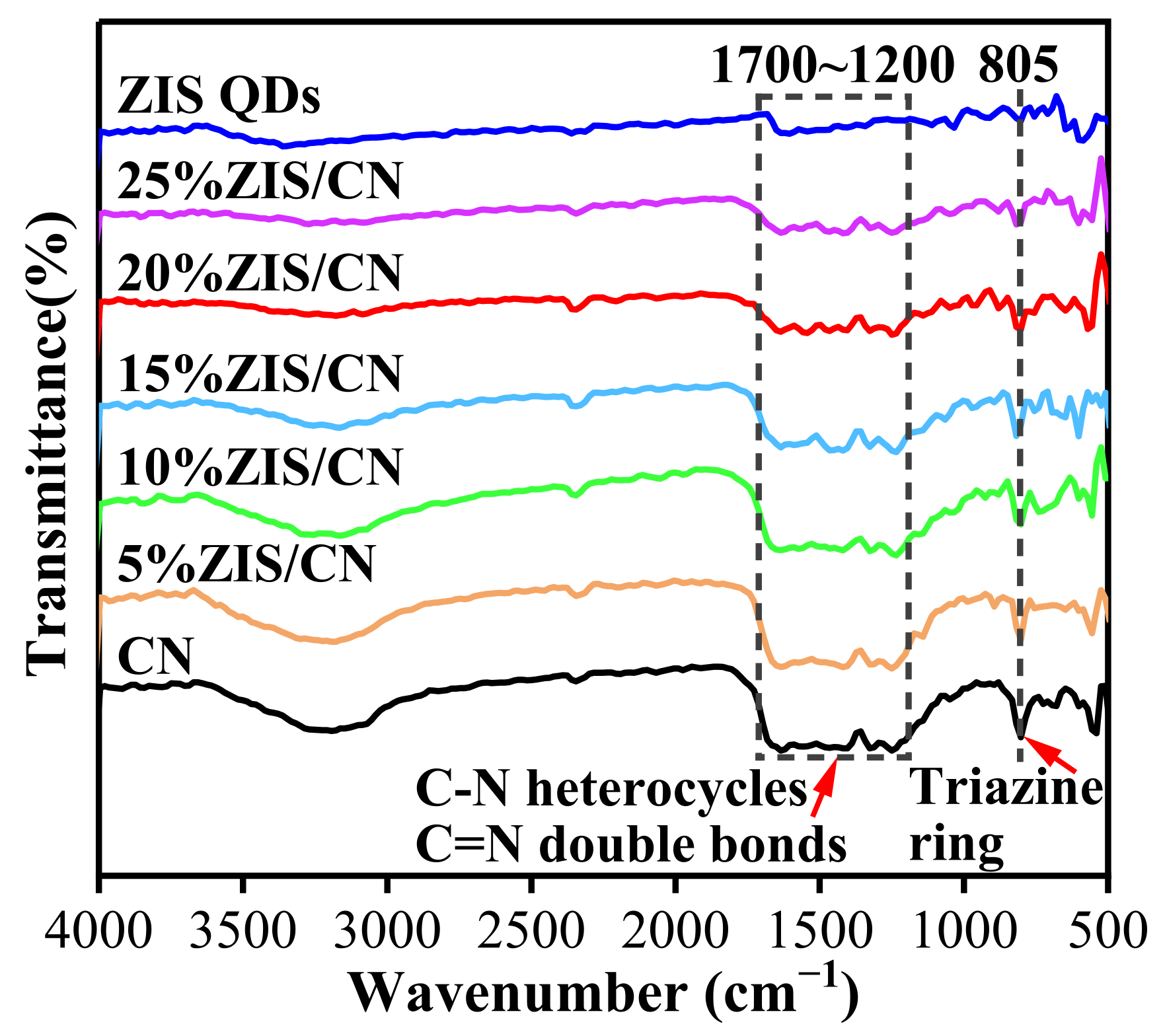
3.2. FTIR
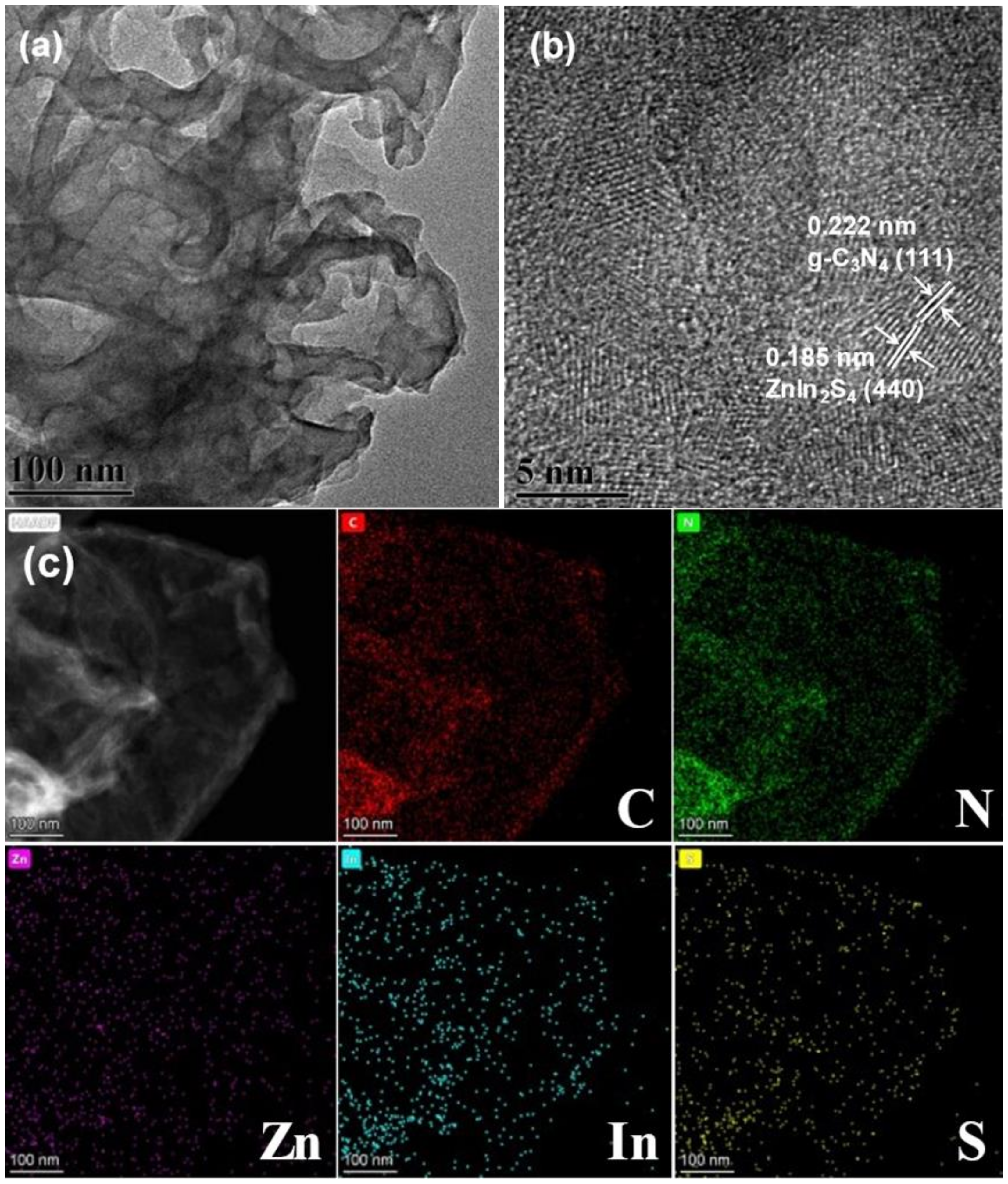
3.3. TEM
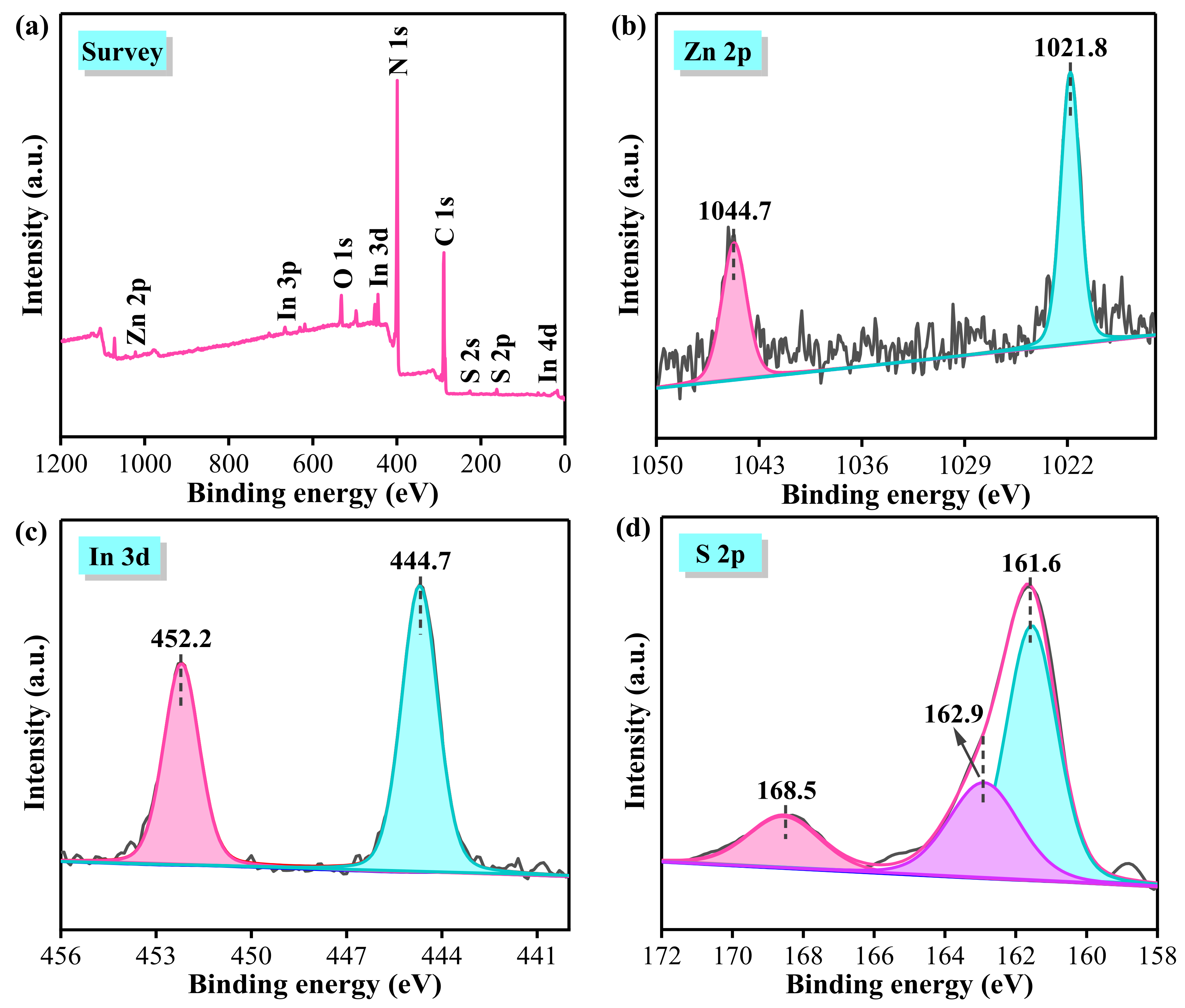
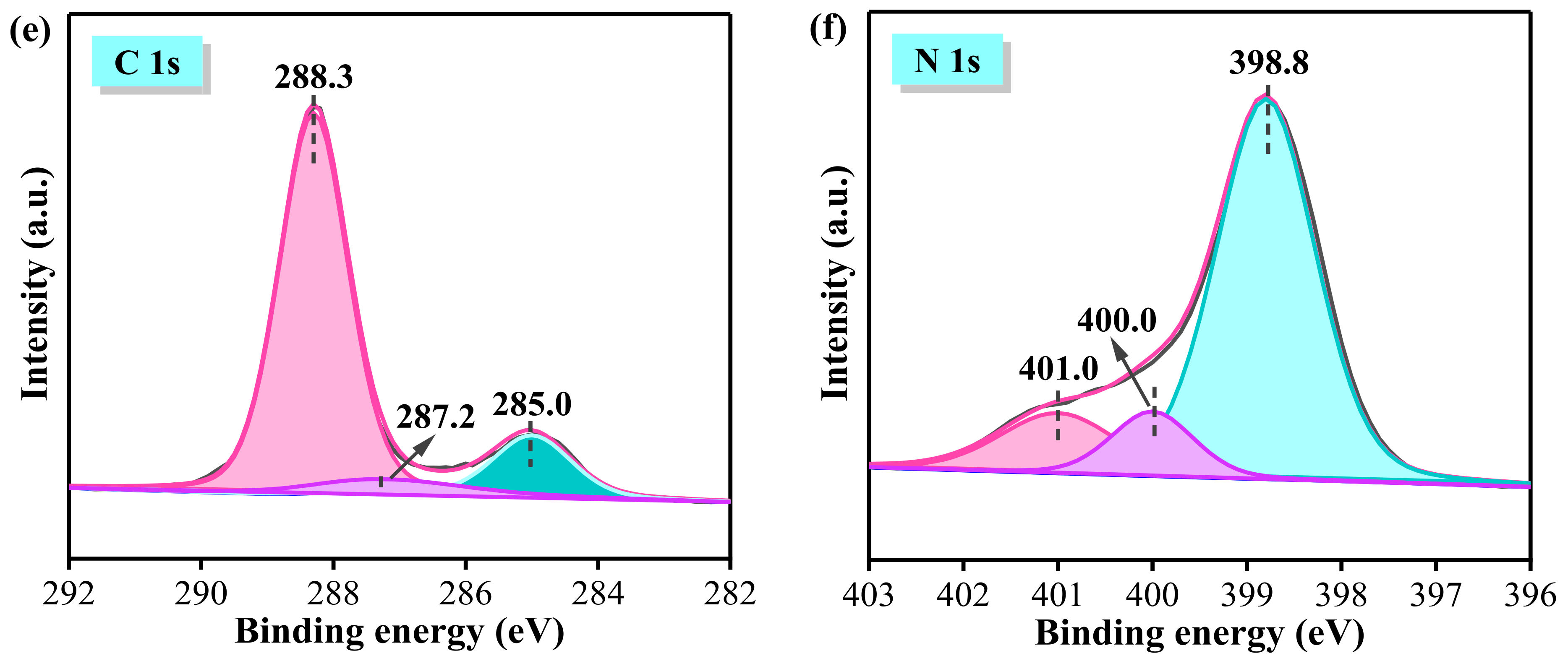
3.4. XPS
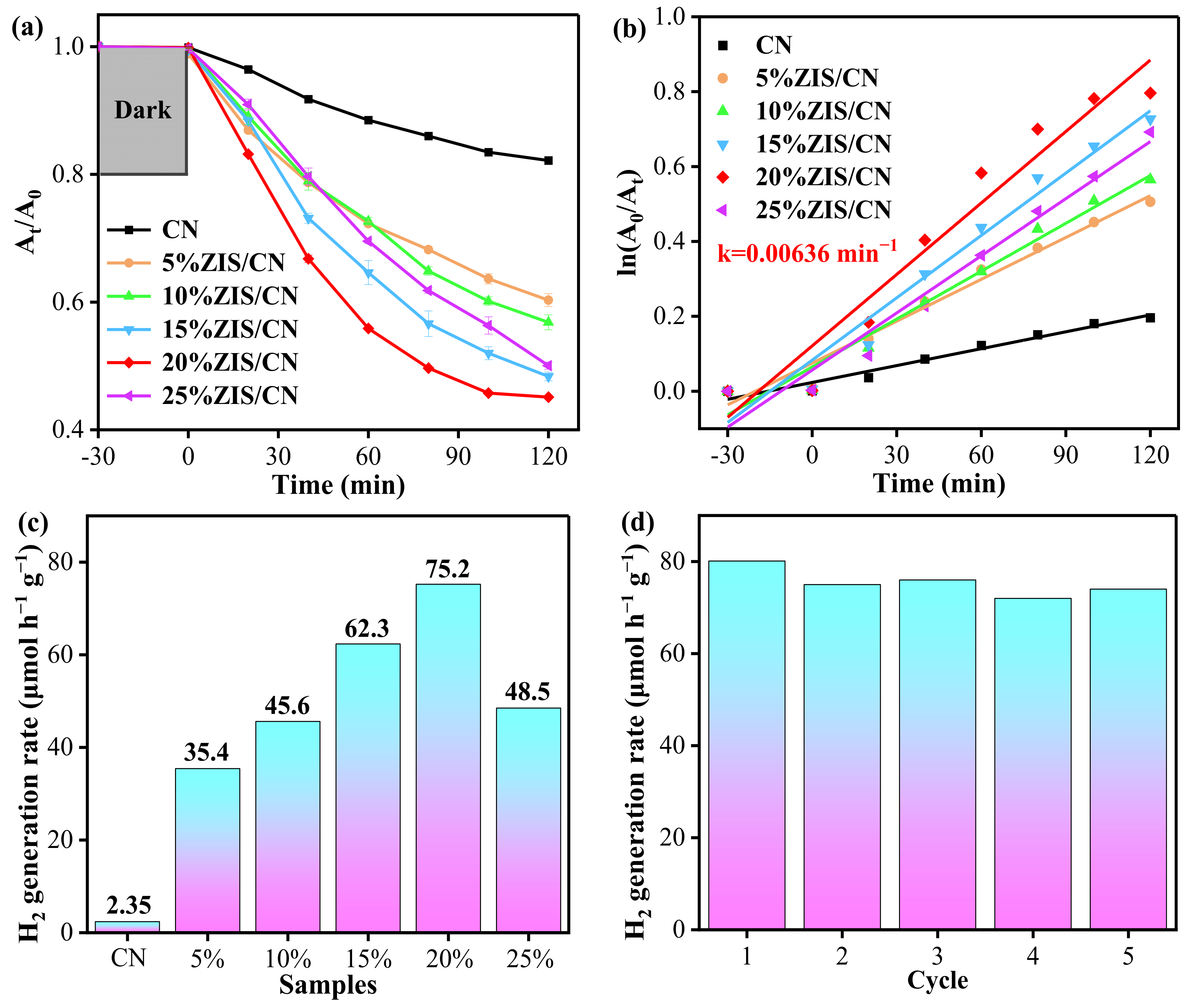
3.5. Photocatalytic Activity
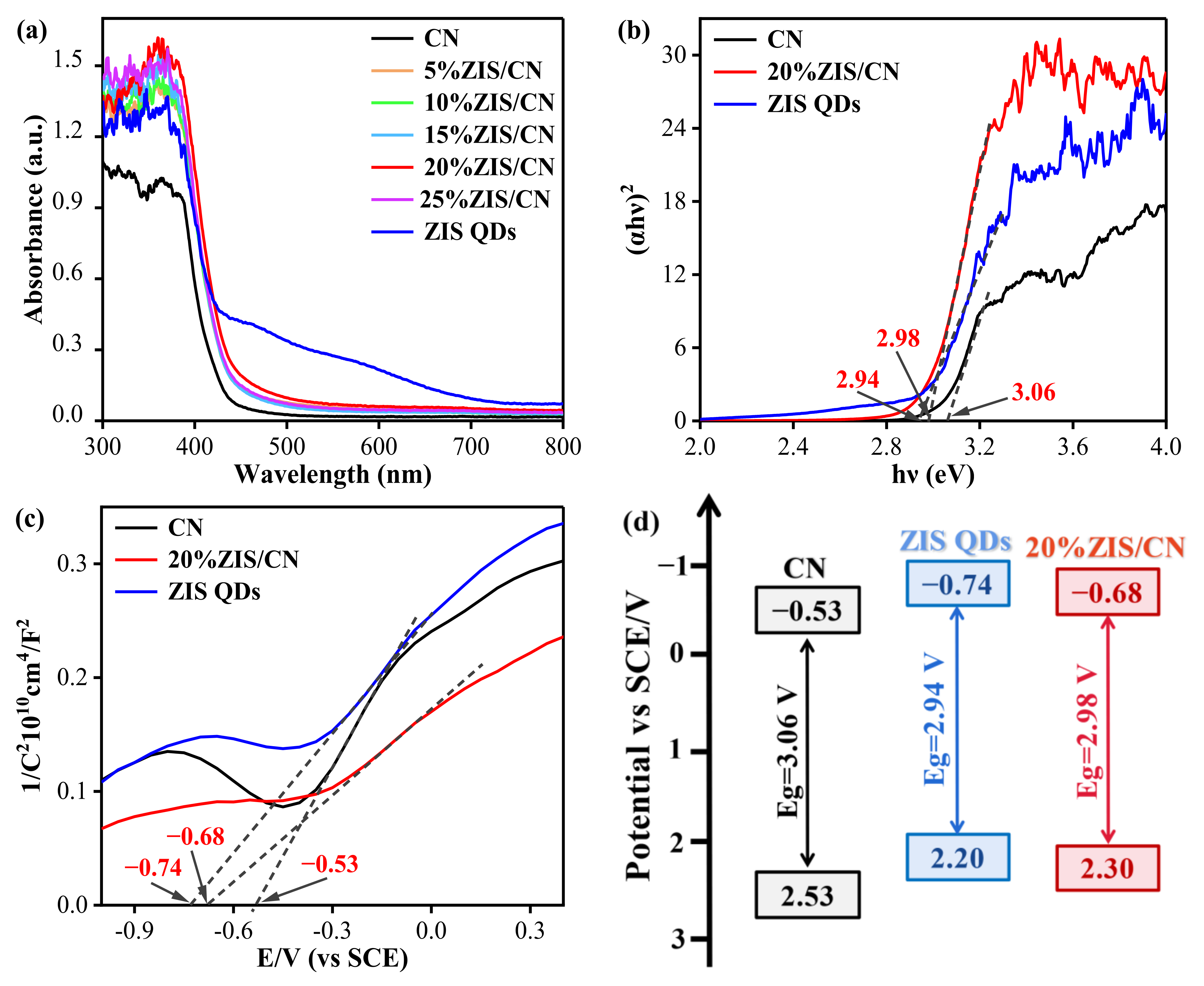
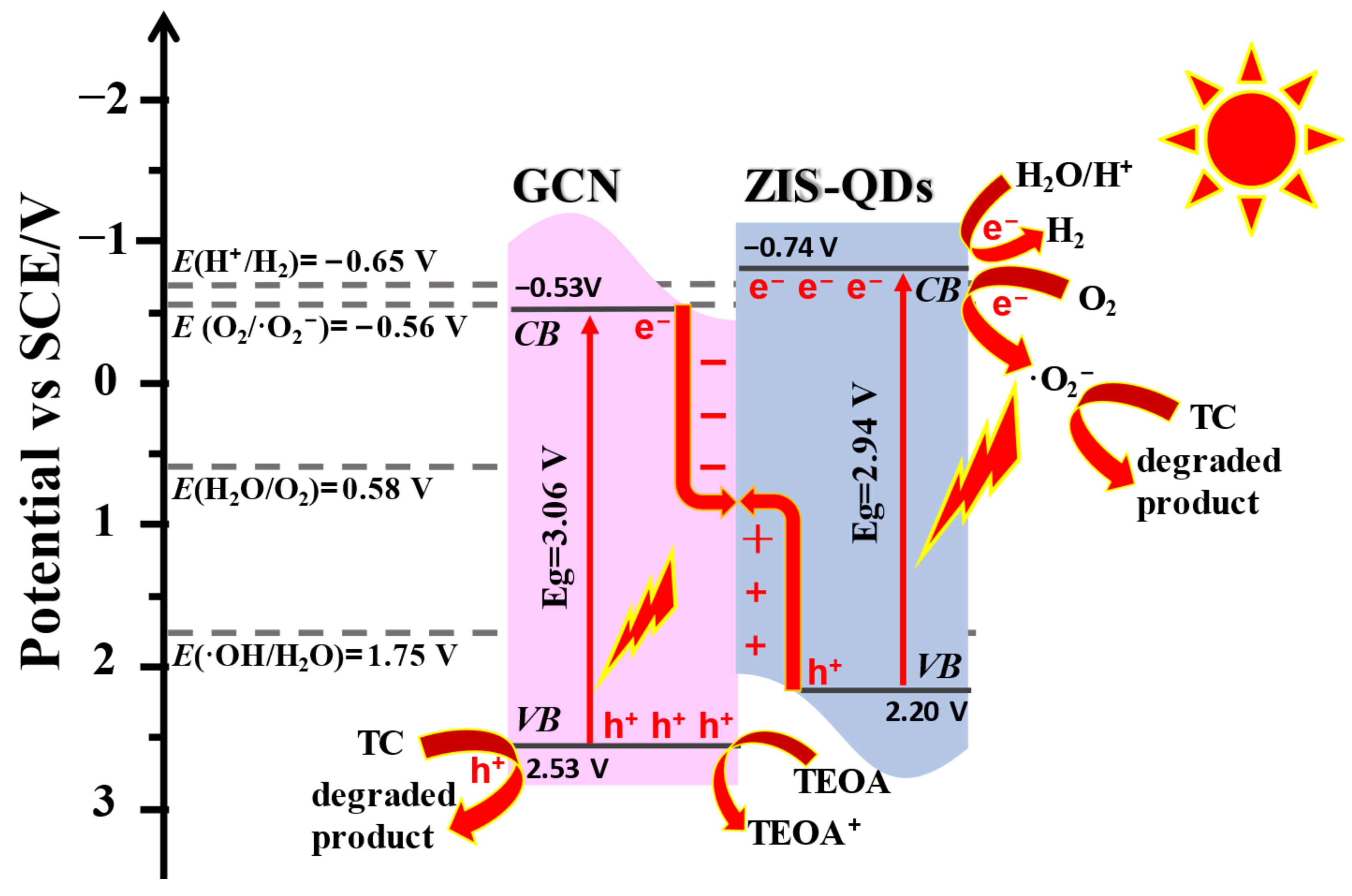
3.6. Energy Band Structure
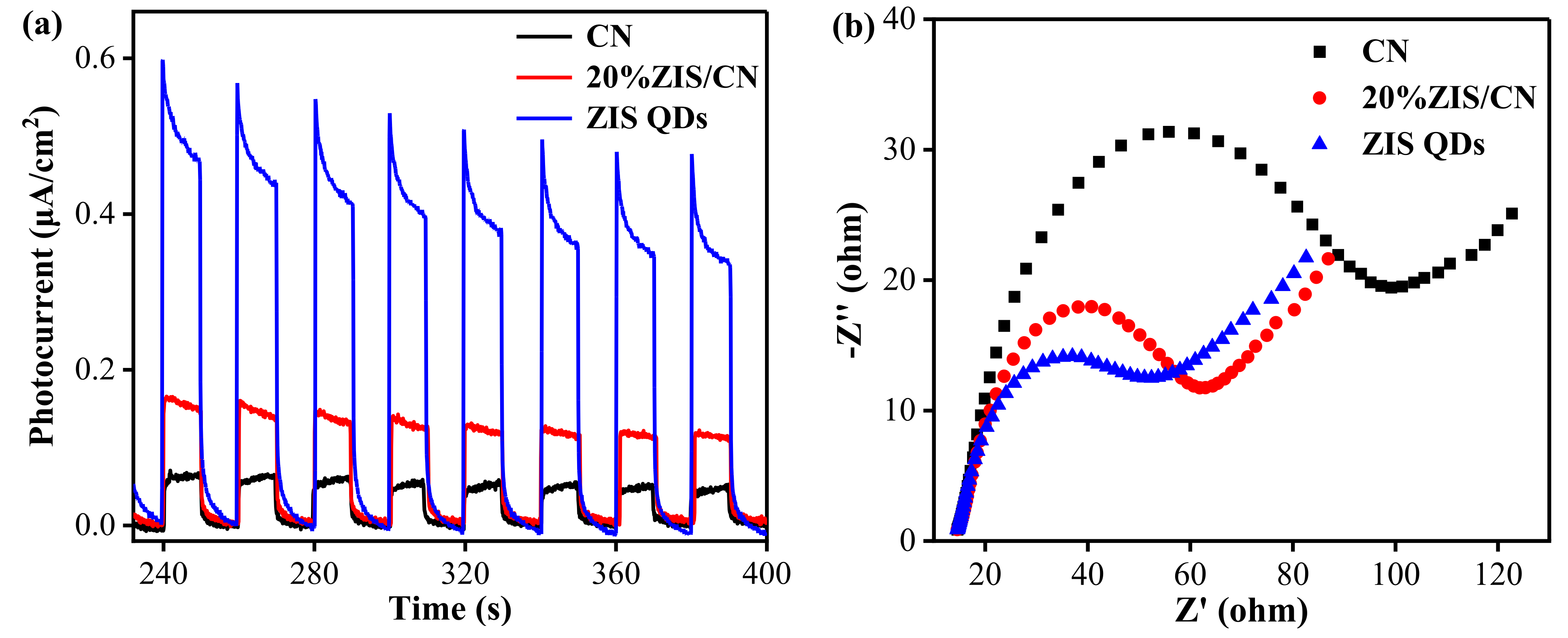
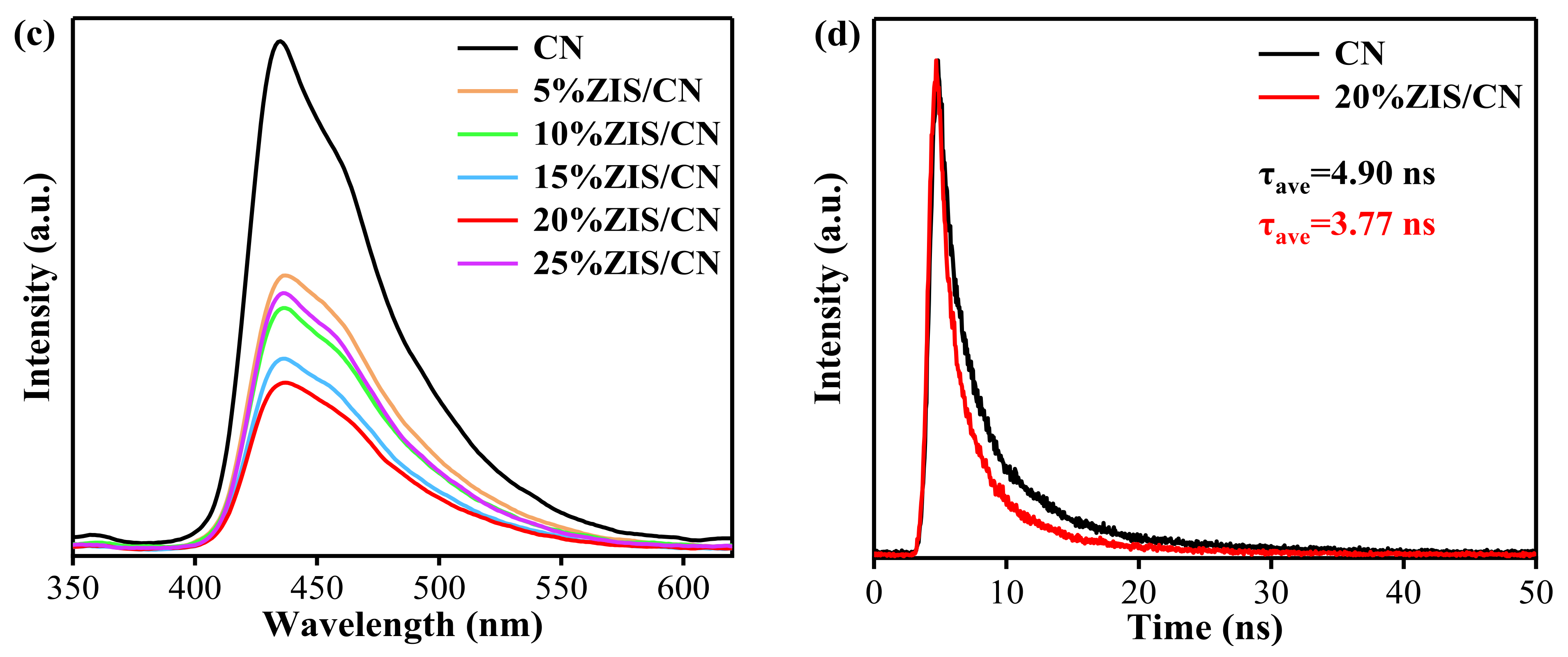
3.7. Photoelectric Performance
3.8. Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zuttel, A.; Remhof, A.; Borgschulte, A.; Friedrichs, O. Hydrogen: The future energy carrier. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 3329–3342. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Xing, X.; Zada, A.; Li, M.; Wang, Q.; Liu, S.-Y.; Lin, H.; Wang, G. Transition metal doped ZnO nanoparticles with enhanced photocatalytic and antibacterial performances: Experimental and DFT studies. Ceram. Int. 2020, 46, 1494–1502. [Google Scholar] [CrossRef]
- Wang, L.; Meng, M.; Zheng, R.; Li, X.; Yuan, H. Synthesis of Two Porous CdS Rods by Anion Exchange Method and Their Photocatalytic Properties. Nanomaterials 2022, 12, 3190. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Zhuang, C.; Zhang, M.; Gholami, P.; Khataee, A. Sonochemical synthesis of photocatalysts and their applications. J. Mater. Sci. Technol. 2022, 123, 243–256. [Google Scholar] [CrossRef]
- Faisal Al, M.; Al Ismail, K.; Qi, K.; Liu, S.-Y.; Selvaraj, R. Microwave Assisted Synthesis of CdS Sub-microspheres for Degradation of Chlorophenols under Solar Light Irradiation. Russ. J. Phys. Chem. A 2019, 93, 2167–2173. [Google Scholar] [CrossRef]
- Gu, X.; Tan, C.; He, L.; Guo, J.; Zhao, X.; Qi, K.; Yan, Y. Mn2+ doped AgInS2 photocatalyst for formaldehyde degradation and hydrogen production from water splitting by carbon tube enhancement. Chemosphere 2022, 304, 135292. [Google Scholar] [CrossRef]
- Nawaz, A.; Kuila, A.; Mishra, N.S.; Leong, K.H.; Sim, L.C.; Saravanan, P.; Jang, M. Challenges and implication of full solar spectrum-driven photocatalyst. Rev. Chem. Eng. 2021, 37, 533–560. [Google Scholar] [CrossRef]
- Buzzetti, L.; Crisenza, G.E.M.; Melchiorre, P. Mechanistic Studies in Photocatalysis. Angew. Chem. Int. Ed. Engl. 2019, 58, 3730–3747. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Qi, K.; Cui, N.; Zhang, M.; Ma, Y.; Wang, G.; Zhao, Z.; Khataee, A. Ionic liquid-assisted synthesis of porous boron-doped graphitic carbon nitride for photocatalytic hydrogen production. Chemosphere 2021, 272, 129953. [Google Scholar] [CrossRef]
- Zhao, Y.; Zada, A.; Yang, Y.; Pan, J.; Wang, Y.; Yan, Z.; Xu, Z.; Qi, K. Photocatalytic Removal of Antibiotics on g-C3N4 Using Amorphous CuO as Cocatalysts. Front. Chem. 2021, 9, 797738. [Google Scholar] [CrossRef]
- Song, J.; Zhao, K.; Yin, X.; Liu, Y.; Khan, I.; Liu, S.Y. Photocatalytic degradation of tetracycline hydrochloride with g-C3N4/Ag/AgBr composites. Front. Chem. 2022, 10, 1069816. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zada, A.; Yu, X.; Liu, F.; Jin, G. NiFe2O4/g-C3N4 heterostructure with an enhanced ability for photocatalytic degradation of tetracycline hydrochloride and antibacterial performance. Chemosphere 2022, 307, 135717. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Hussain, M.I.; Zhou, W.; Chen, Y.; Wang, L.N. g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials 2021, 12, 121. [Google Scholar] [CrossRef]
- Qi, K.; Liu, S.-Y.; Zada, A. Graphitic carbon nitride, a polymer photocatalyst. J. Taiwan Inst. Chem. Eng. 2020, 109, 111–123. [Google Scholar] [CrossRef]
- Zhu, A.; Qiao, L.; Tan, P.; Pan, J. Interfaces of graphitic carbon nitride-based composite photocatalysts. Inorg. Chem. Front. 2020, 7, 4754–4793. [Google Scholar] [CrossRef]
- Qi, K.; Li, Y.; Xie, Y.; Liu, S.Y.; Zheng, K.; Chen, Z.; Wang, R. Ag Loading Enhanced Photocatalytic Activity of g-C3N4 Porous Nanosheets for Decomposition of Organic Pollutants. Front. Chem. 2019, 7, 91. [Google Scholar] [CrossRef]
- Wang, L.; Fan, Z.; Cao, X.; Fan, P.; Xie, Y.; Sun, Q.; Zhao, J. Template-Free Synthesis of g-C3N4 Nanoball/BiOCl Nanotube Heterojunction with Enhanced Photocatalytic Activity. Nanomaterials 2022, 12, 2569. [Google Scholar] [CrossRef]
- Qi, K.; Liu, S.-Y.; Wang, R.; Chen, Z.; Selvaraj, R. Pt/g-C3N4 composites for photocatalytic H2 production and ·OH formation. Desalin. Water Treat. 2019, 154, 312–319. [Google Scholar] [CrossRef]
- Dai, A.; Huang, Z.; Tian, L.; Zhang, Z.; Guan, X.; Guo, L. Phenyl-incorporated carbon nitride photocatalyst with extended visible-light-absorption for enhanced hydrogen production from water splitting. J. Colloid Interface Sci. 2022, 622, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Xie, Y.; Wang, R.; Liu, S.-Y.; Zhao, Z. Electroless plating Ni-P cocatalyst decorated g-C3N4 with enhanced photocatalytic water splitting for H2 generation. Appl. Surf. Sci. 2019, 466, 847–853. [Google Scholar] [CrossRef]
- Xiao, Y.; Yao, B.; Wang, Z.; Chen, T.; Xiao, X.; Wang, Y. Plasma Ag-Modified alpha-Fe2O3/g-C3N4 Self-Assembled S-Scheme Heterojunctions with Enhanced Photothermal-Photocatalytic-Fenton Performances. Nanomaterials 2022, 12, 4212. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Long, X.; Qi, K.; Dang, S.; Zhong, M.; Xiao, S.; Zhou, T. Two-dimensional CdS/g-C6N6 heterostructure used for visible light photocatalysis. Appl. Surf. Sci. 2019, 471, 162–167. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)-Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef]
- Qi, K.; Lv, W.; Khan, I.; Liu, S.-Y. Photocatalytic H2 generation via CoP quantum-dot-modified g-C3N4 synthesized by electroless plating. Chin. J. Catal. 2020, 41, 114–121. [Google Scholar] [CrossRef]
- Han, G.; Xu, F.; Cheng, B.; Li, Y.; Yu, J.; Zhang, L. Enhanced Photocatalytic H2O2 Production over Inverse Opal ZnO@ Polydopamine S-Scheme Heterojunctions. Acta Phys. Chim. Sin. 2022, 38, 2112037. [Google Scholar] [CrossRef]
- Liu, B.; Bie, C.; Zhang, Y.; Wang, L.; Li, Y.; Yu, J. Hierarchically Porous ZnO/g-C3N4 S-Scheme Heterojunction Photocatalyst for Efficient H2O2 Production. Langmuir 2021, 37, 14114–14124. [Google Scholar] [CrossRef]
- Yao, L.; Wei, D.; Ni, Y.; Yan, D.; Hu, C. Surface localization of CdZnS quantum dots onto 2D g-C3N4 ultrathin microribbons: Highly efficient visible light-induced H2-generation. Nano Energy 2016, 26, 248–256. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, J.-W.; Ma, D.; Fan, Z.; He, C.; Cheng, L.; Sun, D.; Li, J.; Wang, Z.; Niua, C. Efficient spatial charge separation and transfer in ultrathin g-C3N4 nanosheets modified with Cu2MoS4 as noble-metal-free cocatalyst for superior visible-light-driven photocatalytic water splitting. Catal. Sci. Technol. 2018, 8, 3883–3893. [Google Scholar] [CrossRef]
- Li, X.; Xie, K.; Song, L.; Zhao, M.; Zhang, Z. Enhanced Photocarrier Separation in Hierarchical Graphitic-C3N4-Supported CuInS2 for Noble-Metal-Free Z-Scheme Photocatalytic Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 24577–24583. [Google Scholar] [CrossRef]
- Ni, S.; Fu, Z.; Li, L.; Ma, M.; Liu, Y. Step-scheme heterojunction g-C3N4/TiO2 for efficient photocatalytic degradation of tetracycline hydrochloride under UV light. Colloids Surf. A 2022, 649, 129475. [Google Scholar] [CrossRef]
- Cao, J.; Pan, C.; Ding, Y.; Li, W.; Lv, K.; Tang, H. Constructing nitrogen vacancy introduced g-C3N4 p-n homojunction for enhanced photocatalytic activity. J. Environ. Chem. Eng. 2019, 7, 102984. [Google Scholar] [CrossRef]
- Qian, H.; Liu, Z.; Guo, Z.; Ruan, M.; Ma, J. Hexagonal phase/cubic phase homogeneous ZnIn2S4 n-n junction photoanode for efficient photoelectrochemical water splitting. J. Alloys Compd. 2020, 830, 154639. [Google Scholar] [CrossRef]
- She, X.; Liu, L.; Ji, H.; Mo, Z.; Li, Y.; Huang, L.; Du, D.; Xu, H.; Li, H. Template-free synthesis of 2D porous ultrathin nonmetal-doped g-C3N4 nanosheets with highly efficient photocatalytic H2 evolution from water under visible light. Appl. Catal. B 2016, 187, 144–153. [Google Scholar] [CrossRef]
- Tay, Q.; Kanhere, P.; Ng, C.F.; Chen, S.; Chakraborty, S.; Huan, A.; Sum, T.C.; Ahuja, R.; Chen, Z. Defect Engineered g-C3N4 for Efficient Visible Light Photocatalytic Hydrogen Production. Chem. Mater. 2015, 27, 4930–4933. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, B.; Surendar, T.; Shanker, V. g-C3N4/NaTaO3 organic–inorganic hybrid nanocomposite: High-performance and recyclable visible light driven photocatalyst. Mater. Res. Bull. 2014, 49, 310–318. [Google Scholar] [CrossRef]
- Chen, Y.; He, J.; Li, J.; Mao, M.; Yan, Z.; Wang, W.; Wang, J. Hydrilla derived ZnIn2S4 photocatalyst with hexagonal-cubic phase junctions: A bio-inspired approach for H2 evolution. Catal. Commun. 2016, 87, 1–5. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Chen, X.-T.; Xue, Z.-L. Bi2MoO6microstructures: Controllable synthesis, growth mechanism, and visible-light-driven photocatalytic activities. CrystEngComm 2013, 15, 498–508. [Google Scholar] [CrossRef]
- Liu, H.; Jin, Z.; Xu, Z.; Zhang, Z.; Ao, D. Fabrication of ZnIn2S4–g-C3N4 sheet-on-sheet nanocomposites for efficient visible-light photocatalytic H2-evolution and degradation of organic pollutants. RSC Adv. 2015, 5, 97951–97961. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, M.-Q.; Xu, Y.-J. A low-temperature and one-step method for fabricating ZnIn2S4–GR nanocomposites with enhanced visible light photoactivity. J. Mater. Chem. A 2014, 2, 14401–14412. [Google Scholar] [CrossRef]
- Cui, R.-R.; Guo, X.; Gong, X.-Y.; Deng, C.-Y. Photoluminescence properties and energy transfer of novel orange–red emitting phosphors: Ba3Bi2(PO4)4: Sm3+, Eu3+ for white light-emitting diodes. Rare Met. 2021, 40, 2882–2891. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Yang, B.; Xu, J.; Yan, Y.; He, J. Synthesis of Zn2+ doped AgInxSy sub-microspheres and its visible light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2019, 30, 15257–15266. [Google Scholar] [CrossRef]
- Kadi, M.W.; Mohamed, R.M.; Bahnemann, D.W. Controlled synthesis of Ag2O/g-C3N4 heterostructures using soft and hard templates for efficient and enhanced visible-light degradation of ciprofloxacin. Ceram. Int. 2021, 47, 31073–31083. [Google Scholar] [CrossRef]
- Wang, L.; Bie, C.; Yu, J. Challenges of Z-scheme photocatalytic mechanisms. Trends Chem. 2022, 4, 973–983. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Gu, X.; Zhao, Y.; Zhang, K.; Yan, Y.; Qi, K. Photocatalytic Hydrogen Production and Tetracycline Degradation Using ZnIn2S4 Quantum Dots Modified g-C3N4 Composites. Nanomaterials 2023, 13, 305. https://doi.org/10.3390/nano13020305
Zhang J, Gu X, Zhao Y, Zhang K, Yan Y, Qi K. Photocatalytic Hydrogen Production and Tetracycline Degradation Using ZnIn2S4 Quantum Dots Modified g-C3N4 Composites. Nanomaterials. 2023; 13(2):305. https://doi.org/10.3390/nano13020305
Chicago/Turabian StyleZhang, Jingjing, Xinyue Gu, Yue Zhao, Kai Zhang, Ya Yan, and Kezhen Qi. 2023. "Photocatalytic Hydrogen Production and Tetracycline Degradation Using ZnIn2S4 Quantum Dots Modified g-C3N4 Composites" Nanomaterials 13, no. 2: 305. https://doi.org/10.3390/nano13020305
APA StyleZhang, J., Gu, X., Zhao, Y., Zhang, K., Yan, Y., & Qi, K. (2023). Photocatalytic Hydrogen Production and Tetracycline Degradation Using ZnIn2S4 Quantum Dots Modified g-C3N4 Composites. Nanomaterials, 13(2), 305. https://doi.org/10.3390/nano13020305






