Highly Sensitive Multi-Channel Biosensor for Low-Interference Simultaneous Detection
Abstract
1. Introduction
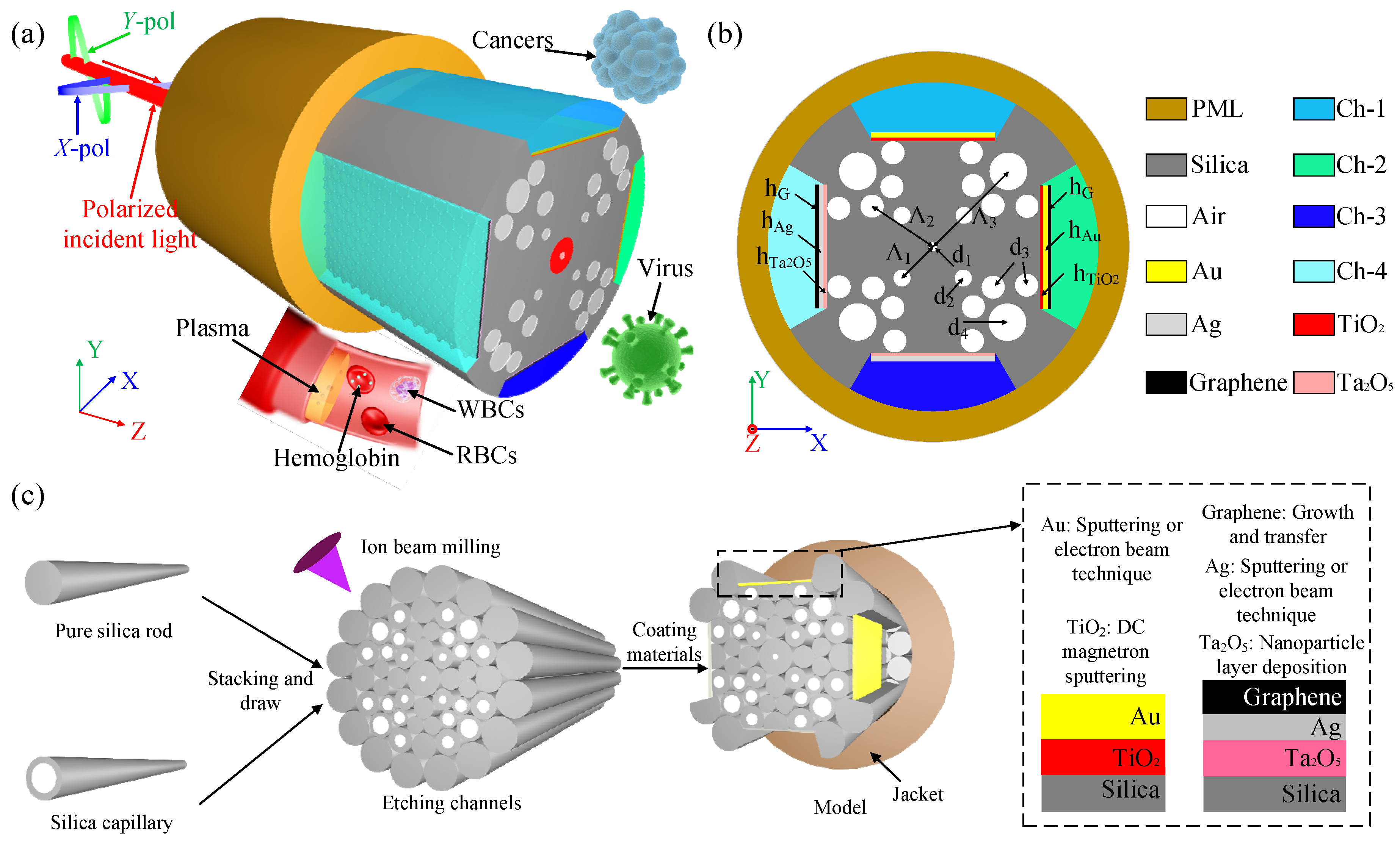
2. Sensor Design and Theory
3. Simulation Results and Discussions
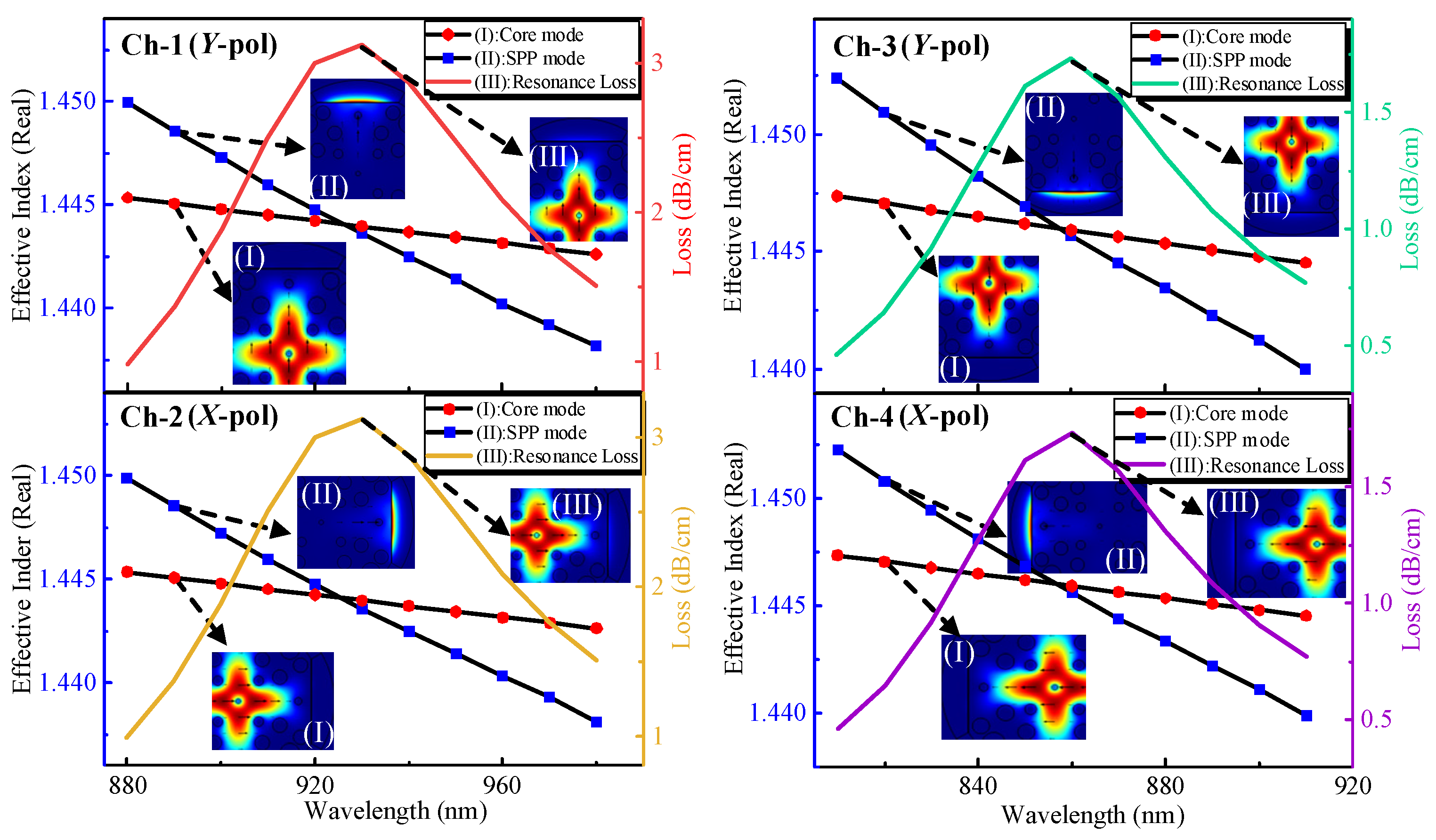
3.1. Modes Analysis
3.2. Interference Analysis
3.3. Materials Selection
3.4. Detection Area Optimization
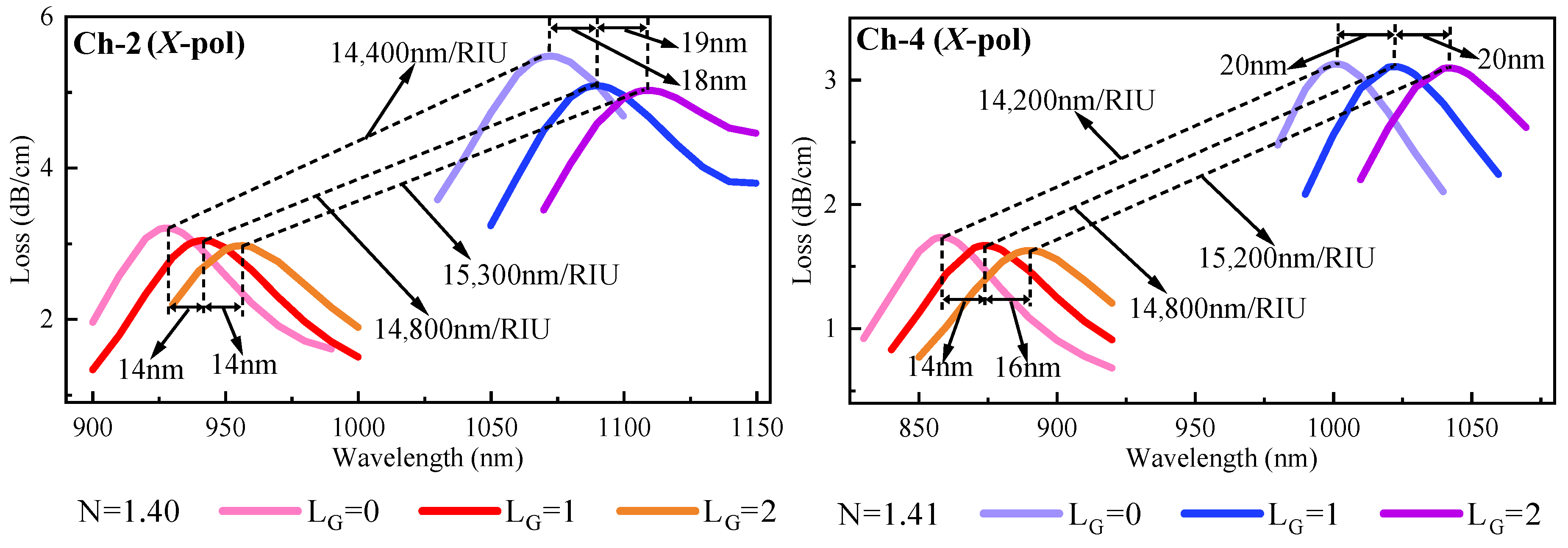
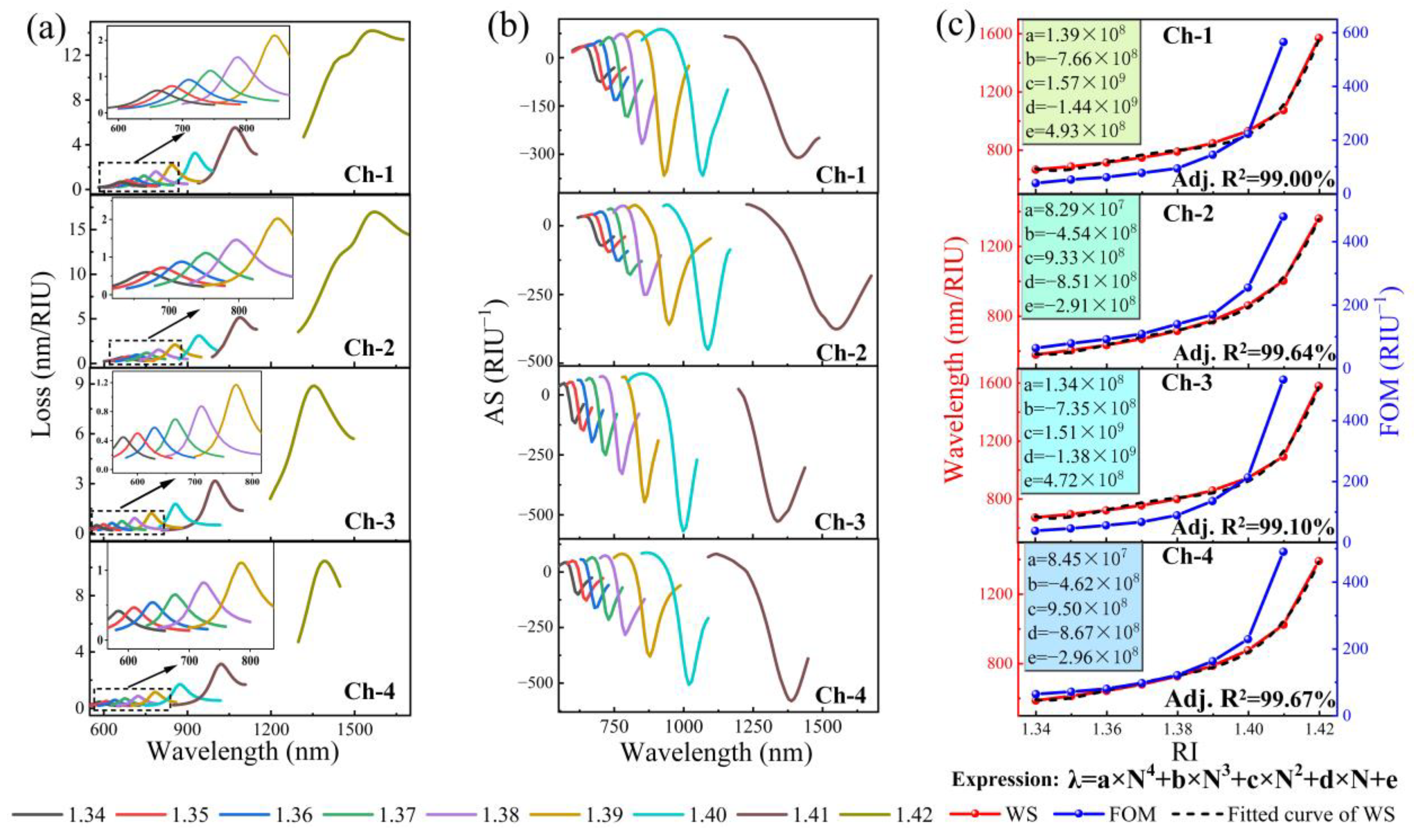
3.5. Sensor Performance
3.6. Bio-Detection Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, D.; Zhang, H.; Wang, G.; Xue, L.; Yang, X.; Shi, W.; Yao, J. Sensitivity and linearity enhanced wide-detection range surface-plasmon-resonance photonic-crystal-fiber sensor. Results Phys. 2021, 29, 104707. [Google Scholar] [CrossRef]
- Luo, W.; Meng, J.; Li, X.; Xie, Q.; Yi, D.; Wang, Y.; Hong, X. Temperature effects on surface plasmon resonance sensor based on side-polished D-shaped photonic crystal fiber. Measurement 2021, 181, 109504. [Google Scholar] [CrossRef]
- De, M.; Gangopadhyay, T.K.; Singh, V.K. Prospects of photonic crystal fiber for analyte sensing applications: An overview. Meas. Sci. Technol. 2020, 31, 042001. [Google Scholar] [CrossRef]
- Rifat, A.A.; Ahmed, R.; Yetisen, A.K.; Butt, H.; Sabouri, A.; Mandiraji, G.A.; Yun, S.H.; Adikan, F.R.M. Photonic crystal fiber based plasmonic sensors. Sens. Actuators B-Chem. 2017, 243, 311–325. [Google Scholar] [CrossRef]
- Shafkat, A.; Reja, M.I.; Miah, M.J.; Fatema, S.; Absar, R.; Akhtar, J. Numerical exploration of external sensing scheme based photonic crystal fiber surface plasmonic sensor with different noble plasmonic materials and their alloys. Optik 2021, 231, 166418. [Google Scholar] [CrossRef]
- Bing, P.; Sui, J.; Wu, G.; Guo, X.; Li, Z.; Tan, L.; Yao, J. Analysis of Dual-Channel Simultaneous Detection of Photonic Crystal Fiber Sensors. Plasmonics 2020, 15, 1071–1076. [Google Scholar] [CrossRef]
- Rahman, K.M.M.; Alam, M.S.; Islam, M.A. Highly Sensitive Surface Plasmon Resonance Refractive Index Multi-Channel Sensor for Multi-Analyte Sensing. IEEE Sens. J. 2021, 21, 27422–27432. [Google Scholar] [CrossRef]
- Islam, M.R.; Iftekher, N.; Hasan, R.; Nayen, J.; Bin Islam, S. Dual-polarized highly sensitive surface-plasmon-resonance-based chemical and biomolecular sensor. Appl. Opt. 2020, 59, 3296–3305. [Google Scholar] [CrossRef]
- Habib, M.A.; Anower, M.S.; Hasan, M.R. Highly birefringent and low effective material loss microstructure fiber for THz wave guidance. Opt. Commun. 2018, 423, 140–144. [Google Scholar] [CrossRef]
- Danlard, I.; Akowuah, E.K. Design and Theoretical Analysis of a Dual-Polarized Quasi D-Shaped Plasmonic PCF Microsensor for Back-to-Back Measurement of Refractive Index and Temperature. IEEE Sens. J. 2021, 21, 9860–9868. [Google Scholar] [CrossRef]
- Hasan, M.R.; Akter, S.; Rifat, A.A.; Rana, S.; Ahmed, K.; Ahmed, R.; Subbaraman, H.; Abbott, D. Spiral Photonic Crystal Fiber-Based Dual-Polarized Surface Plasmon Resonance Biosensor. IEEE Sens. J. 2018, 18, 133–140. [Google Scholar] [CrossRef]
- Paul, A.K.; Habib, M.S.; Nguyen Hoang, H.; Razzak, S.M.A. An air-core photonic crystal fiber based plasmonic sensor for high refractive index sensing. Opt. Commun. 2020, 464, 125556. [Google Scholar] [CrossRef]
- Hu, D.J.J.; Ho, H.P. Recent advances in plasmonic photonic crystal fibers: Design, fabrication and applications. Adv. Opt. Photonics 2017, 9, 257–314. [Google Scholar] [CrossRef]
- Liu, C.; Su, W.; Liu, Q.; Lu, X.; Wang, F.; Sun, T.; Chu, P.K. Symmetrical dual D-shape photonic crystal fibers for surface plasmon resonance sensing. Opt. Express 2018, 26, 9039–9049. [Google Scholar] [CrossRef]
- Liu, C.; Yang, L.; Lu, X.; Liu, Q.; Wang, F.; Lv, J.; Sun, T.; Mu, H.; Chu, P.K. Mid-infrared surface plasmon resonance sensor based on photonic crystal fibers. Opt. Express 2017, 25, 14227–14237. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Wang, F.; Su, W.; Yang, L.; Lv, J.; Fu, G.; Li, X.; Liu, Q.; Sun, T.; et al. Surface plasmon resonance (SPR) infrared sensor based on D-shape photonic crystal fibers with ITO coatings. Opt. Commun. 2020, 464, 125496. [Google Scholar] [CrossRef]
- Zhao, F.; Yi, Y.; Lin, J.; Yi, Z.; Qin, F.; Zheng, Y.; Liu, L.; Zheng, F.; Li, H.; Wu, P. The better photoelectric performance of thin-film TiO2/c-Si heterojunction solar cells based on surface plasmon resonance. Results Phys. 2021, 28, 104628. [Google Scholar] [CrossRef]
- Das, S.; Singh, V.K. The role of Ta2O5 thin film on a plasmonic refractive index sensor based on photonic crystal fiber. Photonics Nanostructures-Fundam. Appl. 2021, 44, 100904. [Google Scholar] [CrossRef]
- Shakya, A.K.; Ramola, A.; Singh, S.; Van, V. Design of an ultra-sensitive bimetallic anisotropic PCF SPR biosensor for liquid analytes sensing. Opt. Express 2022, 30, 9233–9255. [Google Scholar] [CrossRef]
- Kamrunnahar, Q.M.; Haider, F.; Aoni, R.A.; Mou, J.R.; Shifa, S.; Begum, F.; Abdul-Rashid, H.A.; Ahmed, R. Plasmonic Micro-Channel Assisted Photonic Crystal Fiber Based Highly Sensitive Sensor for Multi-Analyte Detection. Nanomaterials 2022, 12, 1444. [Google Scholar] [CrossRef]
- Singh, S.; Prajapati, Y.K. Highly sensitive dual-core symmetrical side-polished modified D-shaped SPR based PCF refractive index sensor with deeply etched micro openings. Optik 2021, 235, 1444. [Google Scholar] [CrossRef]
- Esfahani Monfared, Y.; Qasymeh, M. Plasmonic Biosensor for Low-Index Liquid Analyte Detection Using Graphene-Assisted Photonic Crystal Fiber. Plasmonics 2021, 16, 881–889. [Google Scholar] [CrossRef]
- Cheng, T.; Li, X.; Li, S.; Yan, X.; Zhang, X.; Wang, F. Surface plasmon resonance temperature sensor based on a photonic crystal fiber filled with silver nanowires. Appl. Opt. 2020, 59, 5108–5113. [Google Scholar] [CrossRef]
- Markin, A.V.; Markina, N.E.; Goryacheva, I.Y. Raman spectroscopy based analysis inside photonic-crystal fibers. Trac-Trends Anal. Chem. 2017, 88, 185–197. [Google Scholar] [CrossRef]
- Paul, A.K.; Sarkar, A.K.; Rahman, A.B.S.; Khaleque, A. Twin Core Photonic Crystal Fiber Plasmonic Refractive Index Sensor. Ieee Sens. J. 2018, 18, 5761–5769. [Google Scholar] [CrossRef]
- Yasli, A.; Ademgil, H. Effect of plasmonic materials on photonic crystal fiber based surface plasmon resonance sensors. Mod. Phys. Lett. B 2019, 33, 1950157. [Google Scholar] [CrossRef]
- Yasli, A.; Ademgil, H.; Haxha, S.; Aggoun, A. Multi-Channel Photonic Crystal Fiber Based Surface Plasmon Resonance Sensor for Multi-Analyte Sensing. IEEE Photonics J. 2020, 12, 1–5. [Google Scholar] [CrossRef]
- Kaur, V.; Singh, S. A dual-channel surface plasmon resonance biosensor based on a photonic crystal fiber for multianalyte sensing. J. Comput. Electron. 2019, 18, 319–328. [Google Scholar] [CrossRef]
- Haider, F.; Aoni, R.A.; Ahmed, R.; Mahdiraji, G.A.; Azman, M.F.; Adikan, F.R.M. Mode-multiplex plasmonic sensor for multi-analyte detection. Opt. Lett. 2020, 45, 3941–3944. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mou, F.A.; Bhuiyan, M.I.H.; Islam, M.R. Refractometric THz Sensing of Blood Components in a Photonic Crystal Fiber Platform. Braz. J. Phys. 2022, 52, 1–12. [Google Scholar] [CrossRef]
- Chaudhary, V.S.; Kumar, D.; Kumar, S. Gold-Immobilized Photonic Crystal Fiber-Based SPR Biosensor for Detection of Malaria Disease in Human Body. IEEE Sens. J. 2021, 21, 17800–17807. [Google Scholar] [CrossRef]
- Chaudhary, V.S.; Kumar, D.; Mishra, G.P.; Sharma, S.; Kumar, S. Plasmonic Biosensor with Gold and Titanium Dioxide Immobilized on Photonic Crystal Fiber for Blood Composition Detection. IEEE Sens. J. 2022, 22, 8474–8481. [Google Scholar] [CrossRef]



| RI | RW (nm) | Loss (dB/cm) | WS (nm/RIU) | AS (RIU−1) | FOM (RIU−1) | RI | RW (nm) | Loss (dB/cm) | WS (nm/RIU) | AS (RIU−1) | FOM (RIU−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.34 | 661 | 0.615 | 2200 | −75 | 36 | 1.34 | 575 | 0.446 | 2500 | −118 | 62 | ||
| 1.35 | 683 | 0.739 | 2700 | −100 | 44 | 1.35 | 600 | 0.500 | 3000 | −150 | 77 | ||
| 1.36 | 710 | 0.911 | 3400 | −132 | 54 | 1.36 | 630 | 0.578 | 3600 | −198 | 90 | ||
| 1.37 | 744 | 1.159 | 4200 | −183 | 65 | 1.37 | 666 | 0.695 | 4600 | −251 | 107 | ||
| Ch-1 | 1.38 | 786 | 1.530 | 5800 | −268 | 87 | Ch-2 | 1.38 | 712 | 0.874 | 6100 | −330 | 138 |
| 1.39 | 844 | 2.132 | 8400 | −367 | 134 | 1.39 | 773 | 1.169 | 8600 | −448 | 168 | ||
| 1.40 | 928 | 3.200 | 14,400 | −368 | 212 | 1.40 | 859 | 1.732 | 14,200 | −572 | 254 | ||
| 1.41 | 1072 | 5.580 | 49,800 | −312 | 534 | 1.41 | 1001 | 3.131 | 35,900 | −530 | 479 | ||
| 1.42 | 1570 | 14.193 | NA | NA | NA | 1.42 | 1360 | 8.890 | NA | NA | NA | ||
| 1.34 | 668 | 0.597 | 2300 | −73 | 36 | 1.34 | 575 | 0.415 | 2600 | −101 | 63 | ||
| 1.35 | 691 | 0.715 | 2700 | −96 | 44 | 1.35 | 610 | 0.465 | 3000 | −128 | 70 | ||
| 1.36 | 718 | 0.877 | 3400 | −128 | 54 | 1.36 | 640 | 0.540 | 3700 | −161 | 79 | ||
| 1.37 | 752 | 1.098 | 4400 | −180 | 65 | 1.37 | 677 | 0.650 | 4700 | −216 | 96 | ||
| Ch-3 | 1.38 | 796 | 1.456 | 5900 | −253 | 87 | Ch-4 | 1.38 | 724 | 0.821 | 6100 | −284 | 120 |
| 1.39 | 855 | 2.021 | 8700 | −362 | 134 | 1.39 | 785 | 1.100 | 8900 | −382 | 162 | ||
| 1.40 | 942 | 3.036 | 14,800 | −453 | 212 | 1.40 | 874 | 1.665 | 14,800 | −510 | 228 | ||
| 1.41 | 1090 | 5.087 | 49,000 | −378 | 534 | 1.41 | 1022 | 3.110 | 36,800 | −584 | 491 | ||
| 1.42 | 1580 | 16.670 | NA | NA | NA | 1.42 | 1890 | 10.44 | NA | NA | NA |
| Design | Wav. Range (nm) | RI Range | Max WS (nm/RIU) | Max AS (RIU−1) | Max FOM (RIU−1) |
|---|---|---|---|---|---|
| Dual Channels Au/Ag [27] | 400–800 | 1.33–1.37 | 2500 (Ch-1) | NA | NA |
| 3083 (Ch-2) | |||||
| Dual Channels Au [28] | 813–1100 | 1.30–1.40 | 1000 (Ch-1) | 0.5 (Ch-1) | NA |
| 3750 (Ch-2) | 40 (Ch-2) | ||||
| Three Channels Au [29] | 500–1045 | 1.33–1.35 | 2000 (Ch-1) | 95 (Ch-1) | 34 (Ch-1) |
| 1.36–1.38 | 3000 (Ch-2) | 184 (Ch-2) | 65 (Ch-2) | ||
| 1.39–1.42 | 18,000 (Ch-3) | 427 (Ch-3) | 200 (Ch-3) | ||
| Three Channels Au and Ta2O5/TiO2 [7] | 690–1440 | 1.37–1.42 | 38,100 (Ch-1) | 582 (Ch-1) | 635 (Ch-1) |
| 690–1250 | 1.37–1.42 | 21,600 (Ch-2) | 969 (Ch-2) | 432 (Ch-2) | |
| 690–1650 | 1.36–1.42 | 45,800 (Ch-3) | 1230 (Ch-3) | 572 (Ch-3) | |
| Four Channels Au/Ag and TiO2/Ta2O5/graphene (Our work) | 661–1570 | 1.36–1.42 (Ch-1) | 49,800 (Ch-1) | 312 (Ch-1) | 565 (Ch-1) |
| 668–1580 | 1.36–1.42 (Ch-2) | 49,000 (Ch-2) | 378 (Ch-2) | 534 (Ch-2) | |
| 575–1360 | 1.36–1.42 (Ch-3) | 35,900 (Ch-3) | 530 (Ch-3) | 479 (Ch-3) | |
| 584–1390 | 1.36–1.42 (Ch-4) | 36,800 (Ch-4) | 584 (Ch-4) | 491 (Ch-4) |
| Type of Test | RI | RW (nm) | Loss (dB/cm) | WS (nm/RIU) | AS (RIU−1) | FOM (RIU−1) |
|---|---|---|---|---|---|---|
| Plasma (Ch-3) | 1.35 | 600 | 0.500 | 3000 | −132 | 70 |
| Hemoglobin (Ch-1) | 1.38 | 786 | 1.530 | 5500 | −221 | 84 |
| WBCs (Ch-4) | 1.36 | 640 | 0.540 | 3500 | −143 | 75 |
| RBCs (Ch-2) | 1.40 | 942 | 3.036 | 11,500 | −398 | 176 |
| RBCS at ring phase (Ch-1) | 1.395 | 877 | 2.608 | 8400 | −371 | 147 |
| RBCS at trophozoite phase (Ch-3) | 1.383 | 726 | 0.958 | 5900 | −310 | 130 |
| RBCS at Schizont phase (Ch-4) | 1.373 | 689 | 0.700 | 4600 | −197 | 102 |
| HeLa Normal (Ch-4) | 1.368 | 666 | 0.619 | 4000 | −176 | 90 |
| HeLa Cancerous (Ch-1) | 1.392 | 855 | 2.246 | 8100 | −346 | 156 |
| MCF-7 Normal (Ch-3) | 1.387 | 747 | 1.037 | 6300 | −350 | 144 |
| MCF-7 Cancerous (Ch-2) | 1.401 | 953 | 3.165 | 12,200 | −409 | 185 |
| Type of Test | RI | Reference | RW (nm) | Δλpeak (nm) | Reference | RW (nm) | Δλpeak (nm) |
|---|---|---|---|---|---|---|---|
| Plasma | 1.35 | [31] | 666 | 30 | Our work | 600 | 40 |
| WBCs | 1.36 | 696 | 88 | 640 | 146 | ||
| Hemoglobin | 1.38 | 784 | 248 | 786 | 156 | ||
| RBCs | 1.40 | 1032 | NA | 942 | NA | ||
| RBCS at Schizont phase | 1.373 | [32] | 698 | 48 | Our work | 689 | 37 |
| RBCS at trophozoite phase | 1.383 | 746 | 90 | 726 | 151 | ||
| RBCS at ring phase | 1.395 | 836 | 100 | 877 | 65 | ||
| RBCS at normal | 1.40 | 936 | NA | 942 | NA | ||
| HeLa Normal | 1.368 | [7] | 750 | 120 | Our work | 666 | 189 |
| HeLa Cancerous | 1.392 | 870 | NA | 855 | NA | ||
| MCF-7 Normal | 1.387 | 750 | 80 | 747 | 206 | ||
| MCF-7 Cancerous | 1.401 | 830 | NA | 953 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Xiao, G.; Yang, H.; Chen, J.; Li, H.; Liu, X.; Luo, Y.; Li, J. Highly Sensitive Multi-Channel Biosensor for Low-Interference Simultaneous Detection. Nanomaterials 2023, 13, 246. https://doi.org/10.3390/nano13020246
Su J, Xiao G, Yang H, Chen J, Li H, Liu X, Luo Y, Li J. Highly Sensitive Multi-Channel Biosensor for Low-Interference Simultaneous Detection. Nanomaterials. 2023; 13(2):246. https://doi.org/10.3390/nano13020246
Chicago/Turabian StyleSu, Jiapeng, Gongli Xiao, Hongyan Yang, Jiayu Chen, Haiou Li, Xingpeng Liu, Yunhan Luo, and Jianqing Li. 2023. "Highly Sensitive Multi-Channel Biosensor for Low-Interference Simultaneous Detection" Nanomaterials 13, no. 2: 246. https://doi.org/10.3390/nano13020246
APA StyleSu, J., Xiao, G., Yang, H., Chen, J., Li, H., Liu, X., Luo, Y., & Li, J. (2023). Highly Sensitive Multi-Channel Biosensor for Low-Interference Simultaneous Detection. Nanomaterials, 13(2), 246. https://doi.org/10.3390/nano13020246








