Solvent Influence on the Magnetization and Phase of Fe-Ni Alloy Nanoparticles Generated by Laser Ablation in Liquids
Abstract
1. Introduction
2. Materials and Methods
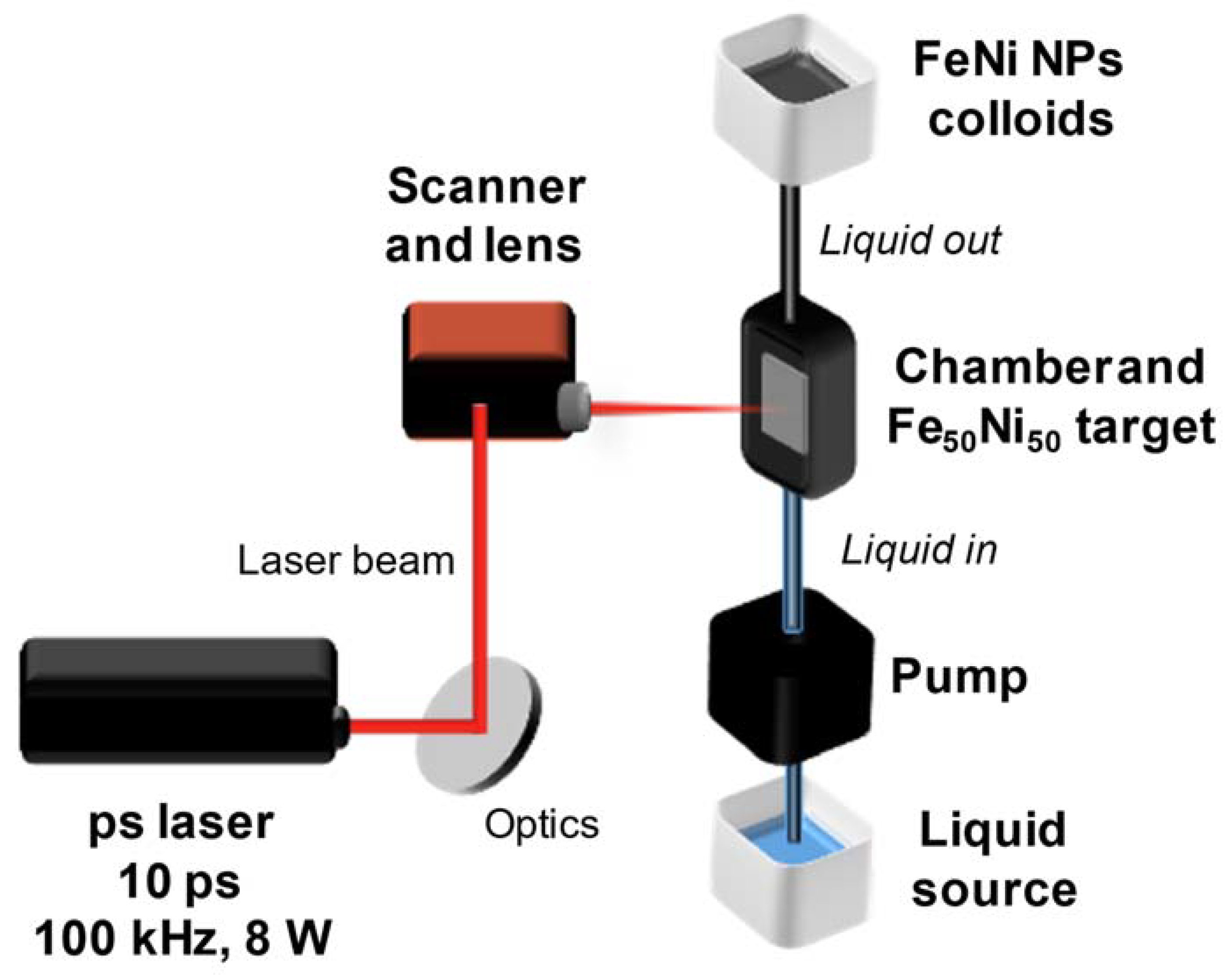
2.1. Fe50Ni50 Colloidal Nanoparticles Production
2.2. Analytical Methods
3. Results and Discussion
3.1. Crystallographic Phases
3.2. Oxide Formation
3.3. Morphology and Particle Size Distribution
3.4. Elemental Composition
3.5. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frey, P.A.; Reed, G.H. The ubiquity of iron. ACS Chem. Biol. 2012, 7, 1477–1481. [Google Scholar] [CrossRef]
- Cui, J.; Kramer, M.; Zhou, L.; Liu, F.; Gabay, A.; Hadjipanayis, G.; Balasubramanian, B.; Sellmyer, D. Current progress and future challenges in rare-earth-free permanent magnets. Acta Mater. 2018, 158, 118–137. [Google Scholar] [CrossRef]
- van Schilfgaarde, M.; Abrikosov, I.A.; Johansson, B. Origin of the Invar effect in iron–nickel alloys. Nature 1999, 400, 46–49. [Google Scholar] [CrossRef]
- Shinjo, T.; Okuno, T.; Hassdorf, R.; Shigeto, K.; Ono, T. Magnetic vortex core observation in circular dots of permalloy. Science 2000, 289, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.S.; Scott, E.R.D. Tetrataenite-ordered FeNi, a new mineral in meteorites. Am. Mineral. 1980, 65, 624–630. [Google Scholar]
- Spooner, T. Current Transformers with Nickel-Iron Cores. Trans. Am. Inst. Electr. Eng. 1926, 45, 701–707. [Google Scholar] [CrossRef]
- Ahn, C.H.; Allen, M.G. Micromachined planar inductors on silicon wafers for MEMS applications. IEEE Trans. Ind. Electron. 1998, 45, 866–876. [Google Scholar] [CrossRef]
- Ahn, C.H.; Kim, Y.J.; Allen, M.G. A fully integrated planar toroidal inductor with a micromachined nickel-iron magnetic bar. IEEE Trans. Compon. Packag. Manuf. Technol. Part A 1994, 17, 463–469. [Google Scholar] [CrossRef]
- Dijith, K.S.; Aiswarya, R.; Praveen, M.; Pillai, S.; Surendran, K.P. Polyol derived Ni and NiFe alloys for effective shielding of electromagnetic interference. Mater. Chem. Front. 2018, 2, 1829–1841. [Google Scholar] [CrossRef]
- Giordano, M.C.; Escobar Steinvall, S.; Watanabe, S.; Fontcuberta i Morral, A.; Grundler, D. Ni 80 Fe 20 nanotubes with optimized spintronic functionalities prepared by atomic layer deposition. Nanoscale 2021, 13, 13451–13462. [Google Scholar] [CrossRef]
- Bokare, A.D.; Chikate, R.C.; Rode, C.V.; Paknikar, K.M. Iron-nickel bimetallic nanoparticles for reductive degradation of azo dye Orange G in aqueous solution. Appl. Catal. B Environ. 2008, 79, 270–278. [Google Scholar] [CrossRef]
- Gong, M.; Dai, H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2014, 8, 23–39. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Li, X.; Li, Y.; Yang, T.; Liang, Y. Facile Synthesis of Nickel-Iron/Nanocarbon Hybrids as Advanced Electrocatalysts for Efficient Water Splitting. ACS Catal. 2016, 6, 580–588. [Google Scholar] [CrossRef]
- Potvin, E.; Brossard, L. Electrocatalytic activity of Ni-Fe anodes for alkaline water electrolysis. Mater. Chem. Phys. 1992, 31, 311–318. [Google Scholar] [CrossRef]
- Lewis, L.H.; Mubarok, A.; Poirier, E.; Bordeaux, N.; Manchanda, P.; Kashyap, A.; Skomski, R.; Goldstein, J.; Pinkerton, F.E.; Mishra, R.K.; et al. Inspired by nature: Investigating tetrataenite for permanent magnet applications. J. Phys. Condens. Matter 2014, 26, 10. [Google Scholar] [CrossRef]
- Lin, Q.; Nadarajah, R.; Hoglund, E.; Semisalova, A.; Howe, J.M.; Gökce, B.; Zangari, G. Towards synthetic L10-FeNi: Detecting the absence of cubic symmetry in Laser-Ablated Fe-Ni nanoparticles. Appl. Surf. Sci. 2021, 567, 150664. [Google Scholar] [CrossRef]
- Chokprasombat, K.; Pinitsoontorn, S.; Maensiri, S. Effects of Ni content on nanocrystalline Fe–Co–Ni ternary alloys synthesized by a chemical reduction method. J. Magn. Magn. Mater. 2016, 405, 174–180. [Google Scholar] [CrossRef]
- Lima, E.; Drago, V.; Bolsoni, R.; Fichtner, P.F.P. Nanostructured Fe50Ni50 alloy formed by chemical reduction. Solid State Commun. 2003, 125, 265–270. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Gökce, B.; Amendola, V.; Barcikowski, S. Opportunities and Challenges for Laser Synthesis of Colloids. ChemPhysChem 2017, 18, 983–985. [Google Scholar] [CrossRef]
- Amendola, V.; Amans, D.; Ishikawa, Y.; Koshizaki, N.; Scirè, S.; Compagnini, G.; Reichenberger, S.; Barcikowski, S. Room-Temperature Laser Synthesis in Liquid of Oxide, Metal-Oxide Core-Shells, and Doped Oxide Nanoparticles. Chem. Eur. J. 2020, 26, 9206–9242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Z.; Sugioka, K. Laser ablation in liquids for nanomaterial synthesis: Diversities of targets and liquids. J. Phys. Photonics 2021, 3, 042002. [Google Scholar] [CrossRef]
- Kalus, M.R.; Lanyumba, R.; Lorenzo-Parodi, N.; Jochmann, M.A.; Kerpen, K.; Hagemann, U.; Schmidt, T.C.; Barcikowski, S.; Gökce, B. Determining the role of redox-active materials during laser-induced water decomposition. Phys. Chem. Chem. Phys. 2019, 21, 18636–18651. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Joshi, M.P.; Mondal, P.; Sinha, A.K.; Srivastava, A.K. Growth of anatase and rutile phase TiO2 nanoparticles using pulsed laser ablation in liquid: Influence of surfactant addition and ablation time variation. Appl. Surf. Sci. 2017, 396, 303–309. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, S.; Gökce, B.; Ma, Z.; Spasova, M.; Yelsukova, A.E.; Farle, M.; Wiedwald, U.; Zhang, D.; Ma, Z.; et al. Formation Mechanism of Laser Synthesized Iron-Manganese Alloy Nanoparticles, Manganese Oxide Nanosheets and Nanofibers. Part. Part. Syst. Charact. 2017, 34, 1600225. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Liu, J.; Chen, Q.; Zhu, X.; Liang, C. Carbon-Encapsulated Metal/Metal Carbide/Metal Oxide Core-Shell Nanostructures Generated by Laser Ablation of Metals in Organic Solvents. ACS Appl. Nano Mater. 2019, 2, 28–39. [Google Scholar] [CrossRef]
- Marzun, G.; Bönnemann, H.; Lehmann, C.; Spliethoff, B.; Weidenthaler, C.; Barcikowski, S. Role of Dissolved and Molecular Oxygen on Cu and PtCu Alloy Particle Structure during Laser Ablation Synthesis in Liquids. ChemPhysChem 2017, 18, 1175–1184. [Google Scholar] [CrossRef]
- Sigma Aldrich. Acetone, ACS Reagent, ≥99.5%. Available online: https://www.sigmaaldrich.com/DE/de/product/sigald/179124 (accessed on 7 September 2022).
- Sigma Aldrich. Acetone, Suitable for HPLC, ≥99.9%. Available online: https://www.sigmaaldrich.com/DE/de/product/sigald/270725 (accessed on 7 September 2022).
- Meeker, R.L.; Critchfield, F.E.; Bishop, E.T. Water Determination by Near Infrared Spectrophotometry. Anal. Chem. 1962, 34, 1510–1511. [Google Scholar] [CrossRef]
- Lin, R.; Ladshaw, A.; Nan, Y.; Liu, J.; Yiacoumi, S.; Tsouris, C.; DePaoli, D.W.; Tavlarides, L.L. Isotherms for Water Adsorption on Molecular Sieve 3A: Influence of Cation Composition. Ind. Eng. Chem. Res. 2015, 54, 10442–10448. [Google Scholar] [CrossRef]
- Davodi, F.; Mühlhausen, E.; Settipani, D.; Rautama, E.L.; Honkanen, A.P.; Huotari, S.; Marzun, G.; Taskinen, P.; Kallio, T. Comprehensive study to design advanced metal-carbide@garaphene and metal-carbide@iron oxide nanoparticles with tunable structure by the laser ablation in liquid. J. Colloid Interface Sci. 2019, 556, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, B.; Xu, X.; Cao, S.; Shi, Y.; Yan, Y.; Song, X.; Hao, C. FeNi Alloy Nanoparticles Encapsulated in Carbon Shells Supported on N-Doped Graphene-Like Carbon as Efficient and Stable Bifunctional Oxygen Electrocatalysts. Chem. Eur. J. 2020, 26, 2890–2896. [Google Scholar] [CrossRef] [PubMed]
- Montes-Arango, A.M.; Marshall, L.G.; Fortes, A.D.; Bordeaux, N.C.; Langridge, S.; Barmak, K.; Lewis, L.H. Discovery of process-induced tetragonality in equiatomic ferromagnetic FeNi. Acta Mater. 2016, 116, 263–269. [Google Scholar] [CrossRef]
- Komabayashi, T.; Hirose, K.; Ohishi, Y. In situ X-ray diffraction measurements of the fcc-hcp phase transition boundary of an Fe-Ni alloy in an internally heated diamond anvil cell. Phys. Chem. Miner. 2012, 39, 329–338. [Google Scholar] [CrossRef]
- Liu, P.; Cao, Y.L.; Cui, H.; Chen, X.Y.; Yang, G.W. Micro- and nanocubes of silicon with zinc-blende structure. Chem. Mater. 2008, 20, 494–502. [Google Scholar] [CrossRef]
- Wang, J.B.; Zhang, C.Y.; Zhong, X.L.; Yang, G.W. Cubic and hexagonal structures of diamond nanocrystals formed upon pulsed laser induced liquid-solid interfacial reaction. Chem. Phys. Lett. 2002, 361, 86–90. [Google Scholar] [CrossRef]
- Patil, P.P.; Phase, D.M.; Kulkarni, S.A.; Ghaisas, S.V.; Kulkarni, S.K.; Kanetkar, S.M.; Ogale, S.B.; Bhide, V.G. Pulsed-laser—Induced reactive quenching at liquid-solid interface: Aqueous oxidation of iron. Phys. Rev. Lett. 1987, 58, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Choi, M.Y. Specific solvent produces specific phase Ni nanoparticles: A pulsed laser ablation in solvents. J. Phys. Chem. C 2014, 118, 14647–14654. [Google Scholar] [CrossRef]
- Lee, S.J.; Theerthagiri, J.; Choi, M.Y. Time-resolved dynamics of laser-induced cavitation bubbles during production of Ni nanoparticles via pulsed laser ablation in different solvents and their electrocatalytic activity for determination of toxic nitroaromatics. Chem. Eng. J. 2022, 427, 130970. [Google Scholar] [CrossRef]
- Tateno, S.; Hirose, K.; Komabayashi, T.; Ozawa, H.; Ohishi, Y. The structure of Fe-Ni alloy in Earth’s inner core. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef]
- Shen, G.; Mao, H.; Hemley, R.J.; Duffy, T.S.; Rivers, M.L. Melting and crystal structure of iron at high pressures and temperatures. Geophys. Res. Lett. 1998, 25, 373–376. [Google Scholar] [CrossRef]
- Torchio, R.; Boccato, S.; Miozzi, F.; Rosa, A.D.; Ishimatsu, N.; Kantor, I.; Sévelin-Radiguet, N.; Briggs, R.; Meneghini, C.; Irifune, T.; et al. Melting Curve and Phase Relations of Fe-Ni Alloys: Implications for the Earth’s Core Composition. Geophys. Res. Lett. 2020, 47, e2020GL088169. [Google Scholar] [CrossRef]
- Lin, J.-F.; Heinz, D.L.; Campbell, A.J.; Devine, J.M.; Mao, W.L.; Shen, G. Iron-Nickel alloy in the Earth’s core. Geophys. Res. Lett. 2002, 29, 109-1–109-3. [Google Scholar] [CrossRef]
- Huang, E.; Bassett, W.A.; Weathers, M.S. Phase relationships in Fe-Ni alloys at high pressures and temperatures. J. Geophys. Res. 1988, 93, 7741. [Google Scholar] [CrossRef]
- Kuwayama, Y.; Hirose, K.; Sata, N.; Ohishi, Y. Phase relations of iron and iron-nickel alloys up to 300 GPa: Implications for composition and structure of the Earth’s inner core. Earth Planet. Sci. Lett. 2008, 273, 379–385. [Google Scholar] [CrossRef]
- Boehler, R. Diamond cells and new materials. Mater. Today 2005, 8, 34–42. [Google Scholar] [CrossRef]
- Soliman, W.; Nakano, T.; Takada, N.; Sasaki, K. Modification of Rayleigh-Plesset theory for reproducing dynamics of cavitation bubbles in liquid-phase laser ablation. Jpn. J. Appl. Phys. 2010, 49, 116202. [Google Scholar] [CrossRef]
- Lam, J.; Lombard, J.; Dujardin, C.; Ledoux, G.; Merabia, S.; Amans, D. Dynamical study of bubble expansion following laser ablation in liquids. Appl. Phys. Lett. 2016, 108, 074104. [Google Scholar] [CrossRef]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Films 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Rebodos, R.L.; Vikesland, P.J. Effects of oxidation on the magnetization of nanoparticulate magnetite. Langmuir 2010, 26, 16745–16753. [Google Scholar] [CrossRef]
- Nadarajah, R.; Tahir, S.; Landers, J.; Koch, D.; Semisalova, A.S.; Wiemeler, J.; El-Zoka, A.; Kim, S.H.; Utzat, D.; Möller, R.; et al. Controlling the oxidation of magnetic and electrically conductive solid-solution iron-rhodium nanoparticles synthesized by laser ablation in liquids. Nanomaterials 2020, 10, 2362. [Google Scholar] [CrossRef] [PubMed]
- Kalus, M.R.; Bärsch, N.; Streubel, R.; Gökce, E.; Barcikowski, S.; Gökce, B. How persistent microbubbles shield nanoparticle productivity in laser synthesis of colloids—Quantification of their volume, dwell dynamics, and gas composition. Phys. Chem. Chem. Phys. 2017, 19, 7112–7123. [Google Scholar] [CrossRef] [PubMed]
- Khairani, I.Y.; Arifiadi, A.N.; Lee, J.-H.; Bhoi, B.; Patel, S.K.S.; Kim, S. Fabrication, Structure, and Magnetic Properties of Pure-Phase BiFeO₃ and MnFe₂O₄ Nanoparticles and their Nanocomposites. J. Magn. 2020, 25, 140–149. [Google Scholar] [CrossRef]
- Arifiadi, A.N.; Kim, K.-T.; Khairani, I.Y.; Park, C.B.; Kim, K.H.; Kim, S.-K. Synthesis and multiferroic properties of high-purity CoFe2O4–BiFeO3 nanocomposites. J. Alloys Compd. 2021, 867, 159008. [Google Scholar] [CrossRef]
- Tao, K.; Dou, H.; Sun, K. Interfacial coprecipitation to prepare magnetite nanoparticles: Concentration and temperature dependence. Colloids Surf. A Physicochem. Eng. Asp. 2008, 320, 115–122. [Google Scholar] [CrossRef]
- Barcikowski, S.; Amendola, V.; Lau, M.; Marzun, G.; Rehbock, C.; Reichenberger, S.; Zhang, D.; Gökce, B. Handbook of Laser Synthesis & Processing of Colloids, 2nd ed.; Duisburg-Essen Publication Online: Essen, Germany, 2019. [Google Scholar]
- Amendola, V.; Riello, P.; Meneghetti, M. Magnetic nanoparticles of iron carbide, iron oxide, iron@iron oxide, and metal iron synthesized by laser ablation in organic solvents. J. Phys. Chem. C 2011, 115, 5140–5146. [Google Scholar] [CrossRef]
- Zhang, D.; Choi, W.; Jakobi, J.; Kalus, M.R.; Barcikowski, S.; Cho, S.H.; Sugioka, K. Spontaneous shape alteration and size separation of surfactant-free silver particles synthesized by laser ablation in acetone during long-period storage. Nanomaterials 2018, 8, 529. [Google Scholar] [CrossRef]
- Jakobi, J.; Menéndez-Manjón, A.; Chakravadhanula, V.S.K.; Kienle, L.; Wagener, P.; Barcikowski, S. Stoichiometry of alloy nanoparticles from laser ablation of PtIr in acetone and their electrophoretic deposition on PtIr electrodes. Nanotechnology 2011, 22, 145601. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Wiley Online Library, Wiley: Weinheim, Germany, 2003; ISBN 9783527302741. [Google Scholar]
- Wareppam, B.; Kuzmann, E.; Garg, V.K.; Singh, L.H. Mössbauer spectroscopic investigations on iron oxides and modified nanostructures: A review. J. Mater. Res. 2022. [Google Scholar] [CrossRef]
- Keller, H.; Kündig, W. Mössbauer studies of Brownian motion. Solid State Commun. 1975, 16, 253–256. [Google Scholar] [CrossRef]
- Landers, J.; Salamon, S.; Remmer, H.; Ludwig, F.; Wende, H. Simultaneous Study of Brownian and Néel Relaxation Phenomena in Ferrofluids by Mössbauer Spectroscopy. Nano Lett. 2016, 16, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Ammar, S.; Jouini, N.; Fiévet, F.; Beji, Z.; Smiri, L.; Moliné, P.; Danot, M.; Grenèche, J.M. Magnetic properties of zinc ferrite nanoparticles synthesized by hydrolysis in a polyol medium. J. Phys. Condens. Matter 2006, 18, 9055–9069. [Google Scholar] [CrossRef]
- Nadarajah, R.; Tasdemir, L.; Thiel, C.; Salamon, S.; Semisalova, A.S.; Wende, H.; Farle, M.; Barcikowski, S.; Erni, D.; Gökce, B. Article formation of fe-ni nanoparticle strands in macroscopic polymer composites: Experiment and simulation. Nanomaterials 2021, 11, 2095. [Google Scholar] [CrossRef] [PubMed]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
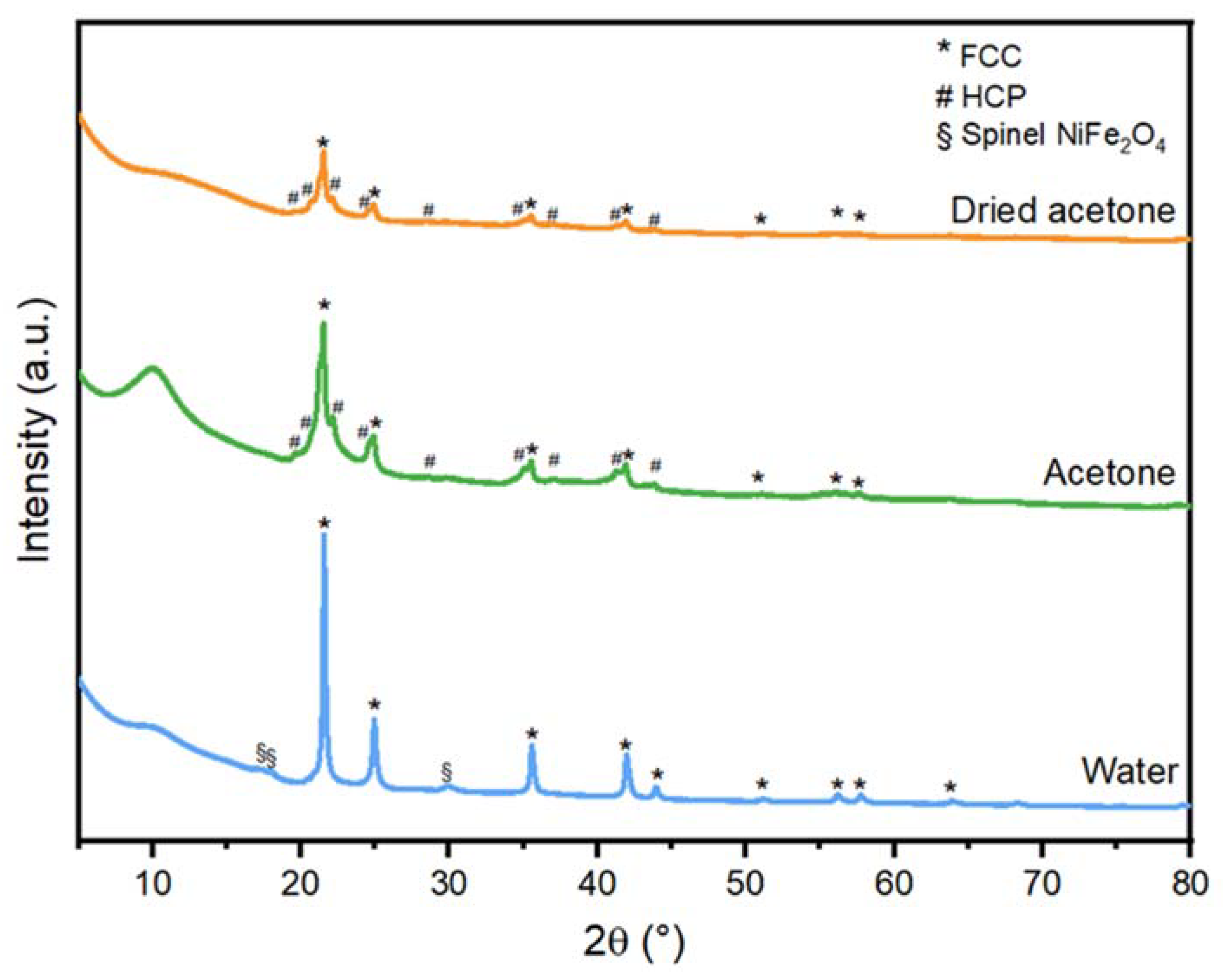





| Ablation liquid | Crystalline Phase Composition | HCP Content (wt%) * |
|---|---|---|
| Dried acetone | FCC FeNi, HCP FeNi, NiFe2O4 | 35.2 ± 1.0 |
| Acetone | FCC FeNi, HCP FeNi, NiFe2O4 | 38.4 ± 0.2 |
| Water | FCC FeNi, NiFe2O4 | 0 |
| Ablation Liquid | Average Particle Size (xc, nm) | Core Phase | Shell Phase | Shell Thickness (nm) | |
|---|---|---|---|---|---|
| Average (Mean) | Range (Min to Max) | ||||
| Dried acetone | 10.2 ± 0.3 | HCP/FCC FeNi | NiFe2O4 | 2.4 | 1.1–4.2 |
| Amorphous carbon | 1.9 | 1.5–2.9 | |||
| Acetone | 12.0 ± 0.2 | HCP/FCC FeNi | NiFe2O4 | 2.3 | 1.4–3.5 |
| Graphitic carbon | 1.2 | 0.7–1.9 | |||
| Water | 17.7 ± 0.6 | FCC FeNi | NiFe2O4 | 4.9 | 2.4–9.8 |
| Ablation Liquid | Whole Particle Composition | Shell Composition * | Core Composition ** | |||||
|---|---|---|---|---|---|---|---|---|
| Fe at% | Ni at% | O at% | Ni at% | Fe2 at% | O4 at% | Fe at% | Ni at% | |
| Dried acetone | 35.1 | 29.3 | 35.6 | 8.9 | 17.8 | 35.6 | 17.3 | 20.4 |
| Acetone | 43.6 | 38.1 | 18.2 | 4.6 | 9.1 | 18.2 | 34.5 | 33.6 |
| Water | 38.7 | 33.6 | 27.7 | 6.9 | 13.9 | 27.7 | 24.9 | 26.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairani, I.Y.; Lin, Q.; Landers, J.; Salamon, S.; Doñate-Buendía, C.; Karapetrova, E.; Wende, H.; Zangari, G.; Gökce, B. Solvent Influence on the Magnetization and Phase of Fe-Ni Alloy Nanoparticles Generated by Laser Ablation in Liquids. Nanomaterials 2023, 13, 227. https://doi.org/10.3390/nano13020227
Khairani IY, Lin Q, Landers J, Salamon S, Doñate-Buendía C, Karapetrova E, Wende H, Zangari G, Gökce B. Solvent Influence on the Magnetization and Phase of Fe-Ni Alloy Nanoparticles Generated by Laser Ablation in Liquids. Nanomaterials. 2023; 13(2):227. https://doi.org/10.3390/nano13020227
Chicago/Turabian StyleKhairani, Inna Y., Qiyuan Lin, Joachim Landers, Soma Salamon, Carlos Doñate-Buendía, Evguenia Karapetrova, Heiko Wende, Giovanni Zangari, and Bilal Gökce. 2023. "Solvent Influence on the Magnetization and Phase of Fe-Ni Alloy Nanoparticles Generated by Laser Ablation in Liquids" Nanomaterials 13, no. 2: 227. https://doi.org/10.3390/nano13020227
APA StyleKhairani, I. Y., Lin, Q., Landers, J., Salamon, S., Doñate-Buendía, C., Karapetrova, E., Wende, H., Zangari, G., & Gökce, B. (2023). Solvent Influence on the Magnetization and Phase of Fe-Ni Alloy Nanoparticles Generated by Laser Ablation in Liquids. Nanomaterials, 13(2), 227. https://doi.org/10.3390/nano13020227







