Crystalline Mesoporous F-Doped Tin Dioxide Nanomaterial Successfully Prepared via a One Pot Synthesis at Room Temperature and Ambient Pressure
Abstract
:1. Introduction
2. Materials and Methods
Characterization
3. Results and Discussion
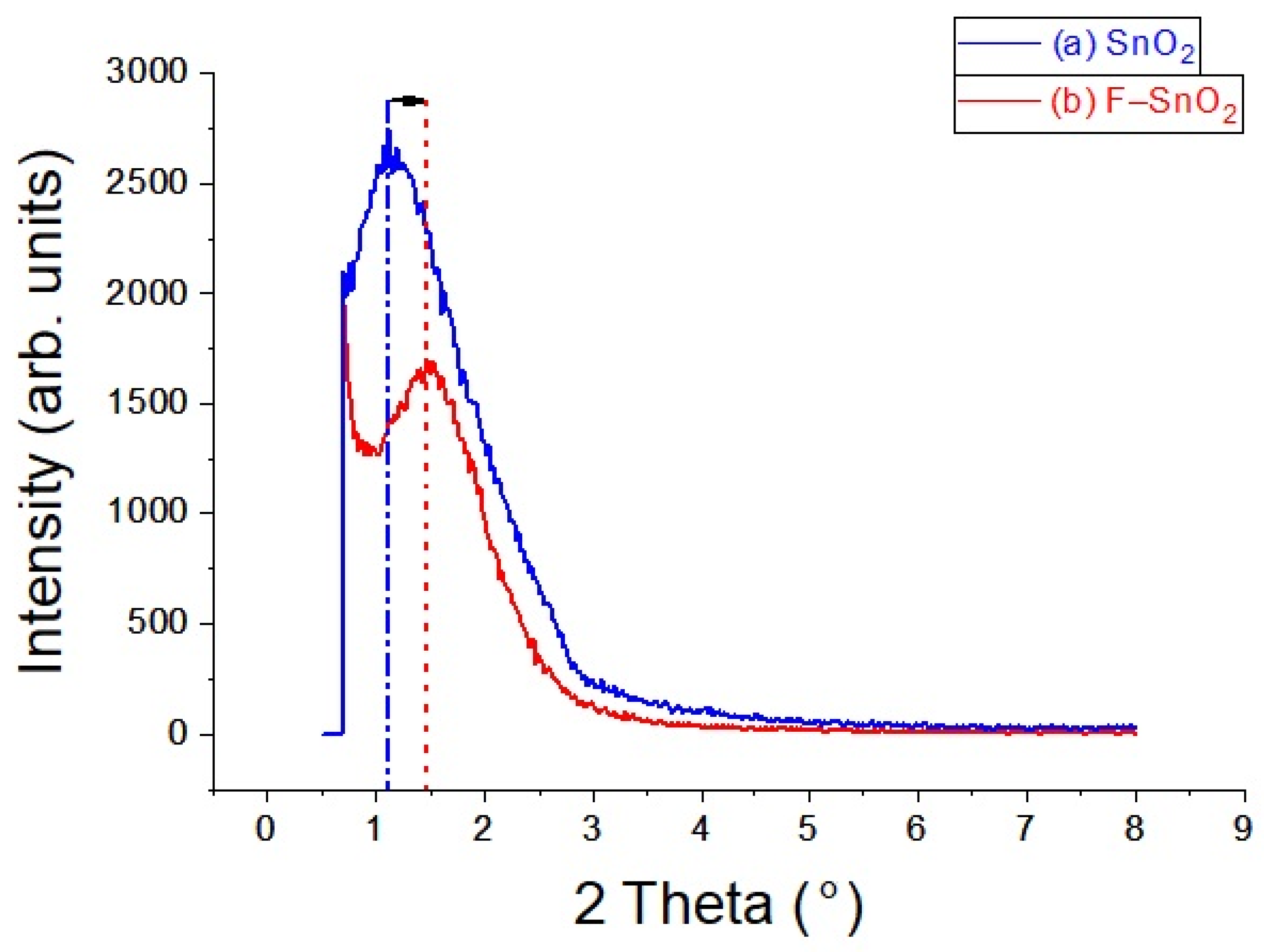
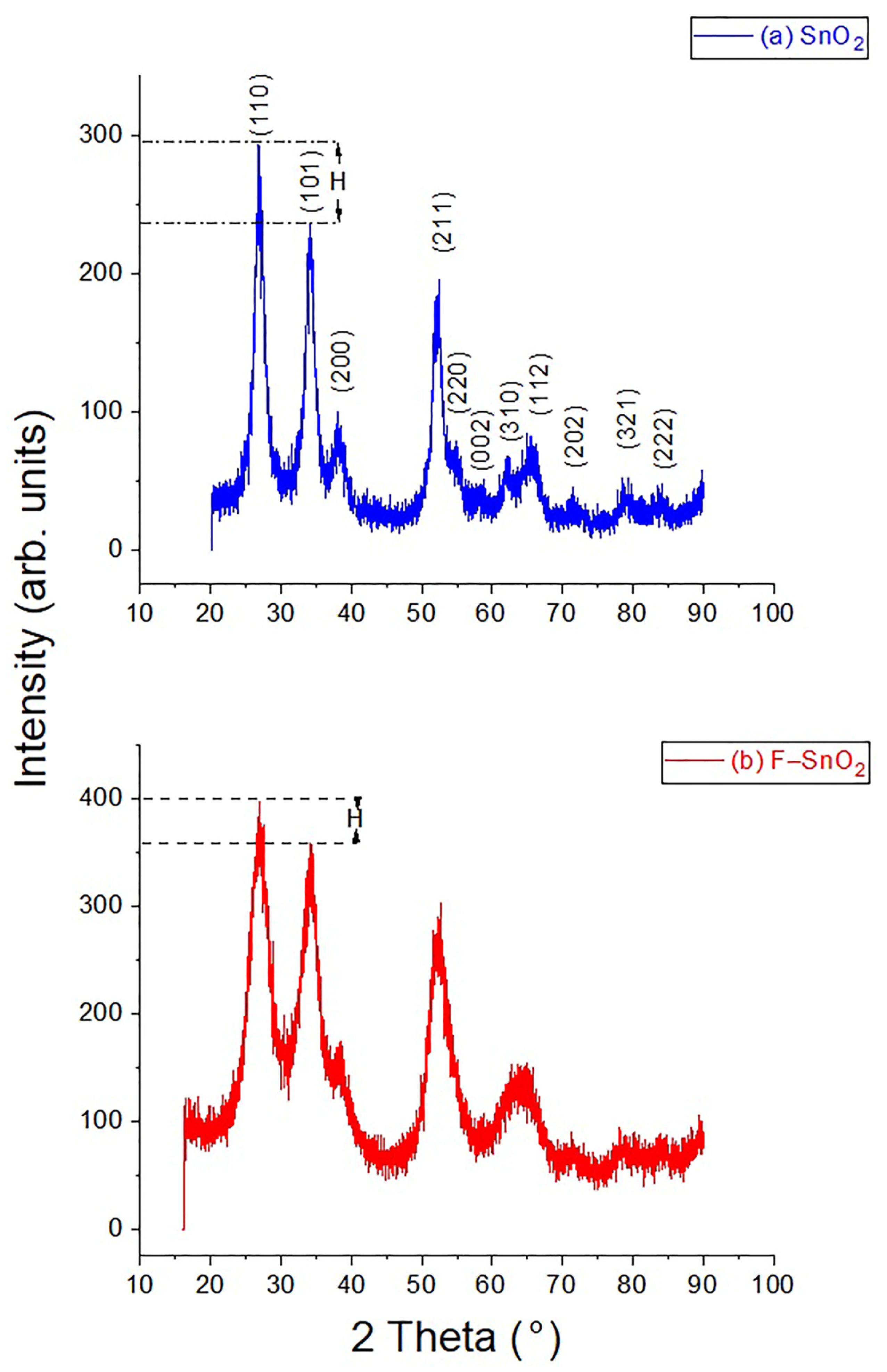
3.1. XRD
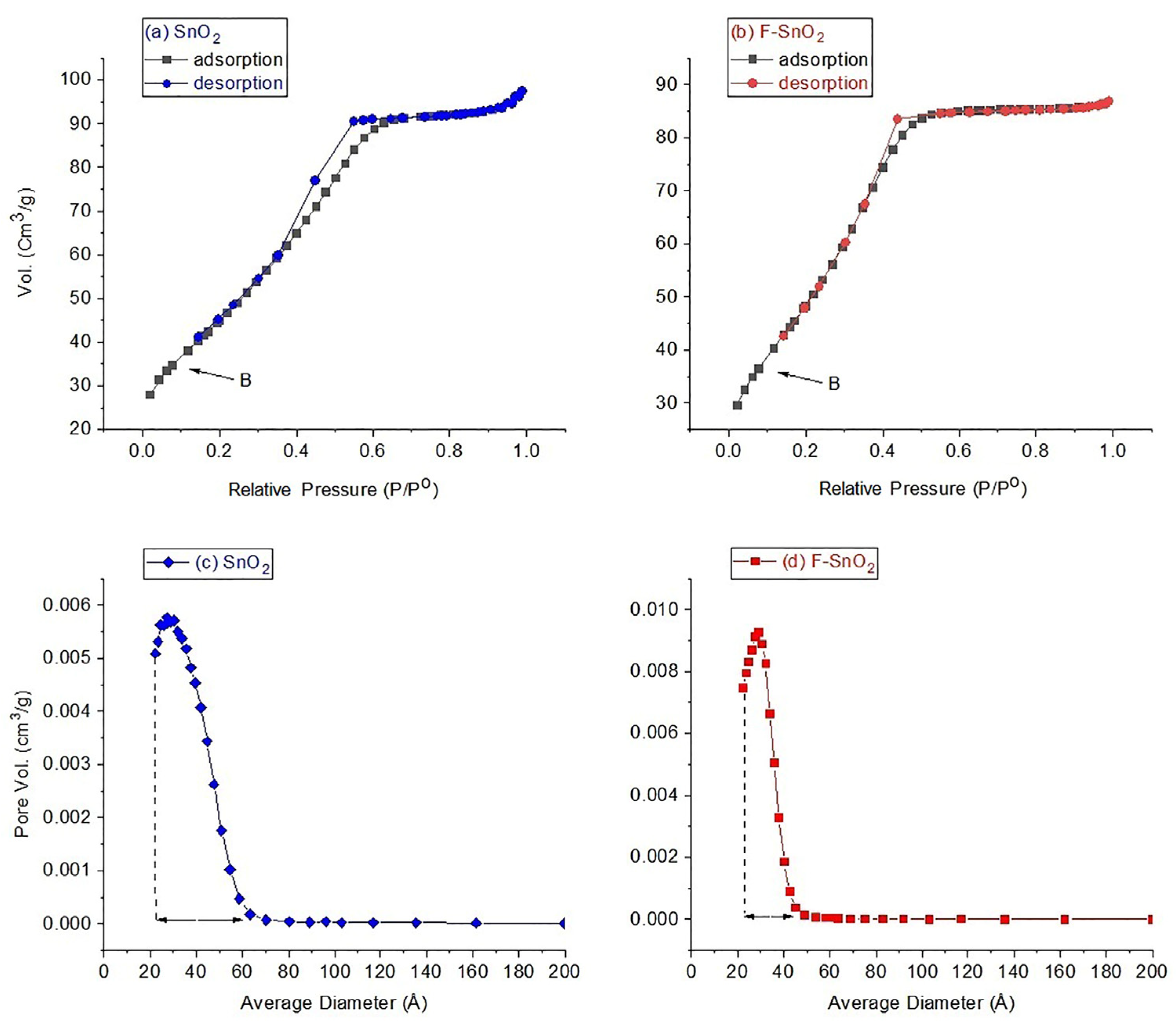
3.2. N2 Sorption Analysis
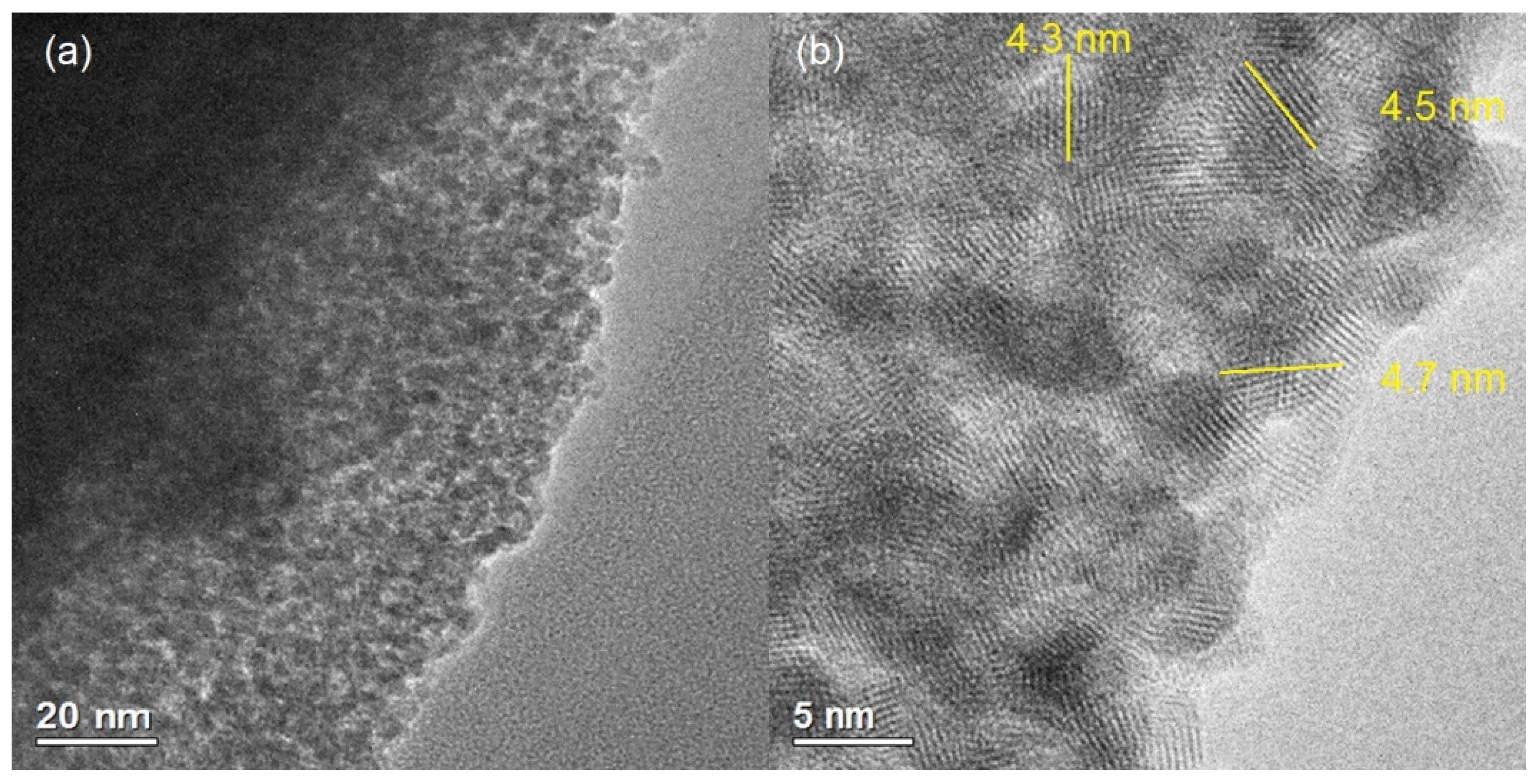
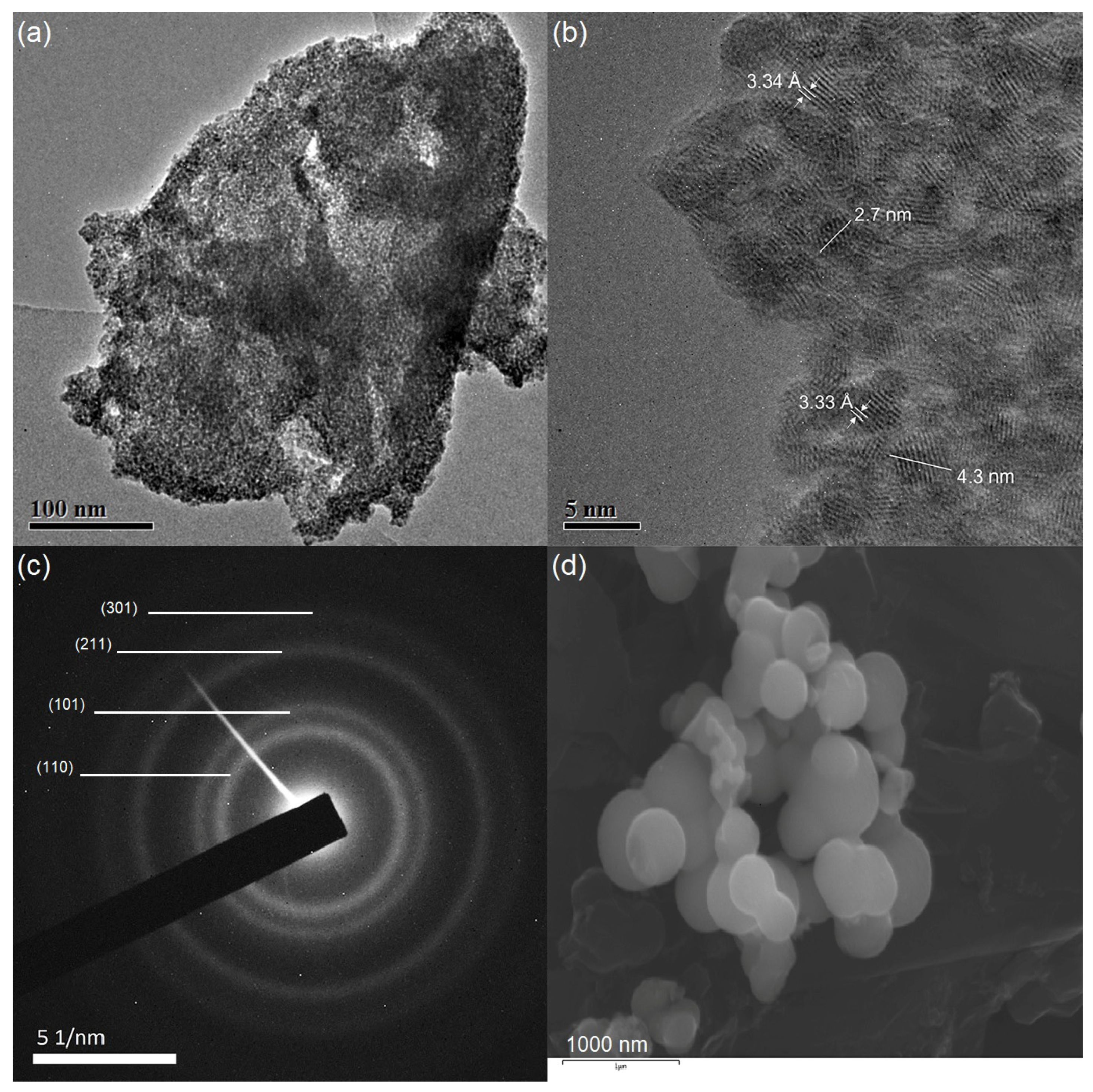
3.3. TEM
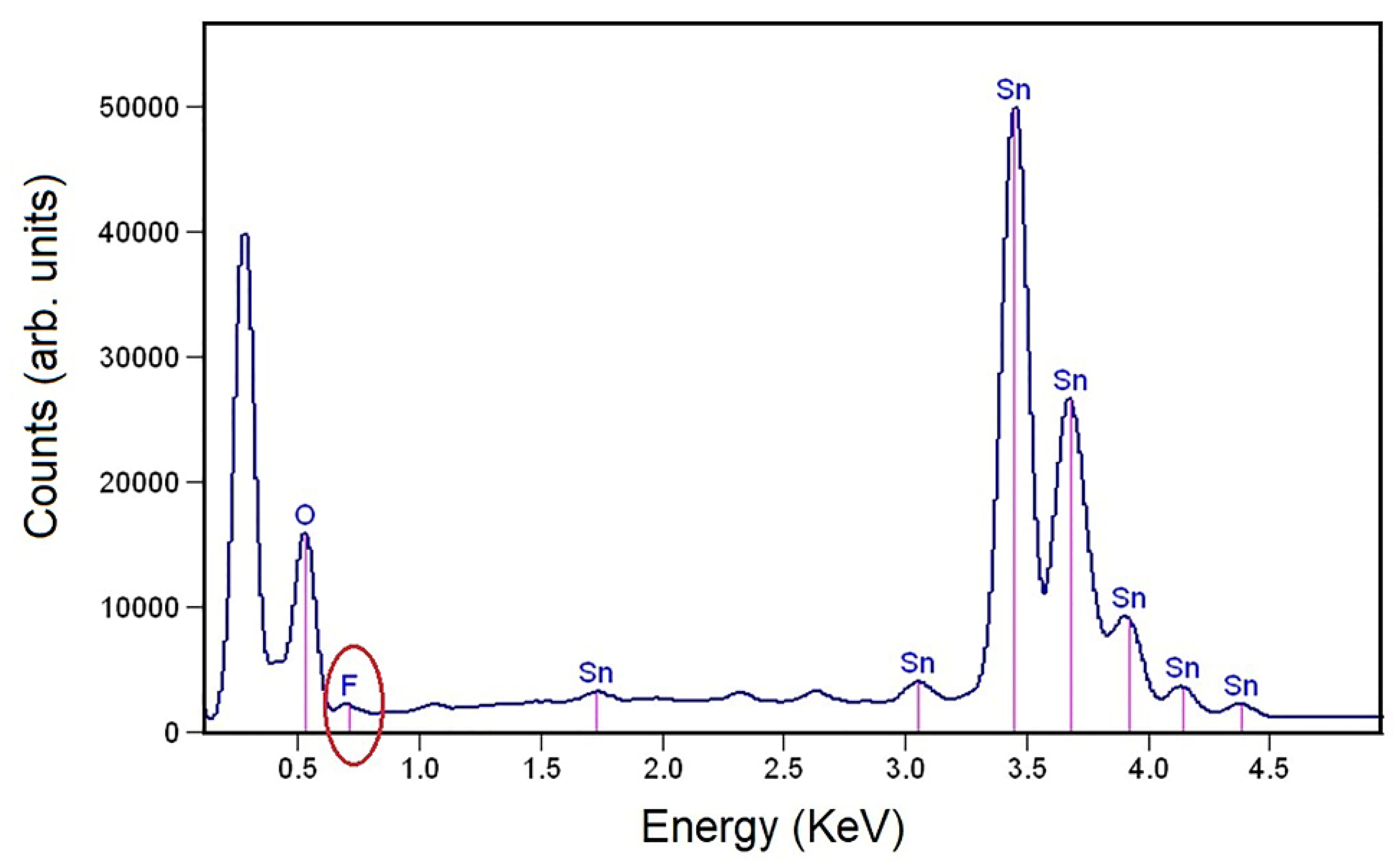
3.4. EDX
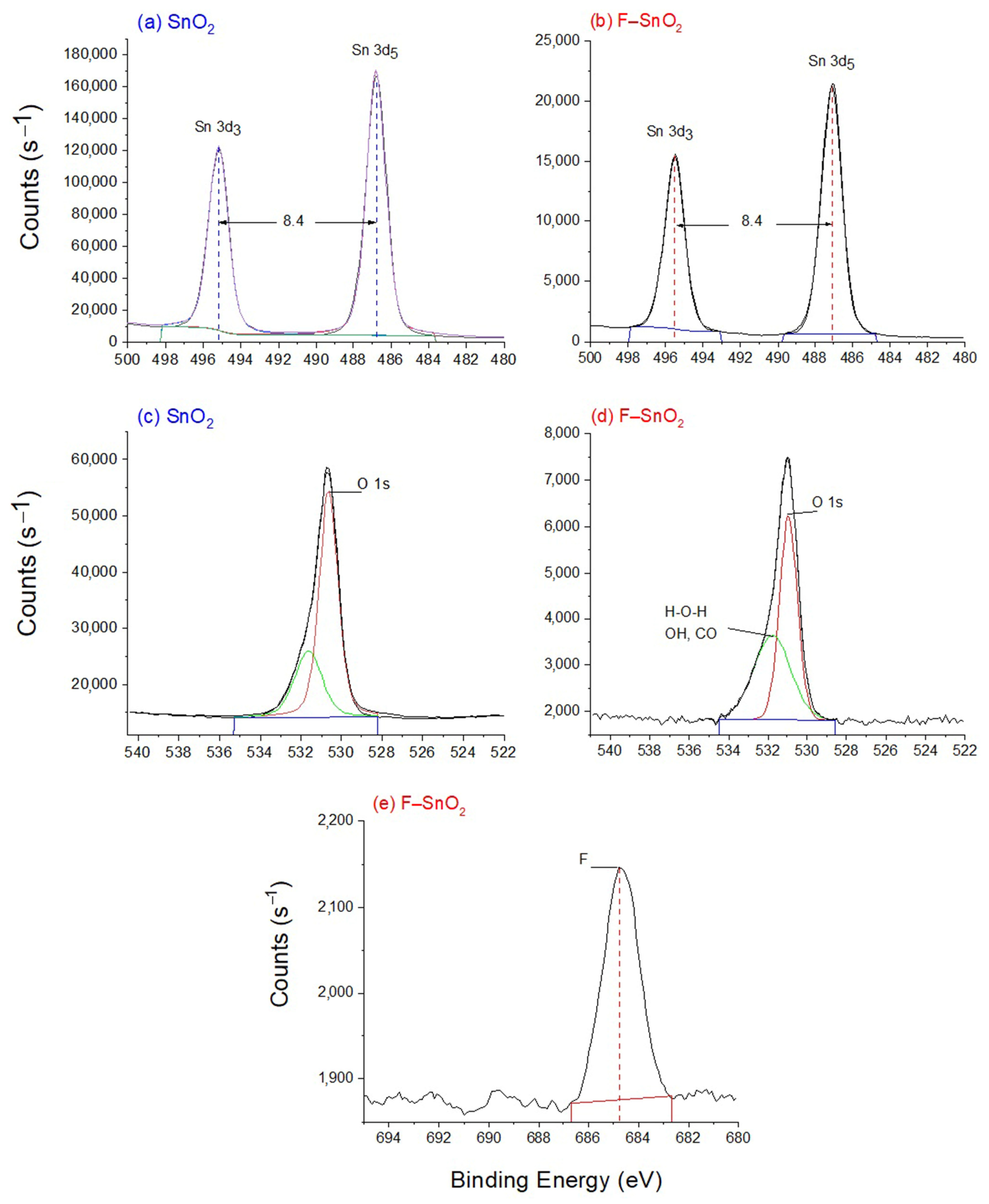
3.5. XPS
3.6. UV-Visible Spectroscopy
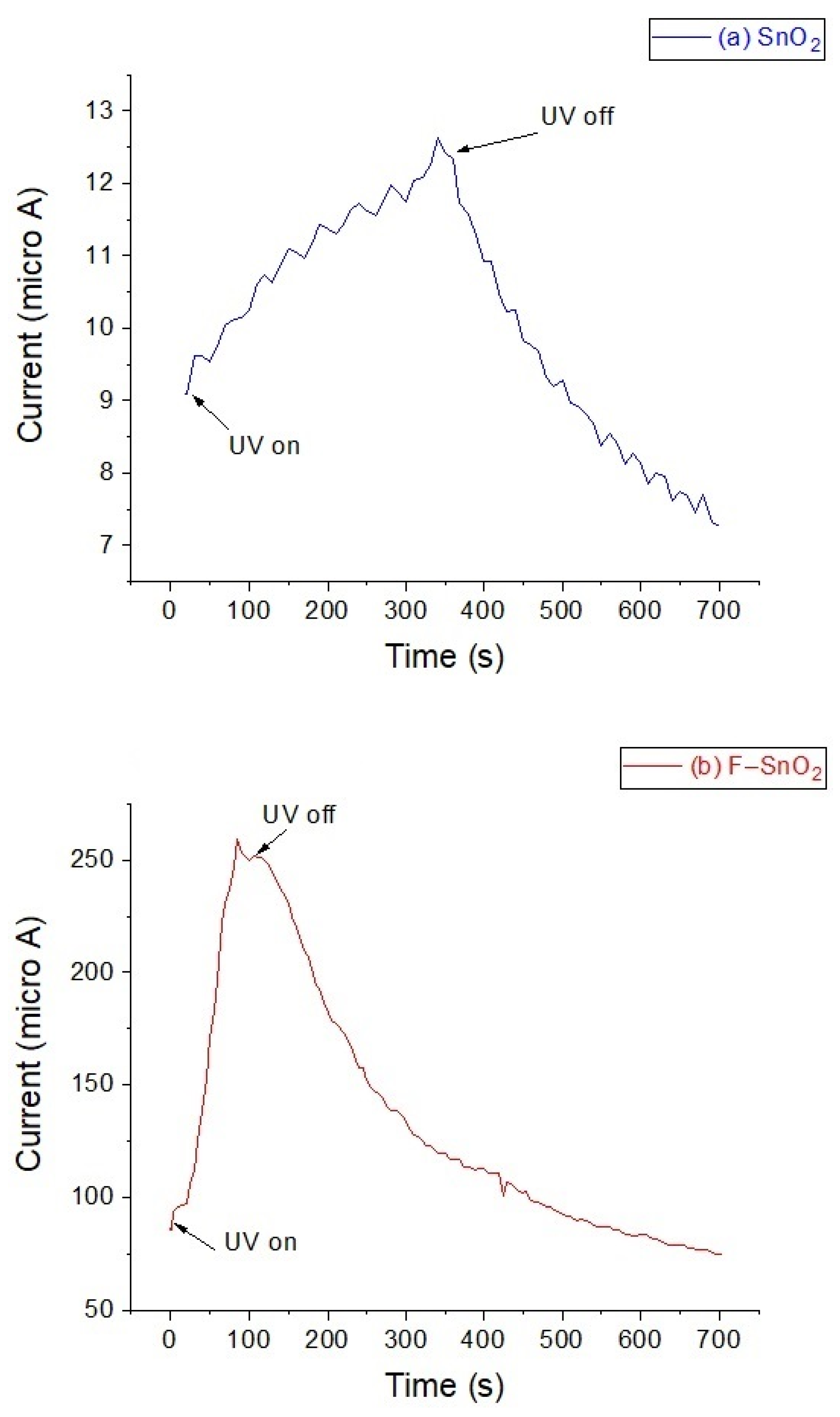
3.7. Conductivity and Opti-Electronic Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aswaghosh, L.; Manoharan, D.; Jaya, N.V. Defect structure and optical phonon confinement in ultrananocrystalline BixSn1-xO2 (x = 0, 0.03, 0.05, and 0.08) synthesized by a sonochemical method. Phys. Chem. Chem. Phys. 2016, 18, 5995–6004. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, N.M.; Hamad, D.; Abdel-Latief, A.Y.; Abdel-Rahim, M.A. Preparation of quantum size of tin oxide: Structural and physical characterization. Prog. Nat. Sci. Mater. Int. 2016, 26, 145–151. [Google Scholar] [CrossRef]
- Wagner, T.; Bauer, M.; Sauerwald, T.; Kohl, C.D.; Tiemann, M. X-ray absorption near-edge spectroscopy investigation of the oxidation state of Pd species in nanoporous SnO2 gas sensors for methane detection. Thin Solid Films 2011, 520, 909–912. [Google Scholar] [CrossRef]
- Li, X.; Cho, J.H.; Kurup, P.; Gu, Z. Novel sensor array based on doped tin oxide nanowires for organic vapor detection. Sens. Actuators B Chem. 2012, 162, 251–258. [Google Scholar] [CrossRef]
- Brunet, E.; Maier, T.; Mutinati, G.C.; Steinhauer, S.; Köck, A.; Gspan, C.; Grogger, W. Comparison of the gas sensing performance of SnO2 thin film and SnO2 nanowire sensors. Sens. Actuators B Chem. 2012, 165, 110–118. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, K.; Wu, J.; Gan, J.; Zhu, M.; Hu, Z.; Chu, J. Electronic structures and excitonic transitions in nanocrystalline iron-doped tin dioxide diluted magnetic semiconductor films: An optical spectroscopic study. Phys. Chem. Chem. Phys. 2011, 13, 6211–6222. [Google Scholar] [CrossRef]
- Ganose, A.M.; Scanlon, D.O. Band gap and work function tailoring of SnO2 for improved transparent conducting ability in photovoltaics. J. Mater. Chem. C 2016, 4, 1467–1475. [Google Scholar] [CrossRef]
- Huang, B.J.; Li, F.; Zhang, C.W.; Li, P.; Wang, P.J. Electronic structure and optical properties of Ag-doped SnO2 nanoribbons. RSC Adv. 2014, 4, 41819–41824. [Google Scholar] [CrossRef]
- Dalapati, G.K.; Sharma, H.; Guchhait, A.; Chakrabarty, N.; Bamola, P.; Liu, Q.; Saianand, G.; Sai Krishna, A.M.; Mukhopadhyay, S.; Dey, A.; et al. Tin oxide for optoelectronic, photovoltaic and energy storage devices: A review. J. Mater. Chem. A 2021, 9, 16621–16684. [Google Scholar] [CrossRef]
- Noor, N.; Parkin, I.P. Enhanced transparent-conducting fluorine-doped tin oxide films formed by Aerosol-Assisted Chemical Vapour Deposition. J. Mater. Chem. C 2013, 1, 984–996. [Google Scholar] [CrossRef]
- Morris, L.; Williams, D.E.; Kaltsoyannis, N.; Tocher, D.A. Surface grafting as a route to modifying the gas-sensitive resistor properties of semiconducting oxides: Studies of Ru-grafted SnO2. Phys. Chem. Chem. Phys. 2001, 3, 132–145. [Google Scholar] [CrossRef]
- Liu, L.; Shu, S.; Zhang, G.; Liu, S. Highly Selective Sensing of C2H6O, HCHO, and C3H6O Gases by Controlling SnO2 Nanoparticle Vacancies. ACS Appl. Nano Mater. 2018, 1, 31–37. [Google Scholar] [CrossRef]
- Manikandan, M.; Tanabe, T.; Ramesh, G.V.; Kodiyath, R.; Ueda, S.; Sakuma, Y.; Homma, Y.; Dakshanamoorthy, A.; Ariga, K.; Abe, H. Tailoring the surface-oxygen defects of a tin dioxide support towards an enhanced electrocatalytic performance of platinum nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 5932–5937. [Google Scholar] [CrossRef] [PubMed]
- Brus, L.E. Electron-electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Martinez, C.J.; Hockey, B.; Montgomery, C.B.; Semancik, S. Porous tin oxide nanostructured microspheres for sensor applications. Langmuir 2005, 21, 7937–7944. [Google Scholar] [CrossRef]
- Smith, A.M.; Nie, S. Bright and compact alloyed quantum dots with broadly tunable near-infrared absorption and fluorescence spectra through mercury cation exchange. J. Am. Chem. Soc. 2011, 133, 24–26. [Google Scholar] [CrossRef]
- Aqeel, T.; Greer, H.F.; Zhou, W.Z.; Bruce, D.W.; Bumajdad, A. Novel Direct Synthesis of Mesoporous Tin Dioxide Network Intact up to 500 °C. J. Nano Res. 2016, 40, 79–89. [Google Scholar] [CrossRef]
- Goebbert, C.; Aegerter, M.A.; Burgard, D.; Nassb, R. Ultrafiltration conducting membranes and coatings from. J. Mater. Chem. 1999, 9, 253–258. [Google Scholar] [CrossRef]
- Oakton, E.; Tillier, J.; Siddiqi, G.; Mickovic, Z.; Sereda, O.; Fedorov, A.; Copéret, C. Structural differences between Sb- and Nb-doped tin oxides and consequences for electrical conductivity. New J. Chem. 2016, 40, 2655–2660. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Sevier, E.I.; Aegerter, M.A.; Reich, A.; Ganz, D.; Gasparro, G.; Piitz, J.; Krajewski, T. Comparative study of SnO2: Sb transparent conducting films produced by various coating and heat treatment techniques. J. Non Cryst. Solids 1997, 218, 123–128. [Google Scholar]
- Zhong, X.; Yang, B.; Zhang, X.; Jia, J.; Yi, G. Effect of calcining temperature and time on the characteristics of Sb-doped SnO2 nanoparticles synthesized by the sol-gel method. Particuology 2012, 10, 365–370. [Google Scholar] [CrossRef]
- Terrier, C.; Chatelon, J.P.; Roger, J.A. Electrical and optical properties of Sb:SnO2 thin films obtained by the sol-gel method. Thin Solid Films 1997, 295, 95–100. [Google Scholar] [CrossRef]
- Noor, N.; Chew, C.K.T.; Bhachu, D.S.; Waugh, M.R.; Carmalt, C.J.; Parkin, I.P. Influencing FTO thin film growth with thin seeding layers: A route to microstructural modification. J. Mater. Chem. C 2015, 3, 9359–9368. [Google Scholar] [CrossRef]
- Burawoy, A. Distance, Bond energy, bond distance, and the nature of the covalent linkage. Trans. Faraday Soc. 1943, 39, 79–90. [Google Scholar] [CrossRef]
- Kojima, M.; Kato, H.; Gatto, M. Microstructure and electrical properties of Sb-Sn-O thin films. J. Non Cryst. Solids 1997, 218, 230–234. [Google Scholar] [CrossRef]
- Severin, K.G.; Abdel-Fattah, T.M.; Pinnavala, T.J. Supramolecular assembly of mesostructured tin oxide. Chem. Commun. 1998, 14, 1471–1472. [Google Scholar] [CrossRef]
- Ponja, S.D.; Williamson, B.A.D.; Sathasivam, S.; Scanlon, D.O.; Parkin, I.P.; Carmalt, C.J. Enhanced electrical properties of antimony doped tin oxide thin films deposited: Via aerosol assisted chemical vapour deposition. J. Mater. Chem. C 2018, 6, 7257–7266. [Google Scholar] [CrossRef]
- Du, Y.; Yan, J.; Meng, Q.; Wang, J.; Dai, H. Fabrication and excellent conductive performance of antimony-doped tin oxide-coated diatomite with porous structure. Mater. Chem. Phys. 2012, 133, 907–912. [Google Scholar] [CrossRef]
- Wang, Y.; Brezesinski, T.; Antonietti, M.; Smarsly, B. Ordered Mesoporous Sb-Nb-Ta. Am. Chem. Soc. Nano 2009, 3, 1373–1378. [Google Scholar]
- Oh, H.S.; Nong, H.N.; Strasser, P. Preparation of mesoporous Sb-, F-, and in-doped SnO2 bulk powder with high surface area for use as catalyst supports in electrolytic cells. Adv. Funct. Mater. 2015, 25, 1074–1081. [Google Scholar] [CrossRef]
- Hao, T.; Cheng, G.; Ke, H.; Zhu, Y.; Fu, Y. Effects of fluorine ions on the formation and photocatalytic activities of SnO2 nanoparticles with small sizes. RSC Adv. 2014, 4, 21548–21552. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Nag, P.; Majumdar, S.; Bumajdad, A.; Devi, P.S. Enhanced gas sensing performance of tin dioxide-based nanoparticles for a wide range of concentrations of hydrogen gas. RSC Adv. 2014, 4, 18512–18521. [Google Scholar] [CrossRef]
- Lee, S.; Galstyan, V.; Ponzoni, A.; Gonzalo-juan, I.; Riedel, R.; Dourges, M.; Nicolas, Y.; Toupance, T. Finely Tuned SnO2 Nanoparticles for Efficient Detection of Reducing and Oxidizing Gases: The Influence of Alkali Metal Cation on Gas- Sensing Properties. ACS Appl. Mater. Interfaces 2018, 10, 10173–10184. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yin, G.; Cai, T.; Yu, W.; Peng, F.; Sun, Y.; Zhang, F.; Lu, J.; Ge, M.; He, D. The role of oxygen vacancies in the sensing properties of Ni substituted SnO2 microspheres. RSC Adv. 2018, 8, 33080–33086. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.T.; Nicolas, Y.; Olivier, C.; Toupance, T.; Servant, L.; Müller, M.M.; Kleebe, H.J.; Ziegler, J.; Jaegermann, W. Nanostructured SnO2-ZnO heterojunction photocatalysts showing enhanced photocatalytic activity for the degradation of organic dyes. Inorg. Chem. 2012, 51, 7764–7773. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Lo, Y.J. High-temperature solid-state reaction induced structure modifications and associated photoactivity and gas-sensing performance of binary oxide one-dimensional composite system. RSC Adv. 2017, 7, 29428–29439. [Google Scholar] [CrossRef]
- Goodall, J.B.M.; Kellici, S.; Illsley, D.; Lines, R.; Knowles, J.C.; Darr, J.A. Optical and photocatalytic behaviours of nanoparticles in the Ti-Zn-O binary system. RSC Adv. 2014, 4, 31799–31809. [Google Scholar] [CrossRef]
- Rashad, M.M.; Ismail, A.A.; Osama, I.; Ibrahim, I.A.; Kandil, A.H.T. Photocatalytic decomposition of dyes using ZnO doped SnO2 nanoparticles prepared by solvothermal method. Arab. J. Chem. 2014, 7, 71–77. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Rabia, M.; Shaban, M.; Aly, A.H.; Ahmed, A.M. Preparation of hexagonal nanoporous Al2O3/TiO2/TiN as a novel photodetector with high efficiency. Sci. Rep. 2021, 11, 17572. [Google Scholar] [CrossRef] [PubMed]
- Nithiyanantham, U.; Ramadoss, A.; Kundu, S. Synthesis and characterization of DNA fenced, self-assembled SnO2 nano-assemblies for supercapacitor applications. Dalton Trans. 2016, 45, 3506–3521. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, Y.; Yang, Y.; Wu, P. Band gap engineering of SnO2 by epitaxial strain: Experimental and theoretical investigations. J. Phys. Chem. C 2014, 118, 6448–6453. [Google Scholar] [CrossRef]
- Popescu, D.A.; Herrmann, J.; Chimie, D.; Paris, U.; Diderot, D.; April, A. Nanosized tin dioxide: Spectroscopic (UV—VIS, NIR, EPR) and electrical conductivity studies. Phys. Chem. Chem. Phys. 2001, 3, 2522–2530. [Google Scholar] [CrossRef]
- Batzill, M.; Diebold, U. Surface studies of gas sensing metal oxides. Phys. Chem. Chem. Phys. 2007, 9, 2307–2318. [Google Scholar] [CrossRef]
- Sergeienko, S.A.; Kukla, A.L.; Yaremov, P.S.; Kiriienko, P.I.; Shvets, A.V.; Nauky, P.; Nauky, P. Effect of synthesis and doping conditions on the physical and chemical properties of mesoporous tin dioxide. Theor. Exp. Chem. 2012, 48, 245–251. [Google Scholar] [CrossRef]
- Sergiienko, S.A.; Kukla, O.L.; Yaremov, P.S.; Solomakha, V.N.; Shvets, O.V. The influence of preparation conditions and doping on the physicochemical and sensor properties of mesoporous tin oxide. Sens. Actuators B Chem. 2013, 177, 643–653. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aqeel, T.; Greer, H.F. Crystalline Mesoporous F-Doped Tin Dioxide Nanomaterial Successfully Prepared via a One Pot Synthesis at Room Temperature and Ambient Pressure. Nanomaterials 2023, 13, 2731. https://doi.org/10.3390/nano13192731
Aqeel T, Greer HF. Crystalline Mesoporous F-Doped Tin Dioxide Nanomaterial Successfully Prepared via a One Pot Synthesis at Room Temperature and Ambient Pressure. Nanomaterials. 2023; 13(19):2731. https://doi.org/10.3390/nano13192731
Chicago/Turabian StyleAqeel, Tariq, and Heather F. Greer. 2023. "Crystalline Mesoporous F-Doped Tin Dioxide Nanomaterial Successfully Prepared via a One Pot Synthesis at Room Temperature and Ambient Pressure" Nanomaterials 13, no. 19: 2731. https://doi.org/10.3390/nano13192731
APA StyleAqeel, T., & Greer, H. F. (2023). Crystalline Mesoporous F-Doped Tin Dioxide Nanomaterial Successfully Prepared via a One Pot Synthesis at Room Temperature and Ambient Pressure. Nanomaterials, 13(19), 2731. https://doi.org/10.3390/nano13192731






