Multifunctional Biotemplated Micromotors for In Situ Decontamination of Antibiotics and Heavy Metals in Soil and Groundwater
Abstract
:1. Introduction
2. Experimental
2.1. Materials
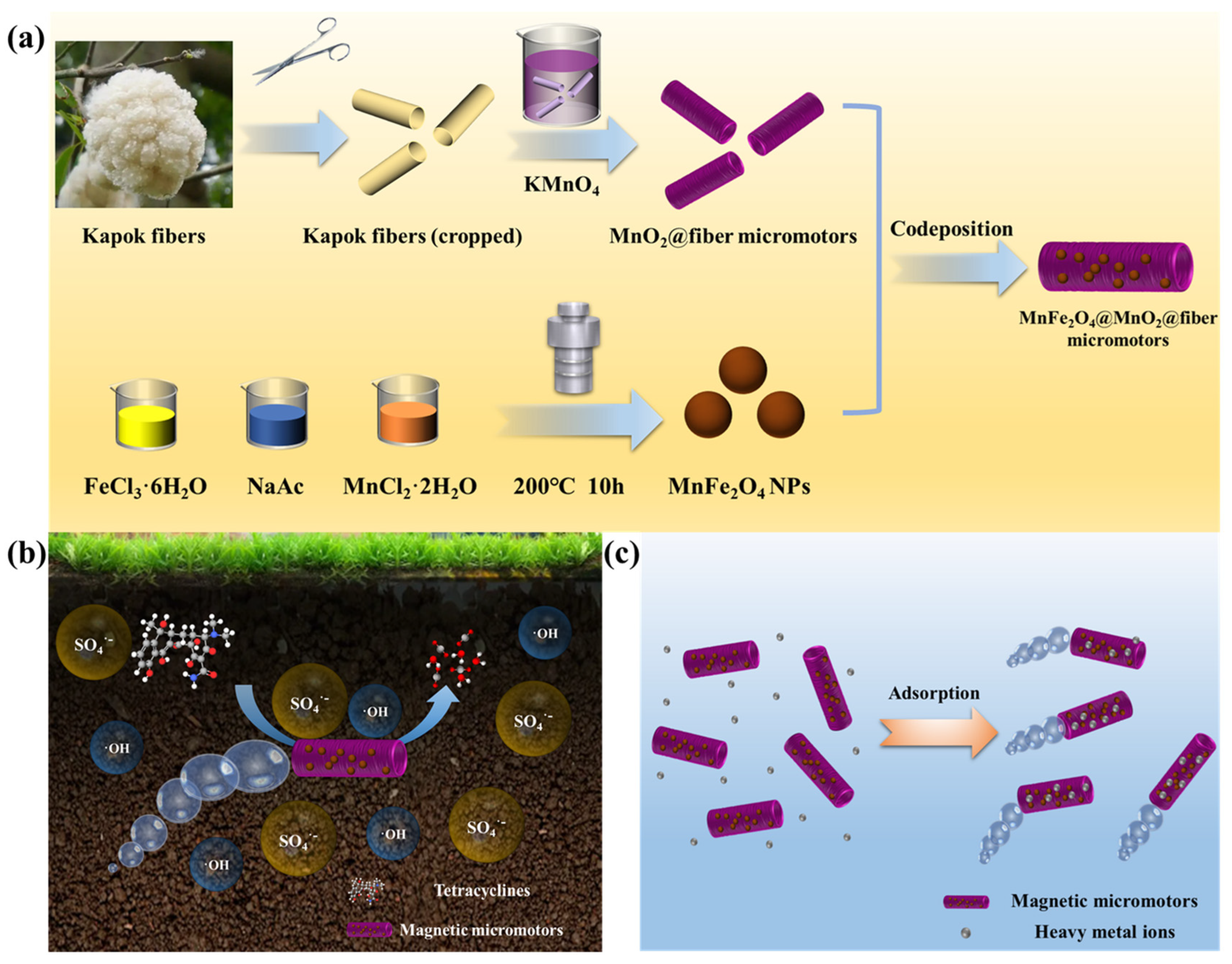
2.2. Preparation of MnO2@fiber Tubular Micromotors
2.3. Preparation of MnFe2O4 Nanoparticles
2.4. Preparation of MnFe2O4@MnO2@fiber Micromotors
2.5. Motion Study of Micromotors
2.6. Preparation of Contaminated Soil
2.7. Extraction of TC from Soil
2.8. Degradation of TC
2.9. Adsorption of Heavy Metal Ions
3. Results and Discussion
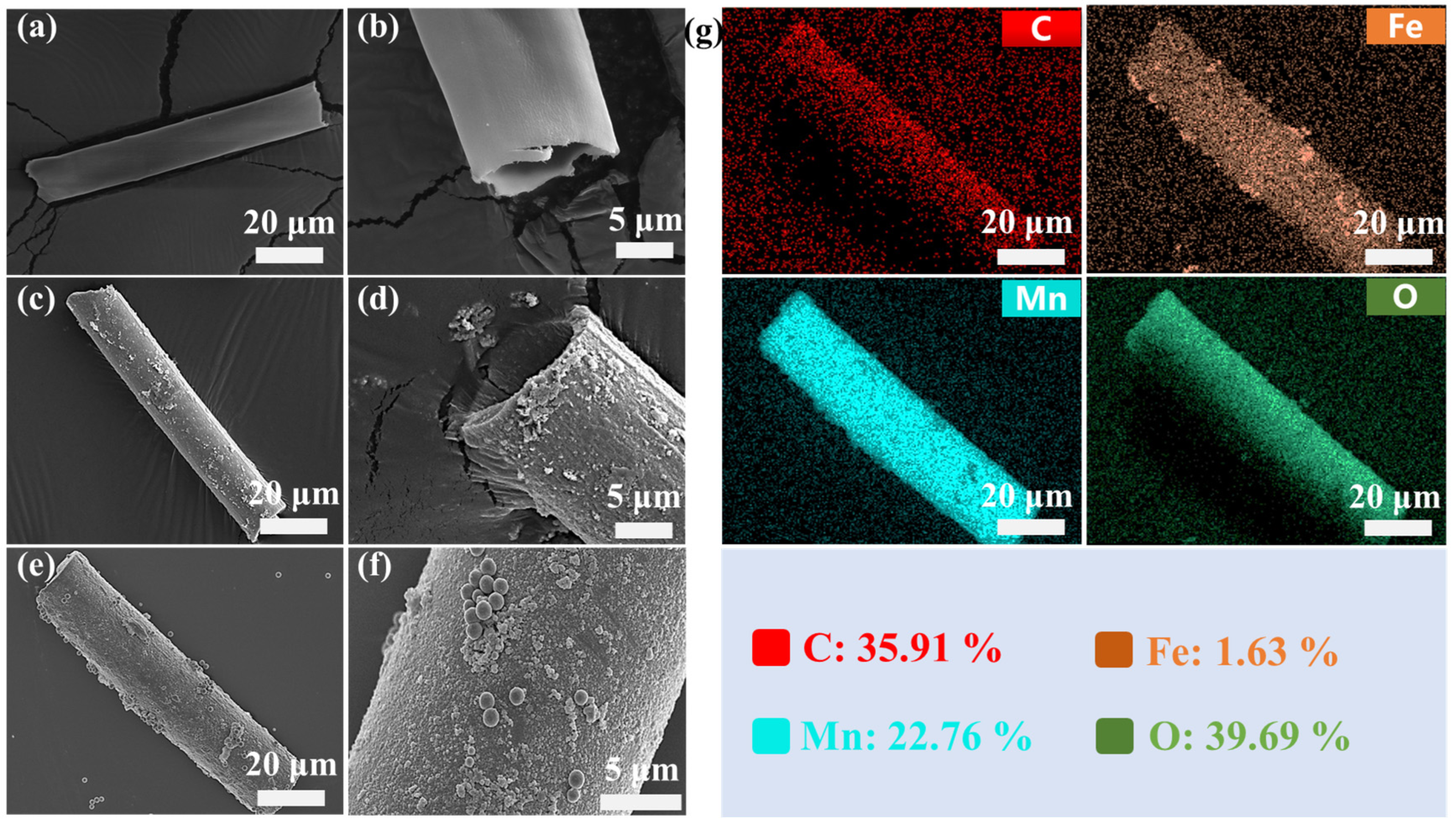
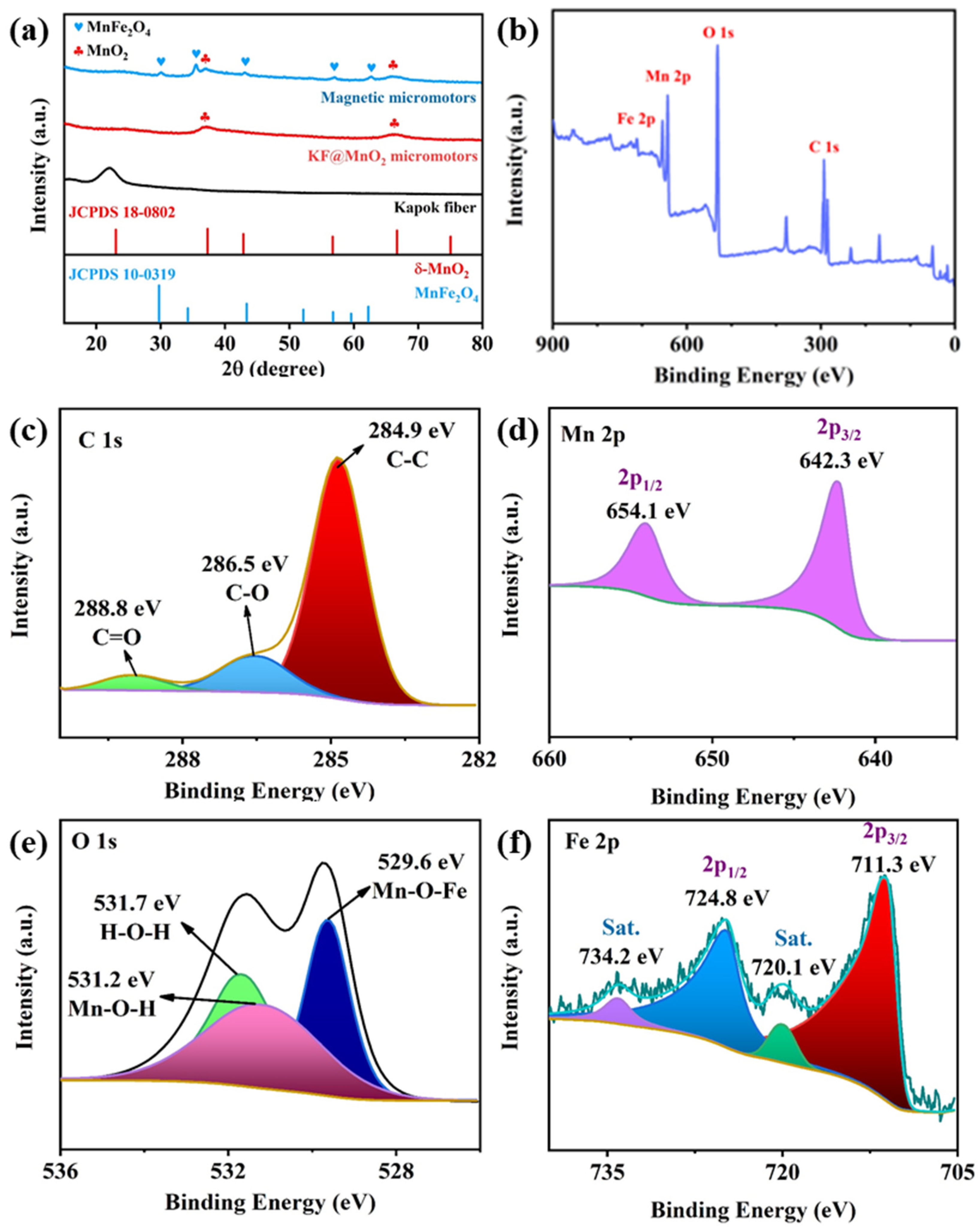
3.1. Fabrication and Characterizations of Micromotors
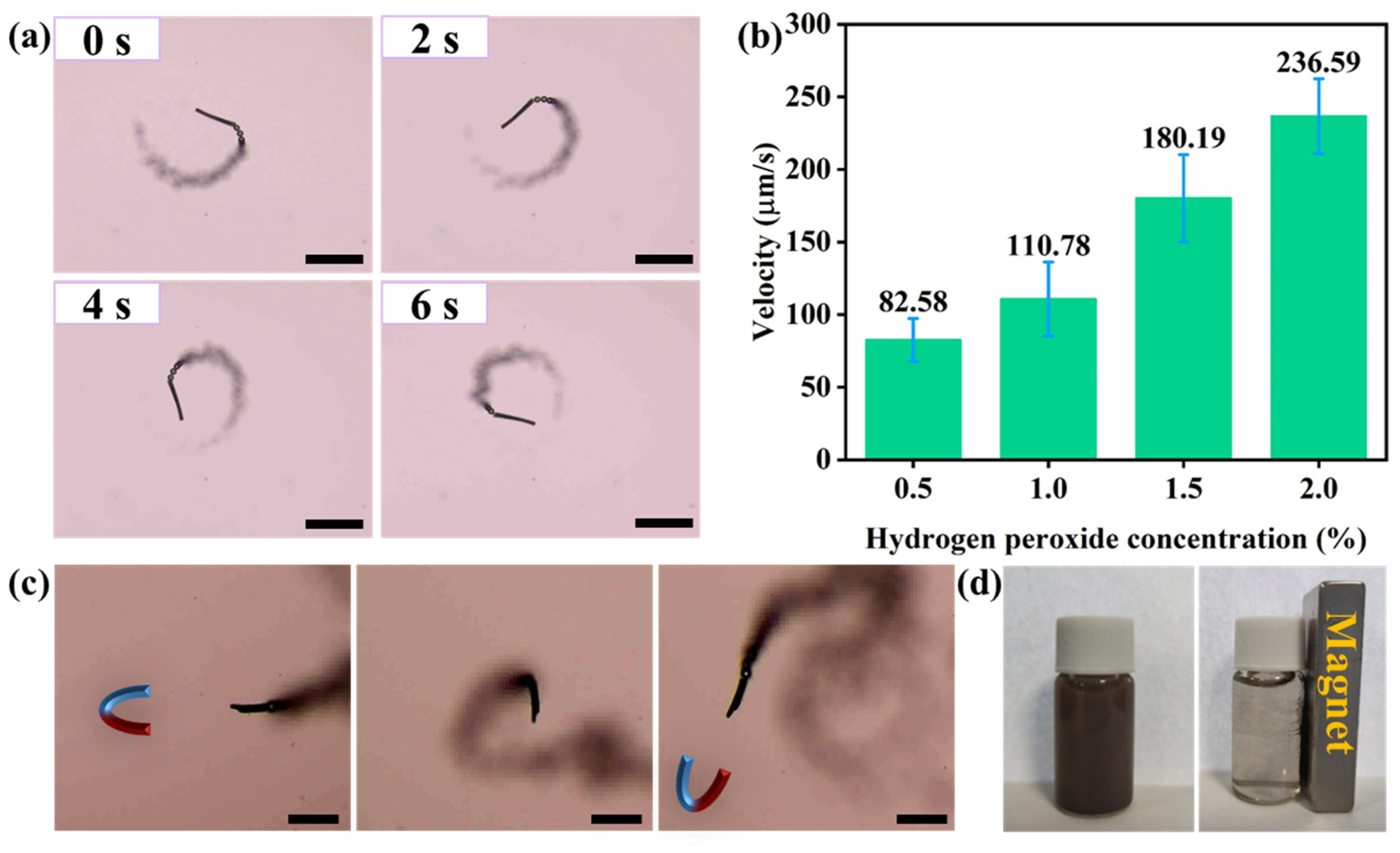
3.2. The Motion Behaviors of Magnetic Micromotors
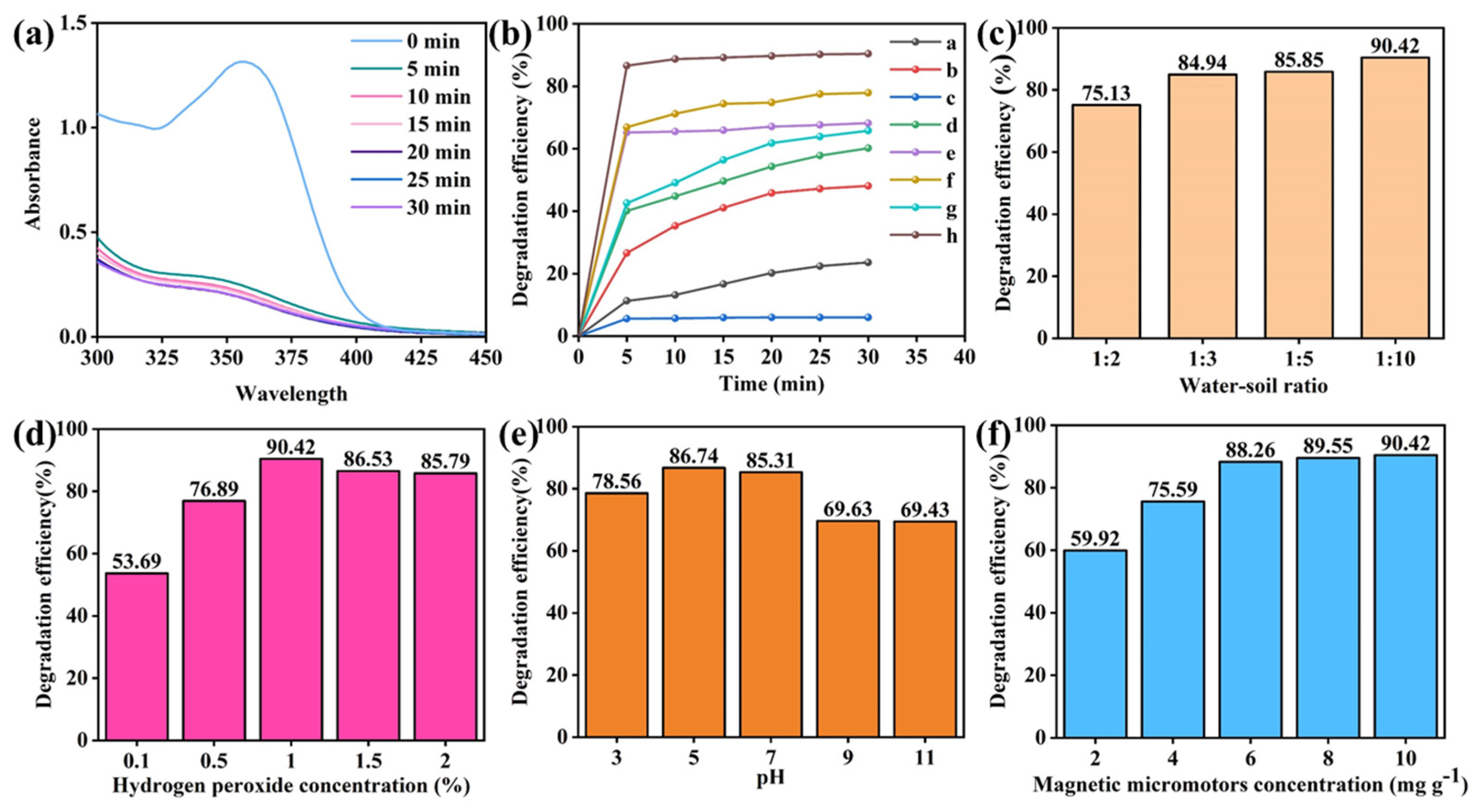
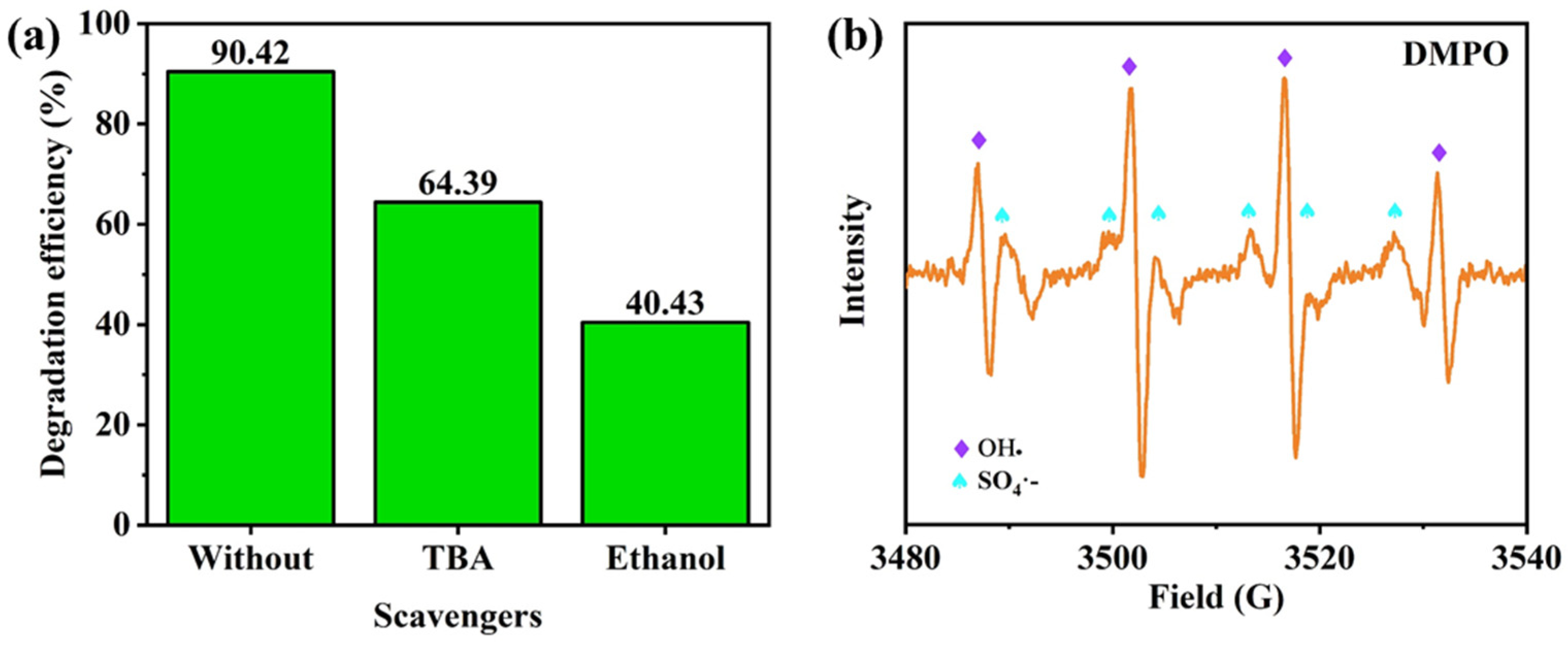
3.3. Degradation Performance of Micromotors
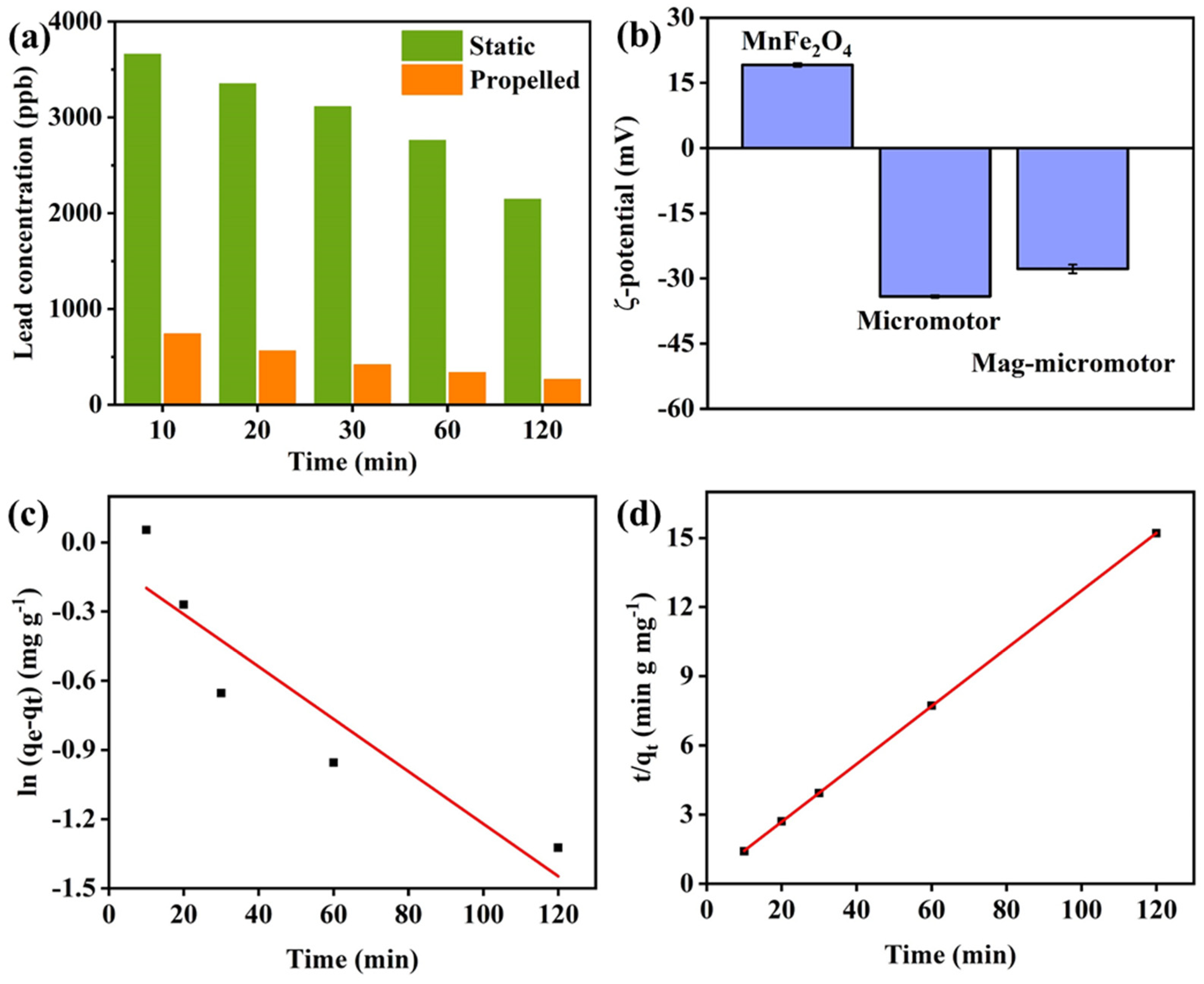
3.4. Adsorption Capacity of Magnetic Micromotors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Besha, A.T.; Bekele, D.N.; Naidu, R.; Chadalavada, S. Recent advances in surfactant-enhanced In-Situ Chemical Oxidation for the remediation of non-aqueous phase liquid contaminated soils and aquifers. Environ. Technol. Innov. 2018, 9, 303–322. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, B.; Chai, X. In Situ Remediation Technology for Heavy Metal Contaminated Sediment: A Review. Int. J. Environ. Res. Public Health 2022, 19, 16767. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Occurrence of veterinary antibiotics and progesterone in broiler manure and agricultural soil in Malaysia. Sci. Total Environ. 2014, 488, 261–267. [Google Scholar] [CrossRef]
- Hamscher, G.; Abu-Quare, A.; Sczesny, S.; Höper, H.; Nau, H. Determination of Tetracyclines and Tylosin in Soil and Water Samples from Agricultural Areas in Lower Saxony. In Proceedings of the Euroresidue IV Conference, Veldhoven, The Netherlands, 8–10 May 2000; pp. 8–10. [Google Scholar]
- Ma, Y.; Xing, S.; Wu, Y.; Gao, Y.; Ma, Z. Lignosulfonate-assisted hydrothermal synthesis of mesoporous MnFe2O4 and Fe3O4 for Pb(II) removal. Nano 2018, 13, 1850024. [Google Scholar] [CrossRef]
- Villa, K.; Parmar, J.; Vilela, D.; Sánchez, S. Metal-Oxide-Based Microjets for the Simultaneous Removal of Organic Pollutants and Heavy Metals. ACS Appl. Mater. Interfaces 2018, 10, 20478–20486. [Google Scholar] [CrossRef]
- Predoi, S.A.; Ciobanu, S.C.; Chifiriuc, M.C.; Motelica-Heino, M.; Predoi, D.; Iconaru, S.L. Hydroxyapatite Nanopowders for Effective Removal of Strontium Ions from Aqueous Solutions. Materials 2023, 16, 229. [Google Scholar] [CrossRef]
- Predoi, S.-A.; Ciobanu, C.S.; Motelica-Heino, M.; Chifiriuc, M.C.; Badea, M.L.; Iconaru, S.L. Preparation of Porous Hydroxyapatite Using Cetyl Trimethyl Ammonium Bromide as Surfactant for the Removal of Lead Ions from Aquatic Solutions. Polymers 2021, 13, 1617. [Google Scholar] [CrossRef]
- Zhang, T.; Lowry, G.V.; Capiro, N.L.; Chen, J.; Chen, W.; Chen, Y.; Dionysiou, D.D.; Elliott, D.W.; Ghoshal, S.; Hofmann, T. In situ remediation of subsurface contamination: Opportunities and challenges for nanotechnology and advanced materials. Environ. Sci. Nano 2019, 6, 1283–1302. [Google Scholar] [CrossRef]
- Devi, P.; Das, U.; Dalai, A.K. In-situ chemical oxidation: Principle and applications of peroxide and persulfate treatments in wastewater systems. Sci. Total Environ. 2016, 571, 643–657. [Google Scholar] [CrossRef]
- Bhat, A.; Megeri, G.B.; Thomas, C.; Bhargava, H.; Jeevitha, C.; Chandrashekar, S.; Madhu, G.M. Adsorption and optimization studies of lead from aqueous solution using γ-Alumina. J. Environ. Chem. Eng. 2015, 3, 30–39. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M. Removal and Oxidation of As(III) from Water Using Iron Oxide Coated CTAB as Adsorbent. Polymers 2020, 12, 1687. [Google Scholar] [CrossRef]
- Yang, W.; Qiang, Y.; Du, M.; Cao, Y.; Wang, Y.; Zhang, X.; Yue, T.; Huang, J.; Li, Z. Self-propelled nanomotors based on hierarchical metal-organic framework composites for the removal of heavy metal ions. J. Hazard. Mater. 2022, 435, 128967. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, X.; Liu, Y.; Li, J.; Liu, W.; Lu, X.; Gu, Z. Highly efficient visible-light-driven Cu2O@CdSe micromotors adsorbent. Appl. Mater. Today 2021, 25, 101200. [Google Scholar] [CrossRef]
- Mayorga-Burrezo, P.; Mayorga-Martinez, C.C.; Kim, J.; Pumera, M. Hybrid magneto-photocatalytic microrobots for sunscreens pollutants decontamination. Chem. Eng. J. 2022, 446, 137139. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Wang, J. Micromotors for environmental applications: A review. Environ. Sci. Nano 2018, 5, 1530–1544. [Google Scholar] [CrossRef]
- Oral, C.M.; Ussia, M.; Pumera, M. Hybrid Enzymatic/Photocatalytic Degradation of Antibiotics via Morphologically Programmable Light-Driven ZnO Microrobots. Small 2022, 18, 2202600. [Google Scholar] [CrossRef]
- Yuan, M.; Gong, M.; Huang, H.; Zhao, Y.; Ying, Y.; Wang, S. Bubble-propelled plasmon-reinforced Pt-ZnIn2S4 micromotors for stirring-free photocatalytic water purification. Inorg. Chem. Front. 2022, 9, 5725–5734. [Google Scholar] [CrossRef]
- Maric, T.; Mayorga-Martinez, C.C.; Nasir, M.Z.M.; Pumera, M. Platinum–halloysite nanoclay nanojets as sensitive and selective mobile nanosensors for mercury detection. Adv. Mater. Technol. 2019, 4, 1800502. [Google Scholar] [CrossRef]
- Yuan, K.; de la Asuncion-Nadal, V.; Li, Y.; Jurado-Sanchez, B.; Escarpa, A. Graphdiyne tubular micromotors: Electrosynthesis, characterization and self-propelled capabilities. Appl. Mater. Today 2020, 20, 100743. [Google Scholar] [CrossRef]
- Ye, H.; Sun, H.; Wang, S. Electrochemical synthesis of graphene/MnO2 in an architecture of bilayer microtubes as micromotors. Chem. Eng. J. 2017, 324, 251–258. [Google Scholar] [CrossRef]
- Maric, T.; Nasir, M.Z.M.; Rosli, N.F.; Budanović, M.; Webster, R.D.; Cho, N.J.; Pumera, M. Microrobots derived from variety plant pollen grains for efficient environmental clean up and as an anti-cancer drug carrier. Adv. Funct. Mater. 2020, 30, 2000112. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, J.; Wang, F. Pore structure of kapok fiber. Cellulose 2018, 25, 3219–3227. [Google Scholar] [CrossRef]
- He, C.; Xie, F. Adsorption behavior of manganese dioxide towards heavy metal ions: Surface zeta potential effect. Water Air Soil Pollut. 2018, 229, 1–13. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, H.; Chen, S.; Zheng, C.; Wu, X.; Li, Z.; Liang, C.; Dai, P.; Wang, Q.; Ma, X. Cost-effective, high-yield production of biotemplated catalytic tubular micromotors as self-propelled microcleaners for water treatment. ACS Appl. Mater. Interfaces 2021, 13, 31226–31235. [Google Scholar] [CrossRef] [PubMed]
- Sari, F.N.I.; So, P.R.; Ting, J.M. MnO2 with controlled phase for use in supercapacitors. J. Am. Ceram. Soc. 2017, 100, 1642–1652. [Google Scholar] [CrossRef]
- Li, Q.; Guo, W.; Kong, X.; Xu, J.; Xu, C.; Chen, J.; Jia, X.; Ding, Y. MnFe2O4/rGO/Diatomite composites with excellent wideband, electromagnetic microwave absorption. J. Alloys Compd. 2023, 941, 168851. [Google Scholar] [CrossRef]
- Xu, L.; Tao, J.; Zhang, X.; Yao, Z.; Wei, B.; Yang, F.; Zhou, C.; Zavabeti, A.; Zuraiqi, K.; Zhou, J. Hollow C@MoS2 nanospheres for microwave absorption. ACS Appl. Nano Mater. 2021, 4, 11199–11209. [Google Scholar] [CrossRef]
- Chen, L.; Ren, S.; Xing, X.; Yang, J.; Li, J.; Yang, J.; Liu, Q. Effect of MnO2 crystal types on CeO2@MnO2 oxides catalysts for low-temperature NH3-SCR. J. Environ. Chem. Eng. 2022, 10, 108239. [Google Scholar] [CrossRef]
- Chigane, M.; Ishikawa, M. Manganese oxide thin film preparation by potentiostatic electrolyses and electrochromism. J. Electrochem. Soc. 2000, 147, 2246. [Google Scholar] [CrossRef]
- Long, F.; Wang, L.; Rehman, S.U.; Zhang, J.; Shen, S.; Peng, B.; Wei, M.; Zhang, W.; Hu, Y.; Liang, T. Double shell structured MnFe2O4@FeO/C derived from MnFe2O4@ZIF-8 for electromagnetic wave absorption. J. Alloys Compd. 2022, 906, 164197. [Google Scholar] [CrossRef]
- Fomin, V.M.; Hippler, M.; Magdanz, V.; Soler, L.; Sanchez, S.; Schmidt, O.G. Propulsion mechanism of catalytic microjet engines. IEEE Trans. Robot. 2013, 30, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Moo, J.G.S.; Pumera, M. From nanomotors to micromotors: The influence of the size of an autonomous bubble-propelled device upon its motion. ACS Nano 2016, 10, 5041–5050. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, X.; Gao, Y.; Zhu, G.; Cheng, Q.; Cheng, X. Novel magnetic MnO2/MnFe2O4 nanocomposite as a heterogeneous catalyst for activation of peroxymonosulfate (PMS) toward oxidation of organic pollutants. Sep. Purif. Technol. 2019, 213, 456–464. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Kou, F.; Ouyang, Q.; Chen, J.; Fang, Z. Removal of norfloxacin by surface Fenton system (MnFe2O4/H2O2): Kinetics, mechanism and degradation pathway. Chem. Eng. J. 2018, 351, 747–755. [Google Scholar] [CrossRef]
- Ren, Z.; Xie, J.; Li, X.; Guo, L.; Zhang, Q.; Wu, J.; Li, Y.; Liu, W.; Li, P.; Fu, Y.; et al. Rational design of graphite carbon nitride-decorated zinc oxide nanoarrays on three-dimensional nickel foam for the efficient production of reactive oxygen species through stirring-promoted piezo–photocatalysis. J. Colloid Interface Sci. 2023, 632, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ren, Z.; Wu, J.; Li, Y.; Liu, W.; Li, P.; Xing, L.; Ma, J.; Wang, H.; Xue, X. Direct Z-scheme heterojunction of ZnO/MoS2 nanoarrays realized by flowing-induced piezoelectric field for enhanced sunlight photocatalytic performances. Appl. Catal. B Environ. 2021, 285, 119785. [Google Scholar] [CrossRef]
- Pan, S.; Huang, G.; Ding, H.; Wang, K.; Wang, H. Polydopamine-Based Surface Modification of Chlorella Microspheres for Multiple Environmental Applications. J. Nanosci. Nanotechnol. 2021, 21, 3065–3071. [Google Scholar] [CrossRef]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V.K. Removal of lead (II) by adsorption using treated granular activated carbon: Batch and column studies. J. Hazard. Mater. 2005, 125, 211–220. [Google Scholar] [CrossRef]
- Solís–Rodríguez, R.; Pérez–Garibay, R.; Alonso–González, O.; Mendieta–George, D. Enhancing the arsenic adsorption by controlling the zeta potential of Zn(OH)2 flocs. J. Environ. Chem. Eng. 2021, 9, 106300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Wang, K.; Ma, E.; Wang, H. Multifunctional Biotemplated Micromotors for In Situ Decontamination of Antibiotics and Heavy Metals in Soil and Groundwater. Nanomaterials 2023, 13, 2710. https://doi.org/10.3390/nano13192710
Cui H, Wang K, Ma E, Wang H. Multifunctional Biotemplated Micromotors for In Situ Decontamination of Antibiotics and Heavy Metals in Soil and Groundwater. Nanomaterials. 2023; 13(19):2710. https://doi.org/10.3390/nano13192710
Chicago/Turabian StyleCui, Haohao, Ke Wang, Enhui Ma, and Hong Wang. 2023. "Multifunctional Biotemplated Micromotors for In Situ Decontamination of Antibiotics and Heavy Metals in Soil and Groundwater" Nanomaterials 13, no. 19: 2710. https://doi.org/10.3390/nano13192710
APA StyleCui, H., Wang, K., Ma, E., & Wang, H. (2023). Multifunctional Biotemplated Micromotors for In Situ Decontamination of Antibiotics and Heavy Metals in Soil and Groundwater. Nanomaterials, 13(19), 2710. https://doi.org/10.3390/nano13192710



