Production of Metallic Alloy Nanowires and Particles Templated Using Tomato Mosaic Virus (ToMV)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Trimetallic ToMV NWs (AuPtPd ToMV NWs)
2.2. Synthesis of Bimetallic ToMV NWs (AuPt, AuPd and PtPd ToMV NWs)
2.3. Recovery of Bi- and Tri-Metallized ToMV Particles
2.4. Electron Microscopy (EM)
2.5. X-ray Photoelectron Spectroscopy (XPS) and Argon (Ar+) ion-Sputtered Sample Analysis
3. Results and Discussion
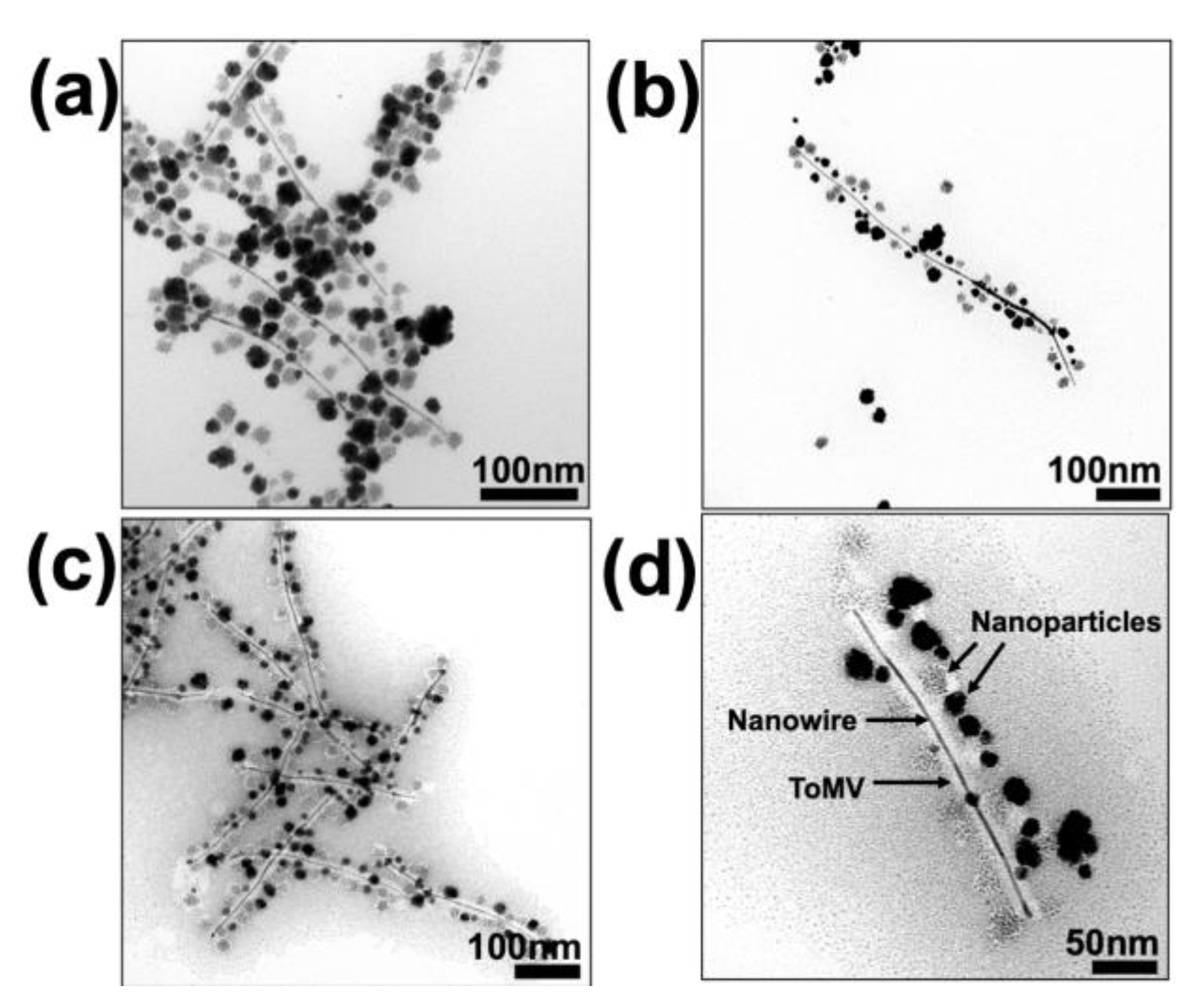
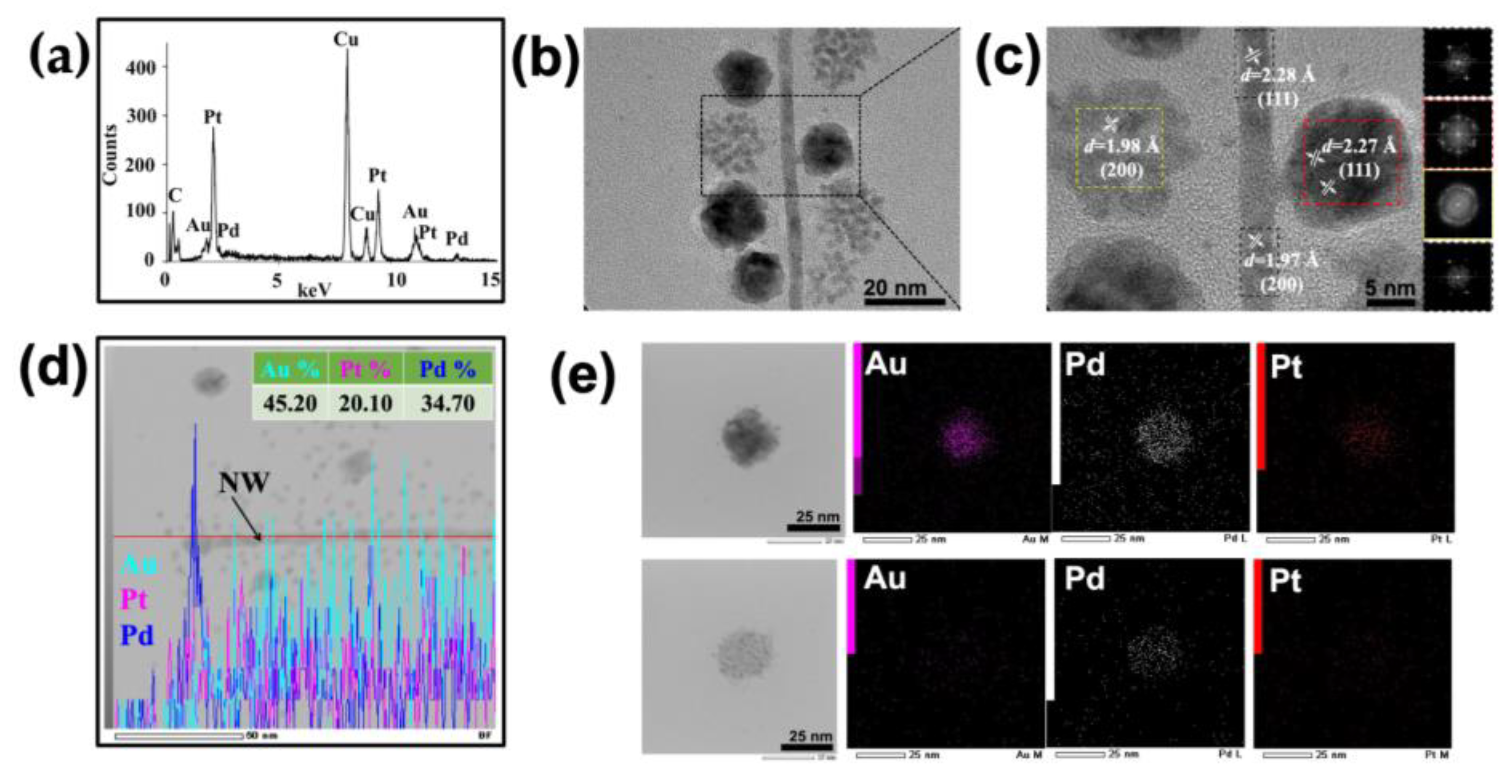
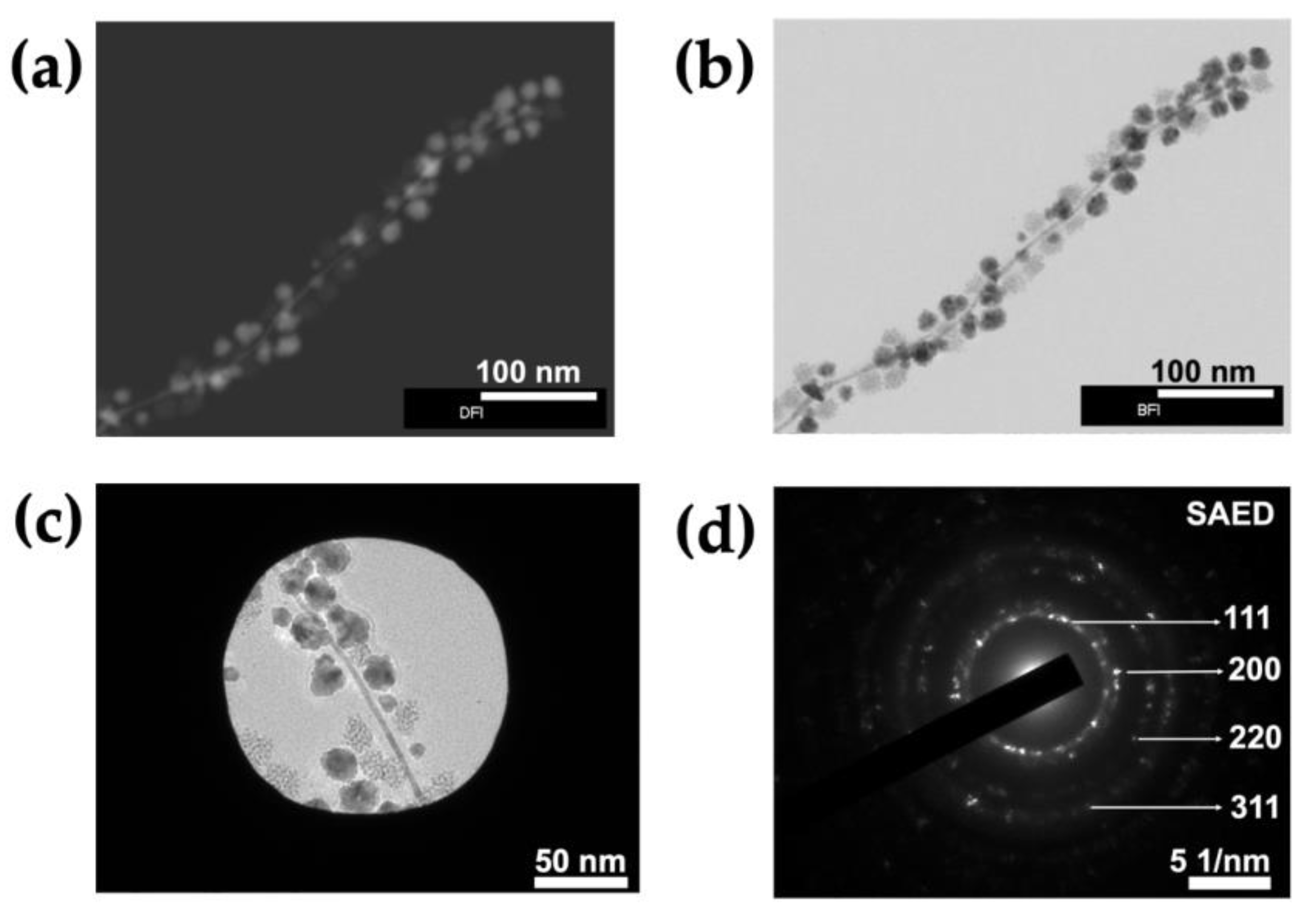
3.1. Synthesis of ToMV Gold–Platinum–Palladium Nanomaterials (ToMV AuPtPd)
3.2. Synthesis of ToMV Bimetallic NWs (ToMV AuPt, AuPd and PtPd NWs)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaleska-Medynska, A.; Marchelek, M.; Diak, M.; Grabowska, E. Noble metal-based bimetallic nanoparticles: The effect of the structure on the optical, catalytic and photocatalytic properties. Adv. Colloid Interface Sci. 2016, 229, 80–107. [Google Scholar] [CrossRef] [PubMed]
- Lamy-Pitara, E.; El Mouahid, S.; Barbier, J. Effect of anions on catalytic and electrocatalytic hydrogenations and on the electrocatalytic oxidation and evolution of hydrogen on platinum. Electrochim. Acta 2000, 45, 4299–4308. [Google Scholar] [CrossRef]
- Zhang, J.; Sasaki, K.; Sutter, E.; Adzic, R.R. Stabilization of Platinum Oxygen-Reduction Electrocatalyst Using Gold Clusters. Science 2007, 315, 220. [Google Scholar] [CrossRef]
- Holst-Olesen, K.; Reda, M.; Hansen, H.A.; Vegge, T.; Arenz, M. Enhanced oxygen reduction activity by selective anion adsorption on non-precious-metal catalysts. ACS Catal. 2018, 8, 7104–7112. [Google Scholar] [CrossRef]
- Shinozaki, K.; Zack, J.W.; Richards, R.M.; Pivovar, B.S.; Kocha, S.S. Oxygen reduction reaction measurements on platinum electrocatalysts utilizing rotating disk electrode technique. J. Electrochem. Soc. 2015, 162, F1144–F1158. [Google Scholar] [CrossRef]
- Bu, L.; Guo, S.; Zhang, X.; Shen, X.; Su, D.; Lu, G.; Zhu, X.; Yao, J.; Guo, J.; Huang, X. Surface engineering of hierarchical platinum-cobalt nanowires for efficient electrocatalysis. Nat. Commun. 2016, 7, 11850. [Google Scholar] [CrossRef]
- Jiang, L.-Y.; Lin, X.-X.; Wang, A.J.; Yuan, J.; Feng, J.J.; Li, X.S. Facile solvothermal synthesis of monodisperse Pt2.6Co1 nanoflowers with enhanced electrocatalytic activity towards oxygen reduction and hydrogen evolution reactions. Electrochim. Acta 2017, 225, 525–532. [Google Scholar] [CrossRef]
- Qian, K.; Huang, W. Au–Pd alloying-promoted thermal decomposition of PdO supported on SiO2 and its effect on the catalytic performance in CO oxidation. Catal. Today 2011, 164, 320–324. [Google Scholar] [CrossRef]
- Antolini, E. Formation, microstructural characteristics and stability of carbon supported platinum catalysts for low temperature fuel cells. J. Mater. Sci. 2003, 38, 2995–3003. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, X.; Su, K.; Wang, S.; Li, J.; Tang, Y. Breaking the lattice match of Pd on Au(111) nanowires: Manipulating the island and epitaxial growth pathways to boost the oxygen reduction reactivity. J. Mater. Chem. A 2020, 37, 19300–19308. [Google Scholar] [CrossRef]
- Bonet, F.; Delmas, V.; Grugeon, S.; Urbina, R.H.; Silvert, P.Y.; Tekaia-Elhsissen, K. Synthesis of monodisperse Au, Pt, Pd, Ru and Ir nanoparticles in ethylene glycol. Nanostruct. Mater. 1999, 11, 1277–1284. [Google Scholar] [CrossRef]
- Lim, B.; Jiang, M.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pippel, E.; Scholz, R.; Gösele, U. Nanoporous Pt−Co alloy nanowires: Fabrication, characterization, and electrocatalytic properties. Nano Lett. 2009, 9, 4352–4358. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Y.; Liu, G.; Dong, B.; Yu, H.; Zhou, J.; Wang, J.; Jin, R. Microbial synthesis of bimetallic PdPt nanoparticles for catalytic reduction of 4-nitrophenol. Environ. Sci. Pollut. Res. 2017, 24, 5249–5258. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, D.; Chen, X.; Zhou, G.; Yu, R.; Li, Y. Defect-Dominated Shape Recovery of Nanocrystals: A New Strategy for Trimetallic Catalysts. J. Am. Chem. Soc. 2013, 135, 12220–12223. [Google Scholar] [CrossRef] [PubMed]
- Kondrat, S.A.; Miedziak, P.J.; Douthwaite, M.; Brett, G.L.; Davies, T.E.; Morgan, D.J.; Edwards, J.K.; Knight, D.W.; Kiely, C.J.; Taylor, S.H.; et al. Base-free oxidation of glycerol using titania-supported trimetallic Au-Pd-Pt nanoparticles. ChemSusChem 2014, 7, 1326–1334. [Google Scholar] [CrossRef]
- Deplanche, K.; Merroun, M.L.; Casadesus, M.; Tran, D.T.; Mikheenko, I.P.; Bennett, J.A.; Zhu, J.; Jones, I.P.; Attard, G.A.; Wood, J.; et al. Microbial synthesis of core/shell gold/palladium nanoparticles for applications in green chemistry. J. R. Soc. Interface 2012, 9, 1705–1712. [Google Scholar] [CrossRef]
- Jena, P.; Bhattacharya, M.; Bhattacharjee, G.; Satpati, B.; Mukherjee, P.; Senapati, D.; Srinivasan, R. Bimetallic gold–silver nanoparticles mediate bacterial killing by disrupting the actin cytoskeleton MreB. Nanoscale 2020, 12, 3731–3749. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Namvar, F.; Moniri, M.; Tahir, P.M.; Azizi, S.; Mohamad, R. Nanoparticles Biosynthesized by Fungi and Yeast: A Review of Their Preparation, Properties, and Medical Applications. Molecules 2015, 20, 16540–16565. [Google Scholar] [CrossRef]
- Govindaraju, K.; Basha, S.K.; Kumar, G.; Singaravelu, G. Silver, gold and bimetallic nanoparticles production using single-cell protein (Spirulina platensis) Geitler. J. Mater. Sci. 2008, 43, 5115–5122. [Google Scholar] [CrossRef]
- Senapati, S.; Ahmad, A.; Khan, M.I.; Sastry, M.; Kumar, R. Extracellular biosynthesis of bimetallic Au-Ag alloy nanoparticles. Small 2005, 1, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Castro-Longoria, E.; Vilchis-Nestor, A.R.; Avalos-Borja, M. Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa. Colloids Surf. B Biointerfaces 2011, 83, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, J.; Yun, D.S.; Nam, Y.S.; Shao-Horn, Y.; Belcher, A.M. Virus-templated Au and Au/Pt core/shell nanowires and their electrocatalytic activitives for fuel cell applications. Energy Environ. Sci. 2012, 5, 8328–8334. [Google Scholar] [CrossRef] [PubMed]
- Young, M.; Debbie, W.; Uchida, M.; Douglas, T. Plant Viruses as Biotemplates for materials and their use in nanotechnology. Annu. Rev. Phytopathol. 2008, 46, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Seki, M.; Tabata, H.; Watanabe, Y.; Yamashita, I. Fabrication of aligned magnetic nanoparticles using tobamoviruses. Nano Lett. 2010, 10, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.J.; Armstrong, J.S.; Gibbs, M.J. A type of nucleotide motif that distinguishes tobamovirus species more efficiently than nucleotide signatures. Arch. Virol. 2004, 149, 1941–1954. [Google Scholar] [CrossRef]
- Shah, S.N.; Khan, A.A.; Espinosa, A.; Garcia, M.A.; Nuansing, W.; Ungureanu, M.; Heddle, J.G.; Chuvilin, A.L.; Wege, C.; Bittner, A.M. Virus-templated near-amorphous iron oxide nanotubes. Langmuir 2016, 32, 5899–5908. [Google Scholar] [CrossRef]
- Van Regenmortel, M.H.V. Tobacco mosaic virus antigenic structure. In The Plant Viruses; Springer: Berlin/Heidelberg, Germany, 1966; pp. 79–104. [Google Scholar]
- Shenton, W.; Douglas, T.; Young, M.; Stubbs, G.; Mann, S. Inorganic-organic nanotubes composites from template mineralization of tobacco mosaic virus. Adv. Mater. 1999, 11, 253–256. [Google Scholar] [CrossRef]
- Dujardin, E.; Peet, C.; Stubbs, G.; Culver, J.N.; Mann, S. Organization of metallic nanoparticles using tobacco mosaic virus templates. Nano Lett. 2003, 3, 413. [Google Scholar] [CrossRef]
- Knez, M.; Bittner, A.M.; Boes, F.; Wege, C.; Jeske, H.; Mai, B.E.; Kern, K. Biotemplate synthesis of 3-nm nickel and cobalt nanowires. Nano Lett. 2003, 3, 1079–1082. [Google Scholar] [CrossRef]
- Knez, M.; Sumser, M.; Bittner, A.M.; Wege, C.; Jeske, H.; Martin, T.P.; Kern, K. Spatially selective nucleation of metal cluster on the tobacco mosaic virus. Adv. Funct. Mater. 2004, 14, 116–124. [Google Scholar] [CrossRef]
- Balci, S.; Bittner, A.M.; Hahn, K.; Scheu, C.; Kenz, M.; Kadri, A.; Wege, C.; Jeske, H.; Kern, K. Copper nanowires within the central channel of tobacco mosaic virus particles. Electrochim. Acta 2006, 51, 6251–6257. [Google Scholar] [CrossRef]
- Balci, S.; Hahn, K.; Kopold, P.; Kadri, A.; Wege, C.; Kern, K.; Bittner, A.M. Electroless synthesis of 3 nm wide alloy nanowires inside tobacco mosaic virus. Nanotechnology 2012, 23, 045603. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-S.; Kim, S.-M.; Lee, S.-Y.; Stach, E.A.; Culver, J.N.; Harris, M.T. Quantitative study of Au(III) and Pd(II) ion adsorption on genetically engineered tobacco mosaic virus. J. Colloid Interface Sci. 2010, 342, 455–461. [Google Scholar] [CrossRef]
- Shah, S.N.; Saunders, K.; Thuenemann, E.C.; Evans, D.J.; Lomonossoff, G.P. Designer-length palladium nanowires can be templated by the central channel of tobacco mosaic virus nanorods. Virology 2022, 577, 155–162. [Google Scholar] [CrossRef]
- Shah, S.N.; Shah, S.S.; Ito, E.; Heddle, J.G. Template-free, hollow and porous platinum nanotubes derived from tobamovirus and their three-dimensional structure at the nanoscale. RSC Adv. 2014, 4, 39305–39311. [Google Scholar] [CrossRef]
- Shah, S.S.; Shah, S.N.; Heddle, J.J. Polymer-mediated dual mineralization of a plant virus: A platinum nanowire encapsulated by iron oxide. Chem. Lett. 2015, 44, 79–81. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation; Physical Electronics Division: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Cai, X.T.; Wang, J.W.; Wang, R.X.; Wang, A.; Zhong, S.X.; Chen, J.R.; Bai, S. Interface engineering on janus Pd-Au heterojunction co-catalysts for selective photocatalytic reduction of CO2 to CH4. J. Mater. Chem. A 2019, 7, 5266–5276. [Google Scholar] [CrossRef]
- Chen, X.; Quan, D.J.; Xu, G.D.; Xu, H.; Dai, J.T.; Du, Y.K. Magnetic Fe3O4 supported PdAu bimetallic nanoparticles with the enhanced catalytic activity for Heck and Suzuki cross-coupling reactions. Colloids Surf. A 2019, 573, 67–72. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Fang, R.; He, L.-L.; Feng, J.-J.; Yuan, J.; Wang, A.-J. Bimetallic PdAu alloyed nanowires: Rapid synthesis via oriented attachment growth and their electrocatalytic activity for methanol oxidation reaction. J. Alloys Compd. 2016, 684, 379–388. [Google Scholar] [CrossRef]
- Qiao, P.; Xu, S.; Zhang, D.; Li, R.; Zou, S.; Liu, J.; Yi, W.; Li, J.; Fan, J. Sub-10 nm Au–Pt–Pd alloy trimetallic nanoparticles with a high oxidation-resistant property as efficient and durable VOC oxidation catalysts. Chem. Commun. 2014, 50, 11713–11716. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzan, L.M.; Philipse, A.P. Stable hydrosols of metallic and bimetallic nanoparticles Immobilized on imogolite fibers. J. Phys. Chem. 1995, 99, 15120–15128. [Google Scholar] [CrossRef]
- Mariscal, M.M.; Dassie, S.A.; Leiva, E.P.M. Collision as a way of forming bimetallic nanoclusters of various structures and chemical compositions. J. Chem. Phys. 2005, 123, 184505–184511. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Sheng, W.; Zhuang, Z.; Xu, B.; Yan, Y. Universal dependence of hydrogen oxidation and evolution reaction activity of platinum-group metals on pH and hydrogen binding energy. Sci. Adv. 2016, 2, e1501602. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.N.; Heddle, J.G.; Evans, D.J.; Lomonossoff, G.P. Production of Metallic Alloy Nanowires and Particles Templated Using Tomato Mosaic Virus (ToMV). Nanomaterials 2023, 13, 2705. https://doi.org/10.3390/nano13192705
Shah SN, Heddle JG, Evans DJ, Lomonossoff GP. Production of Metallic Alloy Nanowires and Particles Templated Using Tomato Mosaic Virus (ToMV). Nanomaterials. 2023; 13(19):2705. https://doi.org/10.3390/nano13192705
Chicago/Turabian StyleShah, Sachin N., Jonathan G. Heddle, David J. Evans, and George P. Lomonossoff. 2023. "Production of Metallic Alloy Nanowires and Particles Templated Using Tomato Mosaic Virus (ToMV)" Nanomaterials 13, no. 19: 2705. https://doi.org/10.3390/nano13192705
APA StyleShah, S. N., Heddle, J. G., Evans, D. J., & Lomonossoff, G. P. (2023). Production of Metallic Alloy Nanowires and Particles Templated Using Tomato Mosaic Virus (ToMV). Nanomaterials, 13(19), 2705. https://doi.org/10.3390/nano13192705





