Fabrication of ZnO/ZnAl2O4/Au Nanoarrays through DC Electrodeposition Utilizing Nanoporous Anodic Alumina Membranes for Environmental Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of AAOM and AAOM/Au
2.2. Deposition of ZnO Nanostructure within the AAOM
2.3. Sample Characterization
2.4. Dye Removal and pH Sensing Applications
3. Results and Discussion
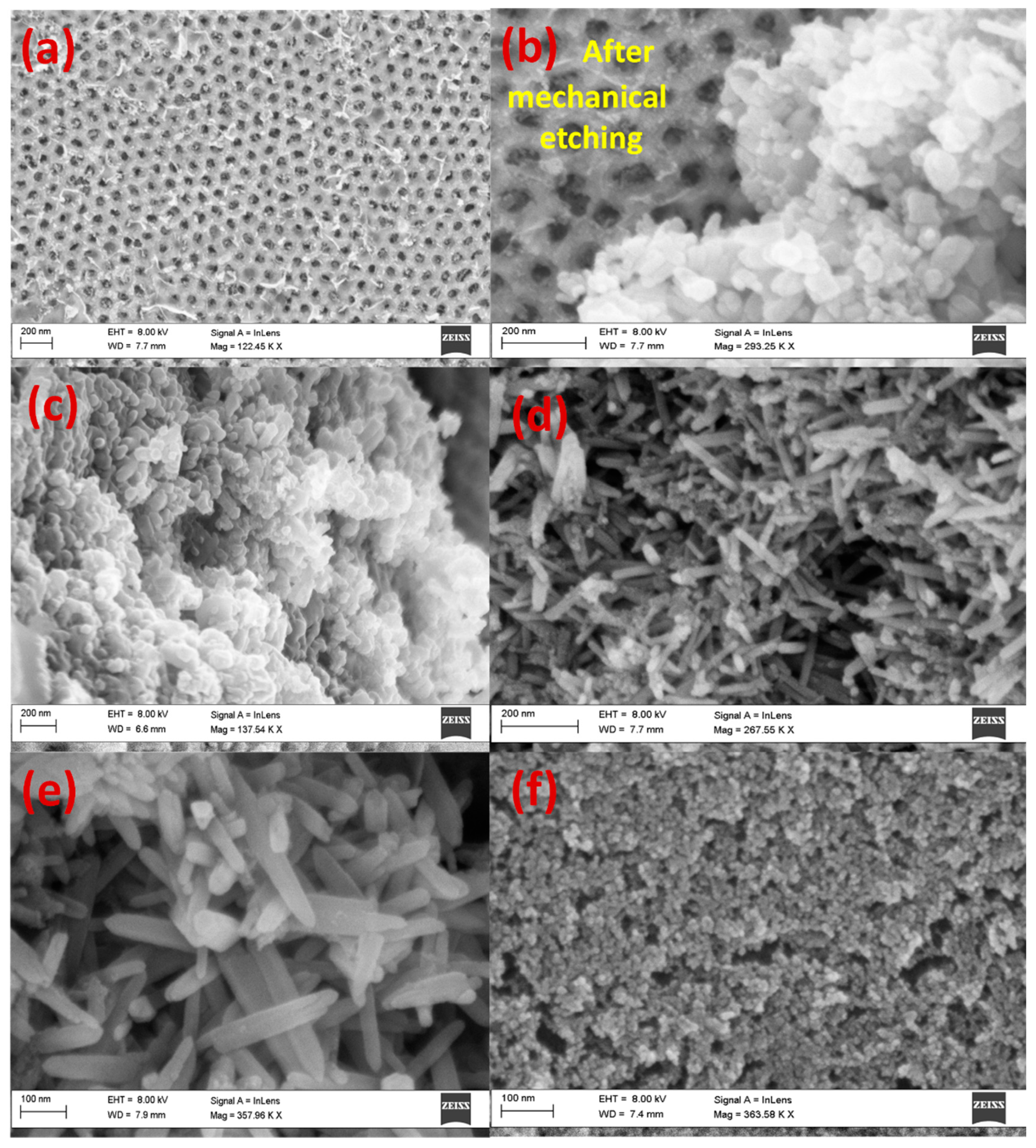
3.1. Morphology and Chemical Composition of AAOM and Au/AAOM
3.2. ZnO-Nanoarray Morphology and Composition
3.3. ZnO Nanorods on the Top Surface of Au/AAOM and AAOM
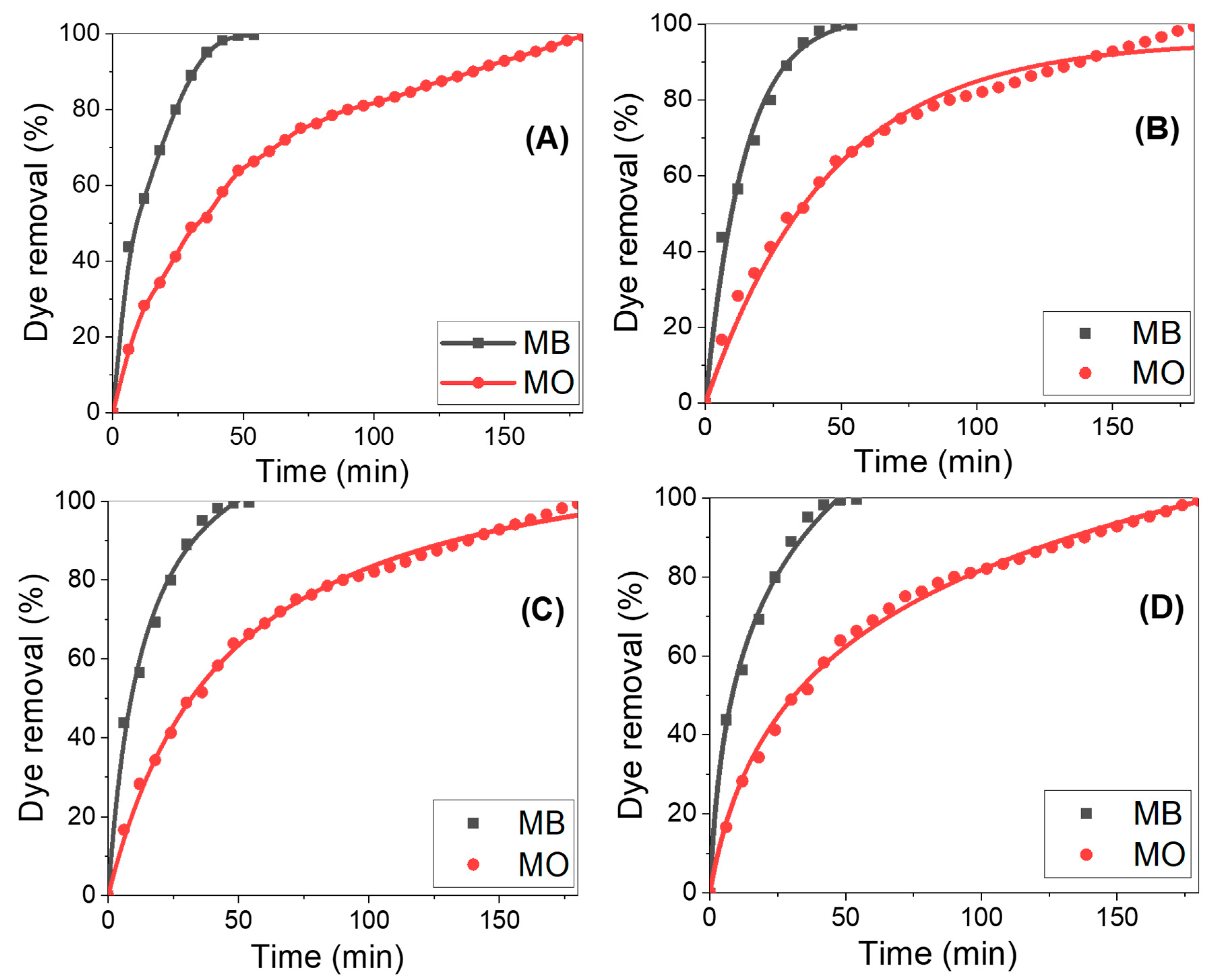
3.4. MB and MO Dye Removal using ZnO/ZnAl2O4/Au
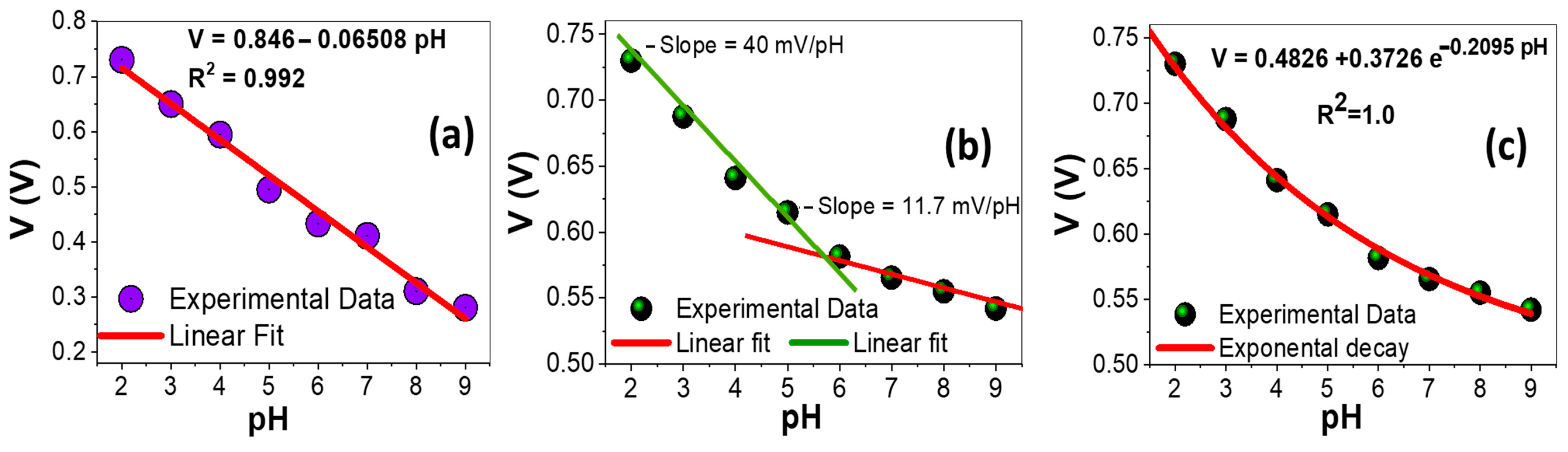
3.5. pH-Sensing Performance of AAOM/Au and ZnO/ZnAl2O4/Au Electrodes
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le, A.T.; Ahmadipour, M.; Pung, S.-Y. A review on ZnO-based piezoelectric nanogenerators: Synthesis, characterization techniques, performance enhancement and applications. J. Alloys Compd. 2020, 844, 156172. [Google Scholar] [CrossRef]
- Mamat, M.H.; Ishak, N.I.; Khusaimi, Z.; Zahidi, M.M.; Abdullah, M.H.; Muhamad, S.; Sin, N.D.M.; Mahmood, M.R. Thickness-dependent characteristics of aluminium-doped zinc oxide nanorod-array-based, ultraviolet photoconductive sensors. Jpn. J. Appl. Phys. 2012, 51, 06FF03. [Google Scholar] [CrossRef]
- Vega-Poot, A.G.; Macías-Montero, M.; Idígoras, J.; Borrás, A.; Barranco, A.; Gonzalez-Elipe, A.R.; Lizama-Tzec, F.I.; Oskam, G.; Anta, J.A. Mechanisms of Electron Transport and Recombination in ZnO Nanostructures for Dye-Sensitized Solar Cells. ChemPhysChem 2014, 15, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.; Nasser, N.; Shaban, M.; Alshaikh, H.; Hamdy, H.; Ahmed, A.M. Effect of morphology and plasmonic on Au/ZnO films for efficient photoelectrochemical water splitting. Nanomaterials 2021, 11, 2338. [Google Scholar] [CrossRef]
- Saad, R.; Gamal, A.; Zayed, M.; Ahmed, A.M.; Shaban, M.; BinSabt, M.; Rabia, M.; Hamdy, H. Fabrication of ZnO/CNTs for Application in CO2 Sensor at Room Temperature. Nanomaterials 2021, 11, 3087. [Google Scholar] [CrossRef]
- Popa, M.L.; Preda, M.D.; Neacșu, I.A.; Grumezescu, A.M.; Ginghină, O. Traditional vs. Microfluidic Synthesis of ZnO Nanoparticles. Int. J. Mol. Sci. 2023, 24, 1875. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the Alcohols on the ZnO Synthesis and Its Properties: The Photocatalytic and Antimicrobial Activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef]
- Motelica, L.; Oprea, O.-C.; Vasile, B.-S.; Ficai, A.; Ficai, D.; Andronescu, E.; Holban, A.M. Antibacterial Activity of Solvothermal Obtained ZnO Nanoparticles with Different Morphology and Photocatalytic Activity against a Dye Mixture: Methylene Blue, Rhodamine B and Methyl Orange. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef]
- Hong, J.I.; Bae, J.; Wang, Z.L.; Snyder, R.L. Room-temperature, texture-controlled growth of ZnO thin films and their application for growing aligned ZnO nanowire arrays. Nanotechnology 2009, 20, 085609. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Awad, M.A.; Shaban, M. Optical constants, photoluminescence and thermogravimetry of ZnS–ZnO hybrid nanowires synthesized via vapor transport. Appl. Phys. A Mater. Sci. Process. 2022, 128, 274. [Google Scholar] [CrossRef]
- Mohamed, F.; Allah, A.E.; Al-Ola, K.A.A.; Shaban, M. Design and Characterization of a Novel ZnO–Ag/Polypyrrole Core–Shell Nanocomposite for Water Bioremediation. Nanomaterials 2021, 11, 1688. [Google Scholar] [CrossRef]
- Shaban, M.; Mohamed, F.; Abdallah, S. Production and Characterization of Superhydrophobic and Antibacterial Coated Fabrics Utilizing ZnO Nanocatalyst. Sci. Rep. 2018, 8, 3925. [Google Scholar] [CrossRef]
- Wang, S.L.; Zhu, H.W.; Tang, W.H.; Li, P.G. Propeller-shaped ZnO nanostructures obtained by chemical vapor deposition: Photoluminescence and photocatalytic properties. J. Nanomater. 2012, 2012, 594290. [Google Scholar] [CrossRef]
- Xu, L.; Guo, Y.; Liao, Q.; Zhang, J.; Xu, D. Morphological control of ZnO nanostructures by electrodeposition. J. Phys. Chem. B 2005, 109, 13519–13522. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Zhang, Q.; Lu, Z.; Song, H.; Wang, Y. Electrodeposition of ZnO Nanorods with Synergistic Photocatalytic and Self-Cleaning Effects. J. Electron. Mater. 2021, 50, 4954–4961. [Google Scholar] [CrossRef]
- Orozco-Messana, J.; Camaratta, R. ZnO Electrodeposition Model for Morphology Control. Nanomaterials 2022, 12, 720. [Google Scholar] [CrossRef] [PubMed]
- Kouhpanji, M.R.Z.; Stadler, B.J.H. A Guideline for Effectively Synthesizing and Characterizing Magnetic Nanoparticles for Advancing Nanobiotechnology: A Review. Sensors 2020, 20, 2554. [Google Scholar] [CrossRef]
- García-Arribas, A.; Martínez, F.; Fernández, E.; Ozaeta, I.; Kurlyandskaya, G.V.; Svalov, A.V.; Berganzo, J.; Barandiaran, J.M. GMI detection of magnetic-particle concentration in continuous flow. Sens. Actuators A Phys. 2011, 172, 103–108. [Google Scholar] [CrossRef]
- Fatimah, S.; Rahman, A.; Yusof, N.A.; Hashim, U.; Nuzaihan, M.; Nor, M. Design and Fabrication of Silicon Nanowire based Sensor. Int. J. Electrochem. Sci. 2013, 8, 10946–10960. [Google Scholar]
- Bennett, T.D.; Coudert, F.-X.; James, S.L.; Cooper, A.I. The changing state of porous materials. Nat. Mater. 2021, 20, 1179–1187. [Google Scholar] [CrossRef]
- Sun, Y.; Rogge, S.M.J.; Lamaire, A.; Vandenbrande, S.; Wieme, J.; Siviour, C.R.; Van Speybroeck, V.; Tan, J.-C. High-rate nanofluidic energy absorption in porous zeolitic frameworks. Nat. Mater. 2021, 20, 1015–1023. [Google Scholar] [CrossRef]
- Saharil, F.; Forsberg, F.; Liu, Y.; Bettotti, P.; Kumar, N.; Niklaus, F.; Haraldsson, T.; Van Der Wijngaart, W.; Gylfason, K.B. Dry adhesive bonding of nanoporous inorganic membranes to microfluidic devices using the OSTE(+) dual-cure polymer. J. Micromech. Microeng. 2013, 23, 025021. [Google Scholar] [CrossRef]
- Shaban, M.; Hady, A.G.A.; Serry, M. A new sensor for heavy metals detection in aqueous media. IEEE Sens. J. 2014, 14, 436–441. [Google Scholar] [CrossRef]
- Safronov, A.P.; Stadler, B.J.H.; Um, J.; Kouhpanji, M.R.Z.; Masa, J.A.; Galyas, A.G.; Kurlyandskaya, G.V. Polyacrylamide Ferrogels with Ni Nanowires. Materials 2019, 12, 2582. [Google Scholar] [CrossRef]
- Shaban, M.; Hamdy, H.; Shahin, F.; Ryu, S.W. Optical properties of porous anodic alumina membrane uniformly decorated with ultra-thin porous gold nanoparticles arrays. J. Nanosci. Nanotechnol. 2011, 11, 941–952. [Google Scholar] [CrossRef]
- Shaban, M. Effect of pore thickness and the state of polarization on the optical properties of hexagonal nanoarray of Au/nanoporous anodic alumina membrane. J. Nanomater. 2015, 2015, 347486. [Google Scholar] [CrossRef]
- Shaban, M.; Hamdy, H.; Shahin, F.; Ryu, S.W. Fabrication of Ordered Cr Nanostructures by Self Agglomeration on Porous Anodic Alumina Membranes. J. Nanosci. Nanotechnol. 2011, 11, 7145–7150. [Google Scholar] [CrossRef]
- Yue, S.; Yan, Z.; Shi, Y.; Ran, G. Synthesis of zinc oxide nanotubes within ultrathin anodic aluminum oxide membrane by sol–gel method. Mater. Lett. 2013, 98, 246–249. [Google Scholar] [CrossRef]
- Zhao, S. Effect of Different Excitation Wavelengths on Luminescent Properties of ZnO/Anodic Alumina Membrane (AAM) Arrays. Nanosci. Nanotechnol. Lett. 2018, 10, 409–412. [Google Scholar] [CrossRef]
- Shaban, M.; Hamdy, H.; Shahin, F.; Park, J.; Ryu, S.W. Uniform and reproducible barrier layer removal of porous anodic alumina membrane. J. Nanosci. Nanotechnol. 2010, 10, 3380–3384. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef]
- Chang, P.C.; Fan, Z.; Wang, D.; Tseng, W.Y.; Chiou, W.A.; Hong, J.; Lu, J.G. ZnO nanowires synthesized by vapor trapping CVD method. Chem. Mater. 2004, 16, 5133–5137. [Google Scholar] [CrossRef]
- Park, W.I.; Kim, J.S.; Yi, G.C.; Bae, M.H.; Lee, H.J. Fabrication and electrical characteristics of high-performance ZnO nanorod field-effect transistors. Appl. Phys. Lett. 2004, 85, 5052–5054. [Google Scholar] [CrossRef]
- Xu, C.; Wu, J.; Desai, U.V.; Gao, D. Multilayer assembly of nanowire arrays for dye-sensitized solar cells. J. Am. Chem. Soc. 2011, 133, 8122–8125. [Google Scholar] [CrossRef]
- Smigelskas, A.; Kirkendall, E. Zinc diffusion in alpha brass. Trans. AIME 1947, 171, 130–142. [Google Scholar]
- Yin, Y.; Rioux, R.M.; Erdonmez, C.K.; Hughes, S.; Somorjai, G.A.; Alivisatos, A.P. Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 2004, 304, 711–714. [Google Scholar] [CrossRef]
- Yang Kim, D.S.; Knez, M.; Scholz, R.; Berger, A.; Pippel, E.; Hesse, D.; Gösele, U.; Zacharias, M. Influence of Temperature on Evolution of Coaxial ZnO/Al2O3 One-Dimensional Heterostructures: From Core-Shell Nanowires to Spinel Nanotubes and Porous Nanowires. J. Phys. Chem. C 2008, 112, 4068–4074. [Google Scholar] [CrossRef]
- Zayed, M.; Ahmed, A.M.; Shaban, M. Synthesis and characterization of nanoporous ZnO and Pt/ZnO thin films for dye degradation and water splitting applications. Int. J. Hydrogen Energy 2019, 44, 17630–17648. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Shaban, M. Highly sensitive Au–Fe2O3–Au and Fe2O3–Au–Fe2O3 biosensors utilizing strong surface plasmon resonance. Appl. Phys. B 2020, 126, 57. [Google Scholar] [CrossRef]
- Abdel-Hady, E.E.; Shaban, M.; Abdel-Hamed, M.O.; Gamal, A.; Yehia, H.; Ahmed, A.M. Synthesis and Characterization of NiCoPt/CNFs Nanoparticles as an Effective Electrocatalyst for Energy Applications. Nanomaterials 2022, 12, 492. [Google Scholar] [CrossRef]
- Zhao, X.; Seo, S.K.; Lee, U.J.; Lee, K.H. Controlled Electrochemical Dissolution of Anodic Aluminum Oxide for Preparation of Open-Through Pore Structures. J. Electrochem. Soc. 2007, 154, C553. [Google Scholar] [CrossRef]
- Altowyan, A.S.; Shaban, M.; Abdelkarem, K.; El Sayed, A.M. The impact of Co doping and annealing temperature on the electrochemical performance and structural characteristics of SnO2 nanoparticulate photoanodes. Materials 2022, 15, 6534. [Google Scholar] [CrossRef] [PubMed]
- Altowyan, A.S.; Shaban, M.; Abdelkarem, K.; El Sayed, A.M. The Influence of Electrode Thickness on the Structure and Water Splitting Performance of Iridium Oxide Nanostructured Films. Nanomaterials 2022, 12, 3272. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, M.; Lupan, O.; Poetica, V.; Wolff, N.; Duppel, V.; Kienle, L.; Tiginyanu, I.; Adelung, R. ZnAl2O4-Functionalized Zinc Oxide Microstructures for Highly Selective Hydrogen Gas Sensing Applications. Phys. Status Solidi 2018, 215, 1700772. [Google Scholar] [CrossRef]
- Khamis Soliman, N.; Moustafa, A.F.; Aboud, A.A.; Halim, K.S.A. Effective utilization of Moringa seeds waste as a new green environmental adsorbent for removal of industrial toxic dyes. J. Mater. Res. Technol. 2019, 8, 1798–1808. [Google Scholar] [CrossRef]
- Mohamed, H.S.; Soliman, N.; Moustafa, A.; Abdel-Gawad, O.F.; Taha, R.R.; Ahmed, S.A. Nano metal oxide impregnated Chitosan-4-nitroacetophenone for industrial dye removal. Int. J. Environ. Anal. Chem. 2019, 101, 1850–1877. [Google Scholar] [CrossRef]
- Sharma, Y.C. Optimization of parameters for adsorption of methylene blue on a low-cost activated carbon. J. Chem. Eng. Data 2009, 55, 435–439. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lee, M.W.; Woo, S.H. Adsorption of congo red by chitosan hydrogel beads impregnated with carbon nanotubes. Bioresour. Technol. 2010, 101, 1800–1806. [Google Scholar] [CrossRef]
- Zare, K.; Sadegh, H.; Shahryari-ghoshekandi, R.; Maazinejad, B.; Ali, V.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Enhanced removal of toxic Congo red dye using multi walled carbon nanotubes: Kinetic, equilibrium studies and its comparison with other adsorbents. J. Mol. Liq. 2015, 212, 266–271. [Google Scholar] [CrossRef]
- Wu, C.H. Adsorption of reactive dye onto carbon nanotubes: Equilibrium, kinetics and thermodynamics. J. Hazard. Mater. 2007, 144, 93–100. [Google Scholar] [CrossRef]
- Hamd, A.; Dryaz, A.R.; Shaban, M.; AlMohamadi, H.; Abu Al-Ola, K.A.; Soliman, N.K.; Ahmed, S. Fabrication and Application of Zeolite/Acanthophora Spicifera Nanoporous Composite for Adsorption of Congo Red Dye from Wastewater. Nanomaterials 2021, 11, 2441. [Google Scholar] [CrossRef]
- Dryaz, A.R.; Shaban, M.; AlMohamadi, H.; Al-Ola, K.A.A.; Hamd, A.; Soliman, N.K.; Ahmed, S.A. Design, characterization, and adsorption properties of Padina gymnospora/zeolite nanocomposite for congo red dye removal from wastewater. Sci. Rep. 2021, 11, 21058. [Google Scholar] [CrossRef]
- Mohamed, F.; Rabia, M.; Shaban, M. Synthesis and characterization of biogenic iron oxides of different nanomorphologies from pomegranate peels for efficient solar hydrogen production. J. Mater. Res. Technol. 2020, 9, 4255–4271. [Google Scholar] [CrossRef]
- Wang, G.; Shi, C.; Zhao, N.; Du, X. Synthesis and characterization of Ag nanoparticles assembled in ordered array pores of porous anodic alumina by chemical deposition. Mater. Lett. 2007, 61, 3795–3797. [Google Scholar] [CrossRef]
- Hopper, A.; Beswick-Jones, H.; Brown, A.M. The Nernst equation: Using physico-chemical laws to steer novel experimental design. Adv. Physiol. Educ. 2022, 46, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Ciribelli, B.N.; Colmati, F.; de Souza, E.C. Nernst equation applied to electrochemical systems and centenary of his Nobel Prize in chemistry. Int. J. Innov. Educ. Res. 2020, 8, 670–683. [Google Scholar] [CrossRef]
- Sayyah, E.S.M.; Shaban, M.; Rabia, M. A sensor of m-cresol nanopolymer/Pt-electrode film for detection of lead ions by potentiometric methods. Adv. Polym. Technol. 2018, 37, 1296–1304. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. Electropolymerization of m-Toluidin on Platinum Electrode from Aqueous Acidic Solution and Character of the Obtained Polymer. Adv. Polym. Technol. 2018, 37, 126–136. [Google Scholar] [CrossRef]
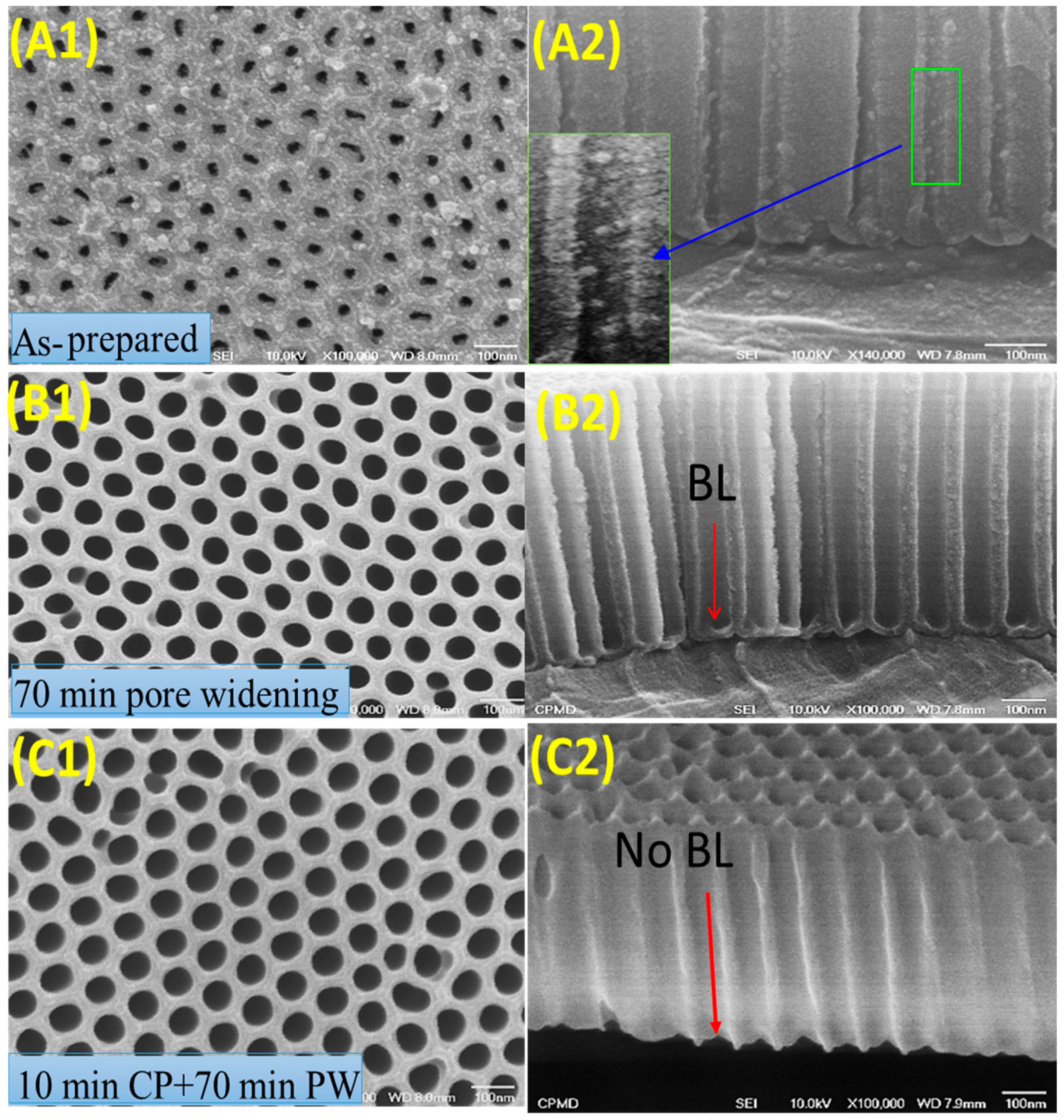
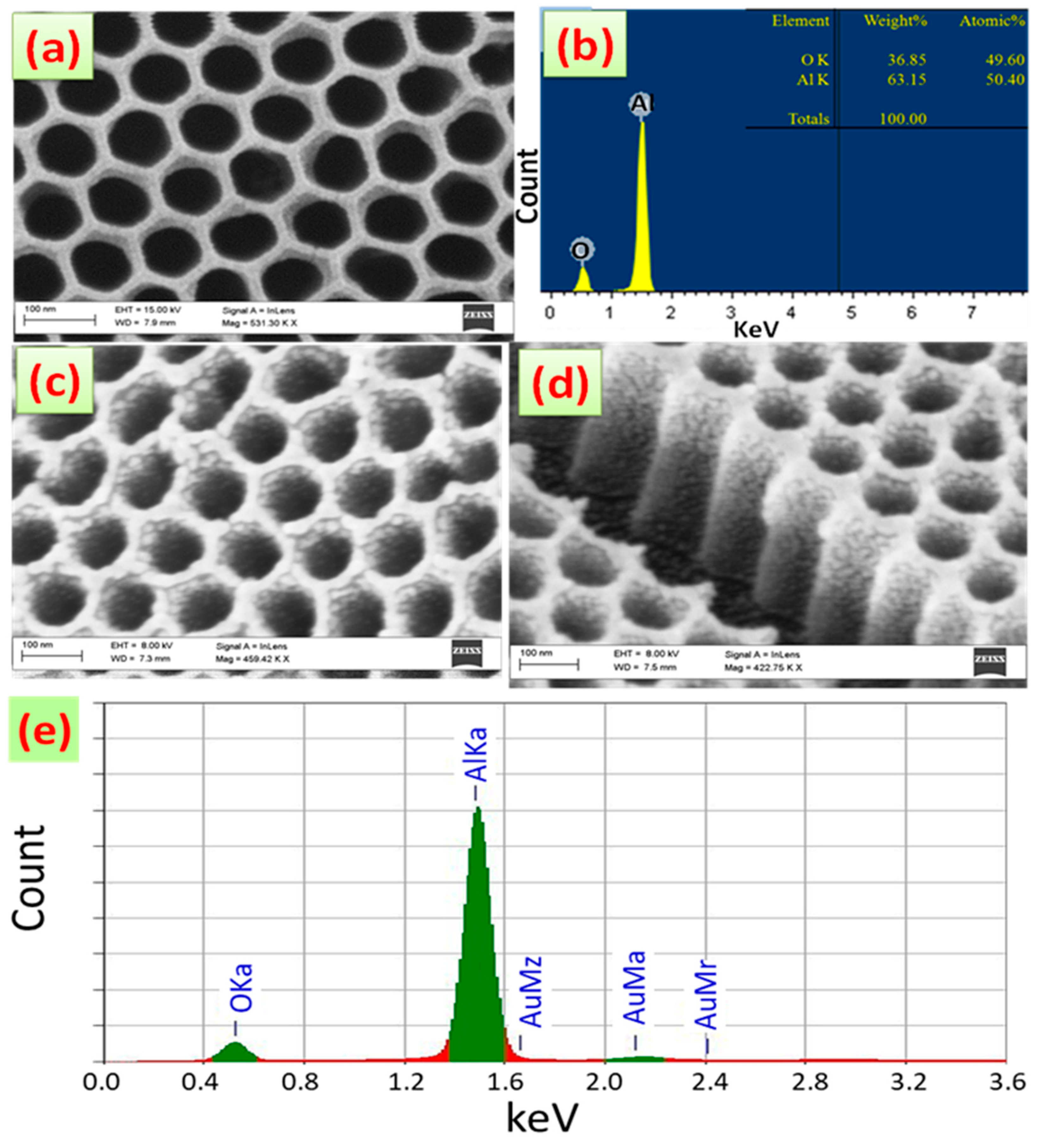
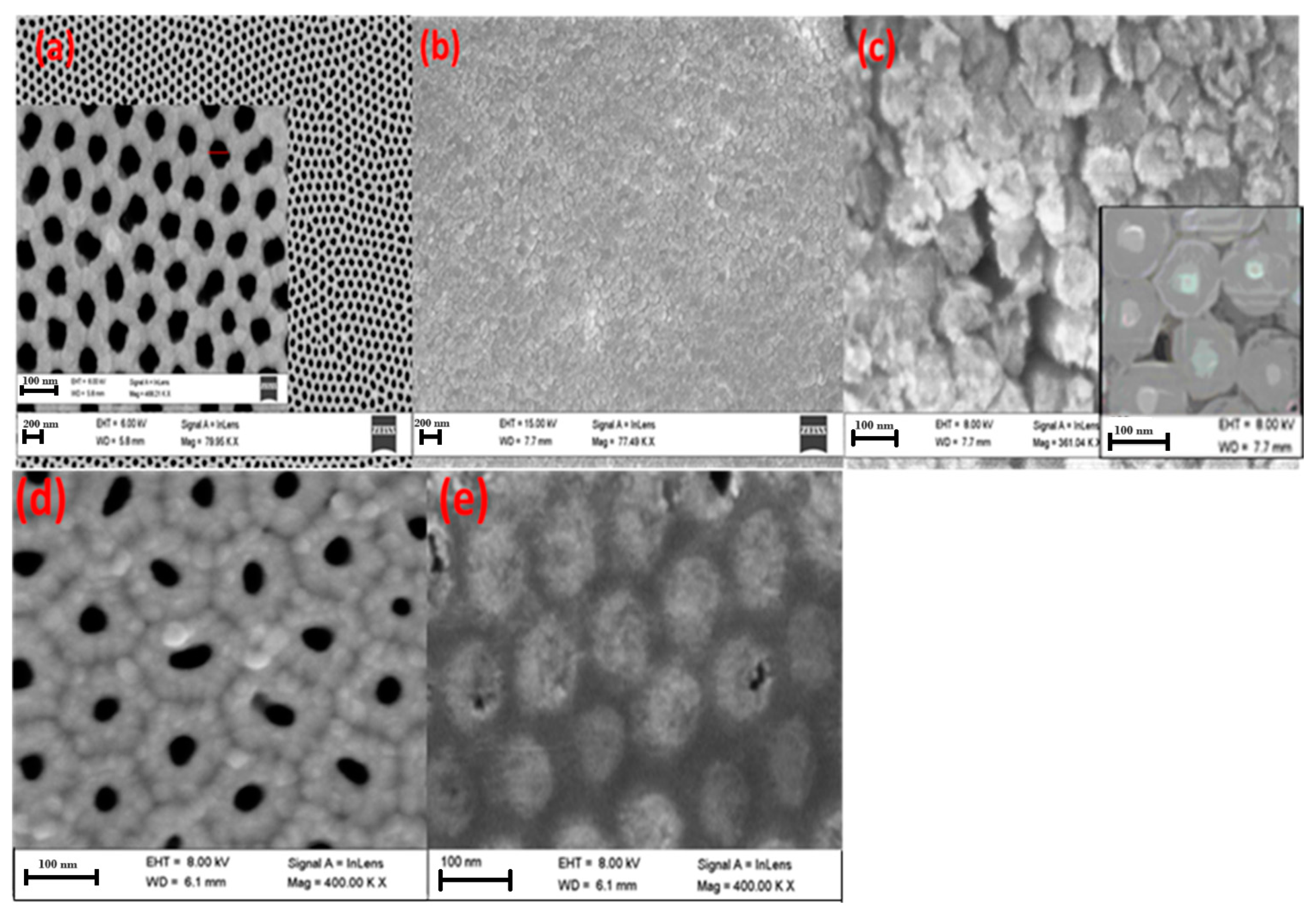
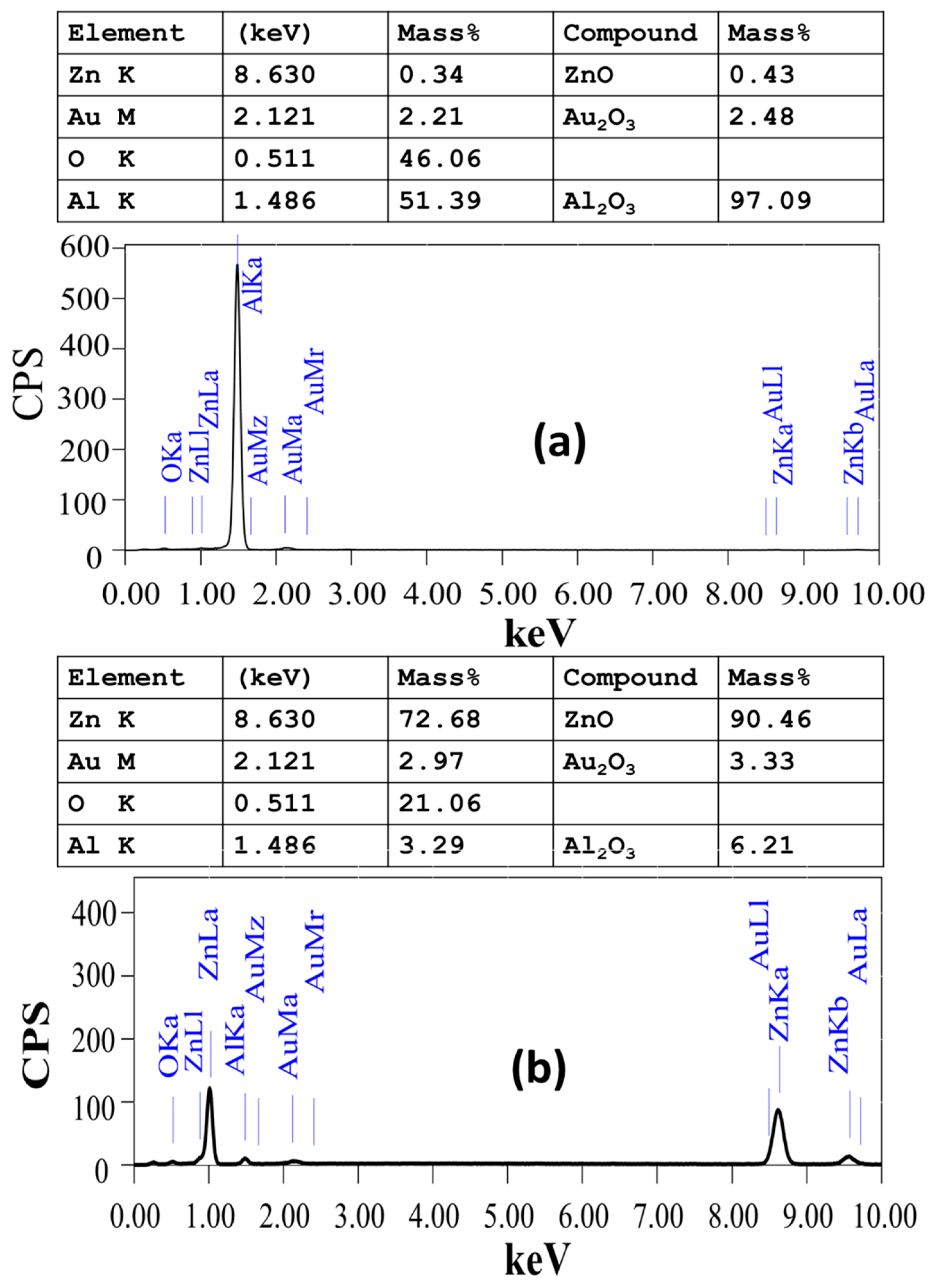



| Pos. [°2Th.] | FWHM [°2Th.] | Rel. Int. [%] | d-Spacing [Å] | Cs [nm] | Dislocation Density [nm−2] |
|---|---|---|---|---|---|
| 38.63 | 0.315 | 5.56 | 2.331 | 27.9 | 1.28 × 10−3 |
| 44.69 | 0.144 | 100.00 | 2.026 | 62.3 | 2.58 × 10−4 |
| 44.81 | 0.096 | 52.86 | 2.026 | 93.5 | 1.14 × 10−4 |
| 78.22 | 0.144 | 4.76 | 1.221 | 74.2 | 1.81 × 10−4 |
| Pseudo-first order: Y = qe [1 − exp(−k1X)] | ||
| MB | (min−1) | 0.070 ± 0.006 |
| maximum amount of CR uptake = qe (mg/g) | 102.018 ± 2.737 | |
| R2 | 0.9883 | |
| MO | k1 | 0.022 ± 8.559 × 10−4 |
| qe | 95.627 ± 1.167 | |
| R2 | 0.9879 | |
| Pseudo-second order: | ||
| MB | (min−1) | 5.717 × 10−4 ± 7.772 × 10−5 |
| qe (mg/g) | 127.695 ± 4.254 | |
| R2 | 0.9933 | |
| MO | k2 | 1.847 × 10−4 ± 7.450 × 10−6 |
| qe | 120.507 ± 1.203 | |
| R2 | 0.9970 | |
| MB | Adsorption rate at 0 min = α (mg/min) | 14.649 ± 1.951 |
| The extent of surface coverage = β (g/mg) | 0.031 ± 0.002 | |
| Correlation Coefficient = R2 | 0.9933 | |
| MO | A | 3.974 ± 0.152 |
| Β | 0.032 ± 6.770 × 10−4 | |
| R2 | 0.99676 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaban, M. Fabrication of ZnO/ZnAl2O4/Au Nanoarrays through DC Electrodeposition Utilizing Nanoporous Anodic Alumina Membranes for Environmental Application. Nanomaterials 2023, 13, 2667. https://doi.org/10.3390/nano13192667
Shaban M. Fabrication of ZnO/ZnAl2O4/Au Nanoarrays through DC Electrodeposition Utilizing Nanoporous Anodic Alumina Membranes for Environmental Application. Nanomaterials. 2023; 13(19):2667. https://doi.org/10.3390/nano13192667
Chicago/Turabian StyleShaban, Mohamed. 2023. "Fabrication of ZnO/ZnAl2O4/Au Nanoarrays through DC Electrodeposition Utilizing Nanoporous Anodic Alumina Membranes for Environmental Application" Nanomaterials 13, no. 19: 2667. https://doi.org/10.3390/nano13192667
APA StyleShaban, M. (2023). Fabrication of ZnO/ZnAl2O4/Au Nanoarrays through DC Electrodeposition Utilizing Nanoporous Anodic Alumina Membranes for Environmental Application. Nanomaterials, 13(19), 2667. https://doi.org/10.3390/nano13192667






