Graphene Oxide Nanostructures as Nanoplatforms for Delivering Natural Therapeutic Agents: Applications in Cancer Treatment, Bacterial Infections, and Bone Regeneration Medicine
Abstract
:1. Introduction
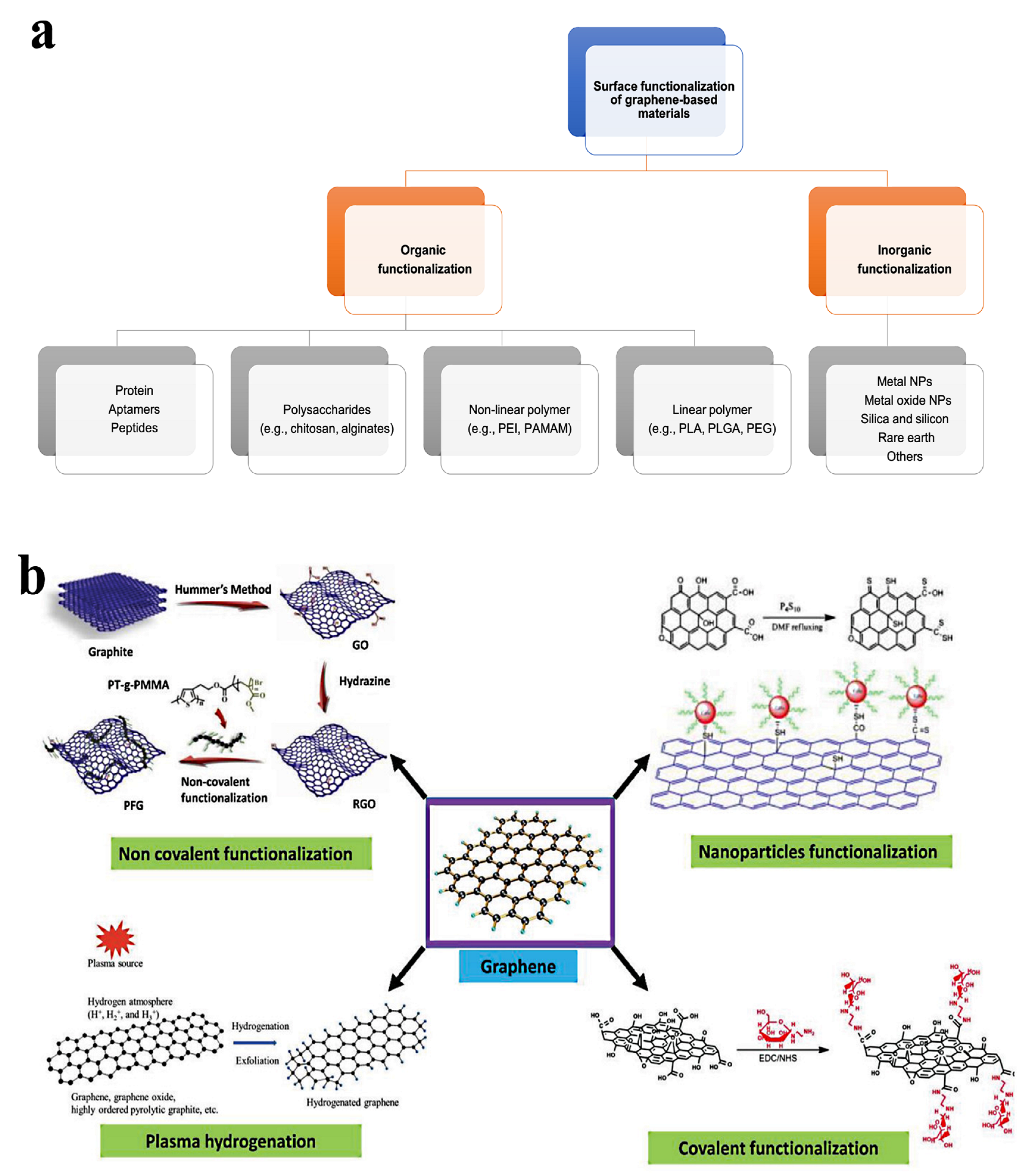
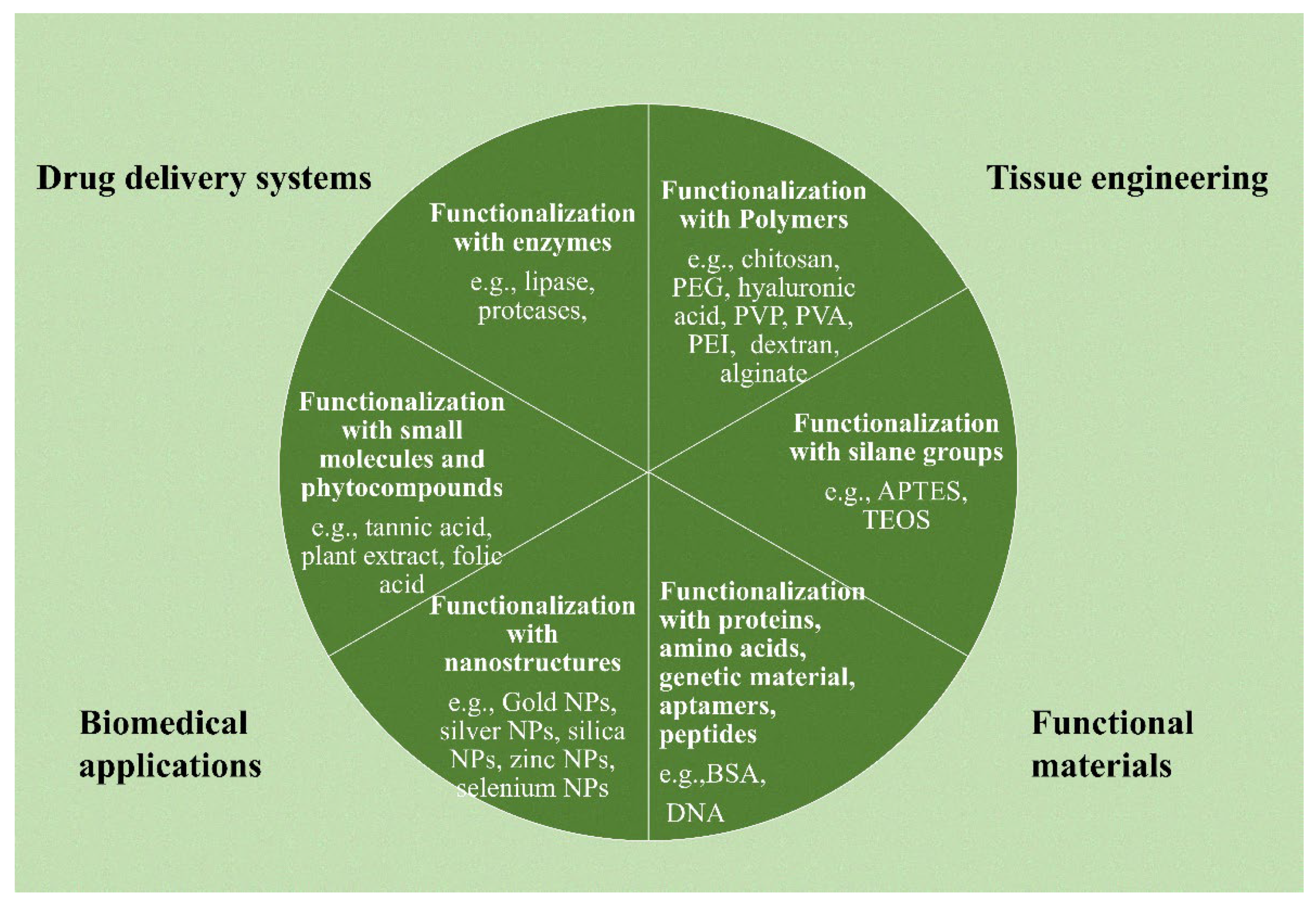
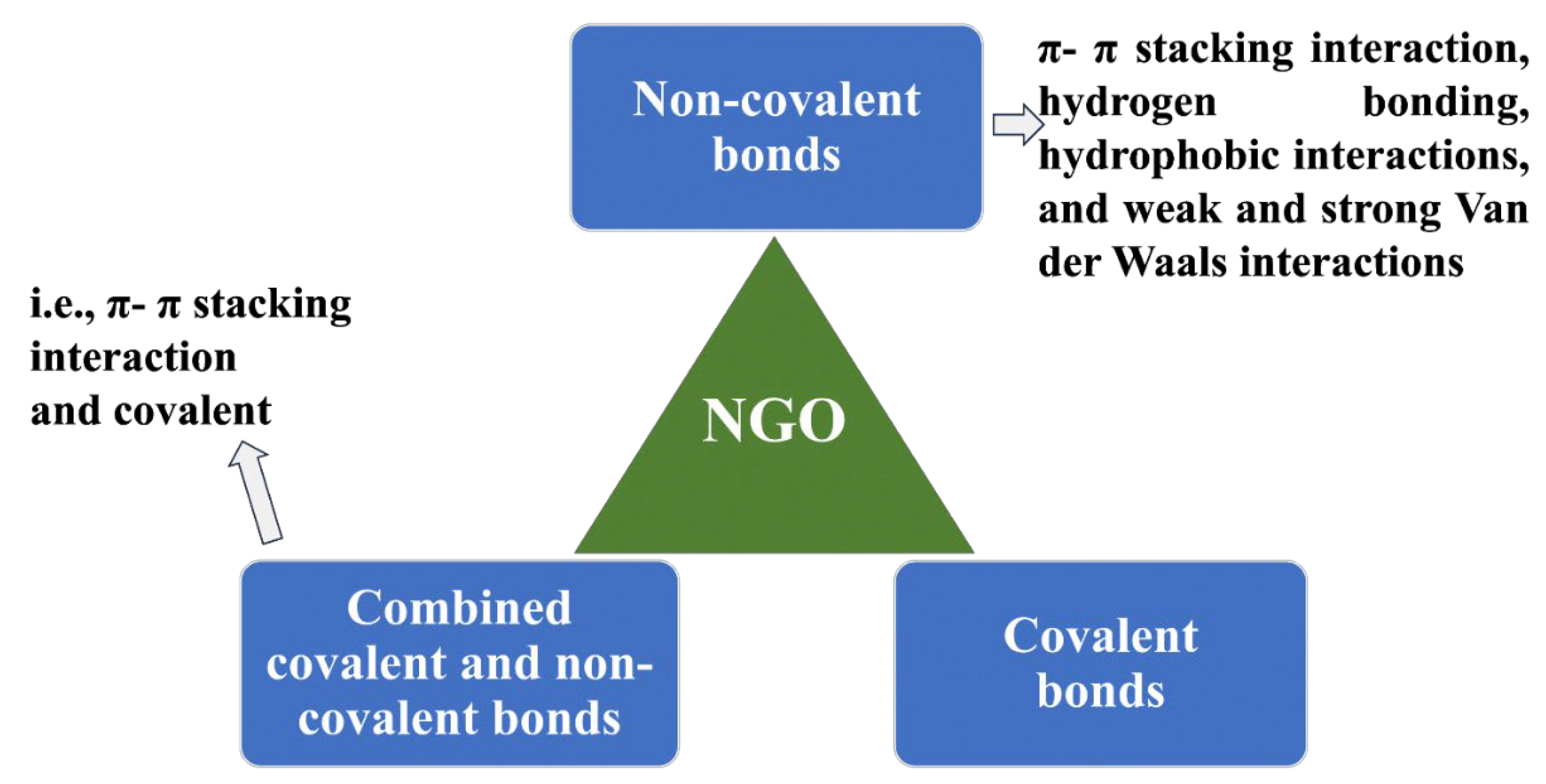
2. Surface Functionality of NGO
3. Drug Loading and Drug Release Profile
3.1. Drug-Loading Strategies
3.1.1. Drug Solubility
3.1.2. Drug-Loading Approaches
3.2. Release from Nanographene Oxide
Triggering Drug Release from Nanographene Oxide
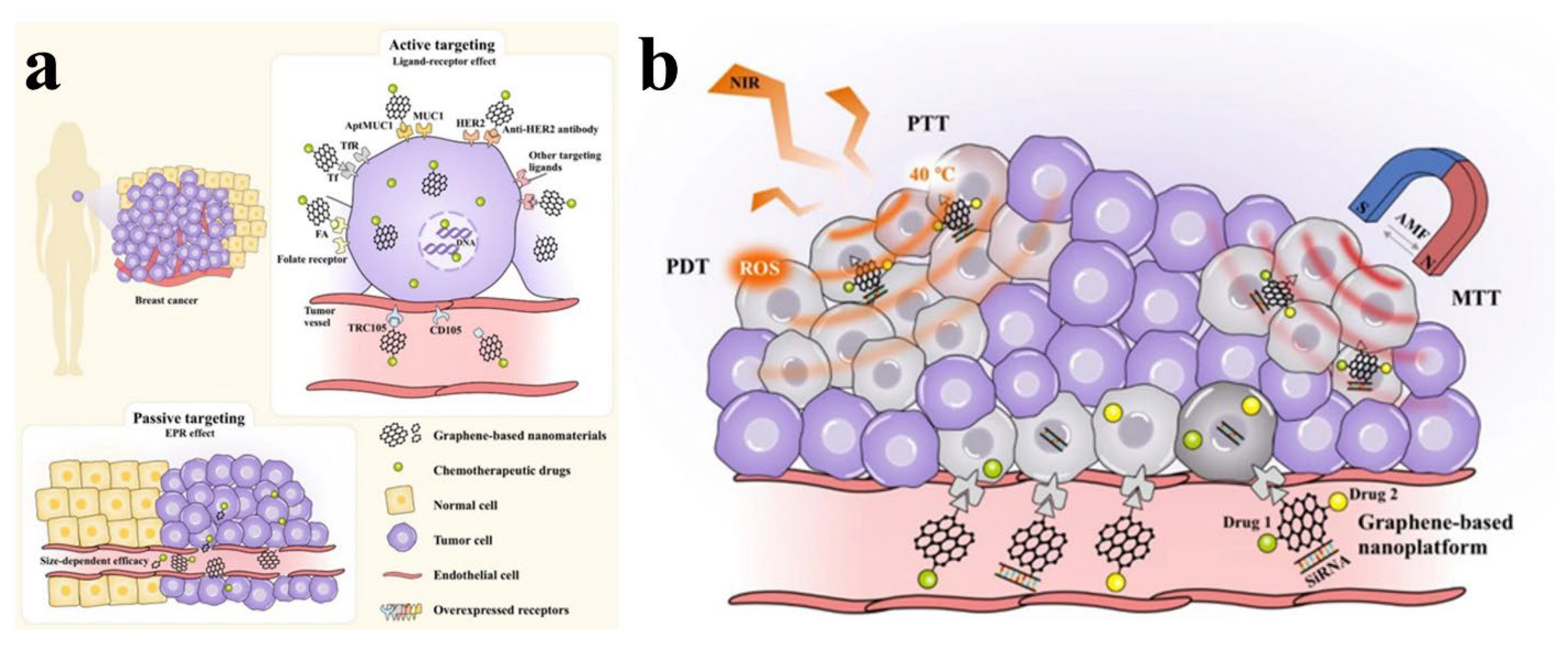
4. Nanographene Oxide for Natural Anticancer Therapy
5. Antibacterial Delivery Systems
6. Bone Regeneration Systems
7. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rauti, R.; Musto, M.; Bosi, S.; Prato, M.; Ballerini, L. Properties and behavior of carbon nanomaterials when interfacing neuronal cells: How far have we come? Carbon 2019, 143, 430–446. [Google Scholar] [CrossRef]
- Silva, G.A. Nanotechnology approaches to crossing the blood-brain barrier and drug delivery to the CNS. BMC Neurosci. 2008, 9 (Suppl. S3), S4. [Google Scholar] [CrossRef] [PubMed]
- Mahor, A.; Singh, P.P.; Bharadwaj, P.; Sharma, N.; Yadav, S.; Rosenholm, J.M.; Bansal, K.K. Carbon-Based Nanomaterials for Delivery of Biologicals and Therapeutics: A Cutting-Edge Technology. C 2021, 7, 19. [Google Scholar] [CrossRef]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Armenta, S.; Esteve-Turrillas, F.A. Carbon-Based Nanomaterials in Analytical Chemistry. In Handbook of Smart Materials in Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2019; pp. 345–374. [Google Scholar]
- Sheoran, K.; Kaur, H.; Siwal, S.S.; Saini, A.K.; Vo, D.-V.N.; Thakur, V.K. Recent advances of carbon-based nanomaterials (CBNMs) for wastewater treatment: Synthesis and application. Chemosphere 2022, 299, 134364. [Google Scholar] [CrossRef]
- Dutta, V.; Verma, R.; Gopalkrishnan, C.; Yuan, M.-H.; Batoo, K.M.; Jayavel, R.; Chauhan, A.; Lin, K.-Y.A.; Balasubramani, R.; Ghotekar, S. Bio-Inspired Synthesis of Carbon-Based Nanomaterials and Their Potential Environmental Applications: A State-of-the-Art Review. Inorganics 2022, 10, 169. [Google Scholar] [CrossRef]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef]
- Kościk, I.; Jankowski, D.; Jagusiak, A. Carbon Nanomaterials for Theranostic Use. C 2022, 8, 3. [Google Scholar] [CrossRef]
- Kumar, N.; Chamoli, P.; Misra, M.; Manoj, M.K.; Sharma, A. Advanced metal and carbon nanostructures for medical, drug delivery and bio-imaging applications. Nanoscale 2022, 14, 3987–4017. [Google Scholar] [CrossRef] [PubMed]
- Chakroborty, S.; Sahoo, S.K. Carbon-Based Nanomaterials in Drug Delivery Systems. In Environmental Applications of Carbon Nanomaterials-Based Devices; Wiley: Hoboken, NJ, USA, 2021; pp. 375–394. [Google Scholar]
- Wu, H.-C.; Chang, X.; Liu, L.; Zhao, F.; Zhao, Y. Chemistry of carbon nanotubes in biomedical applications. J. Mater. Chem. 2010, 20, 1036–1052. [Google Scholar] [CrossRef]
- Shanbhag, V.K.L.; Prasad, K.S. Graphene based sensors in the detection of glucose in saliva—A promising emerging modality to diagnose diabetes mellitus. Anal. Methods 2016, 8, 6255–6259. [Google Scholar] [CrossRef]
- He, Y.; Hu, C.; Li, Z.; Wu, C.; Zeng, Y.; Peng, C. Multifunctional carbon nanomaterials for diagnostic applications in infectious diseases and tumors. Mater. Today Bio 2022, 14, 100231. [Google Scholar] [CrossRef] [PubMed]
- Hoseini-Ghahfarokhi, M.; Mirkiani, S.; Mozaffari, N.; Abdolahi Sadatlu, M.A.; Ghasemi, A.; Abbaspour, S.; Akbarian, M.; Farjadian, F.; Karimi, M. Applications of Graphene and Graphene Oxide in Smart Drug/Gene Delivery: Is the World Still Flat? Int. J. Nanomed. 2020, 15, 9469–9496. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, R.; Singh, D.P. Graphene oxide: Strategies for synthesis, reduction and frontier applications. RSC Adv. 2016, 6, 64993–65011. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.-W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Chang, C.-T. Preparation and Characterization of Graphene Oxide. J. Nanomater. 2014, 2014, 276143. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Lee, D.J.; Park, S.S. Estimation of Number of Graphene Layers Using Different Methods: A Focused Review. Materials 2021, 14, 4590. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yang, S.-T.; Liu, J.-H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Z.; Chu, M. Long-term toxicity of reduced graphene oxide nanosheets: Effects on female mouse reproductive ability and offspring development. Biomaterials 2015, 54, 188–200. [Google Scholar] [CrossRef]
- Wu, W.; Yan, L.; Wu, Q.; Li, Y.; Li, Q.; Chen, S.; Yang, Y.; Gu, Z.; Xu, H.; Yin, Z.Q. Evaluation of the toxicity of graphene oxide exposure to the eye. Nanotoxicology 2016, 10, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Kiew, S.F.; Kiew, L.V.; Lee, H.B.; Imae, T.; Chung, L.Y. Assessing biocompatibility of graphene oxide-based nanocarriers: A review. J. Control. Release 2016, 226, 217–228. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.; Huang, Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, C.; Yang, J.; Lv, M.; Liu, R.; He, D.; Fan, C.; Huang, Q. Uniform Ultrasmall Graphene Oxide Nanosheets with Low Cytotoxicity and High Cellular Uptake. ACS Appl. Mater. Interfaces 2013, 5, 1761–1767. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Chitosan as a Valuable Biomolecule from Seafood Industry Waste in the Design of Green Food Packaging. Biomolecules 2021, 11, 1599. [Google Scholar] [CrossRef] [PubMed]
- Pinho de Aguiar, K.L.N.; Frias de Oliveira, P.; Elias Mansur, C.R. A comprehensive review of in situ polymer hydrogels for conformance control of oil reservoirs. Oil Gas Sci. Technol. Rev. IFP Energ. Nouv. 2020, 75, 8. [Google Scholar] [CrossRef]
- Bie, B.-J.; Zhao, X.-R.; Yan, J.-R.; Ke, X.-J.; Liu, F.; Yan, G.-P. Dextran Fluorescent Probes Containing Sulfadiazine and Rhodamine B Groups. Molecules 2022, 27, 6747. [Google Scholar] [CrossRef]
- Jirkovec, R.; Erben, J.; Samkova, A.; Chaloupek, J.; Chvojka, J. The effect of the electrospinning setup on the surface energy of polycaprolactone nanofibre layers. J. Ind. Text. 2022, 51, 8517S–8527S. [Google Scholar] [CrossRef]
- Yang, K.; Gong, H.; Shi, X.; Wan, J.; Zhang, Y.; Liu, Z. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials 2013, 34, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, K.; Feng, L.; Liu, Z. In vitro and in vivo behaviors of dextran functionalized graphene. Carbon 2011, 49, 4040–4049. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, J.; Wang, F.; Xiong, Y.; Wu, Y.; Wang, Q.; Weng, J.; Zhang, Z.; Chen, W.; Liu, S. Improved In Vitro and In Vivo Biocompatibility of Graphene Oxide through Surface Modification: Poly(Acrylic Acid)-Functionalization is Superior to PEGylation. ACS Nano 2016, 10, 3267–3281. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Portolés, M.T.; Marques, P.A.A.P.; Feito, M.J.; Matesanz, M.C.; Ramírez-Santillán, C.; Gonçalves, G.; Cruz, S.M.A.; Nieto, A.; Vallet-Regi, M. Cell uptake survey of pegylated nanographene oxide. Nanotechnology 2012, 23, 465103. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Sanchez Casalongue, H.; Vinh, D.; Dai, H. Ultrasmall Reduced Graphene Oxide with High Near-Infrared Absorbance for Photothermal Therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Chatterjee, K. Poly(Ethylene Glycol) Functionalized Graphene Oxide in Tissue Engineering: A Review on Recent Advances. Int. J. Nanomed. 2020, 15, 5991–6006. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, S.; Li, Y.; Wang, M.; Shi, P.; Huang, X. Covalent Functionalization of Graphene Oxide with Biocompatible Poly(ethylene glycol) for Delivery of Paclitaxel. ACS Appl. Mater. Interfaces 2014, 6, 17268–17276. [Google Scholar] [CrossRef]
- Mao, N.D.; Jeong, H.; Ngan Nguyen, T.K.; Loan Nguyen, T.M.; Vi Do, T.V.; Ha Thuc, C.N.; Perré, P.; Ko, S.C.; Kim, H.G.; Tran, D.T. Polyethylene glycol functionalized graphene oxide and its influences on properties of Poly(lactic acid) biohybrid materials. Compos. Part B Eng. 2019, 161, 651–658. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, H.; Huang, D.; Feng, S.; Fujita, M.; Gao, X.D. Chitosan-Functionalized Graphene Oxide as a Potential Immunoadjuvant. Nanomaterials 2017, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Bussy, C.; Ali-Boucetta, H.; Kostarelos, K. Safety Considerations for Graphene: Lessons Learnt from Carbon Nanotubes. Acc. Chem. Res. 2013, 46, 692–701. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, P.; Zhou, Z.; Wei, T. Interactions of graphene with mammalian cells: Molecular mechanisms and biomedical insights. Adv. Drug Deliv. Rev. 2016, 105, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kim, J.H. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int. J. Nanomed. 2016, 11, 1927–1945. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef]
- Bullock, C.J.; Bussy, C. Biocompatibility Considerations in the Design of Graphene Biomedical Materials. Adv. Mater. Interfaces 2019, 6, 1900229. [Google Scholar] [CrossRef]
- Rhazouani, A.; Gamrani, H.; El Achaby, M.; Aziz, K.; Gebrati, L.; Uddin, M.S.; Aziz, F. Synthesis and Toxicity of Graphene Oxide Nanoparticles: A Literature Review of In Vitro and In Vivo Studies. Biomed Res. Int. 2021, 2021, 5518999. [Google Scholar] [CrossRef]
- Jasim, D.A.; Boutin, H.; Fairclough, M.; Ménard-Moyon, C.; Prenant, C.; Bianco, A.; Kostarelos, K. Thickness of functionalized graphene oxide sheets plays critical role in tissue accumulation and urinary excretion: A pilot PET/CT study. Appl. Mater. Today 2016, 4, 24–30. [Google Scholar] [CrossRef]
- Sasidharan, A.; Swaroop, S.; Koduri, C.K.; Girish, C.M.; Chandran, P.; Panchakarla, L.S.; Somasundaram, V.H.; Gowd, G.S.; Nair, S.; Koyakutty, M. Comparative in vivo toxicity, organ biodistribution and immune response of pristine, carboxylated and PEGylated few-layer graphene sheets in Swiss albino mice: A three month study. Carbon 2015, 95, 511–524. [Google Scholar] [CrossRef]
- Dasari Shareena, T.P.; McShan, D.; Dasmahapatra, A.K.; Tchounwou, P.B. A Review on Graphene-Based Nanomaterials in Biomedical Applications and Risks in Environment and Health. Nano-Micro Lett. 2018, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Ema, M.; Gamo, M.; Honda, K. A review of toxicity studies on graphene-based nanomaterials in laboratory animals. Regul. Toxicol. Pharmacol. 2017, 85, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Perini, G.; De Spirito, M.; Papi, M. Graphene oxide touches blood: In vivo interactions of bio-coronated 2D materials. Nanoscale Horiz. 2019, 4, 273–290. [Google Scholar] [CrossRef]
- Bai, R.G.; Husseini, G.A. Chapter 11—Graphene-based drug delivery systems. In Biomimetic Nanoengineered Materials for Advanced Drug Delivery; Unnithan, A.R., Sasikala, A.R.K., Park, C.H., Kim, C.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–168. [Google Scholar]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Lim, K.T. Graphene Oxide-Based Stimuli-Responsive Platforms for Biomedical Applications. Molecules 2021, 26, 2797. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Liu, Z. Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv. Drug Deliv. Rev. 2016, 105, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Seo, Y.-R.; Lim, K.-T. Stimuli-Responsive Graphene Nanohybrids for Biomedical Applications. Stem Cells Int. 2019, 2019, 9831853. [Google Scholar] [CrossRef]
- Delgoda, R. Pharmacognosy: Fundamentals, Applications and Strategies; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Stella Omokhefe, B. Secondary Metabolites from Natural Products. In Secondary Metabolites; Ramasamy, V., Suresh Selvapuram Sudalaimuthu, R., Eds.; IntechOpen: Rijeka, Croatia, 2022; Chapter 4. [Google Scholar]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Andrade, M.A.; Braga, M.A.; Cesar, P.H.S.; Trento, M.V.C.; Espósito, M.A.; Silva, L.F.; Marcussi, S. Anticancer Properties of Essential Oils: An Overview. Curr. Cancer Drug Targets 2018, 18, 957–966. [Google Scholar] [CrossRef]
- Herr, I.; Büchler, M.W. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat. Rev. 2010, 36, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Mahavy, C.E.; Duez, P.; ElJaziri, M.; Rasamiravaka, T. African Plant-Based Natural Products with Antivirulence Activities to the Rescue of Antibiotics. Antibiotics 2020, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.L.; Guimaraes-Lopes, V.P.; Altoe, L.S.; Sarandy, M.M.; Melo, F.; Novaes, R.D.; Goncalves, R.V. Plant Extracts in the Bone Repair Process: A Systematic Review. Mediat. Inflamm. 2019, 2019, 1296153. [Google Scholar] [CrossRef]
- Mondal, A.; Gandhi, A.; Fimognari, C.; Atanasov, A.G.; Bishayee, A. Alkaloids for cancer prevention and therapy: Current progress and future perspectives. Eur. J. Pharmacol. 2019, 858, 172472. [Google Scholar] [CrossRef]
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.-R.; et al. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2020, 70, 153218. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef]
- Springob, K.; Kutchan, T.M. Introduction to the Different Classes of Natural Products. In Plant-Derived Natural Products: Synthesis, Function, and Application; Osbourn, A.E., Lanzotti, V., Eds.; Springer US: New York, NY, USA, 2009; pp. 3–50. [Google Scholar]
- Thoppil, R.J.; Bishayee, A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011, 3, 228–249. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Cereti, E.; Barile, D.; Coïsson, J.D.; Arlorio, M.; Dessi, S.; Coroneo, V.; Cabras, P. Chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential oil of Rosmarinus officinalis L. J. Agric. Food Chem. 2004, 52, 3530–3535. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 2009, 53, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.S.L.; Tan, L.T.-H.; Chan, K.-G.; Yap, W.H.; Pusparajah, P.; Chuah, L.-H.; Ming, L.C.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Resveratrol-Potential Antibacterial Agent against Foodborne Pathogens. Front. Pharmacol. 2018, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef]
- Hashemi, H.; Hand, A.; Florentinus-Mefailoski, A.; Kerrigan, P.; Samuel, P.; Friedberg, J. Plants as medical tools. Cannabis Med. Asp. 2016, 9, 493–504. [Google Scholar]
- Dembitsky, V.M.; Dzhemileva, L.; Gloriozova, T.; D’Yakonov, V. Natural and synthetic drugs used for the treatment of the dementia. Biochem. Biophys. Res. Commun. 2020, 524, 772–783. [Google Scholar] [CrossRef]
- Khamjan, N.A.; Beigh, S.; Algaissi, A.; Megha, K.; Lohani, M.; Darraj, M.; Kamli, N.; Madkhali, F.; Dar, S.A. Natural and synthetic drugs and formulations for intravaginal HPV clearance. J. Infect. Public Health 2023, 16, 1471–1480. [Google Scholar] [CrossRef]
- Huygens, S.; Vellekoop, H.; Versteegh, M.; Santi, I.; Szilberhorn, L.; Zelei, T.; Nagy, B.; Tsiachristas, A.; Koleva-Kolarova, R.; Wordsworth, S.; et al. Cost-Effectiveness Analysis of Treating Patients With NTRK-Positive Cancer With the Histology-Independent Therapy Entrectinib. Value Health 2023, 26, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Atta, H.; Alzahaby, N.; Hamdy, N.M.; Emam, S.H.; Sonousi, A.; Ziko, L. New trends in synthetic drugs and natural products targeting 20S proteasomes in cancers. Bioorganic Chem. 2023, 133, 106427. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Merzenich, G.S. Combination screening of synthetic drugs and plant derived natural products—Potential and challenges for drug development. Synergy 2014, 1, 59–69. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and Applications of Graphene Oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Han, X.M.; Zheng, K.W.; Wang, R.L.; Yue, S.F.; Chen, J.; Zhao, Z.W.; Song, F.; Su, Y.; Ma, Q. Functionalization and optimization-strategy of graphene oxide-based nanomaterials for gene and drug delivery. Am. J. Transl. Res. 2020, 12, 1515–1534. [Google Scholar] [PubMed]
- Guo, Z.; Chakraborty, S.; Monikh, F.A.; Varsou, D.-D.; Chetwynd, A.J.; Afantitis, A.; Lynch, I.; Zhang, P. Surface Functionalization of Graphene-Based Materials: Biological Behavior, Toxicology, and Safe-By-Design Aspects. Adv. Biol. 2021, 5, 2100637. [Google Scholar] [CrossRef]
- Pei, X.; Zhu, Z.; Gan, Z.; Chen, J.; Zhang, X.; Cheng, X.; Wan, Q.; Wang, J. PEGylated nano-graphene oxide as a nanocarrier for delivering mixed anticancer drugs to improve anticancer activity. Sci. Rep. 2020, 10, 2717. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, K.; Shi, X.; Gao, M.; Liu, J.; Liu, Z. Smart pH-Responsive Nanocarriers Based on Nano-Graphene Oxide for Combined Chemo- and Photothermal Therapy Overcoming Drug Resistance. Adv. Healthc. Mater. 2014, 3, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Masoudipour, E.; Kashanian, S.; Maleki, N. A targeted drug delivery system based on dopamine functionalized nano graphene oxide. Chem. Phys. Lett. 2017, 668, 56–63. [Google Scholar] [CrossRef]
- Alibolandi, M.; Mohammadi, M.; Taghdisi, S.M.; Ramezani, M.; Abnous, K. Fabrication of aptamer decorated dextran coated nano-graphene oxide for targeted drug delivery. Carbohydr. Polym. 2017, 155, 218–229. [Google Scholar] [CrossRef]
- Erdal, N.B.; Yao, J.G.; Hakkarainen, M. Cellulose-Derived Nanographene Oxide Surface-Functionalized Three-Dimensional Scaffolds with Drug Delivery Capability. Biomacromolecules 2019, 20, 738–749. [Google Scholar] [CrossRef]
- Lu, B.-Y.; Zhu, G.-Y.; Yu, C.-H.; Chen, G.-Y.; Zhang, C.-L.; Zeng, X.; Chen, Q.-M.; Peng, Q. Functionalized graphene oxide nanosheets with unique three-in-one properties for efficient and tunable antibacterial applications. Nano Res. 2021, 14, 185–190. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Zhang, H.; Lei, L. Nano-graphene oxide with antisense walR RNA inhibits the pathogenicity of Enterococcus faecalis in periapical periodontitis. J. Dent. Sci. 2020, 15, 65–74. [Google Scholar] [CrossRef]
- Mushtaq, S.; Yasin, T.; Saleem, M.; Dai, T.; Yameen, M.A. Potentiation of Antimicrobial Photodynamic Therapy by Curcumin-loaded Graphene Quantum Dots. Photochem. Photobiol. 2022, 98, 202–210. [Google Scholar] [CrossRef]
- Łoczechin, A.; Séron, K.; Barras, A.; Giovanelli, E.; Belouzard, S.; Chen, Y.-T.; Metzler-Nolte, N.; Boukherroub, R.; Dubuisson, J.; Szunerits, S. Functional Carbon Quantum Dots as Medical Countermeasures to Human Coronavirus. ACS Appl. Mater. Interfaces 2019, 11, 42964–42974. [Google Scholar] [CrossRef]
- Kim, S.; Ryoo, S.-R.; Na, H.-K.; Kim, Y.-K.; Choi, B.-S.; Lee, Y.; Kim, D.-E.; Min, D.-H. Deoxyribozyme-loaded nano-graphene oxide for simultaneous sensing and silencing of the hepatitis C virus gene in liver cells. Chem. Commun. 2013, 49, 8241–8243. [Google Scholar] [CrossRef]
- Nanda, S.S.; Papaefthymiou, G.C.; Yi, D.K. Functionalization of Graphene Oxide and its Biomedical Applications. Crit. Rev. Solid State Mater. Sci. 2015, 40, 291–315. [Google Scholar] [CrossRef]
- Holt, B.D.; Wright, Z.M.; Arnold, A.M.; Sydlik, S.A. Graphene oxide as a scaffold for bone regeneration. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1437. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Samanta, A.; Srivastava, R.K.; Hakkarainen, M. Nano-Graphene Oxide Functionalized Bioactive Poly(lactic acid) and Poly(ε-caprolactone) Nanofibrous Scaffolds. Materials 2018, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiang, S.; Lin, Z.; Li, E.N.; Yagi, H.; Cao, G.; Yocum, L.; Li, L.; Hao, T.; Bruce, K.K.; et al. Graphene oxide-functionalized nanocomposites promote osteogenesis of human mesenchymal stem cells via enhancement of BMP-SMAD1/5 signaling pathway. Biomaterials 2021, 277, 121082. [Google Scholar] [CrossRef]
- Ardeshirzadeh, B.; Anaraki, N.A.; Irani, M.; Rad, L.R.; Shamshiri, S. Controlled release of doxorubicin from electrospun PEO/chitosan/graphene oxide nanocomposite nanofibrous scaffolds. Mater. Sci. Eng. C 2015, 48, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Nan, X.; Shi, W.; Sun, Y.; Su, H.; He, Y.; Liu, X.; Zhang, Z.; Ge, D. Polydopamine-functionalized nanographene oxide: A versatile nanocarrier for chemotherapy and photothermal therapy. Nanotechnology 2017, 28, 295102. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Lee, M.Y.; Kong, W.H.; Do, I.H.; Hahn, S.K. Nano graphene oxide-hyaluronic acid conjugate for target specific cancer drug delivery. RSC Adv. 2014, 4, 14197–14200. [Google Scholar] [CrossRef]
- Campbell, E.; Hasan, M.T.; Pho, C.; Callaghan, K.; Akkaraju, G.R.; Naumov, A.V. Graphene Oxide as a Multifunctional Platform for Intracellular Delivery, Imaging, and Cancer Sensing. Sci. Rep. 2019, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Mahdavian, L. DFT studies of the drug carrier of anti-migraine (sumatriptan) on nano-graphene oxide (NGO) and graphene oxide/polyethylene glycol polymer nano-composite. Diam. Relat. Mater. 2020, 104, 107745. [Google Scholar] [CrossRef]
- Mirhosseini, M.M.; Khordad, R.; Vaseghi, B. Effect of hydrogen bonding on drug loading using a nanographene surface: A molecular dynamics study. Chin. J. Phys. 2019, 62, 99–105. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Khakpour, E.; Salehi, S.; Naghib, S.M.; Ghorbanzadeh, S.; Zhang, W. Graphene-based nanomaterials for stimuli-sensitive controlled delivery of therapeutic molecules. Front. Bioeng. Biotechnol. 2023, 11, 1129768. [Google Scholar] [CrossRef]
- Cho, Y.; Min, S.K.; Yun, J.; Kim, W.Y.; Tkatchenko, A.; Kim, K.S. Noncovalent Interactions of DNA Bases with Naphthalene and Graphene. J. Chem. Theory Comput. 2013, 9, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, L.; Lee, W.B.; Ng, M.C. Structure of graphene and its disorders: A review. Sci. Technol. Adv. Mater. 2018, 19, 613–648. [Google Scholar] [CrossRef]
- Ghawanmeh, A.A.; Ali, G.A.M.; Algarni, H.; Sarkar, S.M.; Chong, K.F. Graphene oxide-based hydrogels as a nanocarrier for anticancer drug delivery. Nano Res. 2019, 12, 973–990. [Google Scholar] [CrossRef]
- Kim, H.; Lee, D.; Kim, J.; Kim, T.-i.; Kim, W.J. Photothermally Triggered Cytosolic Drug Delivery via Endosome Disruption Using a Functionalized Reduced Graphene Oxide. ACS Nano 2013, 7, 6735–6746. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, L.; Sun, Z.; Han, D.; Zhao, L. Research on the Targeted Treatment of Squamous Cell Carcinoma by Nanographene Drug Delivery System. Adv. Mater. Sci. Eng. 2023, 2023, 6336569. [Google Scholar] [CrossRef]
- Zhuang, W.; He, L.; Wang, K.; Ma, B.; Ge, L.; Wang, Z.; Huang, J.; Wu, J.; Zhang, Q.; Ying, H. Combined Adsorption and Covalent Linking of Paclitaxel on Functionalized Nano-Graphene Oxide for Inhibiting Cancer Cells. ACS Omega 2018, 3, 2396–2405. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, Z.; Li, H.; Hao, Y.; Liu, C.; Zhu, L.; Liu, J.; Lu, B.; Li, R. Multifunctional Nanographene Oxide for Targeted Gene-Mediated Thermochemotherapy of Drug-resistant Tumour. Sci. Rep. 2017, 7, 43506. [Google Scholar] [CrossRef]
- Hu, H.; Yu, J.; Li, Y.; Zhao, J.; Dong, H. Engineering of a novel pluronic F127/graphene nanohybrid for pH responsive drug delivery. J. Biomed. Mater. Res. Part A 2012, 100A, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef]
- Alhourani, A.; Førde, J.-L.; Eichacker, L.A.; Herfindal, L.; Hagland, H.R. Improved pH-Responsive Release of Phenformin from Low-Defect Graphene Compared to Graphene Oxide. ACS Omega 2021, 6, 24619–24629. [Google Scholar] [CrossRef]
- Li, R.; Liu, C.; Wan, C.; Liu, T.; Zhang, R.; Du, J.; Wang, X.; Jiao, X.; Gao, R.; Li, B. A Targeted and pH-Responsive Nano-Graphene Oxide Nanoparticle Loaded with Doxorubicin for Synergetic Chemo-Photothermal Therapy of Oral Squamous Cell Carcinoma. Int. J. Nanomed. 2023, 18, 3309–3324. [Google Scholar] [CrossRef]
- Wen, H.; Dong, C.; Dong, H.; Shen, A.; Xia, W.; Cai, X.; Song, Y.; Li, X.; Li, Y.; Shi, D. Engineered Redox-Responsive PEG Detachment Mechanism in PEGylated Nano-Graphene Oxide for Intracellular Drug Delivery. Small 2012, 8, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Lu, T.; Nong, Z.; Li, G.; Pan, X.; Wei, Y.; Yang, Y.; Wu, N.; Huang, J.; Pan, M.; et al. Reductive response and RGD targeting nano-graphene oxide drug delivery system. J. Drug Deliv. Sci. Technol. 2019, 53, 101202. [Google Scholar] [CrossRef]
- Yin, T.; Liu, J.; Zhao, Z.; Zhao, Y.; Dong, L.; Yang, M.; Zhou, J.; Huo, M. Redox Sensitive Hyaluronic Acid-Decorated Graphene Oxide for Photothermally Controlled Tumor-Cytoplasm-Selective Rapid Drug Delivery. Adv. Funct. Mater. 2017, 27, 1604620. [Google Scholar] [CrossRef]
- Havanur, S.; Batish, I.; Cheruku, S.P.; Gourishetti, K.; JagadeeshBabu, P.E.; Kumar, N. Poly(N,N-diethyl acrylamide)/functionalized graphene quantum dots hydrogels loaded with doxorubicin as a nano-drug carrier for metastatic lung cancer in mice. Mater. Sci. Eng. C 2019, 105, 110094. [Google Scholar] [CrossRef]
- Khoee, S.; Karimi, M.R. Dual-drug loaded Janus graphene oxide-based thermoresponsive nanoparticles for targeted therapy. Polymer 2018, 142, 80–98. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, F.; Peng, H.; Zhang, Y.; Li, Y.; Xu, Y.; Xie, J. Layer-by-layer modification of magnetic graphene oxide by chitosan and sodium alginate with enhanced dispersibility for targeted drug delivery and photothermal therapy. Colloids Surf. B Biointerfaces 2019, 176, 462–470. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Hooshyar, Z. Thermo/pH/magnetic-triple sensitive poly(N-isopropylacrylamide-co-2-dimethylaminoethyl) methacrylate)/sodium alginate modified magnetic graphene oxide nanogel for anticancer drug delivery. Polym. Bull. 2018, 75, 5403–5419. [Google Scholar] [CrossRef]
- Wang, L.; Yu, D.; Dai, R.; Fu, D.; Li, W.; Guo, Z.; Cui, C.; Xu, J.; Shen, S.; Ma, K. PEGylated doxorubicin cloaked nano-graphene oxide for dual-responsive photochemical therapy. Int. J. Pharm. 2019, 557, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally Enhanced Photodynamic Therapy Delivered by Nano-Graphene Oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Yan, J.; Long, S.; Xiong, H.; Wen, N.; Cai, S.; Wang, Y.; Peng, D.; Liu, Z.; Liu, Y. Tumor microenvironment and NIR laser dual-responsive release of berberine 9-O-pyrazole alkyl derivative loaded in graphene oxide nanosheets for chemo-photothermal synergetic cancer therapy. J. Mater. Chem. B 2020, 8, 4046–4055. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, C.; Xing, Y.; Fang, W.; Ren, J.; Yu, H.; Wang, G. NIR-Light- and pH-Responsive Graphene Oxide Hybrid Cyclodextrin-Based Supramolecular Hydrogels. Langmuir 2019, 35, 1021–1031. [Google Scholar] [CrossRef]
- Thapa, R.K.; Byeon, J.H.; Choi, H.-G.; Yong, C.S.; Kim, J.O. PEGylated lipid bilayer-wrapped nano-graphene oxides for synergistic co-delivery of doxorubicin and rapamycin to prevent drug resistance in cancers. Nanotechnology 2017, 28, 295101. [Google Scholar] [CrossRef]
- Yang, D.; Feng, L.; Dougherty, C.A.; Luker, K.E.; Chen, D.; Cauble, M.A.; Banaszak Holl, M.M.; Luker, G.D.; Ross, B.D.; Liu, Z.; et al. In vivo targeting of metastatic breast cancer via tumor vasculature-specific nano-graphene oxide. Biomaterials 2016, 104, 361–371. [Google Scholar] [CrossRef]
- Mitra, T.; Manna, P.J.; Raja, S.T.K.; Gnanamani, A.; Kundu, P.P. Curcumin loaded nano graphene oxide reinforced fish scale collagen—A 3D scaffold biomaterial for wound healing applications. RSC Adv. 2015, 5, 98653–98665. [Google Scholar] [CrossRef]
- Charmi, J.; Nosrati, H.; Mostafavi Amjad, J.; Mohammadkhani, R.; Danafar, H. Polyethylene glycol (PEG) decorated graphene oxide nanosheets for controlled release curcumin delivery. Heliyon 2019, 5, e01466. [Google Scholar] [CrossRef]
- Rahman, M.; Singh, J.G.; Afzal, O.; Altamimi, A.S.A.; Alrobaian, M.; Haneef, J.; Barkat, M.A.; Almalki, W.H.; Handa, M.; Shukla, R.; et al. Preparation, Characterization, and Evaluation of Curcumin–Graphene Oxide Complex-Loaded Liposomes against Staphylococcus aureus in Topical Disease. ACS Omega 2022, 7, 43499–43509. [Google Scholar] [CrossRef]
- Hai, L.; He, D.; He, X.; Wang, K.; Yang, X.; Liu, J.; Cheng, H.; Huang, X.; Shangguan, J. Facile fabrication of a resveratrol loaded phospholipid@reduced graphene oxide nanoassembly for targeted and near-infrared laser-triggered chemo/photothermal synergistic therapy of cancer in vivo. J. Mater. Chem. B 2017, 5, 5783–5792. [Google Scholar] [CrossRef]
- Rahmanian, N.; Hamishehkar, H.; Dolatabadi, J.E.N.; Arsalani, N. Nano graphene oxide: A novel carrier for oral delivery of flavonoids. Colloids Surf. B Biointerfaces 2014, 123, 331–338. [Google Scholar] [CrossRef]
- Najafabadi, A.P.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Rahdar, A.; Díez-Pascual, A.M. pH-sensitive ameliorated quercetin delivery using graphene oxide nanocarriers coated with potential anticancer gelatin-polyvinylpyrrolidone nanoemulsion with bitter almond oil. J. Drug Deliv. Sci. Technol. 2023, 82, 104339. [Google Scholar] [CrossRef]
- Rana, A.; Matiyani, M.; Tewari, C.; Negi, P.B.; Chandra Arya, M.; Das, V.; Pal, M.; Sahoo, N.G. Functionalized graphene oxide based nanocarrier for enhanced cytotoxicity of Juniperus squamata root essential oil against breast cancer cells. J. Drug Deliv. Sci. Technol. 2022, 72, 103370. [Google Scholar] [CrossRef]
- Figueroa, T.; Aguayo, C.; Fernández, K. Design and Characterization of Chitosan-Graphene Oxide Nanocomposites for the Delivery of Proanthocyanidins. Int. J. Nanomed. 2020, 15, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Meng, D.; Zheng, X.; Zhang, Y.; Chen, H. Graphene-based materials: A new tool to fight against breast cancer. Int. J. Pharm. 2021, 603, 120644. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Wu, J.; Lin, J.; Liu, W.; Chen, P.; Yu, M.; Zhou, D.; Yao, G. Graphene-based nanomaterials for breast cancer treatment: Promising therapeutic strategies. J. Nanobiotechnol. 2021, 19, 211. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Du, J.; Wang, X.; Duan, A.; Gao, R.; Liu, J.; Li, B. Graphene oxide loaded with tumor-targeted peptide and anti-cancer drugs for cancer target therapy. Sci. Rep. 2021, 11, 1725. [Google Scholar] [CrossRef]
- Qin, X.C.; Guo, Z.Y.; Liu, Z.M.; Zhang, W.; Wan, M.M.; Yang, B.W. Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J. Photochem. Photobiol. B Biol. 2013, 120, 156–162. [Google Scholar] [CrossRef]
- Kumari, S.; Nehra, A.; Gupta, K.; Puri, A.; Kumar, V.; Singh, K.P.; Kumar, M.; Sharma, A. Chlorambucil-Loaded Graphene-Oxide-Based Nano-Vesicles for Cancer Therapy. Pharmaceutics 2023, 15, 649. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-H.; Pi, J.; Jin, H.; Cai, J.-Y. Functional graphene oxide as cancer-targeted drug delivery system to selectively induce oesophageal cancer cell apoptosis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wei, Y.; Zhai, S.; Chen, Q.; Xing, D. Dihydroartemisinin and transferrin dual-dressed nano-graphene oxide for a pH-triggered chemotherapy. Biomaterials 2015, 62, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.T.; Chen, C.-H.; Chen, J.-P. Intratumoral Delivery of Doxorubicin on Folate-Conjugated Graphene Oxide by In-Situ Forming Thermo-Sensitive Hydrogel for Breast Cancer Therapy. Nanomaterials 2017, 7, 388. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Yang, K.; Zhang, Y.; Engle, J.W.; Feng, L.; Yang, Y.; Nayak, T.R.; Goel, S.; Bean, J.; Theuer, C.P.; et al. In Vivo Targeting and Imaging of Tumor Vasculature with Radiolabeled, Antibody-Conjugated Nanographene. ACS Nano 2012, 6, 2361–2370. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Y.; Zheng, L.; Wu, Y.; Wang, T.; Jiang, T.; Liu, X.; Peng, D.; Liu, Y.; Liu, Z. Let-7i miRNA and platinum loaded nano-graphene oxide platform for detection/reversion of drug resistance and synergetic chemical-photothermal inhibition of cancer cell. Chin. Chem. Lett. 2022, 33, 767–772. [Google Scholar] [CrossRef]
- Al-Ani, L.A.; Kadir, F.A.; Hashim, N.M.; Julkapli, N.M.; Seyfoddin, A.; Lu, J.; AlSaadi, M.A.; Yehye, W.A. The impact of curcumin-graphene based nanoformulation on cellular interaction and redox-activated apoptosis: An in vitro colon cancer study. Heliyon 2020, 6, e05360. [Google Scholar] [CrossRef]
- Yaghoubi, F.; Motlagh, N.S.H.; Naghib, S.M.; Haghiralsadat, F.; Jaliani, H.Z.; Moradi, A. A functionalized graphene oxide with improved cytocompatibility for stimuli-responsive co-delivery of curcumin and doxorubicin in cancer treatment. Sci. Rep. 2022, 12, 1959. [Google Scholar] [CrossRef]
- Yaghoubi, F.; Hosseini Motlagh, N.S.; Moradi, A.; Haghiralsadat, F. Carboxylated Graphene Oxide as a Nanocarrier for Drug Delivery of Quercetin as an Effective Anticancer Agent. Iran. Biomed. J. 2022, 26, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, H.; Karki, N.; Pal, M.; Basak, S.; Verma, R.K.; Bal, R.; Kandpal, N.D.; Bisht, G.; Sahoo, N.G. Functionalized graphene oxide as a nanocarrier for dual drug delivery applications: The synergistic effect of quercetin and gefitinib against ovarian cancer cells. Colloids Surf. B Biointerfaces 2019, 178, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Buskaran, K.; Hussein, M.Z.; Moklas, M.A.M.; Masarudin, M.J.; Fakurazi, S. Graphene Oxide Loaded with Protocatechuic Acid and Chlorogenic Acid Dual Drug Nanodelivery System for Human Hepatocellular Carcinoma Therapeutic Application. Int. J. Mol. Sci. 2021, 22, 5786. [Google Scholar] [CrossRef] [PubMed]
- Sontakke, A.D.; Fopase, R.; Pandey, L.M.; Purkait, M.K. Development of graphene oxide nanoscrolls imparted nano-delivery system for the sustained release of gallic acid. Appl. Nanosci. 2022, 12, 2733–2751. [Google Scholar] [CrossRef]
- Bhatt, S.; Punetha, V.D.; Pathak, R.; Punetha, M. Graphene in nanomedicine: A review on nano-bio factors and antibacterial activity. Colloids Surf. B Biointerfaces 2023, 226, 113323. [Google Scholar] [CrossRef] [PubMed]
- Ng, I.M.J.; Shamsi, S. Graphene Oxide (GO): A Promising Nanomaterial against Infectious Diseases Caused by Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2022, 23, 9096. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Dadashpour, M.; Hejazi, M.; Hasanzadeh, M.; Behnam, B.; de la Guardia, M.; Shadjou, N.; Mokhtarzadeh, A. Anti-bacterial activity of graphene oxide as a new weapon nanomaterial to combat multidrug-resistance bacteria. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 568–581. [Google Scholar] [CrossRef]
- Wu, X.; Tan, S.; Xing, Y.; Pu, Q.; Wu, M.; Zhao, J.X. Graphene oxide as an efficient antimicrobial nanomaterial for eradicating multi-drug resistant bacteria in vitro and in vivo. Colloids Surf. B Biointerfaces 2017, 157, 1–9. [Google Scholar] [CrossRef]
- Bugli, F.; Cacaci, M.; Palmieri, V.; Di Santo, R.; Torelli, R.; Ciasca, G.; Di Vito, M.; Vitali, A.; Conti, C.; Sanguinetti, M.; et al. Curcumin-loaded graphene oxide flakes as an effective antibacterial system against methicillin-resistant Staphylococcus aureus. Interface Focus 2018, 8, 20170059. [Google Scholar] [CrossRef] [PubMed]
- Robb, S.M.; Li, M.; Dahl, K.N.; Islam, M.F. Antibacterial Mechanisms of Nano-Graphene Oxide By Measuring Membrane Interactions. Electrochem. Soc. Meet. Abstr. 2016, MA2016-01, 616. [Google Scholar] [CrossRef]
- Pulingam, T.; Thong, K.L.; Ali, M.E.; Appaturi, J.N.; Dinshaw, I.J.; Ong, Z.Y.; Leo, B.F. Graphene oxide exhibits differential mechanistic action towards Gram-positive and Gram-negative bacteria. Colloids Surf. B Biointerfaces 2019, 181, 6–15. [Google Scholar] [CrossRef]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef]
- Mao, J.; Guo, R.; Yan, L.-T. Simulation and analysis of cellular internalization pathways and membrane perturbation for graphene nanosheets. Biomaterials 2014, 35, 6069–6077. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Han, H. A new function of graphene oxide emerges: Inactivating phytopathogenic bacterium Xanthomonas oryzae pv. Oryzae. J. Nanopart. Res. 2013, 15, 1658. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, H.; Liu, D.-E.; An, J.; Gao, H. Construction of biocompatible bovine serum albumin nanoparticles composed of nano graphene oxide and AIEgen for dual-mode phototherapy bacteriostatic and bacterial tracking. J. Nanobiotechnol. 2019, 17, 104. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Etemad-Moghadam, S.; Alaeddini, M.; Miri Mousavi, R.s.; Bahador, A. DNA-aptamer-nanographene oxide as a targeted bio-theragnostic system in antimicrobial photodynamic therapy against Porphyromonas gingivalis. Sci. Rep. 2022, 12, 12161. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, X.; Li, Q.; Zhu, J.; Ding, J.; Jiang, T. Vancomycin modified graphene oxide as carriers to load phthalocyanine for synergistic phototherapy of vancomycin-resistant bacteria. J. Nanopart.Res. 2022, 24, 234. [Google Scholar] [CrossRef]
- Jihad, M.A.; Noori, F.T.M.; Jabir, M.S.; Albukhaty, S.; AlMalki, F.A.; Alyamani, A.A. Polyethylene Glycol Functionalized Graphene Oxide Nanoparticles Loaded with Nigella sativa Extract: A Smart Antibacterial Therapeutic Drug Delivery System. Molecules 2021, 26, 3067. [Google Scholar] [CrossRef]
- Yan, H.; Li, P.; Jiang, X.; Wang, X.; Hu, Y.; Zhang, Y.; Su, R.; Su, W. Preparation of graphene oxide/polydopamine-curcumin composite nanomaterials and its antibacterial effect against Staphylococcus aureus induced by white light. Biomater. Adv. 2022, 139, 213040. [Google Scholar] [CrossRef] [PubMed]
- Cacaci, M.; Squitieri, D.; Palmieri, V.; Torelli, R.; Perini, G.; Campolo, M.; Di Vito, M.; Papi, M.; Posteraro, B.; Sanguinetti, M.; et al. Curcumin-Functionalized Graphene Oxide Strongly Prevents Candida parapsilosis Adhesion and Biofilm Formation. Pharmaceuticals 2023, 16, 275. [Google Scholar] [CrossRef]
- Oves, M.; Ansari, M.O.; Ansari, M.S.; Memić, A. Graphene@Curcumin-Copper Paintable Coatings for the Prevention of Nosocomial Microbial Infection. Molecules 2023, 28, 2814. [Google Scholar] [CrossRef]
- Shamsi, S.; Abdul Ghafor, A.A.H.; Norjoshukrudin, N.H.; Ng, I.M.J.; Abdullah, S.N.S.; Sarchio, S.N.E.; Md Yasin, F.; Abd Gani, S.; Mohd Desa, M.N. Stability, Toxicity, and Antibacterial Potential of Gallic Acid-Loaded Graphene Oxide (GAGO) Against Methicillin-Resistant Staphylococcus aureus (MRSA) Strains. Int. J. Nanomed. 2022, 17, 5781–5807. [Google Scholar] [CrossRef] [PubMed]
- Arfat, Y.A.; Ahmed, J.; Ejaz, M.; Mullah, M. Polylactide/graphene oxide nanosheets/clove essential oil composite films for potential food packaging applications. Int. J. Biol. Macromol. 2018, 107, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, R.; Zhang, X.; Zhang, D. Enhanced antimicrobial activity and pH-responsive sustained release of chitosan/poly (vinyl alcohol)/graphene oxide nanofibrous membrane loading with allicin. Int. J. Biol. Macromol. 2020, 161, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-Y.; Meng, T.; Ye, J.-X.; Yin, H.-B.; Song, D.-W. Enhanced Antibacterial and Osteogenic Properties of Graphene Oxide Loaded with Berberine on Biomedical Titanium. J. Biomed. Nanotechnol. 2022, 18, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Wang, X.; Chen, P.; Liu, S.; Hu, J.; Xiao, R.; Li, L.; Lu, F. Targeted inhibition of methicillin-resistant Staphylococcus aureus biofilm formation by a graphene oxide-loaded aptamer/berberine bifunctional complex. Drug Deliv. 2022, 29, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Park, R.; Jo, H.J.; Shin, Y.C.; Kim, C.S.; Hyon, S.H.; Hong, S.W.; Oh, J.; Han, D.W. Spontaneous Osteogenic Differentiation of Human Mesenchymal Stem Cells by Tuna-Bone-Derived Hydroxyapatite Composites with Green Tea Polyphenol-Reduced Graphene Oxide. Cells 2023, 12, 1448. [Google Scholar] [CrossRef]
- Shen, H.; Lin, H.; Sun, A.X.; Song, S.; Wang, B.; Yang, Y.; Dai, J.; Tuan, R.S. Acceleration of chondrogenic differentiation of human mesenchymal stem cells by sustained growth factor release in 3D graphene oxide incorporated hydrogels. Acta Biomater. 2020, 105, 44–55. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, J.; Lin, X.; Bao, Q. Continuous release of bone morphogenetic protein-2 through nano-graphene oxide-based delivery influences the activation of the NF-κB signal transduction pathway. Int. J. Nanomed. 2017, 12, 1215–1226. [Google Scholar] [CrossRef]
- La, W.-G.; Jin, M.; Park, S.; Yoon, H.-H.; Jeong, G.-J.; Bhang, S.H.; Park, H.; Char, K.; Kim, B.-S. Delivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regeneration. Int. J. Nanomed. 2014, 9, 107–116. [Google Scholar] [CrossRef]
- Faraji, S.; Nowroozi, N.; Nouralishahi, A.; Shabani Shayeh, J. Electrospun poly-caprolactone/graphene oxide/quercetin nanofibrous scaffold for wound dressing: Evaluation of biological and structural properties. Life Sci. 2020, 257, 118062. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Feng, X.; Wang, Y.; Wang, X.; He, Y. Biomimetic and immunomodulatory baicalin-loaded graphene oxide-demineralized bone matrix scaffold for in vivo bone regeneration. J. Mater. Chem. B 2021, 9, 9720–9733. [Google Scholar] [CrossRef] [PubMed]
- Kashte, S.; Sharma, R.K.; Kadam, S. Layer-by-layer decorated herbal cell compatible scaffolds for bone tissue engineering: A synergistic effect of graphene oxide and Cissus quadrangularis. J. Bioact. Compat. Polym. 2020, 35, 57–73. [Google Scholar] [CrossRef]



| Class Type of Natural Compounds | Examples |
|---|---|
| Alkaloids | 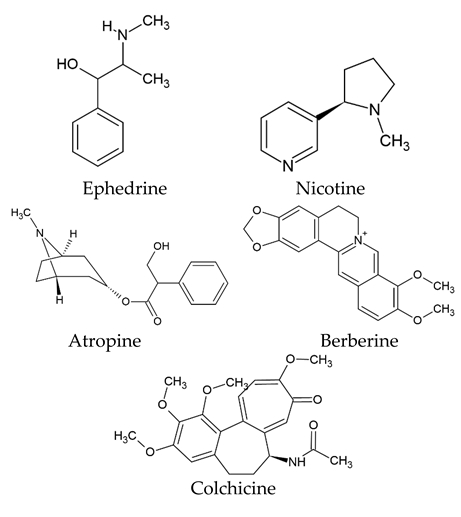 |
| Phenolic compounds | 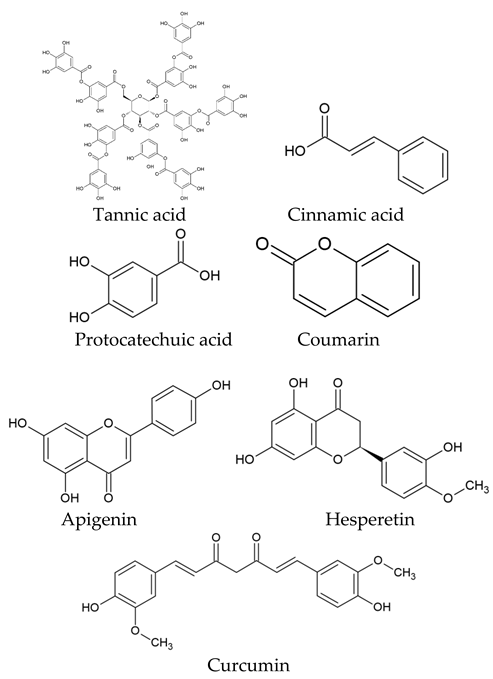 |
| Terpenoids | 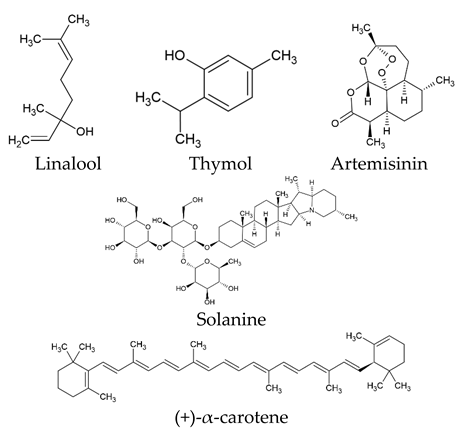 |
| Sulfur-containing compounds |  |
| Natural Agent | Natural Product Class | Plant Source | Trade Name/Year of the Introduction | Therapy |
|---|---|---|---|---|
| Vincristine | Vinca alkaloids | Catharanthus roseus L. formerly Vinca rosea L. | Vincristine/1963/FDA | Cancers |
| Artemisinin | Sesquiterpene lactone | Artemisia annua | Artemisinin/1987/FDA | Malaria |
| Arglabin | Sesquiterpene lactone | Artemisia glabella | Arglabin/1999/FDA | Cancer chemotherapy |
| Capsaicin | Alkaloid | Casicum Annum L. | Qutenza/2010/FDA | Post therapeutic neuralgia |
| Colchicine | Alkaloid | Colichicum | Colcrys/2009/FDA | Gout |
| Dronabinol/ Cannabidiol/Cannabinol | Alkaloid | Cannabis Sativa L. | Sativex/2005/FDA | Chronic neuropathic pain |
| Galanthiamine | Alkaloid | Galanthus Cancasicus | Razadyne/2001/FDA | Dementia associated with Alzheimer’s disease |
| Ingenol mebutate | Diterpene ingenol | Euphorbia peplus L. | Picato/2012/FDA | Actinic keratosis |
| Masoprocol | Phenolic lignan | Larrea tridentata | Actinex/1992/FDA | Cancer chemotherapy |
| Omacetaxine mepesuccinate (Homoharringtonine) | Alkaloid | Cephalotaxus harringtonia | Synribo/2012/FDA | Cancer |
| Paclitaxel | Alkaloid | Taxus brevifolia Nutt. | Taxol/1993/FDA Abraxanec/2005/FDA Nanoxelc/2007/FDA | Cancer chemotherapy |
| Solamargine | Alkaloid | Solanum spp. | Curadermd/1989 | Cancer chemotherapy |
| Ingenol mebutate | Diterpenes | Euphorbia peplus L. | In clinical evaluations | Potent antiproliferative effects for cancers |
| Epigallocatechin-3-O-gallate (EGCG) | Polyphenolic | Camellia sinensis (L.) | In clinical evaluations | Antiviral, Alzheimer’s disease, cardiac amyloid light-chain amyloidosis, others |
| Curcumin | Polyphenolic | Curcuma longa L. | In clinical evaluations | Cancers, Alzheimer’s disease, fibromyalgia, cardiovascular disease |
| Genistein | Phenolic (flavonoid) | Genista tinctoria L. | In clinical evaluations | Cancers |
| Betulinic acid | Triterpene | Gratiola officinalis L. | In clinical evaluations | Cancers |
| Gossypol | Phenolic | Gossypium hirsutum L. | In clinical evaluations | Leukemia cancers |
| Quercetin | Phenolic (flavonoid) | Allium cepa L. and other plants | In clinical evaluations | Cancers, diabetes mellitus, obesity diastolic heart failure, hypertensive heart disease, Alzheimer’s disease, others |
| Resveratrol | Phenolic | Vitis vinifera L. | In clinical evaluations | Diabetes, vascular liver disease, cardiovascular disease, inflammation, insulin resistance, bone disease, coronary artery disease, obesity, oxidative stress, others |
| Natural Drugs | Synthetic Drugs | |
|---|---|---|
| Advantages | - Ease of access - Relative safety - Synergistic effects - Cultural acceptance - Long history of use - Traditional knowledge - Environmentally friendly - Complex chemical composition - Health benefits beyond treatment - Potential for novel drug discovery | - Cost-effectiveness - Potency and efficacy - Targeted drug design - Rapid drug development - Precision and consistency - Controlled side effect profile - Intellectual property protection - Improved stability and shelf life - Reduced contamination and allergenicity |
| Disadvantages | - Limited supply - Variable potency - Limited shelf life - Ethical considerations - Lack of standardization - Potential contamination - Risk of allergic reactions - Lack of rigorous clinical trials - Interaction with other medications - Standardization and regulatory challenges | - Drug resistance - Ethical concerns - Drug interactions - Environmental impact - Side effects and toxicity - Lack of natural synergy - Limited natural diversity - Patent exclusivity and cost - Development time and costs - Unforeseen long-term effects |
| Stimuli Classification | Stimuli Kind | Carrier | Drug/Loading | Release Mechanism | Ref. |
|---|---|---|---|---|---|
| Internal-stimuli release | pH-responsive release | Pluronic NGO-pluronic F127 | Doxorubicin/loaded onto PF127/GO nanohybrid. | A change in pH can cause the release by changing hydrogen bonds and the solubility of the drug. | [118] |
| NGO-sulfonic acid groups-folic acid | Doxorubicin and camptothecin are loaded onto this nanocarrier via π–π stacking and hydrophobic interactions. | These drugs were released from NGO into an aqueous solution as their hydrophilicity increased, making them more water-soluble and hydrophilic. | [119] | ||
| NGO-PEG | Phenformin encapsulated onto NGO through hydrogen bonds and π–π stacking interaction | The drug release varies according to the shifting zeta potential of the prepared loaded material in the surrounding media. At acidic pH levels, improved positive phenformin release results from an electrostatic potential (at the shear planes of PNGS). | [120] | ||
| NGO-PEG | Doxorubicin drug loaded through hydrogen bonding and π–π bonding | The release can take place due to the partial hydrogen bonds dissociation that connects NGO, DOX, and the -OH and -NH2 groups in a low acidic environment, thereby accelerating drug release. | [121] | ||
| Redox-responsive release | NGO-SS-mPEG | Doxorubicin hydrochloride loaded via π–π bonding | Increasing the intracellular GSH concentrations leads to rapid drug release that may relate to drug diffusion from the carriers as well. | [122] | |
| NGO-PEG-NH2-RGD | Doxorubicin | With more GSH reduction, along with evaluated special photothermal performance, the response release happens. | [123] | ||
| NGO-hyaluronic acid | Doxorubicin via π–π stacking and hydrophobic interactions onto NGO sheets | With the presence of GSH at various concentrations, the release accolated through thiol exchange from NGO’s surface. | [124] | ||
| Temperature-responsive release | Poly(N,N-diethyl acrylamide)/ functionalized GQD-thermosensitive hydrogels | Doxorubicin | Drug releases from the nanocomposite at the range of 28–42 °C. The release takes place due to diffusion kinetics. | [125] | |
| NGO-functionalized polymer | Quercetin and 5-FU as hydrophobic and hydrophilic drugs. | These drugs release in response to changing temperature levels. | [126] | ||
| External-stimuli release | Magnetic-responsive release | Magnetic nanoparticles incorporated NGO-chitosan/alginate nanocomposites | Doxorubicin hydrochloride loaded into nanocomposite via π−π stacking and electrostatic attraction | It releases corresponding to magnetically stimulated effect and produces uptake. | [127] |
| Polymeric-magnetic-GO | Doxorubicin | The release effect happens according to the presence of magnetic triggering. | [128] | ||
| Light-responsive release | NGO-PEG | Doxorubicin | NIR and pH dual-responsive affects releasing of DOX loaded by noncovalent bonding modification. | [129] | |
| NGO-PEG | Photosensitizer molecule (Chlorin e6) loaded via non-covalent bonding as a photodynamic therapy | The photothermal effect promotes the delivery and release of Ce6 when exposed to a near-infrared laser. | [130] | ||
| Combined-responsive release | Combined-responsive release with dual or triple effect | Functionalized NGO-based materials | Many therapeutics | Drug molecules release depending on various conditions accelerating their release profiles to produce therapeutic action and cellular uptake. | [129,131,132] |
| Drug Carrier | Drug Model | Loading Method | Drug Loading | Drug Content | Drug Release | Ref. |
|---|---|---|---|---|---|---|
| NGO-polydopamine | Cytarabine hydrochloride-Hydroxycamptothecin | Non-covalent bonds | 35% 43% | 11.3% 19% | 50% 50% | [103] |
| NGO-polydopamine conjugated | Methotrexate | Non-covalent bonds | 81.88% | 19.72% | 80% | [90] |
| NGO-PEGylated | Doxorubicin | Physical adsorption | 90% | 10% | 65% | [133] |
| NGO-conjugated FSHR antibody | Doxorubicin | Adsorption | 75.6% | 8.4% | 69.3% | [134] |
| NGO-PEG | Doxorubicin/ Cisplatin | Combined method | 36.7% 37.6% | 64.6% 65.7% | [88] |
| Drug Carrier | Drug Model | Drug Content | Drug Release | Application | Ref. |
|---|---|---|---|---|---|
| NGO-functionalized collagen scaffold | Curcumin | NA | 82.5% | Antimicrobial and wound healing tissue engineering | [135] |
| NGO-PEG | Curcumin | 4.5% | 60% | NA | [136] |
| GO-liposome complex | Curcumin | NA | 71.2 | Antibacterial in topical disease | [137] |
| Folate-PEG-phospholipid coated RGO nano assembly (FA-PEG-Lip@rGO/Res) | Resveratrol | 69.5 ± 4.3% | Up to 40.57% | Anticancer | [138] |
| NGO | Quercetin | Up to 35% | Negligible at 24 h | Anticancer | [139] |
| NGO-gelatin-polyvinylpyrrolidone (PVP) nanoemulsion | Quercetin | 45% | 91% and 95.5% | Anticancer | [140] |
| NGO-PVP | Essential oil | 87.08% | NA | Anticancer | [141] |
| GO-chitosan nanocomposites | Proanthocyanidins (from grape seed extract) | Aprox. 20% | 28.4% to 100% | NA | [142] |
| Ligands | Cancer Type | Drugs/Therapeutic Agents | Approach | Findings | References |
|---|---|---|---|---|---|
| HN-1 peptide | Oral squamous cell carcinoma (OSCC) | Doxorubicin drug | Through hydrogen and π–π bonds | Due to extensive tumor targeting, it causes higher cellular uptake and cytotoxicity in OSCC cells. | [145] |
| Fibroblast activation protein (FAP, a membrane-bound protease) | Oral squamous cell carcinoma (OSCC) | Doxorubicin drug | Via π–π stacking | It exhibits specific targeting effects for OSCC with improved tumor suppression performance in vivo and in vitro. | [121] |
| Tumor-specific antibody SCCA (8H11) | Squamous cell carcinoma | Cisplatin drug | Non-covalent adsorption | Attaching antibody demonstrates the capacity to target squamous cancer cells with efficient killing of cancer cells combined with limiting the toxicity to non-cancer cells. The data obtained from the nude mouse tumor-bearing model shows the new approach to therapy for squamous cell carcinoma to be both safe and effective. | [115] |
| Folic acid | Cancer cells | Doxorubicin drug | Covalent amide bond | It enhances receptor-mediated endocytosis, which helps the internalization of tumor cells. Additionally, it demonstrates a targeted chemo-photothermal therapy with good anticancer therapeutic effectiveness that precisely delivers medicine and heat to tumor sites. | [146] |
| Folic acid | Human cervical adenocarcinoma cell line | Chlorambucil drug | Covalent amide bond | More cytotoxic on cancer cells. | [147] |
| EGFR targeting GE11 peptide | Esophageal cancer cells | Oridonin natural agent | Covalent bonds | For esophageal cancers, the system exhibits a high ability to target cancer in combination with anticancer efficacy via the EGFR pathway. | [148] |
| Transferrin (Tf) | Murine mammary carcinoma cell line (EMT6) | Dihydroartemisinin (natural agent) and transferrin | Covalent bonds | When compared to the drug alone, the system significantly increases tumor delivery specificity and cytotoxicity. It also shows that it can substantially reduce tumor burden in mice while producing only minor side effects. | [149] |
| Folic acid | Breast cancer | Doxorubicin drug | Covalent bonds | Targeted delivery via FA-conjugated and loaded anticancer drug could be a safe and efficient treatment for breast cancer. | [150] |
| Monoclonal antibody (mAb) against follicle-stimulating hormone receptor (FSHR) | Metastatic breast cancer | Doxorubicin drug | Covalent bonds | This focused system demonstrates an effective tool for early metastasis selective killing in vivo animal model. | [134] |
| TRC105, a monoclonal antibody | Breast cancer | NA | Covalent bonds | A functionalized NGO with active ligands demonstrates specifically targeted cancer cells. | [151] |
| Folic acid | Ovarian cancer cells | miRNA (let-7i) combined platinum | Covalent bonds | The system shows effective action against cisplatin resistant SKOV3 cells. | [152] |
| Ways and Characteristics of Active Cancer Targeting and Drug Loading | Remarks |
|---|---|
| NGO in the form of nanoparticles or nanosheets can connect an active targeting ligand on the surface via adsorption and chemical bonds. |
|
| The conjugation of ligands, e.g., aptamers, antibodies, and small molecules can be achieved before or after the natural agent’s therapeutic encapsulation/loading. |
|
| It is important to know the solubility of natural agents and targeting ligands. |
|
| Most of the chemical bonding can occur according to carboxylic acid groups of NGO. |
|
| To ensure cellular uptake by passive or active targeting, scanning electron microscopy can be employed without fluorescence molecules. In the case of fluorescence dye, confocal laser scanning microscopy can be performed. |
|
| It is necessary to investigate different cancer cells to evaluate the active cancer targeting. |
|
| The conjugation of targeting ligand contents on the surface of NGO may affect drug release percentages and profiles. |
|
| Antibacterial Designs | Remarks |
|---|---|
| GO-based materials |
|
| GO materials have inherent antibacterial effects. |
|
| Many fabricated systems have different mechanisms of actions. |
|
| The delivery systems compared to traditional therapeutic agents can result in high antibacterial activity and selectivity. |
|
| Efficient killing of biofilm bacterial formation on medical devices and different approaches like antibacterial coating |
|
| Disinfection and killing of microorganisms can perform, e.g., nanocomposites coatings. |
|
| Targeting microbial infection and biofilm formation by delivering therapeutics |
|
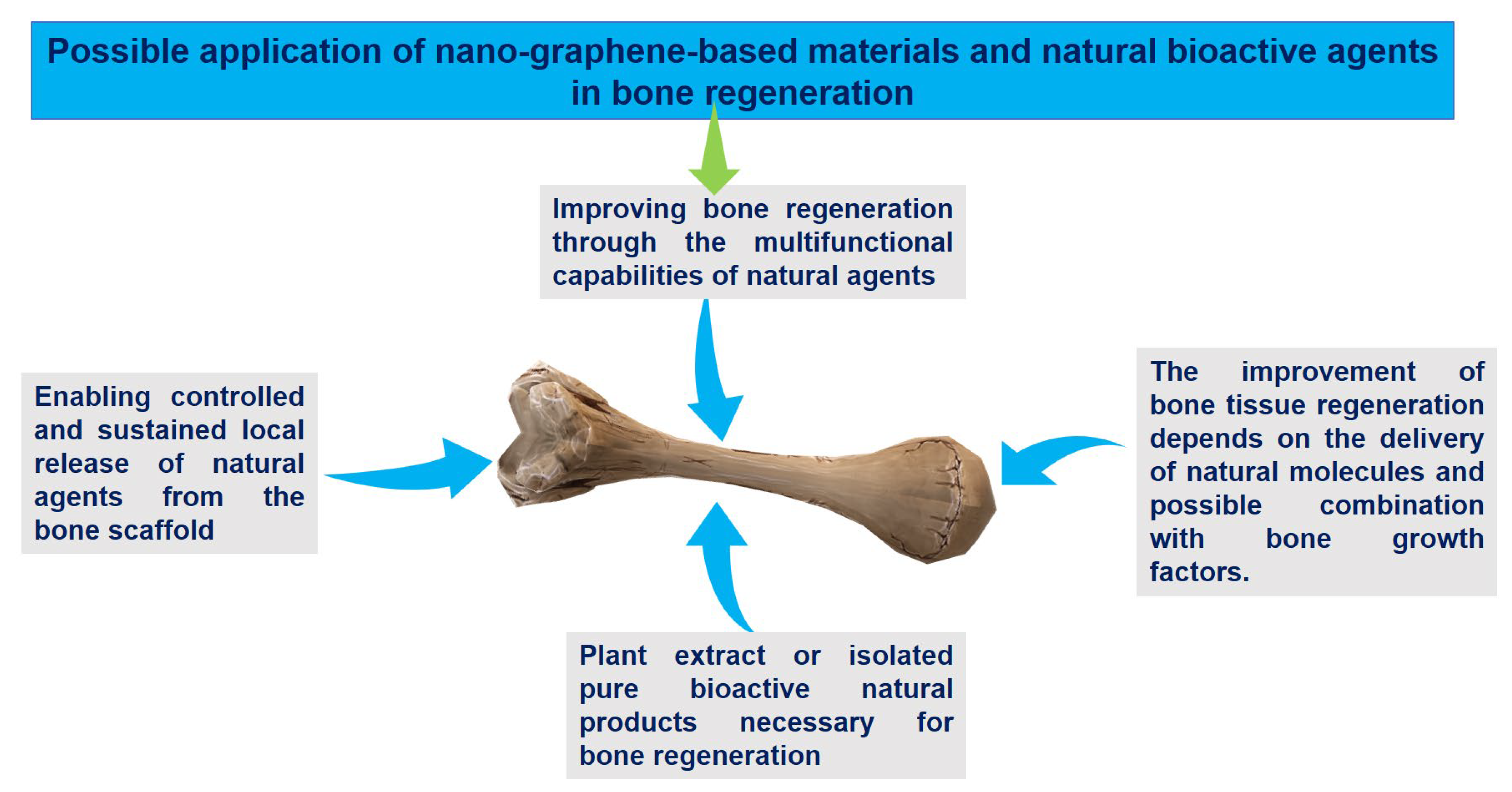
| Advantages | Remarks |
|---|---|
| Improving bone regeneration through the multifunctional capabilities of natural agents |
|
| Enabling controlled and sustained local release of natural agents from the bone scaffold |
|
| Plant extract or isolated pure bioactive natural products necessary for bone regeneration |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AbouAitah, K.; Sabbagh, F.; Kim, B.S. Graphene Oxide Nanostructures as Nanoplatforms for Delivering Natural Therapeutic Agents: Applications in Cancer Treatment, Bacterial Infections, and Bone Regeneration Medicine. Nanomaterials 2023, 13, 2666. https://doi.org/10.3390/nano13192666
AbouAitah K, Sabbagh F, Kim BS. Graphene Oxide Nanostructures as Nanoplatforms for Delivering Natural Therapeutic Agents: Applications in Cancer Treatment, Bacterial Infections, and Bone Regeneration Medicine. Nanomaterials. 2023; 13(19):2666. https://doi.org/10.3390/nano13192666
Chicago/Turabian StyleAbouAitah, Khaled, Farzaneh Sabbagh, and Beom Soo Kim. 2023. "Graphene Oxide Nanostructures as Nanoplatforms for Delivering Natural Therapeutic Agents: Applications in Cancer Treatment, Bacterial Infections, and Bone Regeneration Medicine" Nanomaterials 13, no. 19: 2666. https://doi.org/10.3390/nano13192666
APA StyleAbouAitah, K., Sabbagh, F., & Kim, B. S. (2023). Graphene Oxide Nanostructures as Nanoplatforms for Delivering Natural Therapeutic Agents: Applications in Cancer Treatment, Bacterial Infections, and Bone Regeneration Medicine. Nanomaterials, 13(19), 2666. https://doi.org/10.3390/nano13192666








