Eco-Friendly Dispersant-Free Purification Method of Boron Nitride Nanotubes through Controlling Surface Tension and Steric Repulsion with Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
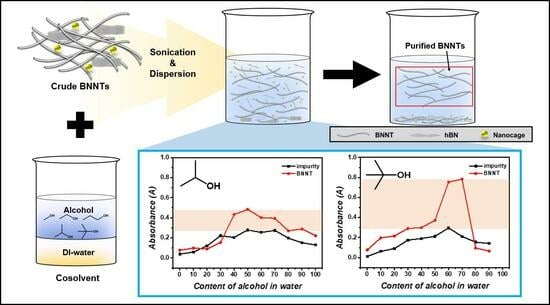
2.2. Purification Processes
2.3. Dispersion Test
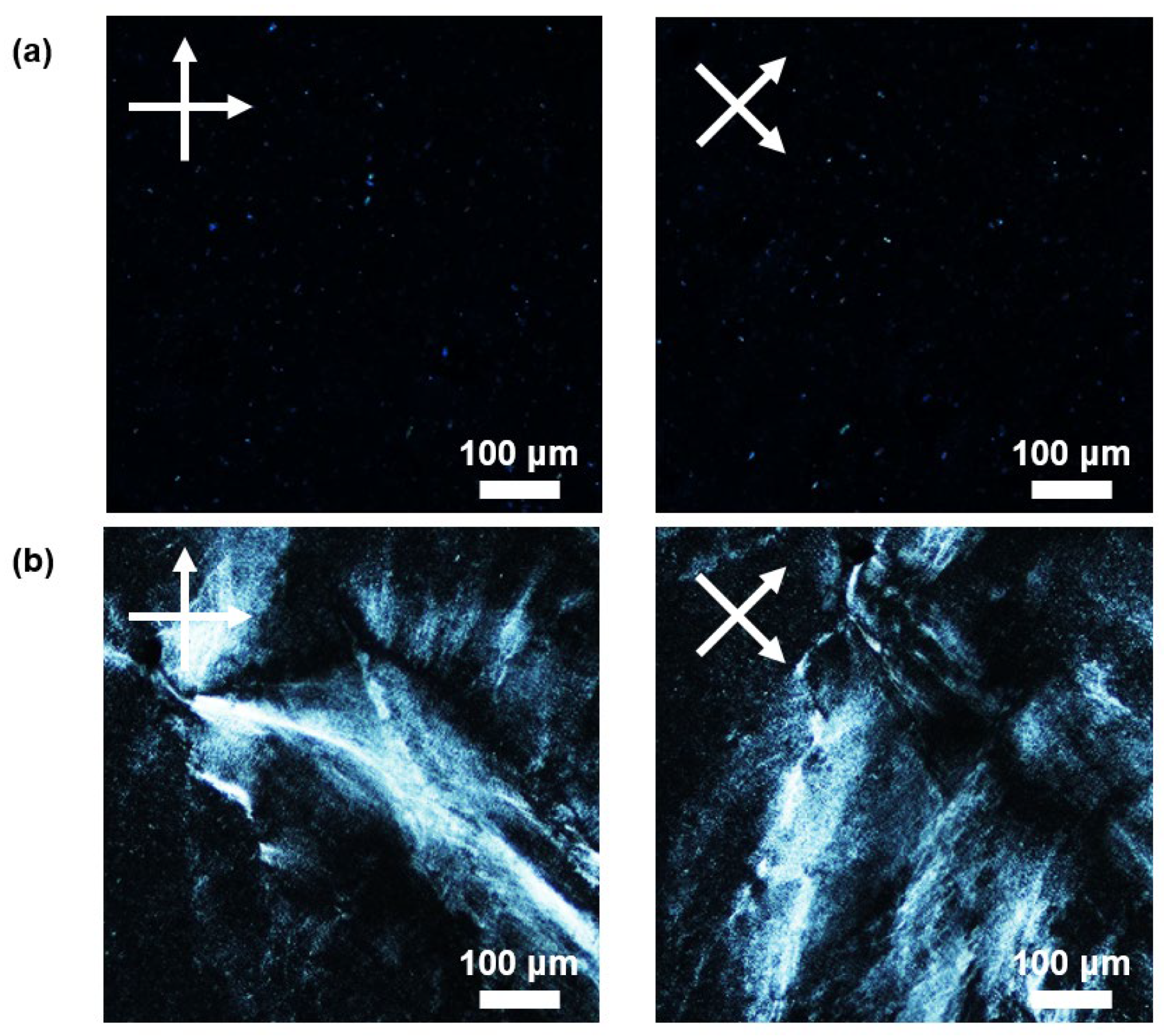
2.4. Liquid Crystal
2.5. Measurements
3. Results and Discussion
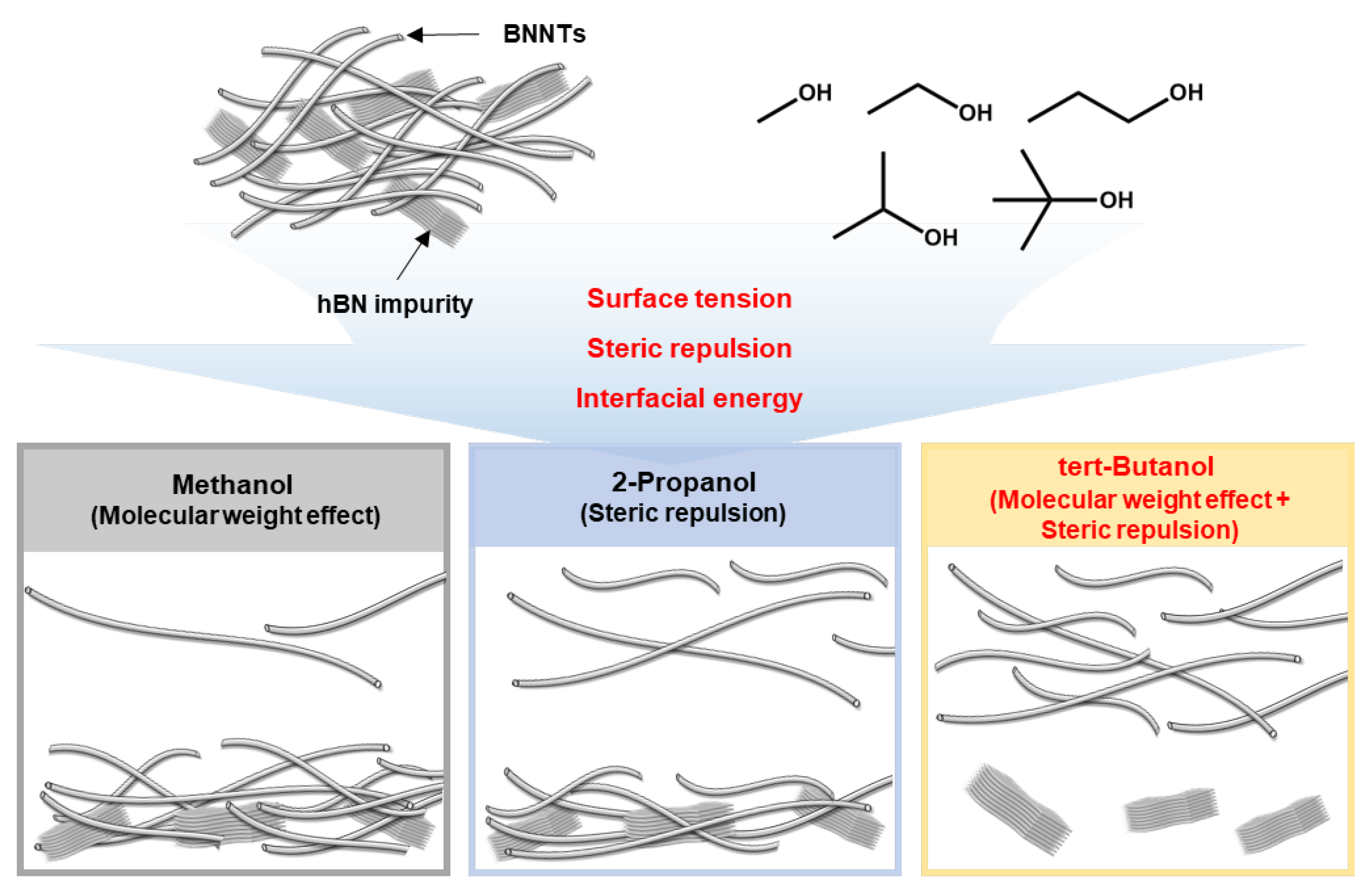
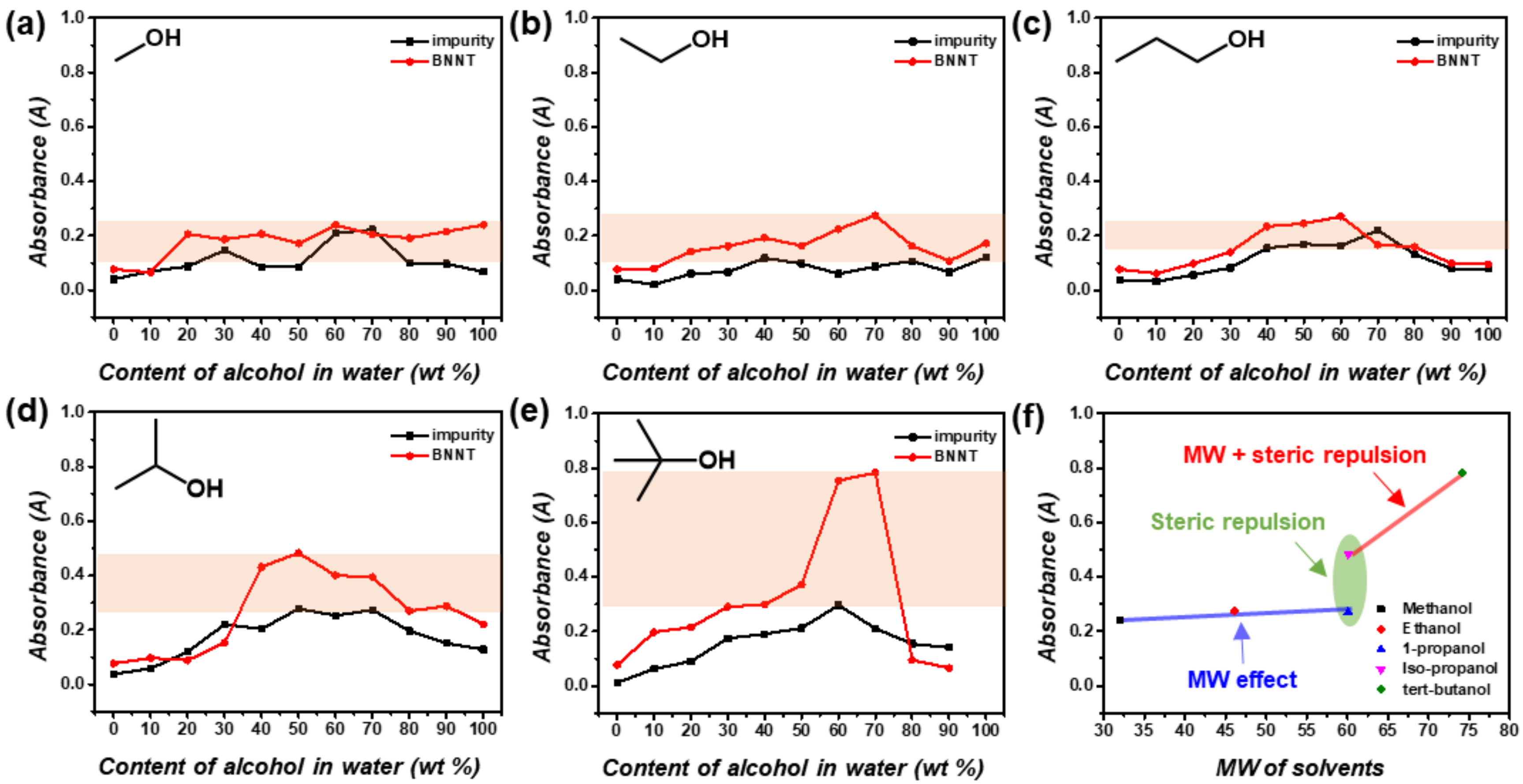
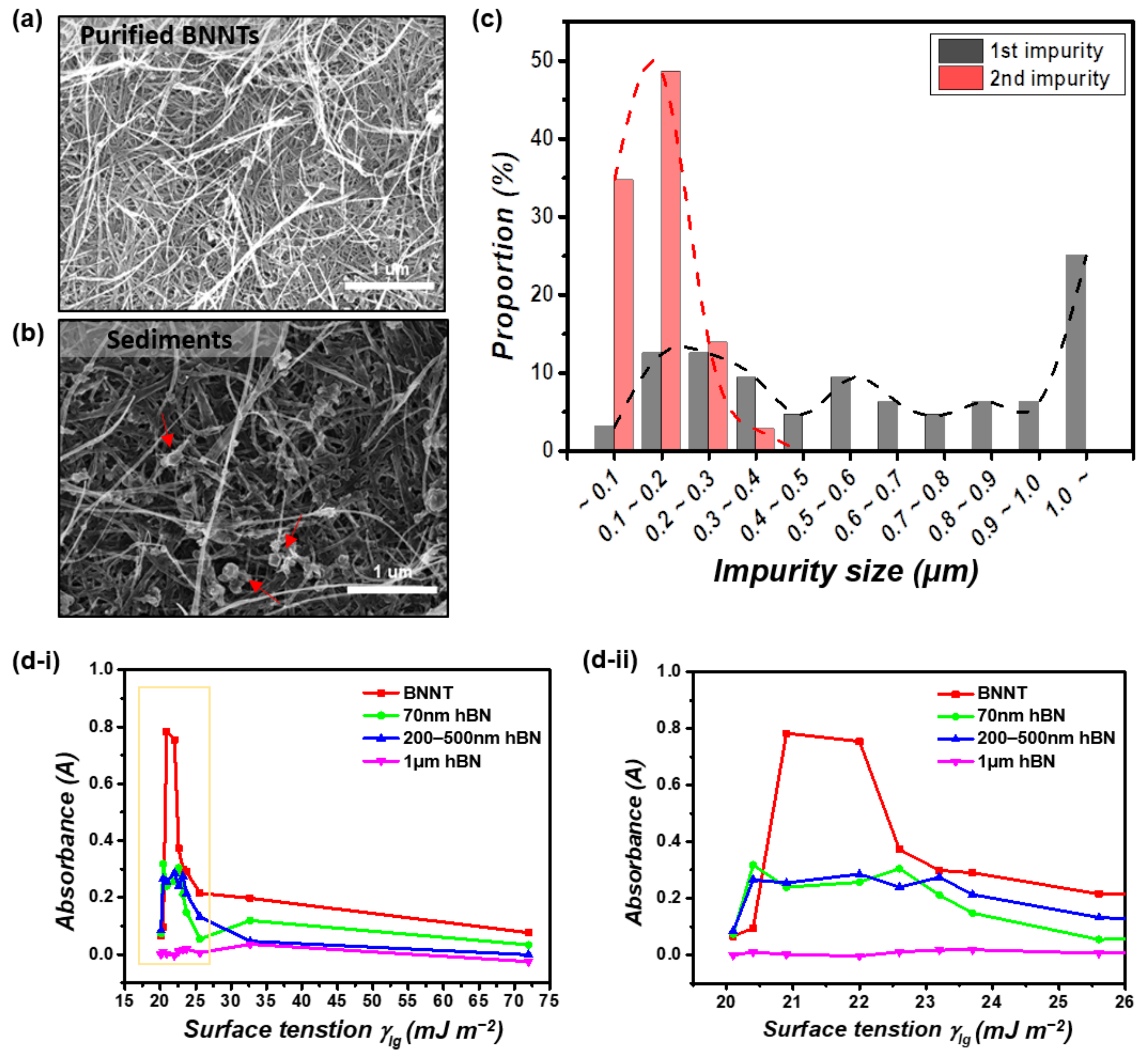
3.1. Optimized Purification Process to Control the Surface Tension and Steric Repulsion of Solvents
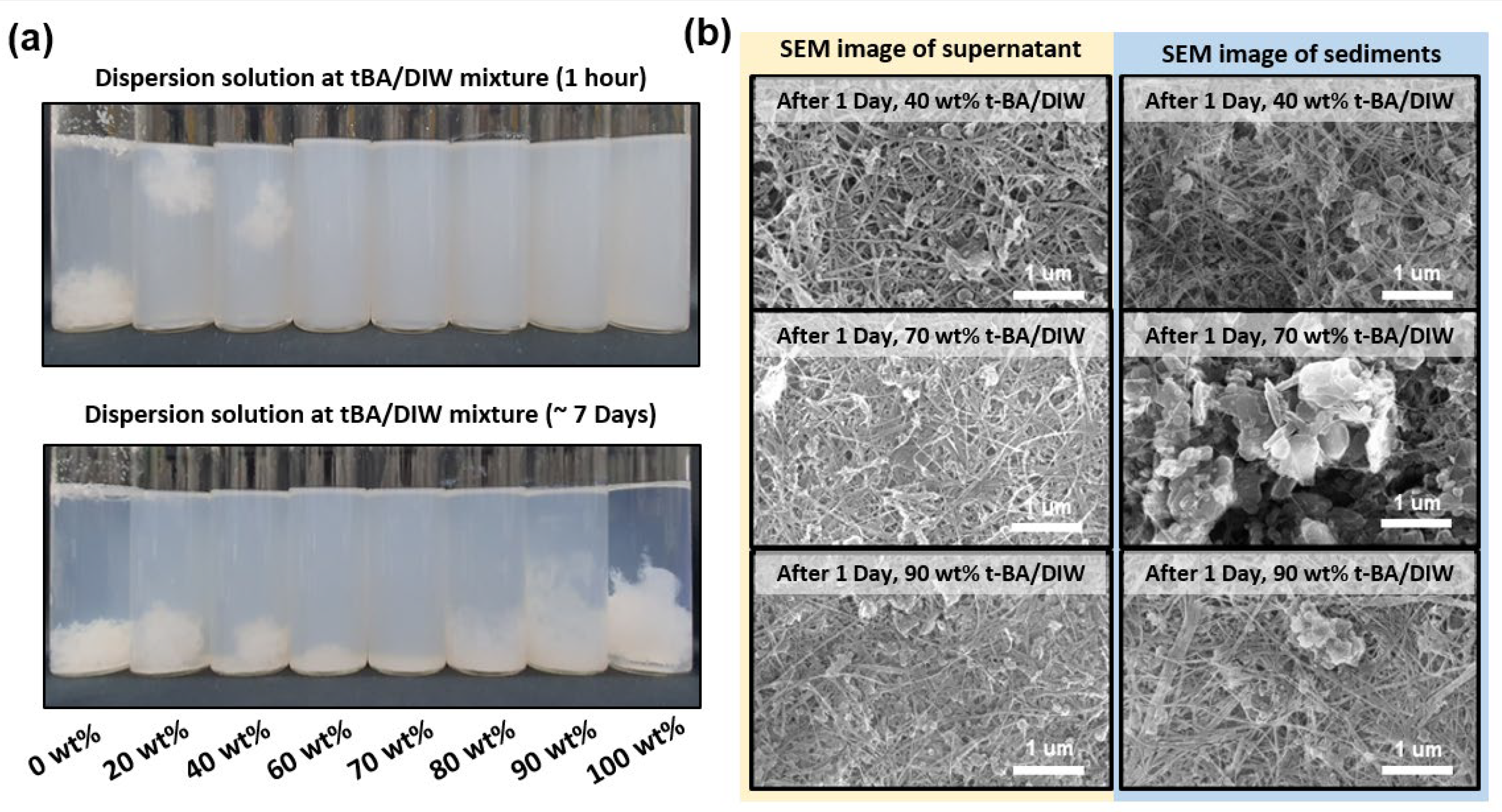
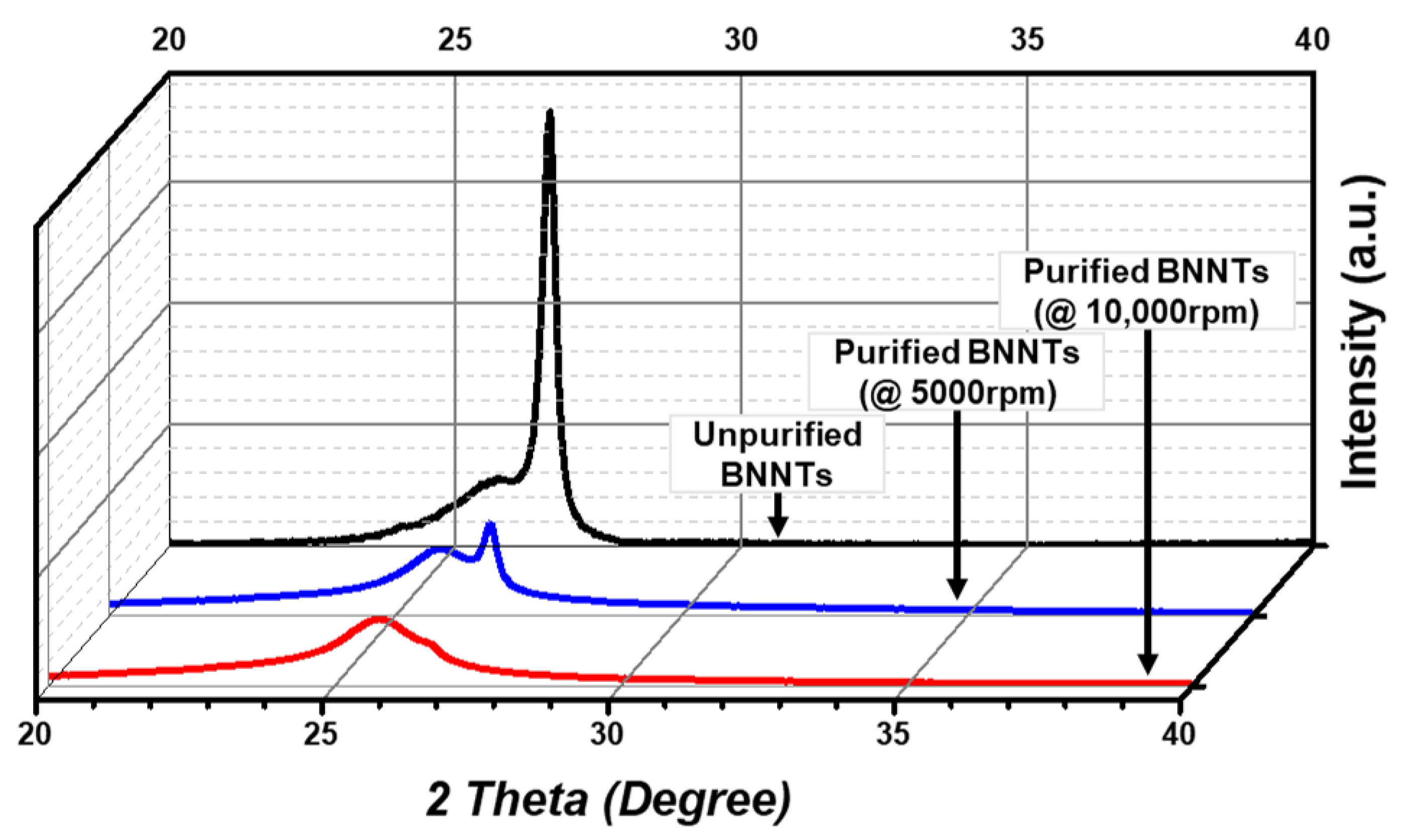
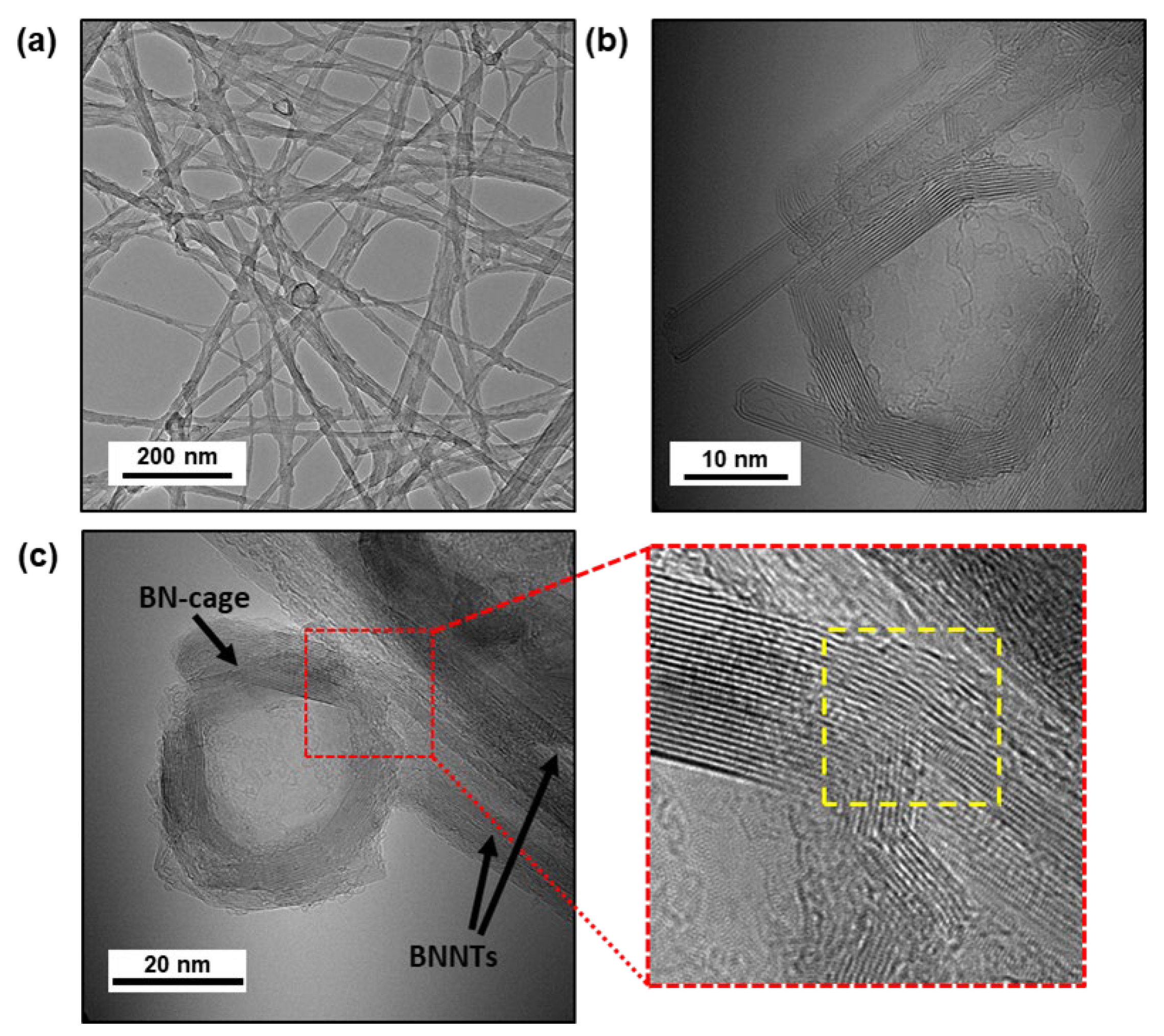
3.2. Characterization and Comparison of Purified BNNTs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chopra, N.G.; Luyken, R.; Cherrey, K.; Crespi, V.H.; Cohen, M.L.; Louie, S.G.; Zettl, A. Boron nitride nanotubes. Science 1995, 269, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Arenal, R.; Wang, M.-S.; Xu, Z.; Loiseau, A.; Golberg, D. Young modulus, mechanical and electrical properties of isolated individual and bundled single-walled boron nitride nanotubes. Nanotechnology 2011, 22, 265704. [Google Scholar] [CrossRef] [PubMed]
- Golberg, D.; Bando, Y.; Kurashima, K.; Sato, T. Synthesis and characterization of ropes made of BN multiwalled nanotubes. Scr. Mater. 2001, 44, 1561–1565. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Lorents, D.C. Mechanical and thermal properties of carbon nanotubes. Carbon 1995, 33, 925–930. [Google Scholar] [CrossRef]
- Ferreira, T.H.; Miranda, M.C.; Rocha, Z.; Leal, A.S.; Gomes, D.A.; Sousa, E.M. An assessment of the potential use of BNNTs for boron neutron capture therapy. Nanomaterials 2017, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Jakubinek, M.B.; Niven, J.F.; Johnson, M.B.; Ashrafi, B.; Kim, K.S.; Simard, B.; White, M.A. Thermal conductivity of bulk boron nitride nanotube sheets and their epoxy-impregnated composites. Phys. Status Solidi A 2016, 213, 2237–2242. [Google Scholar] [CrossRef]
- Kang, J.H.; Sauti, G.; Park, C.; Yamakov, V.I.; Wise, K.E.; Lowther, S.E.; Fay, C.C.; Thibeault, S.A.; Bryant, R.G. Multifunctional electroactive nanocomposites based on piezoelectric boron nitride nanotubes. ACS Nano 2015, 9, 11942–11950. [Google Scholar] [CrossRef]
- Kim, K.S.; Jakubinek, M.B.; Martinez-Rubi, Y.; Ashrafi, B.; Guan, J.; O’Neill, K.; Plunkett, M.; Hrdina, A.; Lin, S.; Dénommée, S. Polymer nanocomposites from free-standing, macroscopic boron nitride nanotube assemblies. RSC Adv. 2015, 5, 41186–41192. [Google Scholar] [CrossRef]
- Fathalizadeh, A.; Pham, T.; Mickelson, W.; Zettl, A. Scaled synthesis of boron nitride nanotubes, nanoribbons, and nanococoons using direct feedstock injection into an extended-pressure, inductively-coupled thermal plasma. Nano Lett. 2014, 14, 4881–4886. [Google Scholar] [CrossRef]
- Kim, K.S.; Kingston, C.T.; Hrdina, A.; Jakubinek, M.B.; Guan, J.; Plunkett, M.; Simard, B. Hydrogen-catalyzed, pilot-scale production of small-diameter boron nitride nanotubes and their macroscopic assemblies. ACS Nano 2014, 8, 6211–6220. [Google Scholar] [CrossRef]
- Cho, H.; Walker, S.; Plunkett, M.; Ruth, D.; Iannitto, R.; Rubi, Y.M.; Kim, K.S.; Homenick, C.M.; Brinkmann, A.; Couillard, M. Scalable gas-phase purification of boron nitride nanotubes by selective chlorine etching. Chem. Mater. 2020, 32, 3911–3921. [Google Scholar] [CrossRef]
- Kleinerman, O.; Adnan, M.; Marincel, D.M.; Ma, A.W.; Bengio, E.A.; Park, C.; Chu, S.-H.; Pasquali, M.; Talmon, Y. Dissolution and characterization of boron nitride nanotubes in superacid. Langmuir 2017, 33, 14340–14346. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Marincel, D.M.; Kleinerman, O.; Chu, S.-H.; Park, C.; Hocker, S.J.; Fay, C.; Arepalli, S.; Talmon, Y.; Pasquali, M. Extraction of boron nitride nanotubes and fabrication of macroscopic articles using chlorosulfonic acid. Nano Lett. 2018, 18, 1615–1619. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Kim, J.; Seo, D.; Seo, Y.-S. Purification of boron nitride nanotubes via polymer wrapping. Mater. Res. Bull. 2013, 48, 1197–1203. [Google Scholar] [CrossRef]
- Kode, V.R.; Thompson, M.E.; McDonald, C.; Weicherding, J.; Dobrila, T.D.; Fodor, P.S.; Wirth, C.L.; Ao, G. Purification and assembly of DNA-stabilized boron nitride nanotubes into aligned films. ACS Appl. Nano Mater. 2019, 2, 2099–2105. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tang, C.; Honda, S.; Sato, K.; Kuwahara, H.; Golberg, D. Purification of boron nitride nanotubes through polymer wrapping. J. Phys. Chem. B 2006, 110, 1525–1528. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, A.D.S.; de Los Reyes, C.A.; Liberman, L.; Ergülen, S.; Talmon, Y.; Pasquali, M.; Martí, A.A. Surfactant-assisted individualization and dispersion of boron nitride nanotubes. Nanoscale Adv. 2019, 1, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-W.; Kang, S.-H.; Choi, J.C.; Kim, T.-H. Dispersion of boron nitride nanotubes by pluronic triblock copolymer in aqueous solution. Polymers 2019, 11, 582. [Google Scholar] [CrossRef]
- Amin, M.S.; Atwater, B.; Pike, R.D.; Williamson, K.E.; Kranbuehl, D.E.; Schniepp, H.C. High-purity boron nitride nanotubes via high-yield hydrocarbon solvent processing. Chem. Mater. 2019, 31, 8351–8357. [Google Scholar] [CrossRef]
- Coleman, J.N. Liquid-phase exfoliation of nanotubes and graphene. Adv. Funct. Mater. 2009, 19, 3680–3695. [Google Scholar] [CrossRef]
- Cunningham, G.; Lotya, M.; Cucinotta, C.S.; Sanvito, S.; Bergin, S.D.; Menzel, R.; Shaffer, M.S.; Coleman, J.N. Solvent exfoliation of transition metal dichalcogenides: Dispersibility of exfoliated nanosheets varies only weakly between compounds. ACS Nano 2012, 6, 3468–3480. [Google Scholar] [CrossRef] [PubMed]
- Bergin, S.D.; Nicolosi, V.; Streich, P.V.; Giordani, S.; Sun, Z.; Windle, A.H.; Ryan, P.; Niraj, N.P.P.; Wang, Z.T.T.; Carpenter, L. Towards solutions of single-walled carbon nanotubes in common solvents. Adv. Mater. 2008, 20, 1876–1881. [Google Scholar] [CrossRef]
- Tiano, A.; Gibbons, L.; Tsui, M.; Applin, S.; Silva, R.; Park, C.; Fay, C. Thermodynamic approach to boron nitride nanotube solubility and dispersion. Nanoscale 2016, 8, 4348–4359. [Google Scholar] [CrossRef] [PubMed]
- Halim, U.; Zheng, C.R.; Chen, Y.; Lin, Z.; Jiang, S.; Cheng, R.; Huang, Y.; Duan, X. A rational design of cosolvent exfoliation of layered materials by directly probing liquid–solid interaction. Nat. Commun. 2013, 4, 2213. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Simmons, T.; Shah, R.; Wolfe, C.; Lewis, K.M.; Washington, M.; Nayak, S.K.; Talapatra, S.; Kar, S. Stable aqueous dispersions of noncovalently functionalized graphene from graphite and their multifunctional high-performance applications. Nano Lett. 2010, 10, 4295–4301. [Google Scholar] [CrossRef] [PubMed]
- Manna, K.; Huang, H.-N.; Li, W.-T.; Ho, Y.-H.; Chiang, W.-H. Toward understanding the efficient exfoliation of layered materials by water-assisted cosolvent liquid-phase exfoliation. Chem. Mater. 2016, 28, 7586–7593. [Google Scholar] [CrossRef]
- Marsh, K.; Souliman, M.; Kaner, R.B. Co-solvent exfoliation and suspension of hexagonal boron nitride. Chem. Commun. 2015, 51, 187–190. [Google Scholar] [CrossRef]
- Kežić, B.; Perera, A. Aqueous tert-butanol mixtures: A model for molecular-emulsions. J. Chem. Phys. 2012, 137, 014501. [Google Scholar] [CrossRef]
- Kiselev, M.; Ivlev, D. The study of hydrophobicity in water–methanol and water–tert-butanol mixtures. J. Mol. Liq. 2004, 110, 193–199. [Google Scholar] [CrossRef]
- Lim, H.; Kim, Y.-K.; Kim, H.-S.; Lee, T.; Hossain, M.M.; Jeong, H.-O.; Lee, H.S.; Cho, H.; Joo, Y.; Lee, S.S. Lyotropic Boron Nitride Nanotube Liquid Crystals: Preparation, Characterization, and Wet-Spinning for Fabrication of Composite Fiber. ACS Appl. Mater. Interfaces 2023, 15, 24681–24692. [Google Scholar] [CrossRef]
- Ko, J.; Kim, D.; Sim, G.; Moon, S.Y.; Lee, S.S.; Jang, S.G.; Ahn, S.; Im, S.G.; Joo, Y. Scalable, Highly Pure, and Diameter-Sorted Boron Nitride Nanotube by Aqueous Polymer Two-Phase Extraction. Small Methods 2023, 7, 2201341. [Google Scholar] [CrossRef] [PubMed]
- Habib, T.; Sundaravadivelu Devarajan, D.; Khabaz, F.; Parviz, D.; Achee, T.C.; Khare, R.; Green, M.J. Cosolvents as liquid surfactants for boron nitride nanosheet (BNNS) dispersions. Langmuir 2016, 32, 11591–11599. [Google Scholar] [CrossRef] [PubMed]
- Harrison, H.; Lamb, J.T.; Nowlin, K.S.; Guenthner, A.J.; Ghiassi, K.B.; Kelkar, A.D.; Alston, J.R. Quantification of hexagonal boron nitride impurities in boron nitride nanotubes via FTIR spectroscopy. Nanoscale Adv. 2019, 1, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Alrebh, A.; Ruth, D.; Plunkett, M.; Gaburici, L.; Couillard, M.; Lacelle, T.; Kingston, C.; Kim, K. Boron nitride nanotubes synthesis from ammonia borane by an inductively coupled plasma. J. Chem. Eng. 2023, 472, 144891. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kang, M.; Lim, H.; Moon, S.Y.; Kim, M.J.; Jang, S.G.; Lee, H.J.; Cho, H.; Ahn, S. Purification of boron nitride nanotubes by functionalization and removal of poly (4-vinylpyridine). Appl. Surf. Sci. 2021, 555, 149722. [Google Scholar] [CrossRef]
- Santra, B.; Ko, H.-Y.; Yeh, Y.-W.; Martelli, F.; Kaganovich, I.; Raitses, Y.; Car, R. Root-growth of boron nitride nanotubes: Experiments and ab initio simulations. Nanoscale 2018, 10, 22223–22230. [Google Scholar] [CrossRef] [PubMed]
- Kleinerman, O.; Liberman, L.; Behabtu, N.; Pasquali, M.; Cohen, Y.; Talmon, Y. Direct imaging of carbon nanotube liquid-crystalline phase development in true solutions. Langmuir 2017, 33, 4011–4018. [Google Scholar] [CrossRef] [PubMed]
- Ginestra, C.J.S.; Martínez-Jiménez, C.; Ya’akobi, A.M.; Dewey, O.S.; McWilliams, A.D.S.; Headrick, R.J.; Acapulco, J.A.; Scammell, L.R.; Smith, M.W.; Kosynkin, D.V. Liquid crystals of neat boron nitride nanotubes and their assembly into ordered macroscopic materials. Nat. Commun. 2022, 13, 3136. [Google Scholar] [CrossRef]
- Gliński, J.; Chavepeyer, G.; Platten, J.K. Surface properties of diluted aqueous solutions of tert-butyl alcohol. J. Chem. Phys. 1995, 102, 2113–2117. [Google Scholar] [CrossRef]
- Balint, M.G.; Petrescu, M.I. An attempt to identify the presence of polytype stacking faults in hBN powders by means of X-ray diffraction. Diam. Relat. Mater. 2009, 18, 1157–1162. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, O.P.; Gusain, R.; Mungse, H.P.; Kukrety, A.; Kumar, N.; Sugimura, H.; Khatri, O.P. Alkyl-chain-grafted hexagonal boron nitride nanoplatelets as oil-dispersible additives for friction and wear reduction. ACS Appl. Mater. Interfaces 2015, 7, 3708–3716. [Google Scholar] [CrossRef]
- Singh, D.K.; Iyer, P.K.; Giri, P.K. Diameter dependence of interwall separation and strain in multiwalled carbon nanotubes probed by X-ray diffraction and Raman scattering studies. Diam. Relat. Mater. 2010, 19, 1281–1288. [Google Scholar] [CrossRef]
| Methods | Materials Used/Solvent | Synthesized BNNTs | Comments | Ref. |
|---|---|---|---|---|
| Polymer wrapping method | Poly(m-phenylenevinylene-co-2,5-dioctoxy-p-phenylenevinylene) (PmPV)/Chloroform | Boron oxide CVD | Centrifugation; Polymer residue | [16] |
| Water-soluble polymer/DIW Polyoxypropylenedimaine/EtOH | Ball milling–annealing | Centrifugation; Polymer residue | [14] | |
| Poly(4-vinylpyridine)/MeOH | Thermal RF plasma | Centrifugation; Almost removable dispersant | [35] Previous work | |
| Surfactant method | DNA/DIW | Laser ablation | Centrifugation; Assemble aligned film; Remain DNA on BNNT surface | [15] |
| CTAB, SDS/DIW | Laser ablation | Centrifugation; Surfactant residue on BNNT surface; | [17] | |
| Sodium cholate, Poly(ethylene glycol), Dextran/DIW | Thermal RF plasma | Two phase extraction; High yield and scalable; Use surfactant | [31] Previous work | |
| Gas etching method | Chlorine gas/None | Thermal RF plasma | Highly toxic gas; Scalable process; High purity | [11] |
| Solution method (w/o dispersant) | None/Chlorosulfonic acid | Laser ablation | Centrifugation; Highly strong acid; Very low yield | [12,13] |
| None/Hydrocarbon solvent | Laser ablation | High purity level and yield | [19] | |
| None/tBA-DIW mixture | Thermal plasma | Centrifugation; Low toxicity; Free dispersant | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.; Kim, J.; Lim, H.; Ko, J.; Kim, H.-S.; Joo, Y.; Moon, S.Y.; Jang, S.G.; Lee, E.; Ahn, S. Eco-Friendly Dispersant-Free Purification Method of Boron Nitride Nanotubes through Controlling Surface Tension and Steric Repulsion with Solvents. Nanomaterials 2023, 13, 2593. https://doi.org/10.3390/nano13182593
Kang M, Kim J, Lim H, Ko J, Kim H-S, Joo Y, Moon SY, Jang SG, Lee E, Ahn S. Eco-Friendly Dispersant-Free Purification Method of Boron Nitride Nanotubes through Controlling Surface Tension and Steric Repulsion with Solvents. Nanomaterials. 2023; 13(18):2593. https://doi.org/10.3390/nano13182593
Chicago/Turabian StyleKang, Minsung, Jungmo Kim, Hongjin Lim, Jaehyoung Ko, Hong-Sik Kim, Yongho Joo, Se Youn Moon, Se Gyu Jang, Eunji Lee, and Seokhoon Ahn. 2023. "Eco-Friendly Dispersant-Free Purification Method of Boron Nitride Nanotubes through Controlling Surface Tension and Steric Repulsion with Solvents" Nanomaterials 13, no. 18: 2593. https://doi.org/10.3390/nano13182593
APA StyleKang, M., Kim, J., Lim, H., Ko, J., Kim, H.-S., Joo, Y., Moon, S. Y., Jang, S. G., Lee, E., & Ahn, S. (2023). Eco-Friendly Dispersant-Free Purification Method of Boron Nitride Nanotubes through Controlling Surface Tension and Steric Repulsion with Solvents. Nanomaterials, 13(18), 2593. https://doi.org/10.3390/nano13182593