Abstract
Nanoparticle deposition on various substrates has gained significant attention due to the potential applications of nanoparticles in various fields. This review paper comprehensively analyzes different nanoparticle deposition techniques on ceramic, polymeric, and metallic substrates. The deposition techniques covered include electron gun evaporation, physical vapor deposition, plasma enriched chemical vapor deposition (PECVD), electrochemical deposition, chemical vapor deposition, electrophoretic deposition, laser metal deposition, and atomic layer deposition (ALD), thermophoretic deposition, supercritical deposition, spin coating, and dip coating. Additionally, the sustainability aspects of these deposition techniques are discussed, along with their potential applications in anti-icing, antibacterial power, and filtration systems. Finally, the review explores the importance of deposition purities in achieving optimal nanomaterial performance. This comprehensive review aims to provide valuable insights into state-of-the-art techniques and applications in the field of nanomaterial deposition.
1. Introduction
The rapid development of nanotechnology has revolutionized research in applied sciences, including biomedical, cosmetic, infrastructure, electronics, and food packaging [1]. The production and use of nanomaterials are growing in various industrial processes [2,3,4]. The global market for these materials was valued at 16.3 billion dollars in 2021 [5], and it is projected to reach between 45 billion [6,7] and 62.8 billion dollars in 2031–2032 [5].
Nanomaterials are generally selected based on their desired applications. They can be synthesized through chemical, physical, or biological methods. Chemical methods involve co-precipitation, chemical reduction of metal salts, electrochemical processes (electrolysis), microemulsion, pyrolysis, photochemical reactions (irradiation), sonochemistry, sol–gel, and solvothermal techniques. Physical methods include arc discharge, electron beam lithography, ion implantation, inert gas condensation, mechanical crushing, grinding, and spray pyrolysis. Biological methods usually rely on biosynthesis approaches using plant extracts, bacteria, fungi, algae, enzymes, and biomolecules [8]. The choice of a specific method often dictates the properties of the particles and the resulting characteristics. Nanomaterials can be synthesized using a bottom-up or top-down approach [9].
Nanomaterials are generally classified based on their production into carbon-based materials, metals, dendrimers, and compounds [10]. Carbon-based nanomaterials typically contain carbon and exist in morphologies such as hollow tubes, ellipsoids, or spheres.
On the other hand, inorganic nanoparticles are produced from carbon-free materials and are classified into two major categories: metals and metal oxide nanoparticles. In most cases, nanoparticles are produced from metals such as platinum (Pt), silver (Ag), gold (Au), cadmium (Cd), cobalt (Co), iron (Fe), copper (Cu), and zinc (Zn) [9,11].
Metal-based nanomaterials are valuable and have a wide range of applications in various industries, including electronics, medical, dental, textiles, coatings, food packaging, and wastewater treatment [1,6]. Other emerging potential uses include dispersions and coatings, consolidated materials, biomedicine, and nanodevices [5], and in recent years, the development of nanomaterial applications in consumer products, such as UV filters in sunscreen, odor-resistant textiles, tumor therapies [12], lithium-ion batteries for electric vehicles, and solar panels [13].
Water treatment applications will likely increase the global demand for nanomaterials [6] and the growing demand for electronic products, mainly due to their superparamagnetic properties [14]. With the increasing application of nanomaterials, the industry is also growing, with an estimated direct employment of 300,000 to 400,000 in Europe. These materials are dominant primarily in tire manufacturing as a polymer filler, in toothpaste, or as an anticoagulant in powdered foods [13]. These applications stem from their physicochemical properties and their small size, which result in a high surface area-to-volume ratio, increased reactivity, and ideal optical properties [1], offering a larger surface area compared to the same mass of bulk materials, making them more chemically reactive [15].
Reports by Harish et al. show that nanomaterials exhibit various functionalities such as dimensionality (0–3D), morphology (low and high aspect ratio), porosity (macro-, nano-, mesoporous), composition (carbon, inorganic, organic, and hybrid), origin (natural, incidental, designed, bioinspired), phase (monophasic, multiphasic), and dispersion state (dispersed or aggregated) [16].
Studies by Chaudhary demonstrate that adding nanomaterials modifies the fundamental properties of materials, such as flexibility, durability, flame resistance, barrier properties, and recycling properties [17]. Additionally, material properties can be modified through deposition or doping [18]. This fact can lead to performance improvements, such as the deposition of an anti-reflective coating on the front surface of solar cells, reducing reflection losses by 8% [19], increasing the conductivity of semiconductors [20], improving the barrier properties of bio-based packaging materials [17], enhancing the photocatalytic activity of TiO2 [1], and strengthening materials [21]. Moreover, as nanoparticles are added to a common material, they refine the grain to some extent, forming an intragranular or intergranular structure, thus improving the grain boundary and promoting the mechanical properties of the materials. For example, adding 3% by weight of nano-SiO2 to concrete can improve its compressive strength, flexural strength, and tensile strength by splitting. Adding 3% nano-empty fruit bunch fibers to kenaf epoxy composites can significantly enhance their tensile strength, elongation at break, and impact resistance [22].
Deposition of sacrificial and structural material on the substrate is the first step in surface micromachining and can be achieved through chemical processes such as chemical vapor deposition (CVD), which is commonly used for nanomaterial manufacturing, electrodeposition, vapor phase epitaxy (VPE), and thermal oxidation, or through physical processes such as physical vapor deposition (PVD) and casting [23].
Vapor deposition methods enable a higher-purity surface coating without organometallic compounds. Currently, CVD is the most promising technology for the functionalization of nanoparticles on an industrially relevant scale. This method has multiple variations, including thermally activated, plasma-enhanced, photo-initiated, and oxidative CVD, to name just a few [24].
This review offers an in-depth analysis of the advancements in nanoparticle deposition techniques for diverse substrates, encompassing ceramic, polymeric, and metallic matrices. Additionally, it emphasizes the importance of sustainability in nanomaterial deposition and highlights potential applications in anti-icing, antibacterial power, and filtration systems. Moreover, it underscores the significance of deposition purities in attaining the optimal performance of nanomaterials. By providing a comprehensive overview of these topics, this review aims to contribute to advancing nanoparticle deposition techniques and inspire further research and innovation in this field.
2. Deposition of Nanoparticles on Ceramic Substrates
The deposition of nanoparticles on different substrates has garnered attention due to the changes it generates in material properties. The functionalization of ceramic substrates has enabled new applications in optical and medical fields, among others. These substrates exhibit different mechanical, optical, electrical, catalytic, and magnetic properties depending on the type of nanoparticle used for functionalization. As a result, new nanoparticle deposition techniques on these substrates have been developed, among which [25,26,27,28,29] stand out.
2.1. Deposition by Electron Gun Evaporation
Electron beam deposition is used to grow thin films of metals with very high melting points or highly pure metals. The precursor is evaporated with gas and then deposited onto the substrate as a film. This method has gained popularity due to its rapid deposition and ability to generate different metal or non-metal film types [30].
In the study conducted by Somayeh Jalilpour et al. [25], tantalum oxide nanolayers were prepared on a glass substrate (1 × 20 × 20) mm3 using an ETS160 system at a pressure of 3 × 10−7 Torr. The layers were obtained under a high vacuum using the electron gun evaporation method (Edwards E19A3). The purity of tantalum oxide was 99.9%, and the deposition rate was 0.2 Å/s. The substrate temperature was kept constant at 300 K. Before the deposition process, the substrates were cleaned with deionized water, acetone, and ethanol for 15 min each using an ultrasonic method. The thickness of the layers (30, 60, 90, and 120 nm) was measured using a quartz crystal technique (Sigma Instrument, SQM–160, Fort Collins, CO, USA) from the deposition angle of the layers, which was vertically oriented.
The study on the coatings’ crystalline nature and morphological evolution was characterized using X-ray diffraction (XRD) and field emission scanning electron microscopy (FESEM), respectively. Surface morphology and roughness were obtained through analysis using an atomic force microscope (AFM) [25].
2.2. Physical Vapor Deposition (PVD)
Physical vapor deposition is a vaporization coating technique that involves the transfer of material at the atomic level under vacuum conditions. This technique can be applied to metals, ceramics, and semiconductors. The process is similar to chemical vapor deposition (CVD), except that physical vapor deposition uses a solid precursor instead of a gas-phase precursor in CVD. The process consists of four stages: the evaporation of the material to be deposited or the precursor using a high-energy source such as an electron beam, the transport of vapor to the substrate, the reaction between the substrate atoms and the reactive gas, and finally, the deposition of the coating onto the substrate surface [31] (See Figure 1).
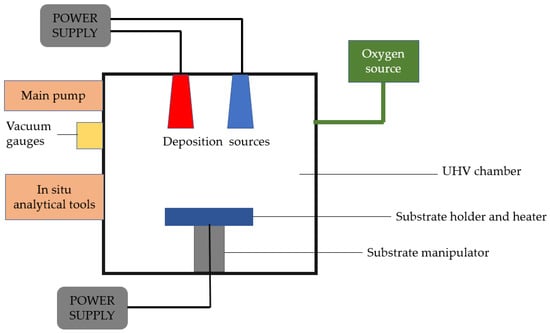
Figure 1.
Schematic of a fundamental physical vapor deposition system, reinterpreted from [32].
In the study conducted by Sanath Kumar Honnali et al. [33], thermoelements were fabricated using glass as the substrate. The substrate underwent a wet chemical cleaning process followed by steam degreasing. Subsequently, the chamber was loaded with O2 to perform a 15-min plasma cleaning, varying the pressure from 8 × 10−2 mbar to 1 × 10−1 mbar. This plasma cleaning increases the Si–O functional groups, thus enhancing the adhesion of thin films on the substrate. Then, ZnO was deposited in the cathodic sputtering with pure zinc as the target at a substrate-reactive distance of 5 cm. The chamber was evacuated to a base pressure of 2 × 10−5 mbar to minimize residual background impurities. Several deposition cycles were performed at different oxygen partial pressures. The pressure ranged from 9 × 10−4 mbar to 3 × 10−3 mbar, maintaining a total working pressure (O2 + Ar) of 8 × 10−3 mbar for each deposition cycle. A power of 16 W with a deposition rate of 10 nm/min and a deposition time of 10 min resulted in a film thickness of 100 ± 10 nm.
2.3. Plasma-Enhanced Chemical Vapor Deposition (PECVD)
Plasma-enhanced chemical vapor deposition is known as a fast and clean technique for surface modification, where plasma is implemented to achieve more localized and controlled deposition on the substrate. This technique is commonly used on silicon templates to coat them at low temperatures with the desired material and can be applied also to ceramics and polymers. Figure 2 shows a schematic of the plasma-enhanced chemical vapor deposition, where, from the equipment perspective, an inert gas flow is used to prevent secondary reactions, along with a vacuum system and a magnetic field. From the material perspective, the precursor is to be deposited, followed by the target substrate [34].
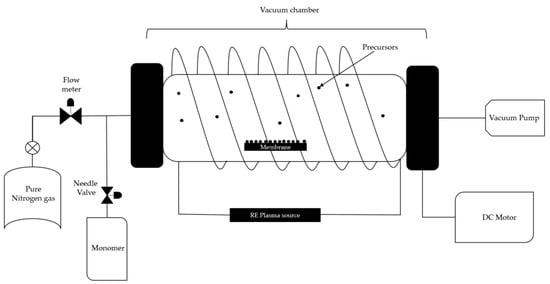
Figure 2.
Chemical vapor deposition with enriched plasma. Reinterpreted from [35].
In the study conducted by Noresah et al. [35], they report the functionalization of an ultrafiltration membrane by depositing two monomers onto the membrane surface under vacuum conditions. A cylindrical quartz vacuum chamber measuring 30 cm in length and 6 cm in diameter was used as the reactor. The UF membrane sample (Dimensions: 6 cm × 15 cm) was placed on flat silicon wafers before being transferred to the chamber. A copper coil antenna connected to a 13.56 MHz radiofrequency plasma generator was positioned through the quartz window of the chamber. Firstly, a vacuum condition was created inside the chamber by turning on the vacuum pump to remove the air from the reactor. The monomer 2-hydroxyethyl methacrylate was vaporized in a stainless steel flask before being delivered to the vacuum chamber by controlling the fine metering valve. A back cooling plate was installed as a substrate support to maintain the membrane temperature. The chamber’s desired operating pressure (<100 mtorr) was maintained using a proportional integral derivative-controlled butterfly valve connected to a capacitance manometer. The optimized flow rate and power of acrylic acid plasma (0.75 sccm; 40 W) and the deposition of 2-hydroxyethyl methacrylate (0.4 sccm; 50 W) were used throughout the modification process.
There are additional deposition methods besides those mentioned previously. Table 1 summarizes ceramic substrates’ most relevant nanoparticle deposition methods, including the Tollen reaction, electrochemical deposition, electron beam gun evaporation, spray deposition, and electrolytic deposition. The structural properties of the functionalized substrate are reported, along with the different techniques, their respective variables, and their applications in the industry.

Table 1.
Deposition of nanomaterials on ceramic substrates.
Some methods stand out for their low cost, such as electrochemical deposition or spray deposition. Electron beam gun evaporation allows the production of nanolayers, which are widely used for their nonlinear optical properties that change depending on the thickness of the obtained film. This method, in turn, modifies the material’s dielectric properties since increasing the film’s thickness increases the material’s bandgap [25]. The electrochemical deposition also imparts materials with nonlinear optical and photoelectronic properties, which depend on the morphology of the obtained nanowires. These properties enable applications in sensors, solar cells, and thin film transistors; this technique is highlighted as a low-cost alternative [27].
3. Deposition of Nanomaterials on Polymeric Substrates
The functionalization of polymeric substrates, similar to ceramic substrates, has allowed for new applications of these materials in various areas, including medicine, photonics, and sensors. These substrates exhibit different thermal, mechanical, optical, and catalytic properties depending on the nanomaterial used for functionalization. This fact has led to the development of new nanomaterial deposition techniques on this type of substrate [36,37,38,39,40,41,42,43,44,45,46]. The most prominent techniques are presented below.
3.1. Simultaneous and Consecutive Electrochemical Deposition
Nanoparticle electrochemical deposition can be performed through two different pathways, as shown in Figure 3; they are known as simultaneous electrochemical deposition and consecutive electrochemical deposition [46]. This technique is applicable also to metal and to ceramic matrices.
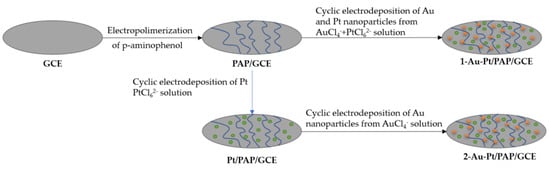
Figure 3.
Procedure to design the Au Pt/PAP/GCE. Reinterpreted from [46].
3.1.1. Simultaneous
In this method, nanoparticles of two different types are simultaneously deposited, as the name implies. As reported by Zekerya Dursun et al. [46], electrodes are doped with gold and silver nanoparticles by immersing two electrodes in a solution of chloroauric acid, which serves as the precursor for gold nanoparticles, and potassium hexachloroplatinate, which serves as the precursor for silver nanoparticles. A potential of 0.8 V to −1.1 V is then applied for ten cycles to produce the doping.
3.1.2. Consecutive
In this technique, unlike simultaneous deposition, the process is performed in stages, doping first with one material and then with the other. Taking the case of the study by Zekerya Dursun et al. [46], for this method, immersion in a solution of potassium hexachloroplatinate is performed with a potential of 0.8 V to −1.1 V, followed by immersion in a solution of chloroauric acid with a potential of 0.1 V to −0.9 V, each for ten cycles.
3.2. Chemical Vapor Deposition (CVD)
Chemical vapor deposition follows the same principle as Enriched Chemical Vapor Deposition, the difference being that PECVD introduces precursors with a plasma gas capable of ionizing the precursors. In contrast, CVD introduces chemical precursors into the reaction chamber that thermally decompose to form solid films without plasma [34].
The experimental system reported in the study by Hui Kun et al. [39] for this technique consists of six parts, as shown in Figure 4: a gas device, a high-temperature furnace, a graphite reaction chamber, a carbon layer sample, a vacuum pump, and a tail gas treatment device. The gas system is divided into two parts, one for argon and the other for propylene, each equipped with a flow meter. The graphite reaction chamber comprises a front graphite channel, a front reaction chamber, a rear reaction chamber, and a rear graphite channel. This design ensures that the reaction gas passes through the porous structure of the carbon layer sample completely.
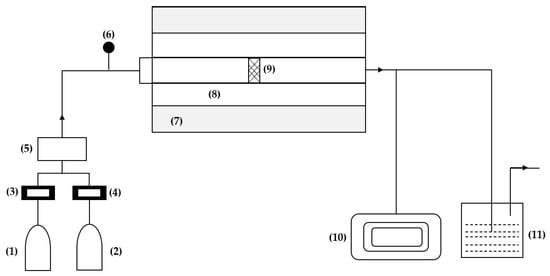
Figure 4.
Experimental deposition system: (1) argon, (2) propylene, (3) flow meter, (4) manometer, (5) mixing cylinder, (6) manometer, (7) furnace, (8) graphite reaction chamber, (9) carbon layer, (10) vacuum pump, (11) off-gas treatment device. Reinterpreted from [37].
In the study by Hui Kun et al. [39], a temperature of 1600 °C is reported inside the furnace, which is maintained stable for 30 min, and the temperature in the graphite reaction chamber is approximately 50 °C lower than that of the furnace. A high-temperature chemical reaction will cause mass loss in the carbon layer, so comparative experiments are performed to reduce the error.
The process is divided into four stages. First, the furnace is evacuated using the vacuum pump, then argon is introduced until the pressure exceeds atmospheric pressure. Once reached, the outlet valve is opened, and the furnace is heated to the reaction temperature with the reaction gas. At this point, the argon valve is closed. At the end of the reaction, the reaction gas valve is closed, and the argon valve is opened again [39].
3.3. Electrophoretic Deposition
Electrophoretic deposition is a widely used technique that promises cost-effective deposition of nanoparticles with relevant applications, such as obtaining antibacterial properties and improving the biocompatibility of a ceramic compound. It is used to uniformly coat an electrode immersed in a stable suspension. The cathode and anode are subjected to an electric field, inducing the movement of precursor particles to the substrate [47]. The electrophoretic deposition can be applied to ceramic, metal, and polymer substrates.
Figure 5 illustrates the setup used by Huanyu Li et al. [40] for electrophoretic deposition, where doping of nano-silica onto carbon fibers is performed. A carbon fiber anode is immersed in distilled water containing nano-silica colloids, and a graphite cylinder is used as the counter electrode. In this technique, voltage and time parameters can be varied, typically ranging from 0 V to 3 V throughout 0 to 15 min, depending on the desired doping, followed by drying of the specimens.
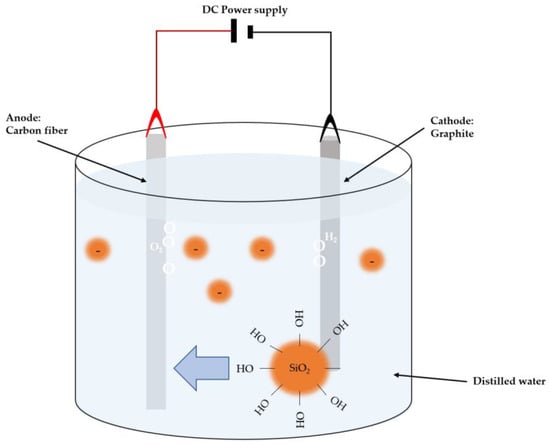
Figure 5.
Schematic illustration of EPD configuration for CF surface modification.
There are more deposition methods in addition to those previously mentioned. Table 2 reports different techniques with their respective variables and applications in the industry.

Table 2.
Deposition of nanomaterials on polymeric substrates.
The application techniques employed showcase the strategic deployment of nanomaterial deposition to achieve tailored functional enhancements. Notably, Chemical Vapor Deposition (CVD) demonstrates the potential to create nanoporous structures in polymer composites, substantially elevating mechanical properties and resistance to erosion [39]. On the other hand, Electrophoretic Deposition (EPD) stands out for its adeptness in enhancing bond properties between nano-silica and carbon fiber surfaces [40]. It stands out for its low cost, simplicity of operation, and the possibility of applying different coating materials. It is also used to improve the performance of interfacial bonds between a substrate and a precursor, thus improving the mechanical properties.
In situ deposition techniques, including creating antimicrobial properties through nano-Cu2O on chitosan nanofibrous scaffolds, exemplify the versatility of these methodologies. Additionally, the ingenious application of the sol–gel method to deposit NiO nanoparticles on cotton fibers imparts notable photocatalytic activation properties, opening avenues for environmental conservation [41,42]. Pulsed Laser Deposition (PLD) emerges as a promising contributor to wound healing, with the deposition of silver nanoparticles onto specific scaffolds showcasing enhanced antibacterial activity [43].
The strategic selection of nanomaterials and matrices further underlines the potency of these techniques, with examples such as nano-silica on carbon fiber offering improved mechanical properties, nano-Cu2O on chitosan nanofibrous scaffolds displaying potential for wound healing, and NiO nanoparticles on cotton contributing to environmental conservation.
A notable method involves depositing photocatalytic micron-thick TiO2 coatings using a microblast technique on titanium metal and FTO-coated glass substrates. This technique demonstrates scalability and cost-effectiveness, showing potential for practical applications. In enhancing adhesion and inter-particle connectivity of TiO2 coatings, both furnace and microwave plasma treatments have been explored. Plasma-treated TiO2 coatings exhibit increased particle packing density, resulting in substantially improved photocurrent density measurements compared to their as-deposited counterparts, thus elevating the application possibilities of this technology.
In a separate study, {[PMo12O40]3−/PAMAM}n multilayer films are synthesized through an LBL electrostatic assembly technique. These films, marked by uniformity and homogeneity, provide a matrix for the in situ electro-deposition of Pt micro–nano clusters. The resulting Pt micro–nano clusters with a unique flower-like structure become immobilized on the surface of the {[PMo12O40]3−/PAMAM}n multilayer films. Electro-deposition conditions, such as deposition potential and time, influence the morphology of Pt micro-nano clusters. The resultant Pt-clusters–{PMo12/PAMAM}3 composite films exhibit robust electrocatalytic activities, particularly in methanol oxidation and improved CO tolerance [45].
Furthermore, the direct electrochemical oxidation of sodium borohydride is investigated through Au–Pt-nanoparticles-decorated poly(p-aminophenol) (PAP) films. These bimetallic surfaces, prepared using simultaneous and successive electro-deposition procedures, serve as catalysts for borohydride electrochemical oxidation. Simultaneous electro-deposition of Au–Pt onto the polymer structure enhances oxidation potential and current density. Extensive characterization, including AFM, SEM, XPS, XRD, and CV, sheds light on these modified surfaces’ structural and morphological properties. The outcomes underscore the potential of these bimetallic catalysts for advancing electrochemical applications, offering insights into their catalytic activities and electron exchange numbers [46].
4. Deposition of Nanomaterials on Metallic Substrates
The functionalization of metallic substrates has allowed for new applications of these materials in optical, medical, environmental, and other fields. These substrates exhibit different mechanical, optical, electrical, catalytic, and magnetic properties depending on the type of nanoparticle used for functionalization. As a result, new nanoparticle deposition techniques have been developed for this type of substrate [10,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. The most prominent ones are presented below.
4.1. Laser Metal Deposition
This technique is mainly based on a fiber laser with a power of ~6000 W that irradiates the substrate while directing the powder of the desired doping material, including metal, ceramic and polymer substrates. Figure 6 shows the laser metal deposition (LMD) process diagram. This technique provides high precision and a rapid deposition rate [48].
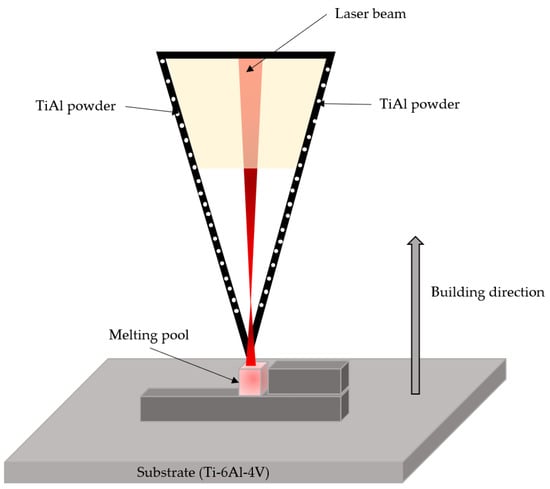
Figure 6.
Schematic illustration of the LMD process. Adapted from [49].
The study by Qi Zhang et al. [49] reports doping a Ti–6Al substrate with a mixture of TiB2/Ti64 powders. The system consisted of a diode laser, a rotating powder feeder, and an industrial robot. The laser power used was 1300 W, with a beam diameter of 3.5 mm and a scanning speed of 10 mm/s. The scanning overlap distance between adjacent paths was 1.92 mm, corresponding to a 40% overlap ratio. The powder feed rate and corresponding layer height were 6.5 g/min and 0.7 mm, respectively. Additionally, the temperature of the Ti64 substrate was 480 °C. All parameters were optimized to prevent cracking of the titanium matrix.
4.2. Electrochemical Deposition
Electrochemical deposition is similar to electrophoretic deposition as it occurs in an electrochemical cell. It is carried out using different configurations with electrodes submerged in an electrolyte mixed with the precursor, which is then subjected to a voltage to induce doping. In the study by C. Augello et al. [50], the substrate was immersed in a solution of monomers acting as precursors. When the voltage was applied, the monomers underwent chemical polymerization on the substrate. This method offers advantages such as improving the interfacial bond between the coating material and the substrate.
4.3. Atomic Layer Deposition (ALD)
ALD is a particular variant of the CVD technique. In ALD, gas-phase reactants are also introduced into a reaction chamber to form the desired material through surface chemical reactions. The difference is that, in ALD, the precursors are pulsed alternately, one at a time, with an intermediate purge of inert gas [51]. This technique is based on sequential and self-limiting surface reactions. Most ALD processes involve binary reaction sequences where two surface reactions occur to form the layer. This method offers advantages such as precise control over film thickness and can be applied to metal and ceramic substrates [52].
In the study by Sheng-Hao et al. [53], the substrate was deposited in a commercial ALD system with the precursor and H2O at a chamber temperature of 200 °C. The precursor container was heated to 70 °C, and the H2O container was kept at room temperature during the ALD processing. A cycle consisted of six steps: precursor 1/dosing time, soak time, evacuation time; precursor 2/dosing time, soak time. During ALD processing, there is a constant flow of carrier gas.
In addition to the methods mentioned above, there are other deposition techniques. Table 3 presents different techniques and their respective variables and applications in the industry. These techniques include electrophoretic deposition, chemical vapor deposition, electrochemical deposition, atomic layer deposition, and laser metal deposition, among others.

Table 3.
Deposition of nanomaterials on metallic substrates.
From Table 3, valuable insights were obtained regarding applying various nanomaterial deposition techniques and their impact on different matrices. Electrostatic layer-by-layer deposition utilizing diamond nanoparticles (SiO2, TiO2) on a Si substrate has shown promise in improving mechanical properties. Plasma-assisted filament evaporation with silver nanoparticles on an indium tin oxide (ITO) matrix demonstrates the ability to enhance optical and electrical properties. Direct laser deposition employing nano-TiB whiskers on Ti–6Al–4 V exhibits improved mechanical properties. Electrochemical deposition of Si nanospheres on Au and graphene-coated Cu showcases the potential for high reproducibility.
Furthermore, chemical vapor deposition of carbon nano-onions on Fe/Fe3C matrices offers corrosion protection, while atomic layer deposition of nano Sn–O2 on titanium demonstrates biocompatibility. Laser metal deposition with nano-SiCp on AlSi10Mg alloy influenced the microstructure, decreasing SiCp size and intensifying the reaction between SiC and the aluminum matrix. Other notable findings include electrophoretic deposition of chitosan-reinforced Baghdadite ceramic nanoparticles on stainless steel 316 L to improve biological and physical characteristics, electrophoretic deposition of polyaniline (PANI) nanofibers on copper for corrosion protection, and in situ deposition of Pt nanoparticles on nanopore stainless steel for high active hydrogen evolution reaction. These findings contribute to a comprehensive understanding of nanomaterial deposition techniques and their potential applications, facilitating the development of advanced materials with tailored properties in nanotechnology.
The CVD stands out as an economical and effective alternative for the deposition of nanomaterials. It allows for the modification of different materials by providing nanostructures on their surface. For example, nitride semiconductors like gallium nitrides and aluminum nitrides can be produced using CVD, forming nanostructures known as quantum confinement points. These nanostructures allow the materials to be utilized in a wide range of optical and microelectronic devices [87].
5. Other Techniques for Nanomaterials Deposition
5.1. Thermophoretic Deposition
Thermophoretic deposition brings forth advantages that enhance its applicability and versatility. One key advantage is the capability for selective deposition, driven by the distinct thermophoretic behaviors exhibited by nanoparticles of differing sizes, compositions, and surface chemistries. This fact allows researchers to intentionally deposit specific nanoparticle types onto substrates, tailoring the composition for targeted functionalities. Moreover, the non-contact nature of thermophoretic deposition proves advantageous, as it sidesteps the need for physical contact between nanoparticles and the substrate. This characteristic becomes particularly valuable when dealing with delicate or sensitive substrates that direct contact may adversely affect.
Thermophoresis facilitates the adhesion of small particles onto a chilled surface. In their study, Alam et al. [94] investigated the effects of thermophoresis and turbulent suction on a 2D (two-dimensional) steady magnetofluid flow over an inclined semi-infinite plate. In a study by Rahman [95], the interplay between thermophoretic particle deposition and magnetic effects was showcased within a nanofluid flowing through a rotating system. The research revealed that the particle deposition rate driven by thermophoresis is substantially shaped by factors encompassing thermal diffusion, slip mechanisms, magnetic fields, diffusion-thermo, and radiation. Additionally, recent investigations [96,97] have further elucidated the ramifications of thermophoretic particle deposition across a spectrum of distinct scenarios.
5.2. Supercritical Deposition
Depositing metals and metal oxides onto surfaces using supercritical fluids (SCFs), particularly supercritical carbon dioxide (scCO2), has gained significant attention due to SCFs’ appealing properties. The advantages of utilizing scCO2 as a deposition medium include adjustable solvent power via pressure and temperature changes, absence of liquid waste, no solvent residue on substrates, rapid mass transfer rates facilitating swift deposition, low surface tension, and seamless miscibility with reactive gases like hydrogen or oxygen.
A broad range of metals and metal oxide nanoparticles or films, such as Cu, Co, Ni, Mo, Pt, Pd, Ru, Ag, Au, Rh, and various oxides like HfO2, ZrO2, yttria-stabilized zirconia, ceria, titanium, tantalum, niobium, and bismuth oxide, have been successfully deposited on diverse support materials including polymers, carbon nanotubes, graphene, carbon blacks, aerogels, alumina, silica, and silicon. Among these, Pt has been extensively studied due to its high catalytic activity and wide use in fuel cells and hydrogenation. In particular, carbon-supported Pt electrocatalysts prepared through supercritical deposition exhibit exceptional efficiency in oxygen reduction and hydrogen oxidation reactions. Comprehensive reviews of supercritical deposition research have been conducted over the past decade, underscoring its significance [98]. This technique is applied to ceramic, polymer, and metal substrates.
5.3. Spin Coating
The spin-coating method is an effective technique for depositing nanoparticles onto various substrates. Gorji [99] found that precise control of spin-coating parameters, such as spinning speed and duration, is necessary to optimize the density and distribution of gold nanoparticles on silicon substrates. Colson [100] applied an experimental design to spin coating and found that the degree of ordering in nanoparticle monolayers is positively correlated with ramp time and negatively correlated with the first rotation speed. Weiss [101] demonstrated that spin coating can produce rough surfaces of uniform nanoparticles. At the same time, Bräuer [102] showed that spin coating can deposit thin films of magnetic transition metal complexes with preserved molecular structure.
5.4. Dip Coating
The nanoparticle deposition by dip coating is a viable method for creating coatings. Sinturel [103] found that the presence of PLGA nanoparticles in PVA and PVP water solutions did not modify the film deposition process by dip coating. Prevo [104] reviewed a convective assembly technique for rapid deposition of structured micro- and nanoparticle coatings, which can be applied to various colloidal systems. Chun [105] developed a nanoparticle deposition system (NPDS) for ceramic and metal coating at room temperature and low vacuum conditions. This fact provides a new coating method for ceramic and metal materials with a large surface area. Shukla [106] investigated a cold gas dynamic spray (CGDS) technique for nanopowder deposition, which produced copper and nano-WC/10% Co coatings on steel and aluminum substrates.
6. Overview of the Driving Forces for the Deposition and Transformation of Nanoparticles
The successful implementation of nanoparticle deposition techniques relies on a nuanced understanding of the underlying forces that drive nanoparticle deposition and subsequent transformation. These driving forces can be broadly categorized into several key mechanisms, each influencing the spatial arrangement and properties of the deposited nanoparticles.
- Surface Interaction Forces: Surface interaction forces govern the affinity of nanoparticles for a substrate’s surface. Van der Waals forces, electrostatic interactions, and capillary forces are common surface-driven mechanisms. Van der Waals forces arise from temporary fluctuations in electron distribution, leading to attractive interactions between particles and substrates. Electrostatic interactions, influenced by the charge distribution of particles and substrate, contribute to adsorption or repulsion. Due to surface tension gradients, capillary forces direct nanoparticles to specific locations on the substrate [107].
- Chemical Affinity: The chemical affinity between nanoparticles and substrates arises from the compatibility of their surface chemistry. In cases where nanoparticles and substrates possess complementary functional groups, chemical bonds can form, enhancing adhesion. Such interactions are vital in processes like plasma-enhanced chemical vapor deposition (PECVD) and atomic layer deposition (ALD), where precursor molecules chemically react on the substrate’s surface to yield conformal coatings [108,109].
- Electric Fields: Electric fields are potent tools for nanoparticle manipulation and deposition. Electrostatic deposition methods, such as electrophoretic deposition, exploit the attraction of charged nanoparticles to oppositely charged electrodes. This method allows for controlled positioning and uniform deposition of nanoparticles on substrates [110,111].
- Thermal Effects: Temperature gradients and localized heating can induce thermal motion of nanoparticles, influencing their deposition. Techniques like laser metal deposition and electron gun evaporation utilize thermal energy to vaporize or melt nanoparticles, which then condense onto the substrate surface [112,113].
- Fluid Dynamics: Fluid flow and dynamics play a significant role in spin- and dip-coating techniques. The controlled spreading and receding of liquid suspensions on substrates lead to an even distribution of nanoparticles. Fluid dynamics also underlies thermophoretic and supercritical deposition methods, where carrier gases or fluids carry nanoparticles to specific deposition sites [99,101,102,103].
- Kinetic Factors: Deposition kinetics determine the rate and extent of nanoparticle assembly. Factors like deposition time, precursor concentration, and growth rate influence the morphology and coverage of deposited layers. In techniques such as physical vapor deposition (PVD), carefully controlling these parameters is essential to achieve desired film characteristics [31,32,33].
Table 4 summarizes the driving forces for the deposition and transformation of nanoparticles for each discussed technique.

Table 4.
Summary of driving forces for the deposition and transformation of nanoparticles.
7. Sustainable Approaches for Nanomaterial Synthesis
The irregularities in nanomaterials’ shape, size, and chemical composition raise concerns about their adverse effects on the environment and human health. The fate, transport, and transformation of nanoparticles released into the environment are critical areas of study [114].
There are sustainable approaches for nanomaterial deposition. Varma [115] highlights using naturally occurring biodegradable materials as reducing and capping agents for nanoparticles, which can reduce toxicity. Xu [116] discusses various green methods for producing photo-active nanomaterials, such as hydrothermal methods and ultrasound sonication, which minimize the use and generation of hazardous substances during manufacturing. Singh [117] emphasizes the need for sustainable nanomaterials to overcome the challenges associated with engineered nanomaterials and discusses their applications in catalysis and corrosion control. Luque [118] advocates for more thoughtful and carefully designed methodologies that consider environmentally sound protocols for nanomaterials development, such as low-temperature ambient pressure methods and the avoidance of hazardous chemicals.
Recognizing the potential advantages and unintended risks associated with nanomaterials synthesis is crucial for their responsible development. While nanotechnology has shown promise in numerous applications, the increasing presence of nanomaterials in the environment raises environmental pollution concerns. Preliminary studies indicate that nanomaterials can affect air, water, and soil quality and disrupt the environmental system’s life cycle [114].
When synthesizing nanomaterials, a significant amount of chemical waste is generated, which poses environmental pollution concerns. Conventional methods like coagulation, filtration, and electrocoagulation have been developed to remove impurities, including chemicals and heavy metals, released into the environment to address this issue. However, these methods could be more efficient and require modifications to eliminate smaller particles effectively. Therefore, the sustainability of synthesis methods becomes crucial to minimize waste generation and enhance efficiency in nanomaterial fabrication and waste treatment processes [119].
Specific nanomaterials have been employed in water desalination applications, necessitating low-cost methods to combat the global challenge of water salinity impacting communities worldwide. Scalable solutions that can meet the global demand are imperative, leading to the utilization of non-permeable membrane solutions incorporating nanomaterials obtained through sol–gel and chemical vapor deposition (CVD) techniques. These methods have been recognized as the most scalable and cost-effective approaches for such applications [120].
Moreover, a cost-effective plasma cannon method has been employed to deposit a novel compound onto a commercial cotton surface. This method is considered low-cost as it aims to reduce energy consumption and the typically high temperatures associated with such processes. The objective is to develop an innovative, single-step method capable of depositing nanoparticles onto flexible surfaces to create electrically conductive coatings [121].
The CVD method holds promise for reducing manufacturing costs due to its applicability to large surfaces and the potential for adjusting working parameters to minimize energy consumption and improve deposition efficiency [122].
Coating-based methods, such as dip coating and spin coating, are frequently employed to modify electrodes with nanomaterials, serving as cost-effective approaches for biosensor fabrication, as they do not require complex equipment. Direct deposition methods encompass electrochemical, electrospinning, electrospray, sputtering, and chemical vapor deposition. These methods offer better control over output parameters and do not require a vacuum pump. Furthermore, the printing-based method represents a novel technique for depositing nanomaterials onto rigid and flexible substrates, enabling large-scale production of devices at a low cost (e.g., screen printing, inkjet printing) [123].
It is essential to highlight that the abovementioned methods effectively address various challenges associated with conventional approaches, including high energy requirements, complex equipment, and low efficiency. While methods like atomic layer deposition (ALD) offer precise control over deposition, the methods discussed above are more efficient and cost-effective for large-scale production [95]. Sustainability plays a vital role in these applications, as the products must be affordable without incurring high production costs.
8. Potential Applications in the Deposition of Nanomaterials
As shown in this study, it is evident that nanomaterial deposition on different types of substrates is often carried out to enhance a specific property (mechanical, electrical, corrosion resistance, photocatalytic, and biocompatibility, among others) of the material and adapt it to a specific application. Therefore, some applications that utilize deposition methods to improve properties directly influencing composite material behavior will be mentioned below.
8.1. Sensing
The development of modified electrodes has been crucial in creating a new generation of analysis and sensing devices with greater sensitivity and selectivity. Several studies mention using nanomaterials for sensing applications where a material with significant changes in response to minimal variations in a medium or stimulus is desired [124,125]. For instance, indium oxide is commonly employed as an n-type semiconductor for detecting pollutant gases. However, previous studies have shown lower selectivity and sensitivity for gases than other elements. A zinc-doped indium oxide nanowires nanocomposite was obtained through CVD to enhance sensitivity and selectivity. The doping with zinc increased electrical resistivity, which is essential for sensing applications [126].
8.2. Anti-Icing
Ice accumulation on brass surfaces can lead to heat transfer inefficiency, equipment degradation, and potential accidents [127]. Superhydrophobic surface technologies are employed to mitigate these effects to create anti-icing surfaces. Laser ablation is utilized to deposit silica nanoparticles, resulting in anti-icing properties. The anti-icing effect is influenced by surface structure, droplet size, and surface temperature. An increase in the apparent contact angle delays the freezing process [127]. The deposition of silica nanoparticles and treatment with a nanolaser achieved an apparent contact angle of 164.5°, leading to a delayed freezing process. However, superhydrophobic coatings are susceptible to damage during repeated ice formation and melting cycles, raising concerns regarding the durability and efficiency of anti-icing coatings [128].
8.3. Antibacterial Power
The issue of bacterial resistance to antibiotics poses a significant problem in human health, leading to research on alternative antibacterial strategies that do not rely on antibiotics [129,130,131]. Carbon nanotubes with vertical alignment are proposed as a nanomorphological design to destroy bacteria [129]. The antibacterial power of vertically aligned carbon nanotubes has been demonstrated, with the ability to achieve 100% inactivation of bacteria. These nanomaterials present a potential alternative to antibiotics and can be applied to self-cleaning surfaces for the prevention of microbial colonization [129].
8.4. Filters
Particulate matter is a critical concern when addressing air pollution. In one study, a compound consisting of lightweight and flexible carbon nanotubes is proposed for use as filters in particulate matter removal [132]. CVD with a floating catalyst is implemented to obtain carbon nanotubes for their application as particulate matter filters. The composite filter composed of carbon nanotubes exhibited an efficiency of over 90% for particles with a size of 2.5 µm. It is a lightweight and flexible filter suitable for textile applications or room filters [132].
9. Purity Requirements for Precursors Used in Deposition Techniques
It is crucial for the precursors in a deposition process to be as pure as possible to prevent impurities from affecting the desired properties. Table 5 presents the required purities of precursors employed in different deposition techniques. These impurities can vary depending on the sintering methods of the nanostructures. Commonly used physical reduction methods for nanoparticle synthesis may introduce traces of other metals from the reduction equipment or the same reduction process. Different types of mills, such as planetary and high-energy ball mills, are commonly used for this purpose. Similarly, chemical reduction synthesis may also contain traces depending on the reactants and washing steps performed during the synthesis [133,134].

Table 5.
Required purities in precursors employed in deposition techniques.
Impurities can significantly affect the quality and properties of the resulting nanomaterials. In vertical deposition, impurities lead to uneven growth, causing thickness, density, and composition variations along the vertical axis, compromising structural integrity and functionality [25]. Plasma-enhanced deposition is affected by impurities altering plasma chemistry, leading to unintended elements in the nanomaterial. This phenomenon changes its properties, making it unsuitable for specific applications [28].
In pulsed laser deposition, impurities disrupt laser interactions, affecting ablation and nanomaterial composition. This results in reduced crystallinity, changed surface structure, and weaker mechanical properties [43]. Physical vapor deposition sees impurities contaminating the deposition chamber and film, causing changes in microstructure, electrical conductivity, and thermal properties, thus limiting potential uses [36].
Electrophoretic deposition with impure suspension leads to uneven particle coating, reduced adhesion, and mechanical stability. Electroless deposition’s catalytic reactions are hindered by impurities, causing incomplete or non-uniform coating and affecting surface properties and catalytic ability [139]. Impurities in atomic layer deposition disrupt self-limiting reactions, causing inconsistent film growth and altering nanomaterial properties [109]. Maintaining high precursor purity is essential for desired nanomaterial characteristics and performance in all cases.
10. Conclusions and Perspectives
In conclusion, the deposition of nanoparticles onto diverse ceramic substrates has unlocked transformative material property changes, driving innovations in optical, medical, and other fields. Notably, electron gun evaporation, physical vapor deposition (PVD), and plasma-enhanced chemical vapor deposition (PECVD) have emerged as potent techniques for controlled deposition, each offering unique advantages. The studies around this topic underscore the significance of substrate-catalyzed reactions and tailored deposition processes. These advancements expand the arsenal of nanoparticle deposition methods, with low-cost alternatives like electrochemical deposition and spray deposition gaining traction, enabling applications in sensors, solar cells, and more. This article opens avenues for developing enhanced materials and devices across industries as these techniques evolve.
Advanced nanoparticle deposition techniques have expanded the use of polymeric substrates in various fields like medicine, photonics, and sensors. Electrochemical deposition methods, including simultaneous and consecutive approaches, enhance materials by introducing multi-metal nanoparticles into electrodes. Chemical vapor deposition (CVD) demonstrates the potential to strengthen polymer composites through nanoporous structures. Electrophoretic deposition (EPD) enhances bonding, while in situ deposition methods like adding nano-Cu2O to chitosan nanofibrous scaffolds demonstrate versatile functional improvements. These techniques highlight the synergy between nanomaterials and matrices, with applications spanning from wound healing to photocatalysis. Innovative methods like microblast deposition and electro-deposition of Pt micro–nano clusters onto multilayer films offer cost-effective routes for practical advancements.
Functionalizing metallic substrates using advanced nanoparticle deposition techniques has revolutionized their applications across diverse sectors such as optics, medicine, and the environment. These substrates exhibit mechanical, optical, electrical, catalytic, and magnetic properties, which can be tailored through innovative deposition methods. Laser metal deposition (LMD) enables precise and rapid material incorporation, while electrochemical deposition enhances interfacial bonds. Atomic layer deposition (ALD) allows for controlled film thickness. The findings from various deposition techniques provide comprehensive insights into their potential applications, such as enhanced mechanical properties, corrosion protection, and improved biocompatibility. Notably, Chemical vapor deposition (CVD) is a versatile and cost-effective approach, offering a broad spectrum of nanostructured surface modifications. Collectively, these advancements hold the key to tailoring material properties for diverse nanotechnological applications, with CVD playing a pivotal role in expanding material functionalities.
In nanomaterial deposition, addressing environmental and health concerns is crucial, and sustainable methods are emerging as effective solutions. These approaches involve using biodegradable materials for reduction and capping alongside eco-friendly techniques. Emphasis is placed on the significance of sustainable nanomaterials to overcome challenges associated with engineered counterparts.
Addressing global issues like water desalination and anti-icing involves adopting scalable, cost-effective methods, such as sol–gel and chemical vapor deposition, and innovative low-energy solutions like plasma cannon deposition. While advanced techniques like atomic layer deposition offer precision, methods like dip coating, spin coating, and printing provide efficient, cost-effective alternatives for large-scale production. Sustainability considerations highlight the importance of affordability and reduced waste in nanomaterial fabrication, driving the responsible evolution of nanotechnology. The diverse applications of nanomaterial deposition, including sensing, anti-icing, antibacterial surfaces, and filters, underscore the versatile potential of tailored nanomaterials in enhancing specific properties. Ensuring high precursor purity is critical, as impurities can negatively affect nanomaterial quality and performance, emphasizing the need to maintain purity for desired characteristics.
Author Contributions
Conceptualization, D.E.-D., S.G.-M., L.R.-C., M.R.-C. and C.O.-L.; validation, D.E.-D., S.G.-M., L.R.-C., M.R.-C. and C.O.-L.; formal analysis, D.E.-D., S.G.-M., L.R.-C., M.R.-C. and C.O.-L.; investigation, D.E.-D., S.G.-M., L.R.-C., M.R.-C. and C.O.-L.; resources, M.R.-C. and L.R.-C.; data curation, D.E.-D., S.G.-M., L.R.-C., M.R.-C. and C.O.-L.; writing—original draft preparation, D.E.-D., S.G.-M., L.R.-C., M.R.-C. and C.O.-L.; writing—review and editing, D.E.-D., S.G.-M., L.R.-C., M.R.-C. and C.O.-L.; visualization, D.E.-D. and S.G.-M.; supervision, L.R.-C., M.R.-C. and C.O.-L.; funding acquisition, M.R.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MINCIENCIAS and SENA, grant number 80740-207-2022. The APC was funded by Universidad Pontificia Bolivariana and Systems and Services Group SAS.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leong, C.Y.; Wahab, R.A.; Lee, S.L.; Ponnusamy, V.K.; Chen, Y.-H. Current Perspectives of Metal-Based Nanomaterials as PhotocatalyticPhotocatalytic Antimicrobial Agents and Their Therapeutic Modes of Action: A Review. Environ. Res. 2023, 227, 115578. [Google Scholar] [CrossRef]
- Bratovcic, A. Different Applications of Nanomaterials and Their Impact on the Environment. Int. J. Mater. Sci. Eng. 2019, 5, 1–7. [Google Scholar]
- Laux, P.; Riebeling, C.; Booth, A.M.; Brain, J.D.; Brunner, J.; Cerrillo, C.; Creutzenberg, O.; Estrela-Lopis, I.; Gebel, T.; Johanson, G.; et al. Biokinetics of Nanomaterials: The Role of Biopersistence. NanoImpact 2017, 6, 69–80. [Google Scholar] [CrossRef]
- Gaffet, E. Nanomaterials: A Review of the Definitions, Applications, Health Effects. Available online: https://arxiv.org/abs/1106.2206 (accessed on 9 July 2023).
- Allied Market Research Nanomaterials Market Research 2031. Available online: https://www.alliedmarketresearch.com/nano-materials-market (accessed on 9 July 2023).
- Future Market Insights Nanomaterials Market to Exceed a Valuation of US$ 45 Billion by 2032 Attributing to Its Rising Use in Healthcare and Automotive Sectors. Available online: https://finance.yahoo.com/news/nanomaterials-market-exceed-valuation-us-150000849.html (accessed on 10 July 2023).
- Future Market Insights Nanomaterials Market. Available online: https://www.futuremarketinsights.com/reports/nanomaterials-market (accessed on 10 July 2023).
- Patra, J.K.; Baek, K.-H. Green Nanobiotechnology: Factors Affecting Synthesis and Characterization Techniques. J. Nanomater. 2014, 2014, 417305. [Google Scholar] [CrossRef]
- Govindaraman, L.T.; Arjunan, A.; Baroutaji, A.; Robinson, J.; Ramadan, M.; Olabi, A.-G. Nanomaterials Theory and Applications. In Encyclopedia of Smart Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 302–314. [Google Scholar]
- Saleh, T.A. Nanomaterials for Pharmaceuticals Determination. Bioenergetics 2016, 5, 1000226. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review of the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Li, L.; Wang, T.; Wang, B.; Che, R.; Zhai, Y.; Zhang, J.; Li, W. Metal Selenide Nanomaterials for Biomedical Applications. Colloids Surf. B Biointerfaces 2023, 225, 113220. [Google Scholar] [CrossRef]
- European Commission Internal Market, Industry, Entrepreneurship and SMEs: Nanomaterials. Available online: https://single-market-economy.ec.europa.eu/sectors/chemicals/reach/nanomaterials_en (accessed on 10 July 2023).
- Grand View Research Nanomaterials Market Size, Share & Growth. Available online: https://www.grandviewresearch.com/industry-analysis/nanotechnology-and-nanomaterials-market (accessed on 10 July 2023).
- Bondavalli, P.; Pribat, D.; Legagneux, P.; Martin, M.-B.; Hamidouche, L.; Qassym, L.; Feugnet, G.; Trompeta, A.-F.; Charitidis, C.A. Deposition of Graphene and Related Nanomaterials by Dynamic Spray-Gun Method: A New Route to Implement Nanomaterials in Real Applications. J. Phys. Mater. 2019, 2, 032002. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Gaur, M.; Yadav, A.B.; García-Betancourt, M.-L.; Abdel-Haleem, F.M.; Bechelany, M.; Barhoum, A. Nanoparticle and Nanostructure Synthesis and Controlled Growth Methods. Nanomaterials 2022, 12, 3226. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Fatima, F.; Kumar, A. Relevance of Nanomaterials in Food Packaging and Its Advanced Future Prospects. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5180–5192. [Google Scholar] [CrossRef]
- Yoo, H.; Heo, K.; Ansari, M.H.R.; Cho, S. Recent Advances in Electrical Doping of 2D Semiconductor Materials: Methods, Analyses, and Applications. Nanomaterials 2021, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Diop, M.M.; Diaw, A.; Mbengue, N.; Ba, O.; Diagne, M.; Niasse, O.A.; Ba, B.; Sarr, J. Optimization and Modeling of Antireflective Layers for Silicon Solar Cells: In Search of Optimal Materials. Mater. Sci. Appl. 2018, 9, 705–722. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, S.W.; Gao, G.Y. Design Ambipolar Conductivity on Wide-Gap Semiconductors: The Case of Al- and Na-Doped CaS. Mater. Sci. Semicond. Process. 2022, 151, 107024. [Google Scholar] [CrossRef]
- Aghababai Beni, A.; Jabbari, H. Nanomaterials for Environmental Applications. Results Eng. 2022, 15, 100467. [Google Scholar] [CrossRef]
- Wu, Q.; Miao, W.; Zhang, Y.; Gao, H.; Hui, D. Mechanical Properties of Nanomaterials: A Review. Nanotechnol. Rev. 2020, 9, 259–273. [Google Scholar] [CrossRef]
- Pokropivny, V.; Lohmus, R.; Hussainova, I.; Pokropivny, A.; Vlassov, S. Introduction in Nanomaterials and Nanotechnology, 1st ed.; University of Tartu, Ed.; University of Tartu: Tartu, Estonia, 2007; Volume 1. [Google Scholar]
- Dorval Dion, C.A.; Tavares, J.R. Photo-Initiated Chemical Vapor Deposition as a Scalable Particle Functionalization Technology: A Practical Review. Powder Technol. 2013, 239, 484–491. [Google Scholar] [CrossRef]
- Darghlou, S.J.; Kangarlou, H.; Razzazi, V. Effect of Vertical Deposition Angle on Structural and Optical Properties of Tantalum Oxide Nano Layers Deposited by Electron Gun Evaporation. Chin. J. Phys. 2021, 74, 226–238. [Google Scholar] [CrossRef]
- Nagi, C.S.; Ogin, S.L.; Mohagheghian, I.; Crean, C.; Foreman, A.D. Spray Deposition of Graphene Nanoplatelets for Modifying Interleaves in Carbon Fibre Reinforced Polymer Laminates. Mater. Des. 2020, 193, 108831. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, W.; Lu, G. Ordered ZnO Nanorod Array Films Prepared by Low Cost Electrochemical Deposition and Its Optical Properties. Mater. Lett. 2015, 138, 262–264. [Google Scholar] [CrossRef]
- Jiang, H.; Manolache, S.; Wong, A.C.L.; Denes, F.S. Plasma-Enhanced Deposition of Silver Nanoparticles onto Polymer and Metal Surfaces for the Generation of Antimicrobial Characteristics. J. Appl. Polym. Sci. 2004, 93, 1411–1422. [Google Scholar] [CrossRef]
- Bruno, L.; Urso, M.; Shacham-Diamand, Y.; Priolo, F.; Mirabella, S. Role of Substrate in Au Nanoparticle Decoration by Electroless Deposition. Nanomaterials 2020, 10, 2180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Z. Electron Beam Evaporation Deposition. In Advanced Nano Deposition Methods; Chemical Industry Press: Beijing, China, 2016; pp. 33–58. [Google Scholar] [CrossRef]
- Makhlouf, A.S.H. Current and Advanced Coating Technologies for Industrial Applications. In Nanocoatings and Ultra-Thin Films; Woodhead Publishing Ltd.: Cambridge, UK, 2011; pp. 3–23. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Wang, B.; Zheng, Y. Ultrathin Ferroelectric Films: Growth, Characterization, Physics and Applications. Materials 2014, 7, 6377–6485. [Google Scholar] [CrossRef] [PubMed]
- Saravanavel, G.; Honnali, S.K.; Lourdes, K.S.; John, S.; Gunasekhar, K.R. Study on the Thermoelectric Properties of Al-ZnO Thin-Film Stack Fabricated by Physical Vapour Deposition Process for Temperature Sensing. Sens. Actuators A Phys. 2021, 332, 113097. [Google Scholar] [CrossRef]
- Albu, C.; Eremia, S.A.V.; Veca, M.L.; Avram, A.; Popa, R.C.; Pachiu, C.; Romanitan, C.; Kusko, M.; Gavrila, R.; Radoi, A. Dataset on Large Area Nanocrystalline Graphite Film (NCG) Grown on SiO2 Using Plasma-Enhanced Chemical Vapour Deposition. Data Brief 2019, 24, 103923. [Google Scholar] [CrossRef] [PubMed]
- Said, N.; Khoo, Y.S.; Lau, W.J.; Gürsoy, M.; Karaman, M.; Ting, T.M.; Abouzari-Lotf, E.; Ismail, A.F. Rapid Surface Modification of Ultrafiltration Membranes for Enhanced Antifouling Properties. Membranes 2020, 10, 401. [Google Scholar] [CrossRef]
- Kangarlou, H.; Aghgonbad, M.M.; Barjisi, Z. Deposition Angle Dependence of Optical and Structural Properties of Titanium Nano-Layers. Optik 2013, 124, 107–112. [Google Scholar] [CrossRef]
- Darvizeh, A.; Luzi, A.; Amin, A.A.; Oliveira-Ogliari, A.; Ogliari, F.A.; Feitosa, V.P.; García-Esparza, M.A.; Pascual, A.; Sauro, S. In-Situ Nano-Silica Deposition and Air-Abrasion with Bioglass 45S5 or Aluminium Oxide: Effects on Methacrylate Bonding to Yttria-Tetragonal Zirconia Polycrystal. Int. J. Adhes. Adhes. 2015, 62, 32–39. [Google Scholar] [CrossRef]
- Moeinzadeh, S.; Jabbari, E. Nanoparticles and Their Applications. In Springer Handbook of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 335–361. [Google Scholar] [CrossRef]
- Kun, H.; Li, J.; Li, K.; Yan, N.; Bian, C.; Guan, Y.; Yang, Y.; Li, H. Effects of Temperature and Pressure on Chemical Vapour Deposition in Micro-Nano Porous Structure in Char Layer of Polymer Composites. Polym. Degrad. Stab. 2022, 196, 109816. [Google Scholar] [CrossRef]
- Li, H.; Liebscher, M.; Curosu, I.; Choudhury, S.; Hempel, S.; Davoodabadi, M.; Dinh, T.T.; Yang, J.; Mechtcherine, V. Electrophoretic Deposition of Nano-Silica onto Carbon Fiber Surfaces for an Improved Bond Strength with Cementitious Matrices. Cem. Concr. Compos. 2020, 114, 103777. [Google Scholar] [CrossRef]
- Zhou, X.; Yin, A.; Sheng, J.; Wang, J.; Chen, H.; Fang, Y.; Zhang, K. In Situ Deposition of Nano Cu2O on Electrospun Chitosan Nanofibrous Scaffolds and Their Antimicrobial Properties. Int. J. Biol. Macromol. 2021, 191, 600–607. [Google Scholar] [CrossRef]
- Pooyandeh, S.; Shahidi, S.; Khajehnezhad, A.; Mongkholrattanasit, R. In Situ Deposition of NiO Nano Particles on Cotton Fabric Using Sol–Gel Method- PhotocatalyticPhotocatalytic Activation Properties. J. Mater. Res. Technol. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Menazea, A.A.; Abdelbadie, S.A.; Ahmed, M.K. Manipulation of AgNPs Coated on Selenium/Carbonated Hydroxyapatite/ε-Polycaprolactone Nanofibrous via Pulsed Laser Deposition for Wound Healing Applications. Appl. Surf. Sci. 2020, 508, 145299. [Google Scholar] [CrossRef]
- McDonnell, K.A.; English, N.J.; Stallard, C.P.; Rahman, M.; Dowling, D.P. Fabrication of Nano-Structured TiO2 Coatings Using a Microblast Deposition Technique. Appl. Surf. Sci. 2013, 275, 316–323. [Google Scholar] [CrossRef]
- Li, Z.S.; Lin, S.; Chen, Z.L.; Shi, Y.D.; Huang, X.M. In Situ Electro-Deposition of Pt Micro-Nano Clusters on the surface of {[PMo12O40]3−/PAMAM}n Multilayer Composite Films and Their Electrocatalytic Activities Regarding Methanol Oxidation. J. Colloid Interface Sci. 2012, 368, 413–419. [Google Scholar] [CrossRef]
- Karabiberoğlu, Ş.; Dursun, Z. Au-Pt Bimetallic Nanoparticles Anchored on Conducting Polymer: An Effective Electrocatalyst for Direct Electrooxidation of Sodium Borohydride in Alkaline Solution. Mater. Sci. Eng. B 2023, 288, 116158. [Google Scholar] [CrossRef]
- Thinakaran, S.; Loordhuswamy, A.M.; Venkateshwapuram Rengaswami, G.D. Electrophoretic Deposition of Chitosan/Nano Silver Embedded Micro Sphere on Centrifugal Spun Fibrous Matrices—A Facile Biofilm Resistant Biocompatible Material. Int. J. Biol. Macromol. 2020, 148, 68–78. [Google Scholar] [CrossRef]
- Zhai, W.; Wu, N.; Zhou, W. Laser Metal Deposition of Low Carbon 410L Stainless Steel and Heat Treatment. Mater. Sci. Eng. A 2023, 872, 144987. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, W.; Xu, S.; Zhang, X.; Wang, J.; Si, C. Nano-TiB Whiskers Reinforced Ti–6Al–4 V Matrix Composite Fabricated by Direct Laser Deposition: Microstructure and Mechanical Properties. J. Alloys Compd. 2022, 922, 166171. [Google Scholar] [CrossRef]
- Augello, C.; Liu, H. Surface Modification of Magnesium by Functional Polymer Coatings for Neural Applications. Surf. Modif. Magnes. Its Alloys Biomed. Appl. 2015, 2, 335–353. [Google Scholar] [CrossRef]
- Leskelä, M.; Niinistö, J.; Ritala, M. Atomic Layer Deposition. Compr. Mater. Process. 2014, 4, 101–123. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-H.; Liao, H.-T.; Chen, R.-S.; Chiu, S.-C.; Tsai, F.-Y.; Lee, M.-S.; Hu, C.-Y.; Tseng, W.-Y. The Influence on Surface Characteristic and Biocompatibility of Nano-SnO2-Modified Titanium Implant Material Using Atomic Layer Deposition Technique. J. Formos. Med. Assoc. 2022, 122, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Tokuda, N.; Makino, Y.; Tsurui, A.; Ieki, R.; Kojima, R.; Liu, M.; Mahiko, T.; Nishikawa, M. Electrostatic Layer-by-Layer Deposition of Diamond Nanoparticles onto Substrate Surfaces. Carbon Trends 2022, 9, 100202. [Google Scholar] [CrossRef]
- Al-Masoodi, A.H.H.; Goh, B.T.; Al-Masoodi, A.H.H.; Majid, W.H.B.A. Deposition of Silver Nanoparticles on Indium Tin Oxide Substrates by Plasma-Assisted Hot-Filament Evaporation. In Thin Films; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Vanpariya, A.; Lellala, K.; Bhagat, D.; Mukhopadhyay, I. Electrochemical Deposition of Si Nanospheres from Water Contaminated Ionic Liquid at Room Temperature: Structural Evolution and Growth Mechanism. J. Electroanal. Chem. 2022, 910, 116175. [Google Scholar] [CrossRef]
- Wu, H.; Song, J.; He, Y.; Wang, S.; Lei, L.; Wen, J.; Gu, A.; Zhang, H.; Boi, F.S. Magnetic Coupling in Cm-Scale Buckypapers of Self-Organized Fe/Fe3C-Filled Carbon Nano-Onions: A Controlled Chemical Vapour Deposition Approach. Diam. Relat. Mater. 2022, 130, 109503. [Google Scholar] [CrossRef]
- Xi, X.; Chen, B.; Tan, C.; Song, X.; Dong, Z. Influence of Micron and Nano SiCp on Microstructure Evolution and Mechanical Properties of Laser Metal Deposition AlSi10Mg Alloy. J. Mater. Process. Technol. 2022, 306, 117609. [Google Scholar] [CrossRef]
- Xue, H.; Liang, Y.; Peng, H.; Wang, Y.; Lin, J. Additive Manufacturing of Micro/Nano Multiphase Synergistically Reinforced Ti-55Al-7.5Nb with a Reticular Boundary Precipitate via Direct Laser Deposition. Addit. Manuf. 2022, 58, 102989. [Google Scholar] [CrossRef]
- Vaez, S.; Emadi, R.; Sadeghzade, S.; Salimijazi, H.; Kharaziha, M. Electrophoretic Deposition of Chitosan Reinforced Baghdadite Ceramic Nanoparticles on the Stainless Steel 316L Substrate to Improve Biological and Physical Characteristics. Mater. Chem. Phys. 2022, 282, 125991. [Google Scholar] [CrossRef]
- Fuseini, M.; Zaghloul, M.M.Y. Investigation of Electrophoretic Deposition of PANI Nano Fibers as a Manufacturing Technology for Corrosion Protection. Prog. Org. Coat. 2022, 171, 107015. [Google Scholar] [CrossRef]
- Tan, Y.; Wei, Y.; Liang, K.; Wang, L.; Zhang, S. Facile In-Situ Deposition of Pt Nanoparticles on Nanopore Stainless Steel Composite Electrodes for High Active Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2021, 46, 26340–26346. [Google Scholar] [CrossRef]
- Fathyunes, L.; Khalili, V. Effect of Ultrasonic Waves on the Electrochemical Deposition of Calcium Phosphate/Nanosized Silica Composite Coating. J. Mater. Res. Technol. 2021, 14, 2345–2356. [Google Scholar] [CrossRef]
- Vetrivezhan, P.; Ayyanar, C.; Arunraj, P.V.; Vasanthkumar, P.; Ganesan, D. Electroless Deposition of Aluminium Alloy LM25 by SiC and Ni-P Nano Coating. Mater. Today Proc. 2021, 45, 6449–6453. [Google Scholar] [CrossRef]
- Parthiban, K.; Lakshmanan, P.; Palani, S.; Arumugam, A. Electroless Deposition of SiC Nano Coating on Aluminium Alloy and Evaluation of Wear Resistance and Electroless Characteristics. Mater. Today Proc. 2021, 46, 1096–1100. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.H.; Kang, Y.S.; Jang, K.T.; Im, J.; Seong, M.J. Evolution of Amorphous Carbon Films into Nanocrystalline Graphite with Increasing Growth Temperature in Plasma-Enhanced Chemical Vapor Deposition. Curr. Appl. Phys. 2021, 23, 52–56. [Google Scholar] [CrossRef]
- Sammaiah, P.; Sneha, M.; Khaseem, M.; Sudheer Kumar, N. Effect of Heat Treatment & Machining Process for Deposition of Al2O3 Nano Particles on Steel. Mater. Today Proc. 2018, 5, 6453–6460. [Google Scholar] [CrossRef]
- Kini, A.R.; Maischner, D.; Weisheit, A.; Ponge, D.; Gault, B.; Jägle, E.A.; Raabe, D. In-Situ Synthesis via Laser Metal Deposition of a Lean Cu–3.4Cr–0.6Nb (At%) Conductive Alloy Hardened by Cr Nano-Scale Precipitates and by Laves Phase Micro-Particles. Acta Mater. 2020, 197, 330–340. [Google Scholar] [CrossRef]
- Hosseini, M.R.; Ahangari, M.; Johar, M.H.; Allahkaram, S.R. Optimization of Nano HA-SiC Coating on AISI 316L Medical Grade Stainless Steel via Electrophoretic Deposition. Mater. Lett. 2021, 285, 129097. [Google Scholar] [CrossRef]
- Raja, C.A.; Balakumar, S.; Anandkumar, B.; George, R.P.; Mudali, U.K. Formation of Bioactive Nano Hybrid Thin Films on Anodized Titanium via Electrophoretic Deposition Intended for Biomedical Applications. Mater. Today Commun. 2020, 25, 101666. [Google Scholar] [CrossRef]
- Sammaiah, P.; Vineetha, B.; Suresh, A.; Sushanth, C.; Kumar, N.S. Analysis of MgO Nano Particles and Its Deposition on Steel by Cold Spray Process. Mater. Today Proc. 2018, 5, 19262–19269. [Google Scholar] [CrossRef]
- Wu, H.; He, D.; Wang, Y. Electrode Materials of Cobalt@Nitrogen Doped Carbon Nano Rod/Reduced Graphene Oxide on Nickel Foam by Electrophoretic Deposition and 3D RGO Aerogel for a High-Performance Asymmetrical Supercapacitor. Electrochim. Acta 2020, 343, 136117. [Google Scholar] [CrossRef]
- Jimenez, M.J.M.; Antunes, V.; Cucatti, S.; Riul, A.; Zagonel, L.F.; Figueroa, C.A.; Wisnivesky, D.; Alvarez, F. Physical and Micro-Nano-Structure Properties of Chromium Nitride Coating Deposited by RF Sputtering Using Dynamic Glancing Angle Deposition. Surf. Coat. Technol. 2019, 372, 268–277. [Google Scholar] [CrossRef]
- Li, J.; Hui, L.; Zhang, W.; Lu, J.; Yang, Y.; Feng, H. Scalable Production of Ultra Small TiO2 Nano Crystal/Activated Carbon Composites by Atomic Layer Deposition for Efficient Removal of Organic Pollutants. Adv. Powder Technol. 2021, 32, 728–739. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, D.; Tang, Z.; Hu, D.; Geng, H.; Zheng, H.; Zang, S.; Yu, Z.; Peng, P. Preparation of Radial ZnSe-CdS Nano-Heterojunctions through Atomic Layer Deposition Method and Their Optoelectronic Applications. J. Alloys Compd. 2019, 777, 102–108. [Google Scholar] [CrossRef]
- Deepa, K.; Arthoba Nayaka, Y.; Purushothama, H.T.; Yathisha, R.O. Co-Deposition of Micro- and Nanosized SnO2 Particles in the Zn-Matrix Composite Coatings Produced from a Zn-Sulphate Bath by Electroplating. Chem. Data Collect. 2021, 32, 100657. [Google Scholar] [CrossRef]
- Song, K.Y.; Navamathavan, R.; Park, J.H.; Ra, Y.B.; Ra, Y.H.; Kim, J.S.; Lee, C.R. Selective Area Growth of GaN Nanowires Using Metalorganic Chemical Vapor Deposition on Nano-Patterned Si(111) Formed by the Etching of Nanosized Au Droplets. Thin Solid Film. 2011, 520, 126–130. [Google Scholar] [CrossRef]
- Das, S.; Guha, S.; Das, P.P.; Ghadai, R.K. Analysis of Morphological, Microstructural, Electrochemical and Nano Mechanical Characteristics of TiCN Coatings Prepared under N2 Gas Flow Rate by Chemical Vapour Deposition (CVD) Process at Higher Temperature. Ceram. Int. 2020, 46, 10292–10298. [Google Scholar] [CrossRef]
- Wang, H.; Wang, N.; Hang, T.; Li, M. Morphologies and Wetting Properties of Copper Film with 3D Porous Micro-Nano Hierarchical Structure Prepared by Electrochemical Deposition. Appl. Surf. Sci. 2016, 372, 7–12. [Google Scholar] [CrossRef]
- Chen, L.; Lai, J.S.; Fu, X.N.; Sun, J.; Ying, Z.F.; Wu, J.D.; Lu, H.; Xu, N. Growth of ZnSe Nanoneedles by Pulsed Laser Deposition and Their Application in Polymer/Inorganic Hybrid Solar Cells. Thin Solid Film. 2013, 529, 76–79. [Google Scholar] [CrossRef]
- He, S.; Zhang, S.; Lu, C.; Wu, G.; Yang, Y.; An, F.; Guo, J.; Li, H. Polyimide Nanocoating on Carbon Fibers by Electrophoretic Deposition. Colloids Surf. A Physicochem. Eng. Asp. 2011, 381, 118–122. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Kawaguchi, T.; Hai, Z.; Karbalaei Akbari, M.; Heynderickx, P.M. Interfacial Engineering of Two-Dimensional Nanostructured Materials by Atomic Layer Deposition. Appl. Surf. Sci. 2017, 392, 231–243. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.F. Electrolytic Deposition of Ni–Co–SiC Nanocoating for Erosion-Enhanced Corrosion of Carbon Steel Pipes in Oilsand Slurry. Surf. Coat. Technol. 2011, 205, 3198–3204. [Google Scholar] [CrossRef]
- Wang, W.; Ji, L.; Li, H.; Zhou, H.; Chen, J. Self-Organized Formation of Nano-Multilayer Structure in the Carbon-Copper Thin Film during Reactive Magnetron Sputtering Deposition Process. J. Alloys Compd. 2017, 722, 242–249. [Google Scholar] [CrossRef]
- Suresh, S.; Rangarajan, S.; Bera, S.; Krishnan, R.; Kalavathi, S.; Velmurugan, S. Electrochemical Characterization of Nano Zinc Ferrite Coating on Carbon Steel by Pulsed Laser Deposition. Thin Solid Film. 2016, 612, 250–258. [Google Scholar] [CrossRef]
- Xiong, Z.; Zheng, M.; Li, H.; Ma, L.; Shen, W. Fabrication and Optical Properties of Silicon Nanowire/Cu2O Nano-Heterojunctions by Electroless Deposition Technique. Mater. Lett. 2013, 112, 211–214. [Google Scholar] [CrossRef]
- Bouchkour, Z.; Tristant, P.; Thune, E.; Dublanche-Tixier, C.; Jaoul, C.; Guinebretière, R. Aluminum Nitride Nano-Dots Prepared by Plasma Enhanced Chemical Vapor Deposition on Si(111). Surf. Coat. Technol. 2011, 205, S586–S591. [Google Scholar] [CrossRef]
- Abdel-Gawad, S.A.; Sadik, M.A.; Shoeib, M.A. Preparation and Properties of a Novel Nano Ni-B-Sn by Electroless Deposition on 7075-T6 Aluminum Alloy for Aerospace Application. J. Alloys Compd. 2019, 785, 1284–1292. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Wang, W.; Zhang, J.; Zhang, L.; He, F.; Yang, J. Controllable Preparation of a Nano-Hydroxyapatite Coating on Carbon Fibers by Electrochemical Deposition and Chemical Treatment. Mater. Sci. Eng. C 2016, 63, 96–105. [Google Scholar] [CrossRef]
- Singh, S.; Meena, V.K.; Sharma, M.; Singh, H. Preparation and Coating of Nano-Ceramic on Orthopaedic Implant Material Using Electrostatic Spray Deposition. Mater. Des. 2015, 88, 278–286. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Daroonparvar, M.; Saud, S.N.; Abdul-Kadir, M.R. Bi-Layer Nano-TiO2/FHA Composite Coatings on Mg–Zn–Ce Alloy Prepared by Combined Physical Vapour Deposition and Electrochemical Deposition Methods. Vacuum 2014, 110, 127–135. [Google Scholar] [CrossRef]
- Yazdani, A.; Soltanieh, M.; Aghajani, H.; Rastegari, S. A New Method for Deposition of Nanosized Titanium Nitride on Steels. Vacuum 2011, 86, 131–139. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, D.; Wei, Z.; Chen, J.; Jing, J. Fabrication of Superhydrophobic Nano-Aluminum Films on Stainless Steel Meshes by Electrophoretic Deposition for Oil-Water Separation. Appl. Surf. Sci. 2018, 427, 253–261. [Google Scholar] [CrossRef]
- Alam, M.S.; Rahman, M.M.; Sattar, M.A. Effects of Variable Suction and Thermophoresis on Steady MHD Combined Free-Forced Convective Heat and Mass Transfer Flow over a Semi-Infinite Permeable Inclined Plate in the Presence of Thermal Radiation. Int. J. Therm. Sci. 2008, 47, 758–765. [Google Scholar] [CrossRef]
- Rahman, M. Thermophoretic Deposition of Nanoparticles Due to a Permeable Rotating Disk: Effects of Partial Slip, Magnetic Field, Thermal Radiation, Thermal-Diffusion and Diffusion-Thermo. In Proceedings of the International Conference on Computational Fluid Dynamics ICCFD: 2013, Berlin, Germany, 22 May 2013; pp. 651–663. [Google Scholar]
- Punith Gowda, R.J.; Naveen Kumar, R.; Aldalbahi, A.; Issakhov, A.; Prasannakumara, B.C.; Rahimi-Gorji, M.; Rahaman, M. Thermophoretic Particle Deposition in Time-Dependent Flow of Hybrid Nanofluid over Rotating and Vertically Upward/Downward Moving Disk. Surf. Interfaces 2021, 22, 100864. [Google Scholar] [CrossRef]
- Bashir, M.N.; Rauf, A.; Shehzad, S.A.; Ali, M.; Mushtaq, T. Thermophoresis Phenomenon in Radiative Flow about Vertical Movement of a Rotating Disk in Porous Region. Adv. Mech. Eng. 2022, 14, 168781322211150. [Google Scholar] [CrossRef]
- Bozbağ, S.E.; Erkey, C. Supercritical Deposition: Current Status and Perspectives for the Preparation of Supported Metal Nanostructures. J. Supercrit. Fluids 2015, 96, 298–312. [Google Scholar] [CrossRef]
- Gorji, M.S.; Khairunisak, A.R.; Cheong, K.Y. Deposition of Gold Nanoparticles on Linker-Free Silicon Substrate by Spin-Coating. Adv. Mat. Res. 2014, 1024, 124–127. [Google Scholar] [CrossRef]
- Colson, P.; Cloots, R.; Henrist, C. Experimental Design Applied to Spin Coating of 2D Colloidal Crystal Masks: A Relevant Method? Langmuir 2011, 27, 12800–12806. [Google Scholar] [CrossRef]
- Weiss, R.A.; Zhai, X.; Dobrynin, A.V. Nanoparticle-Textured Surfaces from Spin Coating. Langmuir 2008, 24, 5218–5220. [Google Scholar] [CrossRef]
- Bräuer, B.; Zahn, D.R.T.; Rüffer, T.; Salvan, G. Deposition of Thin Films of a Transition Metal Complex by Spin Coating. Chem. Phys. Lett. 2006, 432, 226–229. [Google Scholar] [CrossRef]
- Sinturel, C.; Vayer, M.; Mahut, F.; Bonnier, F.; Chourpa, I.; Munnier, E. Influence of PLGA Nanoparticles on the Deposition of Model Water-Soluble Biocompatible Polymers by Dip Coating. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 608, 125591. [Google Scholar] [CrossRef]
- Prevo, B.G.; Kuncicky, D.M.; Velev, O.D. Engineered Deposition of Coatings from Nano- and Micro-Particles: A Brief Review of Convective Assembly at High Volume Fraction. Colloids Surf. A Physicochem. Eng. Asp. 2007, 311, 2–10. [Google Scholar] [CrossRef]
- Chun, D.-M.; Kim, M.-H.; Lee, J.-C.; Ahn, S.-H. Nano Particle Deposition System (NPDS) for Ceramic and Metal Coating at Room Temperature and Low Vacuum Condition. In Proceedings of the 2008 International Conference on Smart Manufacturing Application, Goyangi, Republic of Korea, 9–11 April 2008; pp. 383–386. [Google Scholar]
- Shukla, V.; Elliott, G.S.; Kear, B.H. Nanopowder Deposition by Supersonic Rectangular Jet Impingement. J. Therm. Spray Technol. 2000, 9, 394–398. [Google Scholar] [CrossRef]
- Homemade, E.; Zigelman, A.; Abezgauz, L.; Manor, O. Signatures of van Der Waals and Electrostatic Forces in the Deposition of Nanoparticle Assemblies. J. Phys. Chem. Lett. 2018, 9, 5226–5232. [Google Scholar] [CrossRef] [PubMed]
- Rianasari, I.; de Jong, M.; Huskens, J.; van der Wiel, W. Covalent Coupling of Nanoparticles with Low-Density Functional Ligands to Surfaces via Click Chemistry. Int. J. Mol. Sci. 2013, 14, 3705–3717. [Google Scholar] [CrossRef] [PubMed]
- Petit, R.R.; Li, J.; Van de Voorde, B.; Van Vlierberghe, S.; Smet, P.F.; Detavernier, C. Atomic Layer Deposition on Polymer Thin Films: On the Role of Precursor Infiltration and Reactivity. ACS Appl. Mater. Interfaces 2021, 13, 46151–46163. [Google Scholar] [CrossRef]
- Qian, F.; Pascall, A.J.; Bora, M.; Han, T.Y.-J.; Guo, S.; Ly, S.S.; Worsley, M.A.; Kuntz, J.D.; Olson, T.Y. On-Demand and Location Selective Particle Assembly via Electrophoretic Deposition for Fabricating Structures with Particle-to-Particle Precision. Langmuir 2015, 31, 3563–3568. [Google Scholar] [CrossRef] [PubMed]
- Amrollahi, P.; Krasinski, J.S.; Vaidyanathan, R.; Tayebi, L.; Vashaee, D. Electrophoretic Deposition (EPD): Fundamentals and Applications from Nano- to Micro-Scale Structures. In Handbook of Nanoelectrochemistry; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–27. [Google Scholar]
- Li, T.; Kumar, R.; Kar, A. Enhanced Heating by Microdroplet Lens in Nanoparticle Electrospray Laser Deposition. J. Laser Appl. 2021, 33, 012012. [Google Scholar] [CrossRef]
- Pereira, J.; Moita, A.; Moreira, A. The Pool-Boiling-Induced Deposition of Nanoparticles as the Transient Game Changer—A Review. Nanomaterials 2022, 12, 4270. [Google Scholar] [CrossRef]
- Kabir, E.; Kumar, V.; Kim, K.H.; Yip, A.C.K.; Sohn, J.R. Environmental Impacts of Nanomaterials. J. Environ. Manag. 2018, 225, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.S. Greener Approach to Nanomaterials and Their Sustainable Applications. Curr. Opin. Chem. Eng. 2012, 1, 123–128. [Google Scholar] [CrossRef]
- Xu, X.; Lyu, Q.; Bader, J.; Accordino, M. Chapter 3: Green Nanomaterials Preparation: Sustainable Methods and Approaches. In Chemical and Materials Engineering; Royal Society of Chemistry: Cambridge, UK, 2015; pp. 34–63. [Google Scholar]
- Singh, S.B. Emerging Sustainable Nanomaterials and Their Applications in Catalysis and Corrosion Control. Curr. Nanosci. 2021, 17, 540–553. [Google Scholar] [CrossRef]
- Luque, R. Sustainable Nanomaterials: A Greener Future Avenue? J. Mater. Sci. Nanotechnol. 2013, 1, e106. [Google Scholar] [CrossRef]
- (PDF) Environmental Impact of Nanomaterials. Available online: https://www.researchgate.net/publication/262932053_Environmental_Impact_of_Nanomaterials (accessed on 26 March 2023).
- Sirohi, R.; Kumar, Y.; Madhavan, A.; Sagar, N.A.; Sindhu, R.; Bharatiraja, B.; Pandey, H.O.; Tarafdar, A. Engineered Nanomaterials for Water Desalination: Trends and Challenges. Environ. Technol. Innov. 2023, 30, 103108. [Google Scholar] [CrossRef]
- Gholipour, S.; Bahari, A.; Sohbatzadeh, F. Deposition of a Conductive Nanocomposite Layer on Cotton Fibers by Injecting Nanomaterial Colloid into an Atmospheric Pressure Plasma Jet. Mater. Today Commun. 2022, 32, 104114. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, U.; Chaudhary, P.; Yadav, B.C. Investigation on Structural and Optical Properties of Porous SnO2 Nanomaterial Fabricated by Direct Liquid Injection Chemical Vapour Deposition Technique. Solid State Commun. 2022, 348–349, 114723. [Google Scholar] [CrossRef]
- Ahmad, R.; Wolfbeis, O.S.; Hahn, Y.B.; Alshareef, H.N.; Torsi, L.; Salama, K.N. Deposition of Nanomaterials: A Crucial Step in Biosensor Fabrication. Mater. Today Commun. 2018, 17, 289–321. [Google Scholar] [CrossRef]
- Jambhulkar, S.; Xu, W.; Ravichandran, D.; Prakash, J.; Mada Kannan, A.N.; Song, K. Scalable Alignment and Selective Deposition of Nanoparticles for Multifunctional Sensor Applications. Nano Lett. 2020, 20, 3199–3206. [Google Scholar] [CrossRef]
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical Deposition of Nanomaterials for Electrochemical Sensing. Sensors 2019, 19, 1186. [Google Scholar] [CrossRef]
- Controlled, K.; Yu, C.-W.; Fu, H.-W.; Yang, S.-M.; Lin, Y.-S.; Lu, K.-C. Controlled Synthesis and Enhanced Gas Sensing Performance of Zinc-Doped Indium Oxide Nanowires. Nanomaterials 2023, 13, 1170. [Google Scholar] [CrossRef]
- Hussain, S.; Muangnapoh, T.; Traipattanakul, B.; Lekmuenwai, M. Anti-Icing Property of Superhydrophobic Nanostructured Brass via Deposition of Silica Nanoparticles and Nanolaser Treatment. Nanomaterials 2023, 13, 1139. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Wang, M.; Li, Y.; Jiang, Z.; Zhang, W.; Yang, H.; Wang, C.; Ho, S.-H. Facile Designing a Superhydrophobic Anti-Icing Surface Applied for Reliable Long-Term De-Icing. Chin. Chem. Lett. 2023, 34, 108353. [Google Scholar] [CrossRef]
- Schifano, E.; Cavoto, G.; Pandolfi, F.; Pettinari, G.; Apponi, A.; Ruocco, A.; Uccelletti, D.; Rago, I. Plasma-Etched Vertically Aligned CNTs with Enhanced Antibacterial Power. Nanomaterials 2023, 13, 1081. [Google Scholar] [CrossRef] [PubMed]
- Peruana, A.M.; Ciro, C.; Vargas, M.; Mendoza, J.G.; Vargas, C.M.; De María González Ponce, F. La Resistencia a Los Antibióticos: Un Problema Muy Serio. Acta Médica Peru. 2019, 36, 145–151. (In Spanish) [Google Scholar]
- Rosenblatt-Farrell, N. El Paisaje de La Resistencia a Los Antibióticos. Salud Publica Mex. 2009, 51, 435–442. (In Spanish) [Google Scholar] [CrossRef]
- Chitranshi, M.; Chen, D.R.; Kosel, P.; Cahay, M.; Schulz, M. Flexible and Lightweight Carbon Nanotube Composite Filter for Particulate Matter Air Filtration. Nanomaterials 2022, 12, 4094. [Google Scholar] [CrossRef] [PubMed]
- Bommakanti, V.; Banerjee, M.; Shah, D.; Manisha, K.; Sri, K.; Banerjee, S. An Overview of Synthesis, Characterization, Applications and Associated Adverse Effects of Bioactive Nanoparticles. Environ. Res. 2022, 214, 113919. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Park, Y.; Kang, H.; Jeong, W.; Son, H.; Ha, D.H. Electrophoretic Deposition of Aged and Charge Controlled Colloidal Copper Sulfide Nanoparticles. Nanomaterials 2021, 11, 133. [Google Scholar] [CrossRef]
- Hosseingholilou, S.; Dorranian, D.; Ghoranneviss, M. Characterization of Gold Nanoparticle Thin Film Prepared by Electrophoretic Deposition Method. Gold Bull. 2020, 53, 1–10. [Google Scholar] [CrossRef]
- Tang, J.; Ou, Q.; Zhou, H.; Qi, L.; Man, S. Seed-Mediated Electroless Deposition of Gold Nanoparticles for Highly Uniform and Efficient SERS Enhancement. Nanomaterials 2019, 9, 185. [Google Scholar] [CrossRef]
- Cao, K.; Zhu, Q.; Shan, B.; Chen, R. Controlled Synthesis of Pd/Pt Core Shell Nanoparticles Using Area-Selective Atomic Layer Deposition. Sci. Rep. 2015, 5, 8470. [Google Scholar] [CrossRef]
- Ghosh, S. Electroless Copper Deposition: A Critical Review. Thin Solid Film. 2019, 669, 641–658. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).