Abstract
Herein, Fe3O4 core-TiO2/mesoSiO2 and Fe3O4 core-mesoSiO2/TiO2 double shell nanoparticles were prepared by first (R1) and second (R2) routes and applied for the removal of methylene blue. The reported adsorption capacities for R1-0.2, R1-0.4 and R2 samples were 128, 118 and 133 mg.g−1, respectively, which were obtained after 80 min as equilibrium contact time, and pH of 6 using a methylene blue concentration of 200 ppm. The adsorption of methylene blue using the prepared Fe3O4 core-meso SiO2/TiO2 double shell was analyzed by kinetic and isotherms models. In addition, thermodynamic investigations were applied to assess the spontaneous nature of the process. The obtained results confirmed that the pseudo-second order model is well fitted with the adsorption data and the Freundlich-isotherm assumption suggested a multilayer adsorption mechanism. In addition, results of the thermodynamic investigation indicated that ΔG° was in the range of −2.3 to −6.8 kJ/mol for R1-0.2, −2.8 to −6.3 kJ/mol for R1-0.4 and −2.0 to −5.2 kJ/mol for R2. In addition, the ΔH° and ΔS° values were found in the range of 26.4 to 36.19 kJ.mol−1 and 94.9 to 126.3 Jmol−1 K−1, respectively. These results confirm that the surfaces of Fe3O4 core-mesoSiO2/TiO2 and Fe3O4 core-TiO2/mesoSiO2 double shell exhibit a spontaneous tendency to adsorb methylene blue from the aqueous solutions. The achieved performance of Fe3O4 core-meso SiO2/TiO2 and Fe3O4 core-TiO2/meso SiO2 double shell as adsorbent for methylene blue removal will encourage future research investigations on the removal of a broad range of contaminants from wastewater.
1. Introduction
The problems related to water pollution have become the most global environmental issue during the recent decades [1,2,3]. These problems are owed to excessive industrial activities, which cause continuous production of polluted liquid effluents. For example, there are many types of dyes that are produced in huge amounts annually and applied in the textile fields. Part of these tremendous tones of dyes are contaminated with discharge effluents [4,5]. These dyes are dispersed in various environmental components and are hazardous to humans and animals. Furthermore, these pollutants have resulted in a bad aesthetic view and damage the water ecosystem [4,6,7]. Methylene blue (MB) dye is known as methylthionium chloride and classified as a cationic dye [8]. The common industrial applications of methylene blue are in the painting and paper industries, textiles fabrication, pesticides production and pharmaceutical products [9]. The water discharge from these industrial sections contains huge amounts of methylene blue dye, which spreads in the environment. Exposure to methylene blue causes negative impacts on human health such as vomiting, headache, cyanosis, jaundice, quadriplegia, shock and others [10]. A large amount of dye >7.0 mg kg−1 causes mental disturbance, abdominal pain and nausea [11].
Currently, the most important methods for methylene blue removal are adsorption, biodegradation, chemical oxidation, photodegradation and membrane filtration. Among these treatment methods, the adsorption process has shown unique advantages such as low cost, easy performance and high removal efficiency [12,13,14]. The common adsorbent materials are zinc oxide, silica and silica-derived materials, alumina and carbon. The broader materials categorized as adsorbents include fly ash, manganese oxide, nickel oxide and transition metal hydroxide, which poses high potential for pollution remediation by adsorption [1,15]. However, the materials in the nanosize exhibit a high surface area and fast dispersion in the adsorption medium, leading to promised adsorption capacity compared with the traditional materials. The application of these non-magnetic nanomaterials is effective wastewater treatment; this has some limitations, such as the need for high speed centrifuge or nanofiltration to separate the adsorbent from the adsorption medium at the end of the treatment process [16,17]. To overcome this limitation, magmatic materials are introduced as adsorbents, which enable high dispersion, porous structures as well as the possibility to be separated by external magnetic field [18]. Furthermore, magnetic-based nanomaterials can be prepared in a core-shell structure, which allows easy functionalization with organic and/or inorganic species. Core-shell-based nanomaterials open the space for tremendous adsorbent materials with amazing abilities regarding adsorption and separation [19,20,21].
Different roots have been developed to prepare core-shell-based magnetic materials; however, the application of Fe3O4 nanoparticles as the core is most effective due to their superior magnetic properties [22,23,24]. The shell structure can be prepared by various coatings of silica, carbon, polymer or titania to protect the magnetic core and enable various functionalizations [25,26,27]. Salamat et al. have synthetized the Fe3O4(np)@TiO2 shell structure for water treatment applications by photocatalytic degradation of organic pollutants [28]. Shi et al. prepared a core-shell structure of Fe3O4@titanate using in situ growth and hydrothermal-assisted etching for application in wastewater treatment [29]. Zheng et al. prepared Fe3O4@ZIF-8 nanoparticles as the core-shell nanostructure and recommended them for the removal of methylene blue with an adsorption capacity of 20.2 mg.g−1 [30]. Saini et al. applied Fe3O4@Ag/SiO2 as a core-shell with excellent adsorption properties for the removal of about 99.6% of methylene blue dye from an aqueous solution of pH 7, and the adsorption mechanism was supported by the Langmuir isotherm assumption, reporting a maximum monolayer adsorption capacity (Qmax) of 128.5 mg/g [31]. Jaseela et al. prepared inorganic–organic adsorbents including TiO2 and PVA for selective adsorption of methylene blue with a removal efficiency of 97.1% of MB. The adsorption kinetic was fitted with a pseudo-second-order-based model [32]. Zhan et al. produced an Fe3O4-derived organic–inorganic hybrid-based adsorbent with various structured magnets (np) by a solvothermal and chemical-based co-precipitation method, naming the products as S-Fe3O4 and C-Fe3O4, respectively. The magnetic materials (S-Fe3O4 and C-Fe3O4) were further functionalized by dopamine (DA) and (3-aminopropyl) triethoxysilane (KH550) to finally produce the core-shell Fe3O4/poly(DA + KH550) adsorbents. The application of these materials for methylene blue removal showed an adsorption capacity higher than 400.00 mg.g−1, which was well fitted for the pseudo-second-order kinetic model and Langmuir isotherm model [33]. Schneider et al. fabricated an adsorbent composite from Fe3O4@SiO2@carbon for methylene blue removal [34]. Akbarbandari et al. developed bi-metallic and tri-metallic metal–organic frameworks (MOFs) supported on magnetic activated carbon (MAC), which were synthesized for methylene blue removal. The adsorption process was reported to follow the pseudo-second-order kinetic model and Langmuir isotherm model with a maximum adsorption capacity of 66.51 and 71.43 mg/g for the bi-metallic- and tri-metallic-based magnetic nanocomposites, respectively [35]. All these studies [28,29,30,31,32,33,34,35] reported effective methods for wastewater treatment. However, research is still continuing to investigate the various roots for building magnetic core-shell-based nanocomposites with porous structures and different shell combinations of metal oxides to tune the properties of core-shell materials and improve their performance as adsorbents. The novelty of this work is to investigate the effect of ordering during coating titania or silica nanoparticles onto the Fe3O4 core in relation to adsorption of methylene blue. Therefore, this work aimed to investigate various roots for fabrication of Fe3O4 core-meso SiO2/TiO2 double shells for methylene blue adsorption. The structure of the fabricated Fe3O4 is designed to achieve coating and protection of the magnetic Fe3O4 core and to produce a double shell around it to enhance the efficiency for methylene removal by the adsorption process. In addition, we study the kinetic, isotherm and thermodynamic properties for the adsorptive removal of methylene blue.
2. Materials and Methods
All applied chemicals were of high-purity analytical grade. Ferric chloride-hexahydrate (FeCl3.6H2O), sodium acetate, sodium citrate, ammonia solution, TBOT, TEOS and cetyltrimethylammonium bromide were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.1. Synthesis of Fe3O4 Magnetic Core
A certain weight of FeCl3·6H2O was dissolved in a certain volume of ethylene glycol; then, calculated amounts of sodium acetate, tri-sodium citrate and polyethylene glycol were added. The mixture was vigorously and continuously stirred to ensure complete mixing; then, it was transferred to an autoclave made of stainless steel, which was lined with Teflon and heated to around 190 °C for a certain time. At the end, after reaching room temperature, the produced Fe3O4 magnetic core was washed three times with ethanol and then dried at 60 °C in an oven for approximately 6 h [36].
2.2. Synthesis of Fe3O4 core-meso SiO2/TiO2 Double Shell Nanoparticles
2.2.1. Coating with Mesoporous Silica
Fe3O4 core magnetic nanoparticles were coated with mesoporous silica shell according to the following procedure. Fe3O4 was dispersed in H2O/ethanol mixture ultrasonically; then, an exact volume of NH4OH was added. Thereafter, a specific weight of the cationic surfactant (cetyltrimethylammonium bromide) was added, followed by the addition of TEOS [37].
2.2.2. Titania Coating
To make a layer of titania onto the magnetic nanocores, the procedure described in the work of Jianping et al. [38] was applied. In detail, the silica-coated magnetic nanocores were dispersed in ethanol and mixed with ammonia solution under ultrasonic stirring. TBOT was then added slowly. The reaction was allowed to continue for 24 h under mechanical stirring. Thereafter, the formed Fe3O4 core-SiO2/TiO2 double shell nanoparticles were separated from the mother solution, washed several times with de-ionized water and then with ethanol, dried and finally calcined at 500 °C for 2 h to form Fe3O4 core-meso SiO2/TiO2 double shell nanoparticles.
2.3. Synthesis of Multifunctional Fe3O4 core-TiO2/meso SiO2 Double Shell Nanoparticles
For fabrication of Fe3O4 core-TiO2/meso SiO2 double shell nanoparticles, the titania layer was coated onto the magnetic Fe3O4 core nanoparticle surface; then, a silica coating was made as a second layer and the samples were finally calcined. In detail, the magnetic nanocores were dispersed in ethanol and mixed with ammonia solution under ultrasonic stirring. Then, TBOT was added slowly (0.2 and 0.4 mL). The reaction was allowed to continue under mechanical stirring for 24 h. Thereafter, the produced Fe3O4 core-TiO2 shell was separated from the mother solution, washed several times with de-ionized water and then with ethanol, dried and finally calcined at 500 °C for 2 h under air condition. The final step for coating meso SiO2 onto Fe3O4 core-TiO2 was applied according to the following procedure: Fe3O4@TiO2 as a core was dispersed in H2O ultrasonically and the exact volume from NH4OH was added. Thereafter, the cationic surfactant (cetyltrimethylammonium bromide) solution was added, followed by the addition of TEOS [38]. The reaction was allowed to continue under mechanical stirring for 6 h. The formed Fe3O4 core-TiO2/mesoSiO2 double shell nanoparticles were separated from the mother solution and washed with ethanol and water. Finally, the samples were calcined at 500 °C for 2 h to form Fe3O4 core-TiO2/meso SiO2 double shell nanoparticles.
2.4. Adsorptive Removal Study for Methylene Blue
To investigate the fabricated Fe3O4 core-meso SiO2/TiO2 double shell for methylene blue uptake, the batch process was applied. A certain weight of fabricated Fe3O4 core-meso SiO2/TiO2 double shell was taken in a 50 mL tube and mixed with 25 mL of the 200 ppm methylene blue dye solution. The mixture was shaken for 80 min; then, the phases were separated by external magnetic field. The concentration of the methylene blue dye was measured by UV–Visible. Blank samples without fabricated Fe3O4 core-meso SiO2/TiO2 double shell were investigated in all experiments. The adsorption capacity (qe) was calculated from Equation (1):
where C0 is the primary concentration of methylene blue solution, Ce is the final methylene blue concentration, V represents the volume of the adsorption solution and M is the adsorbent mass (g) (Fe3O4 core-meso SiO2/TiO2 double shell).
qe = (C0 − Ce) × V/M,
The procedures for the adsorption of methylene blue onto Fe3O4 core-meso SiO2/TiO2 double shell were repeated to investigate the most import factors such as pH, contact time, methylene blue dye concentration and temperature, which significantly affect the uptake of methylene blue onto fabricated Fe3O4 core-meso SiO2/TiO2 double shell. For reusability in investigations, the applied Fe3O4 core-meso SiO2/TiO2 double shells were washed three times each with 3 mL ethanol and reused directly.
3. Results and Discussion
3.1. Characterization of Fe3O4 core-Double Shell
Multiple procedures were applied to prepare Fe3O4 core-meso SiO2/TiO2 double shells with alternative sequences of titania and silica. In the first stage, the homogenous spherical magnetic noncore (Fe3O4) was obtained via a solvothermal process, which resulted in separated particles with an average size between 50 nm and 100 nm (Figure 1a). The obtained magnetic nanocore was applied to fabricate the Fe3O4 core-mesoSiO2/TiO2 double shell or Fe3O4 core-TiO2/mesoSiO2 double shell.
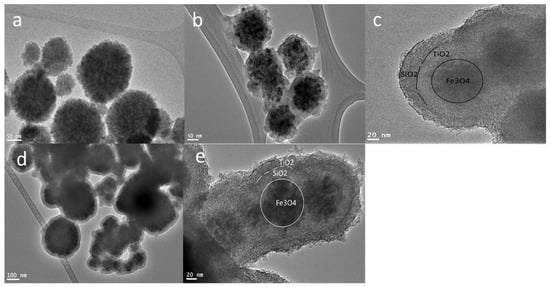
Figure 1.
TEM images of (a) Fe3O4 nanocores prepared by solvothermal method, (b) titania-coated Fe3O4 nanocores and (c) double shell Fe3O4@TiO2@m-SiO2 by first route (R1), and (d) mesoporous silica-coated Fe3O4 nanocores and (e) double shell Fe3O4@m-SiO2@TiO2 by second route (R2).
To fabricate Fe3O4@TiO2@m-SiO2, we used Fe3O4 nanoparticles with the first route. The nanoparticles were firstly coated with TiO2 shell and secondly with mesoporous silica shell; finally, the calcination process was conducted to crystalize the TiO2 shell and to remove surfactants from the silica shell to transform it into a mesoporous one. TiO2 coating onto Fe3O4 nanoparticles was conducted using the Stöber-modified approach, where citrate-modified Fe3O4 nanoparticles were dispersed in ethanol solution, ammonium hydroxide and titanium butoxide were added to the above mixture and then the coating process was conducted at 45 °C for 20 h. The mesoporous silica step was conducted on TiO2-coated Fe3O4 using the Stöber approach by adding a cationic surfactant (Cetyl trimethylammonium bromide (CTAB)). Finally, the calcination process was conducted to ensure the crystallization of the TiO2 shell and to remove CTAB to obtain a mesoporous silica shell. TEM observation (Figure 1b) showed the formation of a ~25 nm TiO2 layer around Fe3O4 nanocores. The TEM image (Figure 1c) revealed that the Fe3O4@TiO2@m-SiO2 structure was formed with a shell thickness of 20 nm. In route R1, two different samples Fe3O4@TiO2@m-SiO2 -0.2(R1-0.2) and Fe3O4@TiO2@m-SiO2 -0.4(R1-0.4) were synthesized by adding 0.2 and 0.4 mL of TEOS, respectively. The thickness of the shell layer was 20 and 45 nm for the R1-0.2 and R2-0.4 samples, respectively.
To fabricate the Fe3O4 core-mesoSiO2/TiO2 double shell using the second route (sample R2), the magnetic nanocores were subjected first to mesoporous silica coating and then to TiO2 coating for the formation of the second shell. To achieve uniform formation of Fe3O4@mesoSiO2, the Stöber method in the presence of cationic surfactant was applied to form a mesoporous silica layer around the magnetic nanocores followed by titania coating, as shown in Figure 1d. The mesoporous silica layer is about 20 nm (Figure 1d). The second step to coat TiO2 on the fabricated Fe3O4 core-meso SiO2 was achieved by hydrolysis of titanium butoxide, which successfully formed a uniform 20 nm layer of TiO2 (Figure 1e) to finally form the Fe3O4 core-meso SiO2/TiO2 double shell (Figure 1). Finally, calcining of the Fe3O4 core-meso SiO2/TiO2 double shell sample at 550 °C was performed to crystallize the TiO2 layer and to remove the surfactant in one single step.
The EDX analysis for both samples, Fe3O4@TiO2@m-SiO2 and Fe3O4@m-SiO2@TiO2, are shown in Figure 2 and Figure 3, respectively. The detected elements indicate the formation of the desired core-double shell structure.
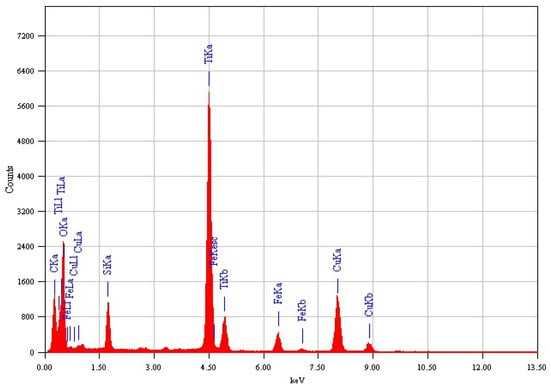
Figure 2.
Elemental EDX analysis of Fe3O4@TiO2@m-SiO2 by first route (R1).
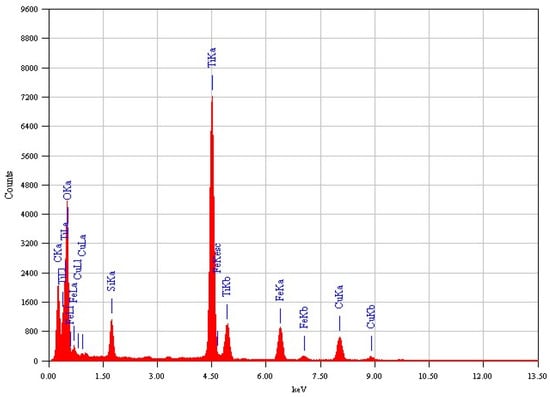
Figure 3.
Elemental EDX analysis of Fe3O4@m-SiO2@TiO2 by second route (R2).
N2 adsorption–desorption isotherms were conducted for core-double shell nanoparticles prepared by route 1 (at 0.2 and 0.4 mL TEOS) and route 2 at 77 K and presented in Figure 4. The prepared core-double shell derived nanoparticles by route 1 and route 2 exhibited a porous structure with type IV isotherm. It is clear that nanoparticles prepared by route 1, with the silica shell outer layer, had higher surface areas as well as larger pore volumes (Table 1 and Figure 4A) when compared with nanoparticles prepared by route 2, where the TiO2 shell is the outer layer. Moreover, these results can be explained based on the porous character of the silica shell compared with the crystalline dense character of the TiO2 one. However, changing the silica content caused a slight increment in the surface area and pore volume of the formed sample. Moreover, the pore size was much bigger in the case of core-double shell nanoparticles prepared by route 1 than in samples by route 2 (Table 1 and Figure 4B). In both samples, Fe3O4@TiO2@m-SiO2 by first route (R1) and Fe3O4@m-SiO2@TiO2 by second route (R2), the heat-treatment process was conducted with an aim to convert amorphous TiO2 to a crystalline one and to remove surfactant molecules from the silica shell to impart it with a mesoporous character. The heat-treatment process is our confirmation of the crystalline character of the TiO2 shell. Second, the amorphous TiO2 shell was built on silica layer by controlled hydrolysis of titanium (IV) butoxide (TBOT). After formation of the amorphous TiO2 shell on the silica middle layer, the heat-treatment process allows us to convert it to a crystalline dense character.
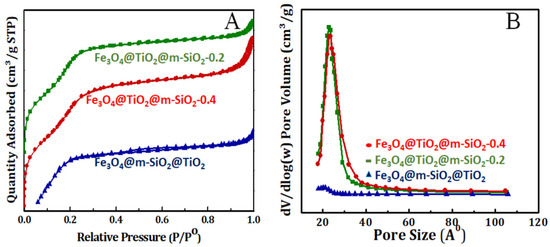
Figure 4.
(A) N2 sorption isotherm and (B) pore size distribution of calcined Fe3O4@TiO2@m-SiO2 at TEOS amounts of 0.2 and 0.4 mL prepared by route 1 (R1) and calcined Fe3O4@m-SiO2@TiO2 prepared by route 2 (R2).

Table 1.
Textural properties for magnetic core-double shell prepared by routes 1 and 2.
FTIR measurements of core-double shell nanoparticles prepared by route1 (at 0.2 and 0.4 mL TEOS) and route 2 are shown in Figure 5. The Si-O peak can be seen at 1050–1250 cm−1. The Fe–O–Si peak that refers to chemical binding between Fe3O4 and silica cannot be seen in the FTIR spectrum because it appears at around 584 cm−1 and, therefore, overlaps with the Fe–O vibration of magnetite nanoparticles. The peaks at 1632 cm−1 and 3425 cm−1 correspond to the vibration of hydroxyl groups (-OH) on the surface of Fe3O4 nanoparticles. The peak at 970 cm−1 can be attributed to the Ti–O–Si bond while the shoulder at 1400 cm−1 can be due to the Ti–O–Ti vibration.
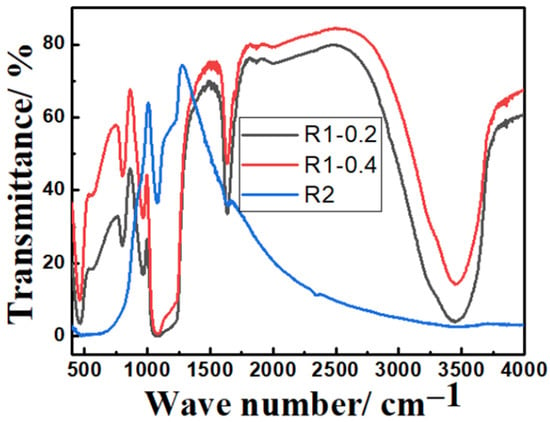
Figure 5.
FTIR spectra of calcined Fe3O4@TiO2@m-SiO2 at TEOS amounts of 0.2 and 0.4 mL prepared by route 1 (R1) and calcined Fe3O4@m-SiO2@TiO2 prepared by route 2 (R2).
3.2. Adsorptive Remediation Investigation
Methylene blue is extensively used in the industrial section for dying and painting, resulting in huge amounts of colored discharge and producing many negative environmental impacts [39,40,41]. Herein, three adsorbent materials including the core-double shell structures from Fe3O4 core-TiO2/mesoSiO2 (R1–0.2), Fe3O4 core-TiO2/mesoSiO2 (R1-0.4) and Fe3O4 core-mesoSiO2/TiO2 (R2) double shell nanoparticles were applied for methylene blue dyes by adsorptive removal. The effect of the pH of the medium was investigated by varying the pH of the methylene blue sample solution from 2 to 7 (Figure 6). In the strong acidic medium, the adsorption capacities for methylene blue removal using R1–0.2, R1-0.4 and R2 had the lowest values; then, they increased with the increasing pH, reaching its maximum value between pH 6 and 7. The lower adsorption capacity at strong pH medium may be owed to the protonation of the adsorbent surfaces [42,43]. The adsorption capacity of methylene blue onto the three tested adsorbents was in the order of R2 > R1–0.2 > R1-0.4. This indicates that the titania as outer shell was more effective for adsorptive removal of methylene blue. This may be attributed to the vacant d orbital in the TiO2 structure, which can correlate and interact with the lone pairs of electrons on the methylene blue structure leading to stronger attractive forces than in the case of silica as the outer shell. The blank experiments that operated in the absence of Fe3O4 core-TiO2/mesoSiO2 or Fe3O4 core-mesoSiO2/TiO2 double shell nanoparticles showed zero efficiency for the adsorption of methylene blue, suggesting that no other mechanisms such as precipitation or coagulation are involved during the developed wastewater treatments. This proves the effective performance of Fe3O4 core-TiO2/mesoSiO2 or Fe3O4 core-mesoSiO2/TiO2 double shell nanoparticles as adsorbents to remove methylene blue dye from wastewater.

Figure 6.
pH investigation for methylene blue adsorption onto R1–0.2, R1-0.4 and R2 (methylene blue concentration 100 mg L−1, adsorbent dose 0.015 g and contact time 120 min).
The effect of contact time on the de-colorization of dyes from aqueous solution by adsorption was investigated to assess the rate and efficiency of the process [44,45]. The effect of time is studied from 5 min to 180 min and the adsorption capacities for R1–0.2, R1-0.4 and R2 for methylene blue uptake are presented in Figure 7. The adsorption capacities after 5 min were 29, 23 and 39 mg.g1 for R1–0.2, R1-0.4 and R2, respectively; then, they increased until reaching equilibrium at 80 min, recording adsorption capacities of 128, 118 and 133 mg.g1. When increasing time from 80 min to 180 min, no noticeable improvements in the adsorption capacities were detected due to the occurrence of the steady state.
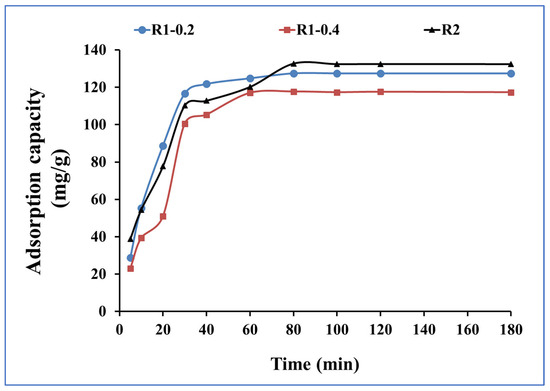
Figure 7.
Time investigation for methylene blue adsorption onto R1–0.2, R1-0.4 and R2 (methylene blue concentration 200 mg L−1, adsorbent dose 0.015 g and pH 7).
The rate of the mass transfer of methylene blue during the adsorption process onto R1–0.2, R1-0.4 and R2 was studied by applying the kinetic models of pseudo first order and pseudo second order [46,47], as presented in Figure 8 and Figure 9, respectively. From the data correlation, the pseudo-second-order kinetic model was found to be more comfortable for describing the rate of the adsorption process. The pseudo-first-order equation of Lagergren is generally expressed in the integrated form of Equation (2):
log(qe − qt) = log qe − k1t/2.303
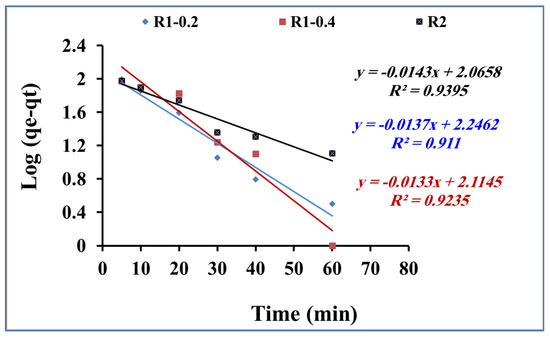
Figure 8.
Pseudo-first-order kinetic model for methylene blue adsorption using R1-0.2, R1-0.4 and R2 (methylene blue concentration 200 mg L−1, adsorbent dose 0.015 g and pH 7).
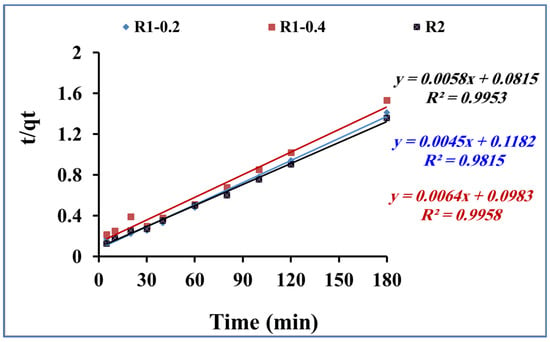
Figure 9.
Pseudo-second-order kinetic model for methylene blue adsorption using R1–0.2, R1-0.4 and R2 (methylene blue concentration 200 mg L−1, adsorbent dose 0.015 g and pH 7).
By plotting log (qe − qt) versus time t (Figure 8), the pseudo-first-order rate constant k1 is calculated and reported in Table 2. In addition, the pseudo-second-order kinetic rate equation is expressed in the integrated form of Equation (3):
where t is the time (min), and qe (mg/g) and qe2 (mg/g) are the quantity of methylene blue adsorbed at equilibrium onto fabricated R1–0.2, R1-0.4 and R2 samples at pH 6 and 25 °C. Figure 9 presents the plotting of t/qt versus t. The qe and k parameters are calculated using the slope and intercept, respectively, according to the second-order kinetic model (Equation (3)).
t/qt = 1/Kqe2 + 1/qe.t

Table 2.
Kinetic constant parameters obtained for methylene blue adsorption using R1–0.2, R1-0.4 and R2 (methylene blue concentration 200 mg L−1, adsorbent dose 0.015 g and pH 7).
Table 2 shows the calculated parameter values and the linear regression correlation coefficient values. The pseudo-second-order kinetic rate equation model fitting was much better than the pseudo-first-order one. The obtained results confirm the assumption related to the second-order kinetic model, including the fast adsorption process. In addition, the adsorption dynamic is dependent on the migration of the methylene blue molecules to the surface of the fabricated Fe3O4 core-meso SiO2/TiO2 double shell adsorbent and, finally, migration of the methylene blue molecules to the entire pores of the fabricated Fe3O4 core-meso SiO2/TiO2 double shell adsorbent [48,49].
3.3. Isotherms Study
The investigation of the effect of the concentration of methylene blue at constant temperatures (isotherms) on the adsorption capacity using fabricated R1-0.2, R1-0.4 and R2 samples is applied to assess the distribution of methylene blue as the adsorbate within the liquid sample solution and the solid fabricated Fe3O4 core-meso SiO2/TiO2 double shell adsorbent at equilibrium [50,51,52,53]. The Langmuir Equation (4) was used to model the adsorption data for methylene blue uptake onto the fabricated Fe3O4 core-meso SiO2/TiO2 double shell adsorbent (R1-0.2, R1-0.4 and R2 samples) [54]:
where Ce is the concentration of methylene blue (mg/L) at equilibrium, Qe is the quantity of methylene blue adsorbed (mg/g), and qmax and b are Langmuir model constants (Figure 10). The correlation coefficients, R2, for adsorption data for methylene blue adsorption onto fabricated R1-0.2, R1-0.4 and R2 samples were low (Table 3), indicating that the adsorption data were not fitted by the Langmuir isotherm.
Ce/Qe = 1/(qmax. b) + Ce/qmax),
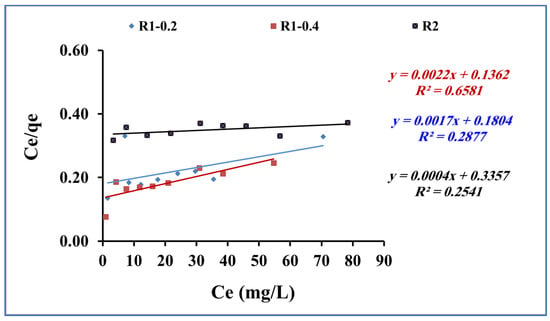
Figure 10.
Langmuir for methylene blue adsorption using R1-0.2, R1-0.4 and R2 (methylene blue concentration 200 mg L−1, adsorbent dose 0.015 g, pH 7 and contact time of 80 min).

Table 3.
Langmuir and Freundlich parameters for methylene blue adsorption using R1-0.2, R1-0.4 and R2 (methylene blue concentration 200 mg L−1, adsorbent dose 0.015 g, pH 7 and contact time of 80 min).
The Freundlich model assumes that the adsorption process occurs as multiple layers of adsorbate molecules (methylene blue) onto the surface of the adsorbent (Fe3O4 core-meso SiO2/TiO2 double shell). The obtained adsorption data for methylene blue adsorption onto fabricated R1-0.2, R1-0.4 and R2 samples were subjected to Freundlich equation [55] (Equation (5)):
where Ce is the concentration of methylene blue (mg/L) at equilibrium and Qe is the quantity of methylene blue adsorbed (mg/g). KF (mg/g) is the Freundlich constant for the adsorbent capacity and n is related to the favorable nature of the adsorption process. Figure 11 shows the plotting of log qe and log Ce. From Freundlich equation and Figure 11, the slope and intercept indicate 1/n and log KF, respectively.
Log qe = log Kf + 1/n log Ce,
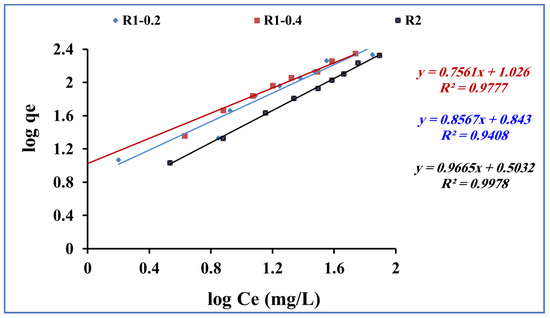
Figure 11.
Freundlich isotherm for methylene blue adsorption onto using R1-0.2, R1-0.4 and R2 (methylene blue concentration 200 mg L−1, adsorbent dose 0.015 g, pH 7 and contact time of 80 min).
The adsorption of methylene blue using Fe3O4 core-meso SiO2/TiO2 double shell (R1-0.2, R1-0.4 and R2 samples) showed agreement with the Freundlich model (R2 > 0.9) for the tested range of concentrations used in this study (Table 3), suggesting a multilayer adsorption process.
3.4. Thermodynamic Studies
The adsorption process is strongly influenced by the temperature of the adsorption medium [56,57,58]. The temperature effect has been studied to evaluate the nature of the adsorption process of methylene blue onto the fabricated Fe3O4 core-meso SiO2/TiO2 double shell. The thermodynamic parameters, including the Gibbs free energy (ΔG°), enthalpy (ΔH°) and entropy (ΔS°) of the adsorption process of methylene blue, onto the fabricated R1-0.2, R1-0.4 and R2 samples are evaluated by Equations (6) and (7):
where Kd refers to the equilibrium partition constant, which is calculated as the ratio between the sorption capacity of Fe3O4 core-meso SiO2/TiO2 double shell (qe) and methylene blue equilibrium concentration (Ce), R represents the gas-constant (8.314 J/mol K) and T is the temperature in Kelvin (K). Equation (6) and the plot of log Kd and 1/T (Figure 12) enable the calculation of ΔH° and ΔS° values.
logKd = ΔS°/2.303R − ΔH°/2.303RT
ΔG° = −RT lnKd,
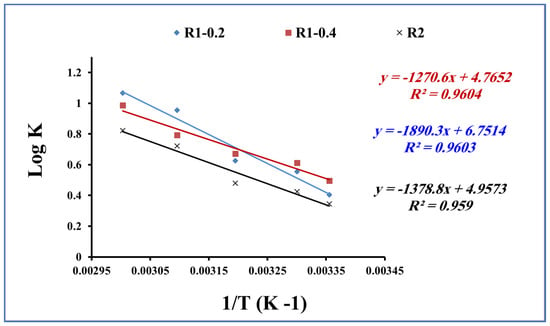
Figure 12.
Thermodynamic parameters for methylene blue adsorption using R1-0.2, R1-0.4 and R2 (methylene blue concentration 200 mg L−1, adsorbent dose 0.015 g, pH 7 and contact time of 80 min).
Gibbs free energy (ΔG°), enthalpy (ΔH°) and entropy (ΔS°) are presented in Table 4. ΔG° was obtained as negative values in the range of −2.3 to −6.8 kJ/mol for R1-0.2, −2.8 to −6.3 kJ/mol for R1-0.4 and −2.0 to −5.2 kJ/mol for R2. In addition, the ΔH° and ΔS° values were found in the range of 26.4 to 36.19 kJ.mol−1 and 94.9 to 126.3 Jmol−1 K−1, respectively. The calculated thermodynamic parameters indicate that the adsorption process of methylene blue onto fabricated R1-0.2, R1-0.4 and R2 samples is spontaneous and physical in nature. Furthermore, the adsorption process of methylene blue increases the degree of freedom during the adsorption interaction process [59,60].

Table 4.
Thermodynamic parameters for methylene blue adsorption onto R1-0.2, R1-0.4 and R2.
The reported adsorption capacity for the removal of methylene blue using the fabricated Fe3O4 core-TiO2/mesoSiO2 and Fe3O4 core-mesoSiO2/TiO2 double shell nanoparticles is compared with other studies from the literature [30,31,35,61,62,63] (Table 5). The obtained adsorption capacity achieved in this work is higher than that reported by Zheng et al. onto Fe3O4@ZIF-8 (20.2 mg.g−1) [30], Saini et al. using Fe3O4@Ag/SiO2 (128.5 mg/g) [31], Akbarbandari et al. (66.51 and 71.43 mg.g−1 onto bi-metallic and tri-metallic metal–organic frameworks, respectively) [35], Bouyahia et al. (7.84 mg.g−1) onto sawdust [61], Kazemi and Sobhani (54.05 mg.g−1) onto CuMn2O4/chitosan micro/nanocomposite [62] and Taweekarn et al. (34.84 mg.g−1) onto Monolithic starch cryogel [63]. The obtained results in this work are lower than those reported by Zhan et al. (higher than 400.00 mg.g−1) onto Fe3O4-derived organic/inorganic (S-Fe3O4 and C-Fe3O4) [33]. The variation in adsorption efficiencies during wastewater treatments is highly influenced by the nature of the adsorbent materials, porosity and nature of adsorbate [64,65].

Table 5.
Comparison of removal of methylene blue between achieved performance in this work and other studies from the literature.
The fabricated Fe3O4 core-TiO2/mesoSiO2 and Fe3O4 core-mesoSiO2/TiO2 double shell nanoparticles were subjected to reactivation and recycling after adsorption of methylene blue (Figure 13). The efficiency during recycling was calculated as percentage from the first usage. Results presented in Figure 13 indicate that the efficiency for the removal of methylene blue was still above 90% for four usages for both adsorbents; then, the efficiency decreased to below 75% in the fifth usage.

Figure 13.
The efficiency of removal of methylene blue using R1-0.2, R1-0.4 and R2 for recycling investigations.
4. Conclusions
Multistep fabrication processes were investigated in this study. Fe3O4 core-TiO2/mesoSiO2 and Fe3O4 core-mesoSiO2/TiO2 double shell nanoparticles were prepared by first (R1) and second (R2) routes as magnetic materials for adsorption of methylene blue. TEM examination showed the successful formation of a magnetic core-double shell structure including silica layer and titania layer of 20 nm thickness. The prepared magnetic core-double shell nanoparticles exhibit surface areas of 1133, 1207 and 52.27 m2/g for R1-0.2, R1-0.4 and R2 samples, respectively. The removal of methylene blue was operated at pH 6 with a contact time of 80 min to reach the steady state with an adsorption capacity of 128, 118 and 133 mg.g−1 for R1-0.2, R1-0.4 and R2, respectively. Upon applying the kinetic models, the pseudo-second-order kinetic model was well fitted with the adsorption data for the removal of methylene blue onto fabricated Fe3O4 core-TiO2/mesoSiO2 and Fe3O4 core-mesoSiO2/TiO2 double shell nanoparticles (R1-0.2, R1-0.4 and R2 samples). The Feundlish isotherm showed good correlation with the adsorption data, suggesting a multilayer adsorption. The thermodynamic parameters confirm that the adsorption process of methylene blue onto the fabricated magnetic core-double shell structure is spontaneous and physical in nature.
Author Contributions
Conceptualization, A.M.E.-T., M.A.H. and A.S.A.-A.; funding acquisition, A.M.E.-T. and Z.A.A.; investigation, A.M.E.-T., J.P.L., M.A.H., M.S., M.E.-M. and A.S.A.-A.; methodology, M.A.H., M.S., M.E.-M. and Z.A.A.; project administration, A.M.E.-T., M.A.H. and Z.A.A.; resources, Z.A.A.; supervision, M.A.H.; validation, A.M.E.-T., J.P.L., M.A.H., M.S., M.E.-M., A.S.A.-A. and Z.A.A.; writing—original draft, M.A.H.; writing—review and editing, M.A.H. and Z.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Plan for Science, Technology, and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia for its grant with award number 14-WAT169-02.
Data Availability Statement
Data and/or Samples of the compounds are available from the authors.
Acknowledgments
Authors acknowledge the National Plan for Science, Technology, and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia for its grant with award number 14-WAT169-02.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E. Adsorption Processes for Water Treatment and Purification; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783319581361. [Google Scholar]
- Azmi, W.; Sani, R.K.; Banerjee, U.C. Biodegradation of Triphenylmethane Dyes. Enzym. Microb. Technol. 1998, 22, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Shukla, S.K.; Singh, N.B. Water Purification by Polymer Nanocomposites: An Overview. Nanocomposites 2017, 3, 47–66. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile Dye Wastewater Characteristics and Constituents of Synthetic Effluents: A Critical Review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Ananthashankar, R.; Alhattab, M.; Ramakrishnan, V.V.; Ghaly, A. Production, Characterization and Treatment of Textile Effluents: A Critical Review. J. Chem. Eng. Process Technol. 2014, 5, 182. [Google Scholar] [CrossRef]
- Li, H.H.; Wang, Y.T.; Wang, Y.; Wang, H.X.; Sun, K.K.; Lu, Z.M. Bacterial Degradation of Anthraquinone Dyes. J. Zhejiang Univ. Sci. B 2019, 20, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Peres, E.C.; Slaviero, J.C.; Cunha, A.M.; Hosseini–Bandegharaei, A.; Dotto, G.L. Microwave Synthesis of Silica Nanoparticles and Its Application for Methylene Blue Adsorption. J. Environ. Chem. Eng. 2018, 6, 649–659. [Google Scholar] [CrossRef]
- Al-Wakeel, K.Z.; Abd El Monem, H.; Khalil, M.M.H. Removal of Divalent Manganese from Aqueous Solution Using Glycine Modified Chitosan Resin. J. Environ. Chem. Eng. 2015, 3, 179–186. [Google Scholar] [CrossRef]
- El-Moselhy, M.M.; Kamal, S.M. Selective Removal and Preconcentration of Methylene Blue from Polluted Water Using Cation Exchange Polymeric Material. Groundw. Sustain. Dev. 2018, 6, 6–13. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.-Q.; Han, B.; Su, C.-L.; Han, Q.; Chen, W.-Z. Biosorption of Copper Ions from Aqueous Solution Using Rape Straw Powders: Optimization, Equilibrium and Kinetic Studies. Ecotoxicol. Environ. Saf. 2018, 150, 251–259. [Google Scholar] [CrossRef]
- Polat, H.; Erdogan, D. Heavy Metal Removal from Waste Waters by Ion Flotation. J. Hazard. Mater. 2007, 148, 267–273. [Google Scholar] [CrossRef]
- Akar, S.T.; Akar, T.; Çabuk, A. Decolorization of a Textile Dye, Reactive Red 198 (RR198), by Aspergillus Parasiticus Fungal Biosorbent. Braz. J. Chem. Eng. 2009, 26, 399–405. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste Materials for Activated Carbon Preparation and Its Use in Aqueous-Phase Treatment: A Review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Hajeb, P.; Sloth, J.J.; Shakibazadeh, S.; Mahyudin, N.A.; Afsah-Hejri, L. Toxic Elements in Food: Occurrence, Binding, and Reduction Approaches. Compr. Rev. Food Sci. Food Saf. 2014, 13, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Qiao, W.; Zhang, P.; Sun, L.; Ma, S.; Xu, W.; Xu, S.; Niu, Y. Adsorption Performance and Mechanism of Schiff Base Functionalized Polyamidoamine Dendrimer/Silica for Aqueous Mn(II) and Co(II). Chin. Chem. Lett. 2020, 31, 2742–2746. [Google Scholar] [CrossRef]
- Zhan, H.; Bian, Y.; Yuan, Q.; Ren, B.; Hursthouse, A.; Zhu, G. Preparation and Potential Applications of Super Paramagnetic Nano-Fe3O4. Processes 2018, 6, 33. [Google Scholar] [CrossRef]
- López, Y.C.; Ortega, G.A.; Martínez, M.A.; Reguera, E. Magnetic Prussian Blue Derivative like Absorbent Cages for an Efficient Thallium Removal. J. Clean. Prod. 2020, 283, 124587. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Pourvala, T.; Motamedi, E.; Fathi, B.; Vashaee, D.; Tayebi, L. Synthesis and Characterization of Encapsulated Nanosilica Particles with an Acrylic Copolymer by in Situ Emulsion Polymerization Using Thermoresponsive Nonionic Surfactant. Materials 2013, 6, 3727–3741. [Google Scholar] [CrossRef]
- De Los Santos Valladares, L.; Bustamante Domínguez, A.; León Félix, L.; Kargin, J.B.; Mukhambetov, D.G.; Kozlovskiy, A.L.; Moreno, N.O.; Flores Santibañez, J.; Castellanos Cabrera, R.; Barnes, C.H.W. Characterization and Magnetic Properties of Hollow α-Fe2O3 Microspheres Obtained by Sol Gel and Spray Roasting Methods. J. Sci. Adv. Mater. Devices 2019, 4, 483–491. [Google Scholar] [CrossRef]
- Jabasingh, A.; Ravi, T.; Yimam, A. Magnetic Hetero-Structures as Prospective Sorbents to Aid Arsenic Elimination from Life Water Streams. Water Sci. 2018, 32, 151–170. [Google Scholar] [CrossRef]
- Zhu, F.; Zheng, Y.M.; Zhang, B.G.; Dai, Y.R. A Critical Review on the Electrospun Nanofibrous Membranes for the Adsorption of Heavy Metals in Water Treatment. J. Hazard. Mater. 2021, 401, 123608. [Google Scholar] [CrossRef]
- Habila, M.A.; Alothman, Z.A.; Mohamed El-Toni, A.; Labis, J.P.; Khan, A.; Al-Marghany, A.; Elafifi, H.E. One-Step Carbon Coating and Polyacrylamide Functionalization of Fe3O4 Nanoparticles for Enhancing Magnetic Adsorptive-Remediation of Heavy Metals. Molecules 2017, 22, 2074. [Google Scholar] [CrossRef] [PubMed]
- El-Toni, A.M.; Habila, M.A.; Labis, J.P.; Alothman, Z.A.; Alhoshan, M.; Elzatahry, A.A.; Zhang, F. Design, Synthesis and Applications of Core-Shell, Hollow Core, and Nanorattle Multifunctional Nanostructures. Nanoscale 2016, 8, 2510–2531. [Google Scholar] [CrossRef] [PubMed]
- Habila, M.A.; ALOthman, Z.A.; El-Toni, A.M.; Labis, J.P.; Li, X.; Zhang, F.; Soylak, M. Mercaptobenzothiazole-Functionalized Magnetic Carbon Nanospheres of Type Fe3O4@SiO2@C for the Preconcentration of Nickel, Copper and Lead Prior to Their Determination by ICP-MS. Microchim. Acta 2016, 183, 2377–2384. [Google Scholar] [CrossRef]
- Salamat, S.; Younesi, H.; Bahramifar, N. Synthesis of Magnetic Core–Shell Fe3O4@TiO2 Nanoparticles from Electric Arc Furnace Dust for Photocatalytic Degradation of Steel Mill Wastewater. RSC Adv. 2017, 7, 19391–19405. [Google Scholar] [CrossRef]
- Shi, L.; Dong, B.; Gao, R.; Su, G.; Liu, W.; Xia, C.; Zhao, F.; Cao, L. Hierarchical Fe3O4@titanate Microspheres with Superior Removal Capability for Water Treatment: In Situ Growth and Structure Tailoring via Hydrothermal Assisted Etching. RSC Adv. 2015, 5, 73126–73132. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, C.; Fang, W.J.; Chen, C.; Yan, R.W.; Huai, H.X.; Wang, C.C. Surfactant-Free Synthesis of a Fe3O4@ZIF-8 Core–Shell Heterostructure for Adsorption of Methylene Blue. CrystEngComm 2014, 16, 3960–3964. [Google Scholar] [CrossRef]
- Saini, J.; Garg, V.K.; Gupta, R.K. Removal of Methylene Blue from Aqueous Solution by Fe3O4@Ag/SiO2 Nanospheres: Synthesis, Characterization and Adsorption Performance. J. Mol. Liq. 2018, 250, 413–422. [Google Scholar] [CrossRef]
- Jaseela, P.K.; Garvasis, J.; Joseph, A. Selective Adsorption of Methylene Blue (MB) Dye from Aqueous Mixture of MB and Methyl Orange (MO) Using Mesoporous Titania (TiO2)—Poly Vinyl Alcohol (PVA) Nanocomposite. J. Mol. Liq. 2019, 286, 110908. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhao, S.; Wan, X.; He, S. Hierarchical Fe3O4-Derived Organic/Inorganic Hybrids Constructed by Modified Bio-Inspired Functionalization: Efficient Adsorbents for Water-Soluble Methylene Blue and Mechanism. J. Chem. Technol. Biotechnol. 2019, 94, 1638–1650. [Google Scholar] [CrossRef]
- Schneider, M.; Ballweg, T.; Groß, L.; Gellermann, C.; Sanchez-Sanchez, A.; Fierro, V.; Celzard, A.; Mandel, K.; Schneider, M.; Ballweg, T.; et al. Magnetic Carbon Composite Particles for Dye Adsorption from Water and Their Electrochemical Regeneration. Part. Part. Syst. Charact. 2019, 36, 1800537. [Google Scholar] [CrossRef]
- Akbarbandari, F.; Zabihi, M.; Faghihi, M. Synthesis of the Magnetic Core–Shell Bi-Metallic and Tri-Metallic Metal–Organic Framework Nanocomposites for Dye Adsorption. Water Environ. Res. 2021, 93, 906–920. [Google Scholar] [CrossRef] [PubMed]
- El-Toni, A.M.; Ibrahim, M.A.; Labis, J.P.; Khan, A.; Alhoshan, M. Optimization of Synthesis Parameters for Mesoporous Shell Formation on Magnetic Nanocores and Their Application as Nanocarriers for Docetaxel Cancer Drug. Int. J. Mol. Sci. 2013, 14, 11496–11509. [Google Scholar] [CrossRef]
- Deng, Y.; Qi, D.; Deng, C.; Zhang, X.; Zhao, D. Superparamagnetic High-Magnetization Microspheres with an Fe3O4@SiO2 Core and Perpendicularly Aligned Mesoporous SiO2 Shell for Removal of Microcystins. J. Am. Chem. Soc. 2008, 130, 28–29. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Wu, Z.; Wang, J.; Li, B.; Feng, S.; Deng, Y.; Zhang, F.; Zhao, D. A Versatile Kinetics-Controlled Coating Method to Construct Uniform Porous TiO 2 Shells for Multifunctional Core-Shell Structures. J. Am. Chem. Soc. 2012, 134, 11864–11867. [Google Scholar] [CrossRef]
- El-Ashtoukhy, E.S.Z.; Fouad, Y.O. Liquid-Liquid Extraction of Methylene Blue Dye from Aqueous Solutions Using Sodium Dodecylbenzenesulfonate as an Extractant. Alex. Eng. J. 2015, 54, 77–81. [Google Scholar] [CrossRef]
- Eslami, H.; Sedighi Khavidak, S.; Salehi, F.; Khosravi, R.; Fallahzadeh, R.A.; Peirovi, R.; Sadeghi, S. Biodegradation of Methylene Blue from Aqueous Solution by Bacteria Isolated from Contaminated Soil; Kurdistan University of Medical Sciences: Sanandaj, Iran, 2016; Volume 5. [Google Scholar]
- Rahman, R.M.; Neethu, M.K. Degradation of Methylene Blue in Textile Waste Water Using Activated Sawdust and Eggshell Biosorbent. Int. J. Adv. Res. Innov. Ideas Educ. 2017, 2, 7–16. [Google Scholar]
- Yan, Y.; Zhang, M.; Gong, K.; Su, L.; Guo, Z.; Mao, L. Adsorption of Methylene Blue Dye onto Carbon Nanotubes: A Route to an Electrochemically Functional Nanostructure and Its Layer-by-Layer Assembled Nanocomposite. Chem. Mater. 2005, 17, 3457–3463. [Google Scholar] [CrossRef]
- Karthik, R.; Muthezhilan, R.; Jaffar Hussain, A.; Ramalingam, K.; Rekha, V. Effective Removal of Methylene Blue Dye from Water Using Three Different Low-Cost Adsorbents. Desalination Water Treat. 2016, 57, 10626–10631. [Google Scholar] [CrossRef]
- Vijayalaks, G.; Ramkumar, B.; Mohan, S.C. Isotherm and Kinetic Studies of Methylene Blue Adsorption Using Activated Carbon Prepared from Teak Wood Waste Biomass. J. Appl. Sci. 2019, 19, 827–836. [Google Scholar] [CrossRef]
- Ghaedi, M.; Nasab, A.G.; Khodadoust, S.; Rajabi, M.; Azizian, S. Application of Activated Carbon as Adsorbents for Efficient Removal of Methylene Blue: Kinetics and Equilibrium Study. J. Ind. Eng. Chem. 2014, 20, 2317–2324. [Google Scholar] [CrossRef]
- AlOthman, Z.A.; Habila, M.A.; Ali, R.; Abdel Ghafar, A.; El-din Hassouna, M.S. Valorization of Two Waste Streams into Activated Carbon and Studying Its Adsorption Kinetics, Equilibrium Isotherms and Thermodynamics for Methylene Blue Removal. Arab. J. Chem. 2014, 7, 1148–1158. [Google Scholar] [CrossRef]
- Al-Othman, Z.A.; Habila, M.A.; Ali, R.; Hassouna, M.S.E.-D. Kinetic and Thermodynamic Studies for Methylene Blue Adsorption Using Activated Carbon Prepared from Agricultural and Municipal Solid Wastes. Asian J. Chem. 2013, 25, 8301–8306. [Google Scholar] [CrossRef]
- El-Toni, A.M.; Habila, M.A.; Ibrahim, M.A.; Labis, J.P.; ALOthman, Z.A. Simple and Facile Synthesis of Amino Functionalized Hollow Core-Mesoporous Shell Silica Spheres Using Anionic Surfactant for Pb(II), Cd(II), and Zn(II) Adsorption and Recovery. Chem. Eng. J. 2014, 251, 441–451. [Google Scholar] [CrossRef]
- Habila, A.M.; Sheikh Moshab, M.; Mohamed El-Toni, A.; Al-Awadi, S.A.; ALOthman, A.Z. Facile Strategy for Fabricating an Organosilica-Modified Fe3O4 (OS/Fe3O4) Hetero-Nanocore and OS/Fe3O4@SiO2 Core–Shell Structure for Wastewater Treatment with Promising Recyclable Efficiency. ACS Omega 2023, 8, 7626–7638. [Google Scholar] [CrossRef]
- Mondal, K.; Lalvani, S.B. Modeling of Mass Transfer Controlled Adsorption Rate Based on the Langmuir Adsorption Isotherm. Sep. Sci. Technol. 2000, 35, 2583–2599. [Google Scholar] [CrossRef]
- Desta, M.B. Batch Sorption Experiments: Langmuir and Freundlich Isotherm Studies for the Adsorption of Textile Metal Ions onto Teff Straw (Eragrostis Tef) Agricultural Waste. J. Thermodyn. 2013, 2013, 375830. [Google Scholar] [CrossRef]
- Moganavally, P.; Deepa, M.; Sudha, P.N.; Suresh, R. Adsorptive Removal of Lead and Cadmium Ions Using Cross-Linked CMC Schiff Base: Isotherm, Kinetics and Catalytic Activity. Orient. J. Chem. 2016, 32, 441–453. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. PART I. SOLIDS. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. A 1906, 57, 385–470. [Google Scholar]
- Ali, I.; Alharbi, O.M.L.; Alothman, Z.A.; Badjah, A.Y. Kinetics, Thermodynamics, and Modeling of Amido Black Dye Photodegradation in Water Using Co/TiO2 Nanoparticles. Photochem. Photobiol. 2018, 94, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Radi, S.; Tighadouini, S.; El Massaoudi, M.; Bacquet, M.; Degoutin, S.; Revel, B.; Mabkhot, Y.N. Thermodynamics and Kinetics of Heavy Metals Adsorption on Silica Particles Chemically Modified by Conjugated β-Ketoenol Furan. J. Chem. Eng. Data 2015, 60, 2915–2925. [Google Scholar] [CrossRef]
- Ghate, E.; Ganjidoust, H.; Ayati, B. The Thermodynamics, Kinetics, and Isotherms of Sulfamethoxazole Adsorption Using Magnetic Activated Carbon Nanocomposite and Its Reusability Potential. Nanotechnol. Environ. Eng. 2021, 6, 32. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Hashem, A.; Habila, M.A. Kinetic, Equilibrium and Thermodynamic Studies of Cadmium (II) Adsorption by Modified Agricultural Wastes. Molecules 2011, 16, 10443–10456. [Google Scholar] [CrossRef]
- Habila, M.A.; ALOthman, Z.A.; Ali, R.; Ghafar, A.A.; Hassouna, M.S.E.-D. Removal of Tartrazine Dye onto Mixed-Waste Activated Carbon: Kinetic and Thermodynamic Studies. Clean Soil Air Water 2014, 42, 1824–1831. [Google Scholar] [CrossRef]
- Bouyahia, C.; Rahmani, M.; Bensemlali, M.; El Hajjaji, S.; Slaoui, M.; Bencheikh, I.; Azoulay, K.; Labjar, N. Influence of Extraction Techniques on the Adsorption Capacity of Methylene Blue on Sawdust: Optimization by Full Factorial Design. Mater. Sci. Energy Technol. 2023, 6, 114–123. [Google Scholar] [CrossRef]
- Samadi Kazemi, M.; Sobhani, A. CuMn2O4/Chitosan Micro/Nanocomposite: Green Synthesis, Methylene Blue Removal, and Study of Kinetic Adsorption, Adsorption Isotherm Experiments, Mechanism and Adsorbent Capacity. Arab. J. Chem. 2023, 16, 104754. [Google Scholar] [CrossRef]
- Taweekarn, T.; Wongniramaikul, W.; Boonkanon, C.; Phanrit, C.; Sriprom, W.; Limsakul, W.; Towanlong, W.; Phawachalotorn, C.; Choodum, A. Starch Biocryogel for Removal of Methylene Blue by Batch Adsorption. Polymers 2022, 14, 5543. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-M.; Liu, L.-X.; Liu, L.; You, Z.-H.; Guo, H.-X.; Chen, Z.-X. Ultrasound Assisted Synthesis of Nanoscale NH2-MIL-53(Fe) for the Adsorption of Dye. Chin. J. Struct. Chem. 2021, 40, 42–46. [Google Scholar]
- Lin, L.; Zhuang, Z.-Y. A Self-growing Porous Calcium-based Adsorbent Derived from Biowaste for Efficient Wastewater Purification. Chin. J. Struct. Chem. 2021, 40, 1328–1336. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).