Nitrogen-Doped Porous Carbon Derived from Coal for High-Performance Dual-Carbon Lithium-Ion Capacitors
Abstract
:1. Introduction
2. Materials and Methods
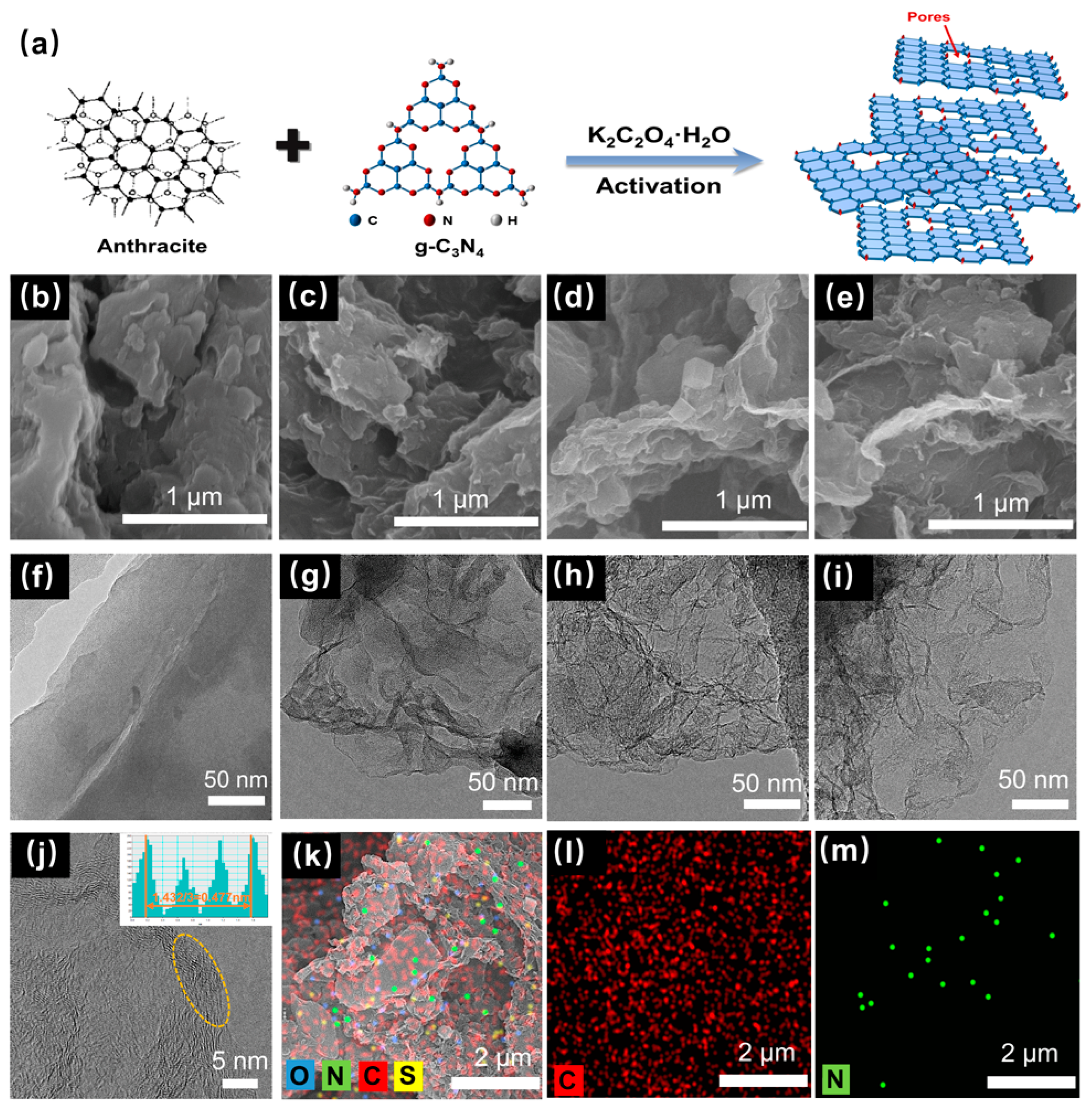
2.1. Synthesis of Coal-Based Porous Carbon Materials
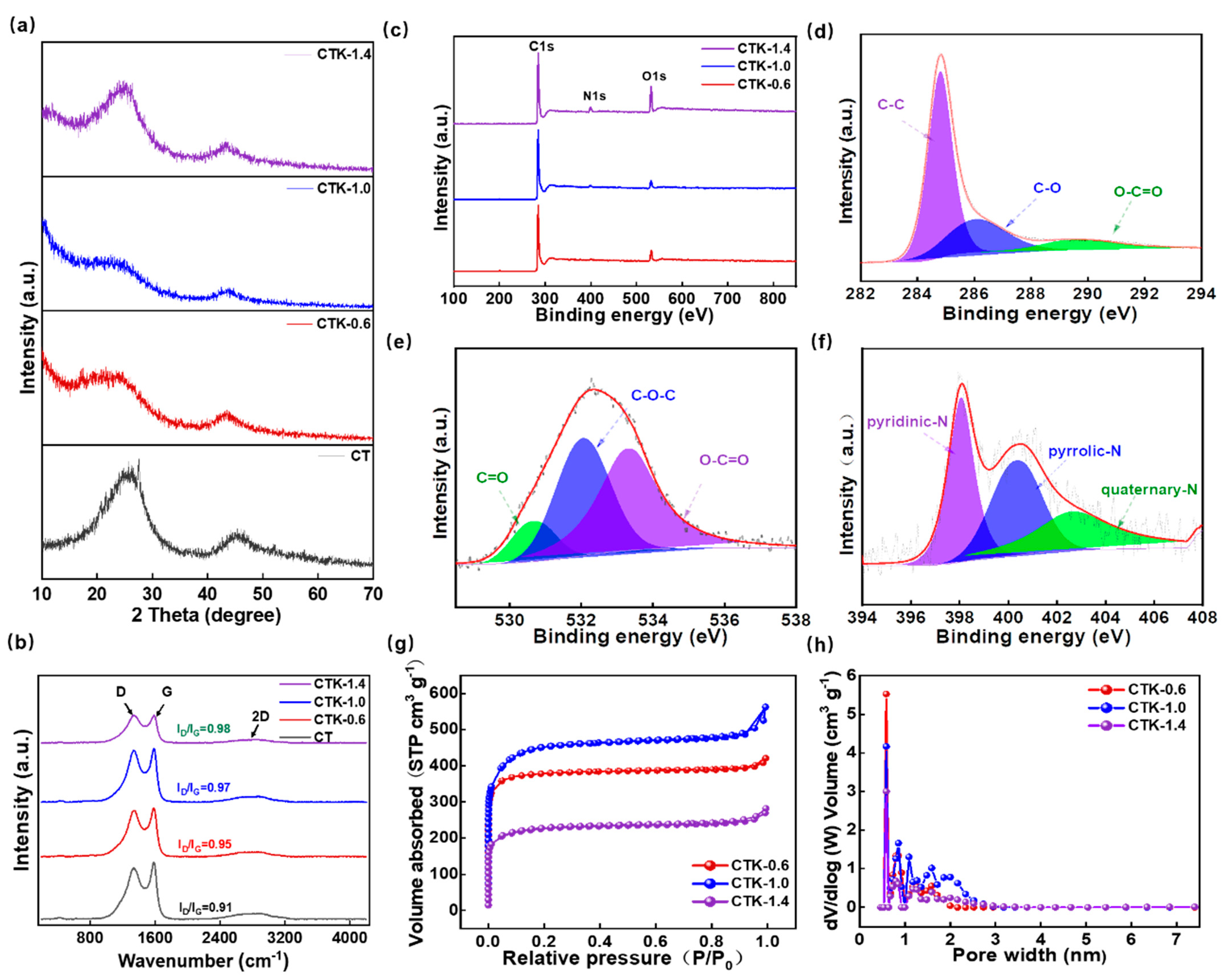
2.2. Material Characterization
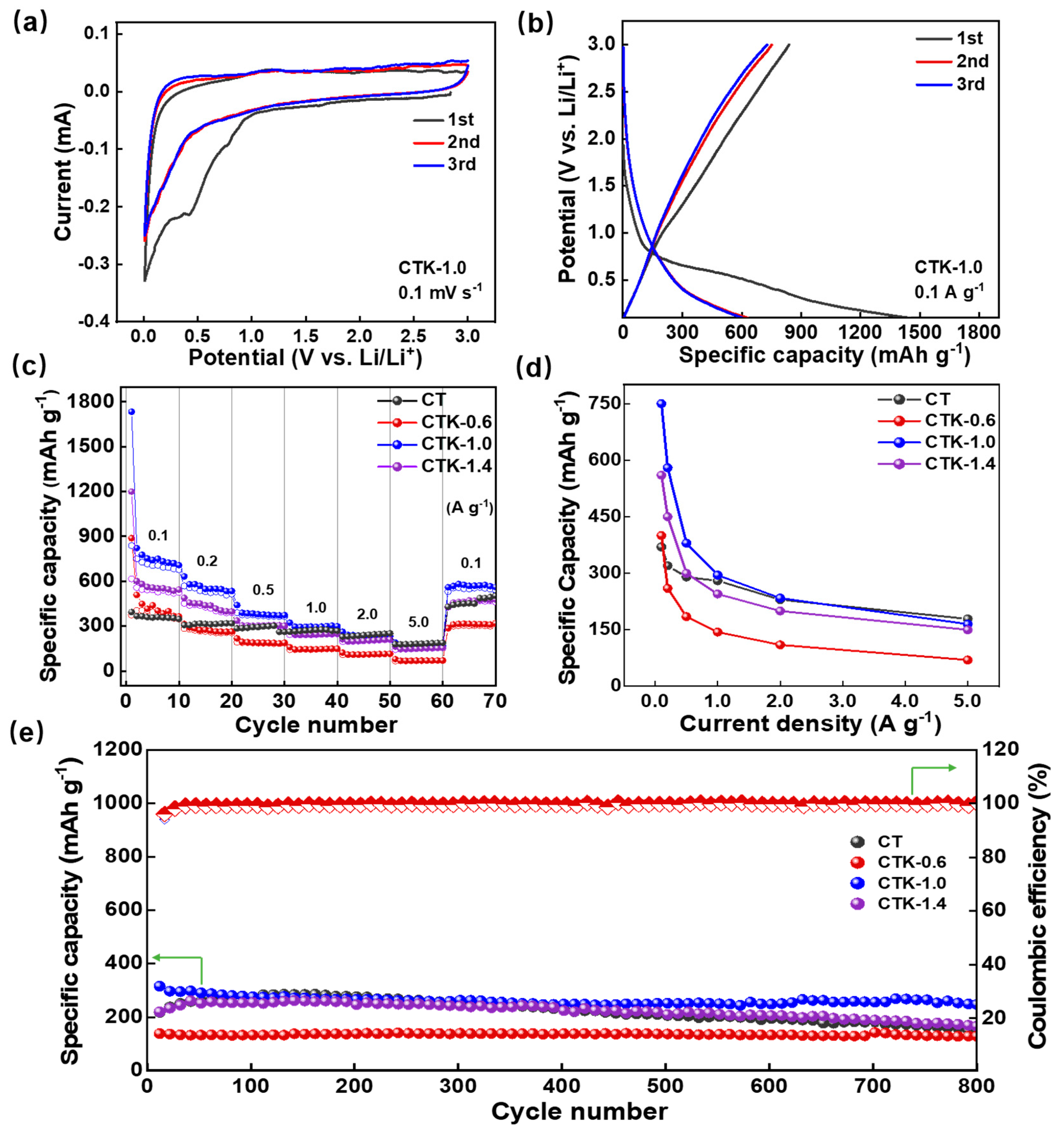
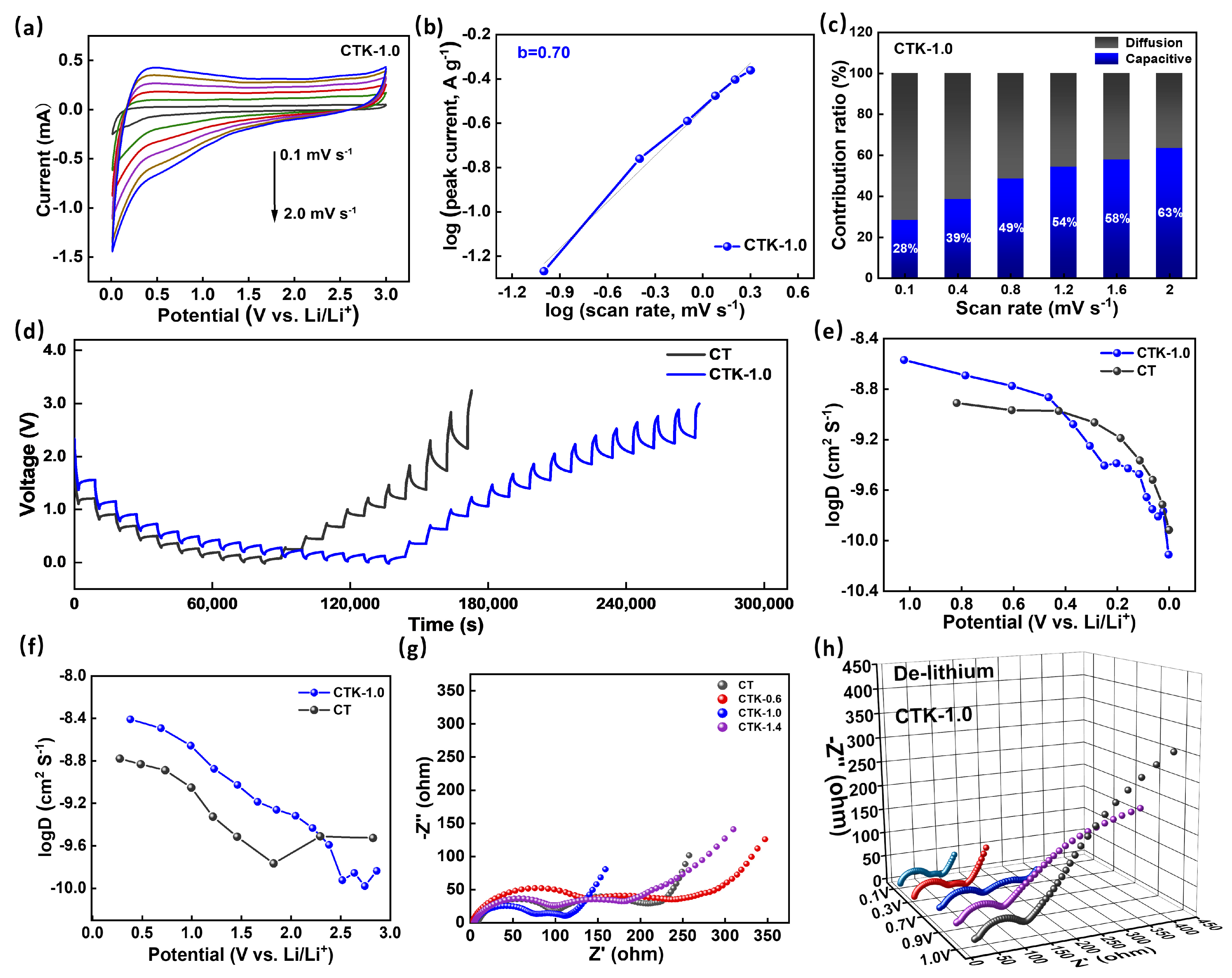
2.3. Electrochemical Measurement
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, P.; Xiong, L.; Li, X.; Guo, Y.; Xie, Y.; Du, X. Research and Application Progress of Conductive Films in Energy Storage Devices. Adv. Mater. Technol. 2023, 8, 2300194. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Li, C.; Zhao, H.; Zhang, J.; Qian, Z.; Yin, L.; Wang, R. Nitrogen and phosphorous co-doped hierarchical meso-microporous carbon nanospheres with extraordinary lithium storage for high-performance lithium-ion capacitors. Sci. China Mater. 2022, 65, 2363–2372. [Google Scholar] [CrossRef]
- Zheng, W.; Li, Z.; Han, G.; Zhao, Q.; Lu, G.; Hu, X.; Sun, J.; Wang, R.; Xu, C. Nitrogen-doped activated porous carbon for 4.5 V lithium-ion capacitor with high energy and power density. J. Energy Storage 2022, 47, 103675. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Liu, C.; Xiong, S.; Feng, J.; Qian, Y. One-Step, Vacuum-Assisted Construction of Micrometer-Sized Nanoporous Silicon Confined by Uniform Two-Dimensional N-Doped Carbon toward Advanced Li Ion and MXene-Based Li Metal Batteries. ACS Nano 2022, 16, 4560–4577. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Xu, Y.; Wang, L.; Li, Z.; Li, C.; Wang, K.; Sun, X.; An, Y.; Wu, Z.; et al. 2D Graphene/MnO Heterostructure with Strongly Stable Interface Enabling High-Performance Flexible Solid-State Lithium-Ion Capacitors. Adv. Funct. Mater. 2022, 32, 2202342. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.; Lu, X.; Choi, D.; Lemmon, J.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Yi, T.; Sari, H.; Li, X.; Wang, F.; Zhu, Y.; Hu, J.; Zhang, J.; Li, X. A review of niobium oxides based nanocomposites for lithium-ion batteries, sodium-ion batteries and supercapacitors. Nano Energy 2021, 85, 105955. [Google Scholar] [CrossRef]
- Yuan, T.; Luo, S.; Soule, L.; Wang, J.; Wang, Y.; Sun, D.; Zhao, B.; Li, W.; Yang, J.; Zheng, S.; et al. A hierarchical Ti2Nb10O29 composite electrode for high-power lithium-ion batteries and capacitors. Mater. Today 2021, 45, 8–19. [Google Scholar] [CrossRef]
- Jagadale, A.; Zhou, X.; Xiong, R.; Dubal, D.; Xu, J.; Yang, S. Lithium ion capacitors (LICs): Development of the materials. Energy Storage Mater. 2019, 19, 314–329. [Google Scholar] [CrossRef]
- Divya, M.; Lee, Y.; Aravindan, V. Carbothermally Synthesized MoO2 as an Insertion Host for High-Performance Li-ion Capacitors. Phys. Rev. Appl. 2023, 19, 034016. [Google Scholar] [CrossRef]
- Bhattacharjee, U.; Bhar, M.; Bhowmik, S.; Martha, S. Upcycling of spent lithium-ion battery graphite anodes for a dual carbon lithium-ion capacitor. Sustain. Energy Fuels 2023, 7, 2104–2116. [Google Scholar] [CrossRef]
- Xu, X.; Cui, Y.; Shi, J.; Liu, W.; Chen, S.; Wang, X.; Wang, H. Sorghum core-derived carbon sheets as electrodes for a lithium-ion capacitor. RSC Adv. 2017, 7, 17178–17183. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, D.; Zhang, G.; Xu, W.; Wang, A.; Sun, K. The effects of doped phosphorus on the electrochemical performance of hard carbon anode for lithium ion capacitors. J. Energy Storage 2023, 57, 106172. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, J.; Shang, Y.; Yang, B.; Han, D.; Du, G.; Su, Q.; Ding, S.; Xu, B.; Cao, A. 3D carbon nanotube-mesoporous carbon sponge with short pore channels for high-power lithium-ion capacitor cathodes. Carbon 2023, 203, 479–489. [Google Scholar] [CrossRef]
- Hu, S.; Ge, Z.; Tan, J.; Lai, H.; Feng, T.; Zhang, S.; Xu, Z.; Zhou, H.; Cao, X.; Zhu, G.; et al. Dual-functionally modified N/S doped hierarchical porous carbon and glycerol-engineered polyacrylonitrile carbon nanofibers combine for high-performance lithium-ion capacitors. J. Power Sources 2023, 558, 232624. [Google Scholar] [CrossRef]
- Li, X.; Sun, M.; Xu, C.; Zhang, X.; Wang, G.; Tian, Y.; Zhu, G. Fast Kinetic Carbon Anode Inherited and Developed from Architectural Designed Porous Aromatic Framework for Flexible Lithium Ion Micro Capacitors. Adv. Funct. Mater. 2023, 33, 2300460. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, J.; Yang, B.; Gao, Z.; Han, D.; Su, Q.; Xu, B.; Du, G. N-Doped Carbon Knotting Carbon Nanotube Sponge Networks for Lithium-Ion Capacitor Anodes. ACS Appl. Nano Mater. 2023, 6, 9306–9314. [Google Scholar] [CrossRef]
- Wang, J.; Meng, Q.; Zhou, X.; Li, X.; Yang, J.; Tang, J.; Zhang, Y. Bamboo-derived flake-layer hierarchically porous carbon coupling nano-Si@porous carbon for advanced high-performance Li-ion capacitor. J. Energy Storage 2023, 63, 107044. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, K.; Liu, Y.; Wu, D.; Zhu, H.; Sun, Y.; Wang, A.; Jiang, J. Double reaction initiated self-assembly process fabricated hard carbon with high power capability for lithium ion capacitor anodes. Appl. Surf. Sci. 2023, 609, 155083. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; Tang, J.; Yang, J.; Luo, C.; Cao, P.; Fan, S.; Ma, Y. High-performance lithium-ion capacitor based on N, S co-doped porous foam carbon derived from bamboo. J. Energy Storage 2022, 49, 104104. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Z.; Chen, W.; Sun, X.; Zhang, X.; Wang, K.; Ma, Y. Battery-Type Lithium-Ion Hybrid Capacitors: Current Status and Future Perspectives. Batteries 2023, 9, 74. [Google Scholar] [CrossRef]
- Yuan, S.; Lai, Q.; Duan, X.; Wang, Q. Carbon-based materials as anode materials for lithium-ion batteries and lithium-ion capacitors: A review. J. Energy Storage 2023, 61, 106716. [Google Scholar] [CrossRef]
- Zeiger, M.; Jäckel, N.; Mochalin, V.; Presser, V. Carbon onions for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 3172–3196. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, F.; Jin, Z.; Qiao, X.; Huang, H.; Chu, X.; Xiong, D.; Zhang, H.; Liu, Y.; Yang, W. Hierarchically divacancy defect building dual-activated porous carbon fibers for high-performance energy-storage devices. Adv. Funct. Mater. 2020, 30, 2002580. [Google Scholar] [CrossRef]
- Banerjee, A.; Upadhyay, K.; Puthusseri, D.; Aravindan, V.; Madhavi, S.; Ogale, S. MOF-derived crumpled-sheet-assembled perforated carbon cuboids as highly effective cathode active materials for ultra-high energy density Li-ion hybrid electrochemical capacitors (Li-HECs). Nanoscale 2014, 6, 4387–4394. [Google Scholar] [CrossRef]
- Lee, J.; Shin, W.; Lim, S.; Kim, B.; Choi, J. Modified graphite and graphene electrodes for high-performance lithium ion hybrid capacitors. Mater. Renew. Sustain. Energy 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, C.; Wang, K.; Chen, J. Recent advances in porous carbons for electrochemical energy storage. New Carbon Mater. 2023, 38, 1518–1529. [Google Scholar] [CrossRef]
- Tan, Z.; Wei, J.; Liu, Y.; Zaman, F.; Rehman, W.; Hou, L.; Yuan, C. V2CTx MXene and its derivatives: Synthesis and recent progress in electrochemical energy storage applications. Rare Met. 2022, 41, 775–797. [Google Scholar] [CrossRef]
- Sun, X.; Geng, L.; Yi, S.; Li, C.; An, Y.; Zhang, X.; Zhang, X.; Wang, K.; Ma, Y. Effects of carbon black on the electrochemical performances of SiOx anode for lithium-ion capacitors. J. Power Sources 2021, 499, 229936. [Google Scholar] [CrossRef]
- Akintola, T.; Shellikeri, A.; Akintola, T.; Zheng, J. The influence of Li4Ti5O12 preparation method on lithium-ion capacitor performance. Batteries 2021, 7, 33. [Google Scholar] [CrossRef]
- Herou, S.; Ribadeneyra, M.; Schlee, P.; Luo, H.; Tanase, L.; Rossberg, C.; Titirici, M. The impact of having an oxygen-rich microporous surface in carbon electrodes for high-power aqueous supercapacitors. J. Energy Chem. 2021, 53, 36–48. [Google Scholar] [CrossRef]
- Jiang, J.; Yuan, J.; Nie, P.; Zhu, Q.; Chen, C.; He, W.; Zhang, T.; Dou, H.; Zhang, X. Hierarchical N-doped hollow carbon microspheres as advanced materials for high-performance lithium-ion capacitors. J. Mater. Chem. A 2020, 8, 3956–3966. [Google Scholar] [CrossRef]
- Duan, Y.; Li, C.; Ye, Z.; Li, H.; Yang, Y.; Sui, D.; Lu, Y. Advances of Carbon Materials for Dual-Carbon Lithium-Ion Capacitors: A Review. Nanomaterials 2022, 12, 3954. [Google Scholar] [CrossRef]
- Zhang, S. Dual-Carbon Lithium-Ion Capacitors: Principle, Materials, and Technologies. Batter. Supercaps 2020, 3, 1137–1146. [Google Scholar] [CrossRef]
- Phattharasupakun, N.; Wutthiprom, J.; Suktha, P.; Ma, N.; Sawangphruk, M. Enhancing the Charge Storage Capacity of Lithium-Ion Capacitors Using Nitrogen-Doped Reduced Graphene Oxide Aerogel as a Negative Electrode: A Hydrodynamic Rotating Disk Electrode Investigation. J. Electrochem. Soc. 2018, 165, A609–A617. [Google Scholar] [CrossRef]
- Sun, F.; Liu, X.; Wu, H.; Wang, L.; Gao, J.; Li, H.; Lu, Y. In Situ High-Level Nitrogen Doping into Carbon Nanospheres and Boosting of Capacitive Charge Storage in Both Anode and Cathode for a High-Energy 4.5 V Full-Carbon Lithium-Ion Capacitor. Nano Lett. 2018, 18, 3368–3376. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Yang, H.; Wang, M.; Yang, M.; Guo, Q.; Wan, L.; Xia, H.; Yu, Y. High Energy and High Power Lithium-Ion Capacitors Based on Boron and Nitrogen Dual-Doped 3D Carbon Nanofibers as Both Cathode and Anode. Adv. Energy Mater. 2017, 7, 1701336. [Google Scholar] [CrossRef]
- Komatsu, T.; Nakamura, T. Polycondensation/pyrolysis of tris-s-triazine derivatives leading to graphite-like carbon nitrides. J. Mater. Chem. 2001, 11, 474–478. [Google Scholar] [CrossRef]
- Zhuo, P.; Jiang, J.; Jiang, Y.; Hao, Y.; He, Q.; Chen, T.; Ding, E.; Zhang, Y.; Han, Y.; Si, W. Biochemical Fulvic Acid Derived Amorphous Carbon Modified Microcrystalline Graphite as Low-cost Anode for Potassium-ion Storage. J. Colloid Interface Sci. 2023, 648, 108–116. [Google Scholar] [CrossRef]
- Jiang, J.; Nie, P.; Ding, B.; Zhang, Y.; Xu, G.; Wu, L.; Dou, H.; Zhang, X. Highly stable lithium ion capacitor enabled by hierarchical polyimide derived carbon microspheres combined with 3D current collectors. J. Mater. Chem. A 2017, 5, 23283–23291. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Zhang, L.; Xie, F.; Vasileff, A.; Qiao, S. Graphitic Carbon Nitride (g-C3N4)-Derived N-Rich Graphene with Tuneable Interlayer Distance as a High-Rate Anode for Sodium-Ion Batteries. Adv. Mater. 2019, 31, e1901261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lei, Y.; Jiang, Q.; Ming, F.; Costa, P.; Aishareef, H. 3D Laser Scribed Graphene Derived from Carbon Nanospheres: An Ultrahigh-Power Electrode for Supercapacitors. Small Methods 2019, 3, 1900005. [Google Scholar] [CrossRef]
- Li, Z.; Cao, L.; Chen, W.; Huang, Z.; Liu, H. Mesh-Like Carbon Nanosheets with High-Level Nitrogen Doping for High-Energy Dual-Carbon Lithium-Ion Capacitors. Small 2019, 15, 1805173. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, K.; Xu, Y.; Zhang, X.; Peng, Q.; Li, S.; Zhang, X.; Sun, X.; Ma, Y. Dehalogenation produces graphene wrapped carbon cages as fast-kinetics and large-capacity anode for lithium-ion capacitors. Carbon 2023, 202, 175–185. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, X.; Huang, Y.; Li, L.; Gao, S.; Tian, Y.; Shen, W.; Zhang, J.; Guo, S. Anthracite-derived carbon-based electrode materials for high performance lithium ion capacitors. Fuel Process. Technol. 2022, 228, 107146. [Google Scholar] [CrossRef]
- Politzer, P.; Lane, P.; Murray, J.; Concha, M. Comparative analysis of surface electrostatic potentials of carbon, boron/nitrogen and carbon/boron/nitrogen model nanotubes. J. Mol. Model. 2005, 11, 1–7. [Google Scholar] [CrossRef]
- Chen, W.; Joly, A.; Morgan, N.; Balandin, A.; Wang, K. Handbook of Semiconductor Nanostructures and Devices; American Scientific Publishers: Los Angeles, CA, USA, 2006; Volume 2, Chapter 7; pp. 295–334. [Google Scholar]
- Jiang, J.; Zhang, Y.; An, Y.; Wu, L.; Zhu, Q.; Dou, H.; Zhang, X. Engineering ultrathin MoS2 nanosheets anchored on N-doped carbon microspheres with pseudocapacitive properties for high-performance lithium-ion capacitors. Small Methods 2019, 3, 1900081. [Google Scholar] [CrossRef]
- Deng, H.; Wang, L.; Li, S.; Zhang, M.; Wang, T.; Zhou, J.; Chen, M.; Chen, S.; Cao, J.; Zhang, Q. Radial pores in nitrogen/oxygen dual-doped carbon nanospheres anode boost high-power and ultrastable potassium-ion batteries. Adv. Funct. Mater. 2021, 31, 2107246. [Google Scholar] [CrossRef]
- Liu, C.; Khosrozadeh, A.; Ren, Q.; Yan, L.; Goh, K.; Li, S.; Liu, J.; Zhao, L.; Gu, D.; Wang, Z. Intercalation-pseudocapacitance hybrid anode for high rate and energy lithium-ion capacitors. J. Energy Chem. 2021, 55, 459–467. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Tian, X.; Liu, Z. Coal-Based Semicoke-Derived Carbon Anode Materials with Tunable Microcrystalline Structure for Fast Lithium-Ion Storage. Nanomaterials 2022, 12, 4067. [Google Scholar] [CrossRef]
- Liu, F.; Lu, P.; Zhang, Y.; Su, F.; Zhang, L.; Zheng, S.; Zhang, X.; Su, F.; Ma, Y.; Wu, Z. Sustainable Lignin-Derived Carbon as Capacity-Kinetics Matched Cathode and Anode towards 4.5 V High-Performance Lithium-Ion Capacitors. Energy Environ. Mater. 2023, 6, e12550. [Google Scholar] [CrossRef]
- Mao, M.; Chen, Q.; Zhang, H.; Hou, Y.; Guo, J. Superior cycling performance of NiC2O4/rGO/NF composites for hybrid supercapacitors. J. Energy Storage 2023, 68, 107702. [Google Scholar] [CrossRef]
- Lei, D.; Hou, Z.; Li, N.; Cao, Y.; Ren, L.; Liu, H.; Zhang, Y.; Wang, J. A homologous N/P-codoped carbon strategy to streamline nanostructured MnO/C and carbon toward boosted lithium-ion capacitors. Carbon 2023, 201, 260–268. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, K.; Zhang, W.; Zhang, X.; Wang, L.; An, Y.; Sun, X.; Li, C.; Wang, K.; Ma, Y. Carbon Nano-Onion-Encapsulated Ni Nanoparticles for High-Performance Lithium-Ion Capacitors. Batteries 2023, 9, 102. [Google Scholar] [CrossRef]
- Natarajan, S.; Akshay, M.; Aravindan, V. MnCO3 Cuboids from Spent LIBs: A New Age Displacement Anode to Build High-Performance Li-Ion Capacitors. Small 2023, 19, 2206226. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, N.; Li, J.; Liu, P.; Zhao, X.; Lin, Y.; Sun, C.; Qin, W. Sb Nanoparticles Embedded in the N-Doped Carbon Fibers as Binder-Free Anode for Flexible Li-Ion Batteries. Nanomaterials 2022, 12, 3093. [Google Scholar] [CrossRef]
- Sui, D.; Chang, M.; Peng, Z.; Li, C.; He, X.; Yang, Y.; Liu, Y.; Lu, Y. Graphene-Based Cathode Materials for Lithium-Ion Capacitors: A Review. Nanomaterials 2021, 11, 2771. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Z.; Zhang, Z.; Wang, S.; Xu, H.; Zheng, X.; Chen, Y.; Ju, Z.; Dou, H.; Zhang, X. Recent advances and perspectives on prelithiation strategies for lithium-ion capacitors. Rare Met. 2022, 41, 3322–3335. [Google Scholar] [CrossRef]
- Ye, J.; Simon, P.; Zhu, Y. Designing ionic channels in novel carbons for electrochemical energy storage. Natl. Sci. Rev. 2020, 7, 191–201. [Google Scholar] [CrossRef]
- Yu, P.; Cao, G.; Yi, S.; Zhang, X.; Li, C.; Sun, X.; Wang, K.; Ma, Y. Binder-free 2D titanium carbide (MXene)/carbon nanotube composites for high-performance lithium-ion capacitors. Nanoscale 2018, 10, 5906–5913. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Li, X.; Wang, Z.; Wang, J.; Wang, Y.; Yan, Z.; Zhang, D. Graphitic carbon balanced between high plateau capacity and high rate capability for lithium ion capacitors. J. Mater. Chem. A 2017, 5, 15302–15309. [Google Scholar] [CrossRef]
- Wang, G.; Lu, C.; Zhang, X.; Wan, B.; Liu, H.; Xia, M.; Gou, H.; Xin, G.; Lian, J.; Zhang, Y. Toward ultrafast lithium ion capacitors: A novel atomic layer deposition seeded preparation of Li4Ti5O12/graphene anode. Nano Energy 2017, 36, 46–57. [Google Scholar] [CrossRef]
- Lim, E.; Jo, C.; Kim, H.; Kim, M.; Mun, Y.; Chun, J.; Ye, Y.; Hwang, J.; Ha, K.; Roh, K. Facile synthesis of Nb2O5@ carbon core-shell nanocrystals with controlled crystalline structure for high-power anodes in hybrid supercapacitors. ACS Nano 2015, 9, 7497–7505. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, Y.; Yin, Z.; Guo, H.; Liu, Y.; Cheng, H.; Wud, Y.; Ji, X.; Wang, J. Defective synergy of 2D graphitic carbon nanosheets promotes lithium-ion capacitors performance. Energy Storage Mater. 2020, 24, 304–311. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Kong, S.; Zhu, K.; Yan, J.; Ye, K.; Wang, G.; Cheng, K.; Zhou, L.; Cao, D. A novel calendula-like MnNb2O6 anchored on graphene sheet as high-performance intercalation pseudocapacitive anode for lithium-ion capacitors. J. Mater. Chem. A 2019, 7, 2855–2863. [Google Scholar] [CrossRef]
- Jin, L.; Gong, R.; Zhang, W.; Xiang, Y.; Zheng, J.; Xiang, Z.; Zhang, C.; Xia, Y.; Zheng, J. Toward high energy-density and long cycling-lifespan lithium ion capacitors: A 3D carbon modified low-potential Li2TiSiO5 anode coupled with a lignin-derived activated carbon cathode. J. Mater. Chem. A 2019, 7, 8234–8244. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Ang, H.; Zhang, Y.; Tan, H.; Zhang, Y.; Guo, Y.; Franklin, J.; Wu, X.; Srinivasan, M.; et al. A High-Energy Lithium-Ion Capacitor by Integration of a 3D Interconnected Titanium Carbide Nanoparticle Chain Anode with a Pyridine-Derived Porous Nitrogen-Doped Carbon Cathode. Adv. Funct. Mater. 2016, 26, 3082–3093. [Google Scholar] [CrossRef]
- Li, G.; Yin, Z.; Guo, H.; Wang, Z.; Yan, G.; Yang, Z.; Liu, Y.; Ji, X.; Wang, J. Metalorganic Quantum Dots and Their Graphene-Like Derivative Porous Graphitic Carbon for Advanced Lithium-Ion Hybrid Supercapacitor. Adv. Energy Mater. 2019, 9, 1802878. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Shen, Q.; Chen, Z.; Wang, S. Nitrogen-Doped Porous Carbon Derived from Coal for High-Performance Dual-Carbon Lithium-Ion Capacitors. Nanomaterials 2023, 13, 2525. https://doi.org/10.3390/nano13182525
Jiang J, Shen Q, Chen Z, Wang S. Nitrogen-Doped Porous Carbon Derived from Coal for High-Performance Dual-Carbon Lithium-Ion Capacitors. Nanomaterials. 2023; 13(18):2525. https://doi.org/10.3390/nano13182525
Chicago/Turabian StyleJiang, Jiangmin, Qianqian Shen, Ziyu Chen, and Shijing Wang. 2023. "Nitrogen-Doped Porous Carbon Derived from Coal for High-Performance Dual-Carbon Lithium-Ion Capacitors" Nanomaterials 13, no. 18: 2525. https://doi.org/10.3390/nano13182525
APA StyleJiang, J., Shen, Q., Chen, Z., & Wang, S. (2023). Nitrogen-Doped Porous Carbon Derived from Coal for High-Performance Dual-Carbon Lithium-Ion Capacitors. Nanomaterials, 13(18), 2525. https://doi.org/10.3390/nano13182525





